Laser Powder Bed Fusion of Precipitation-Hardened Martensitic Stainless Steels: A Review
Abstract
:1. Introduction
2. Microstructure
2.1. Microstructure in as L-PBFed State
- (1)
- Initial Atomizing Media of Powder and Building Chamber Atmosphere
- (2)
- Energy density
- (3)
- Building orientation
- (4)
- Laser scanning pattern
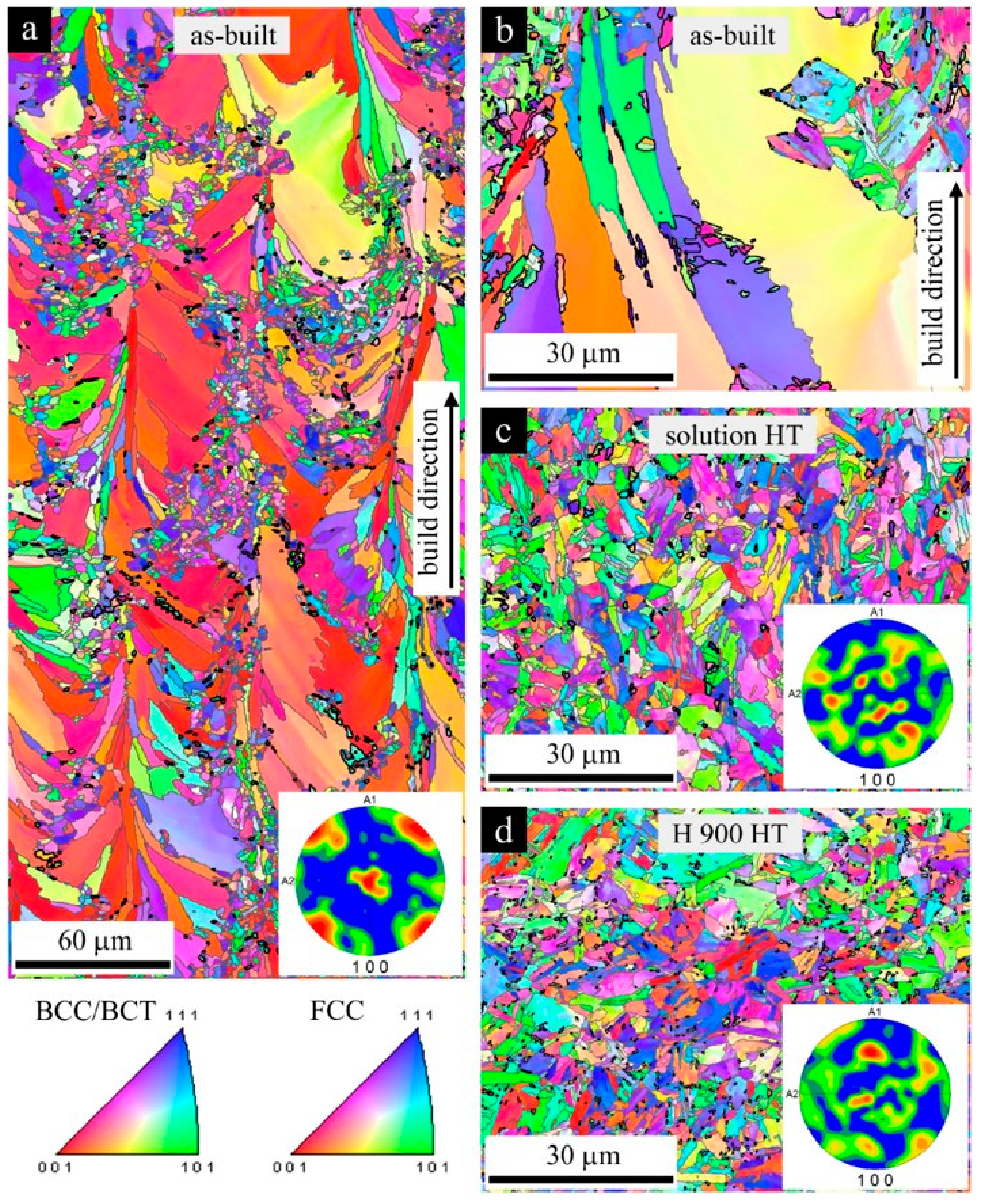
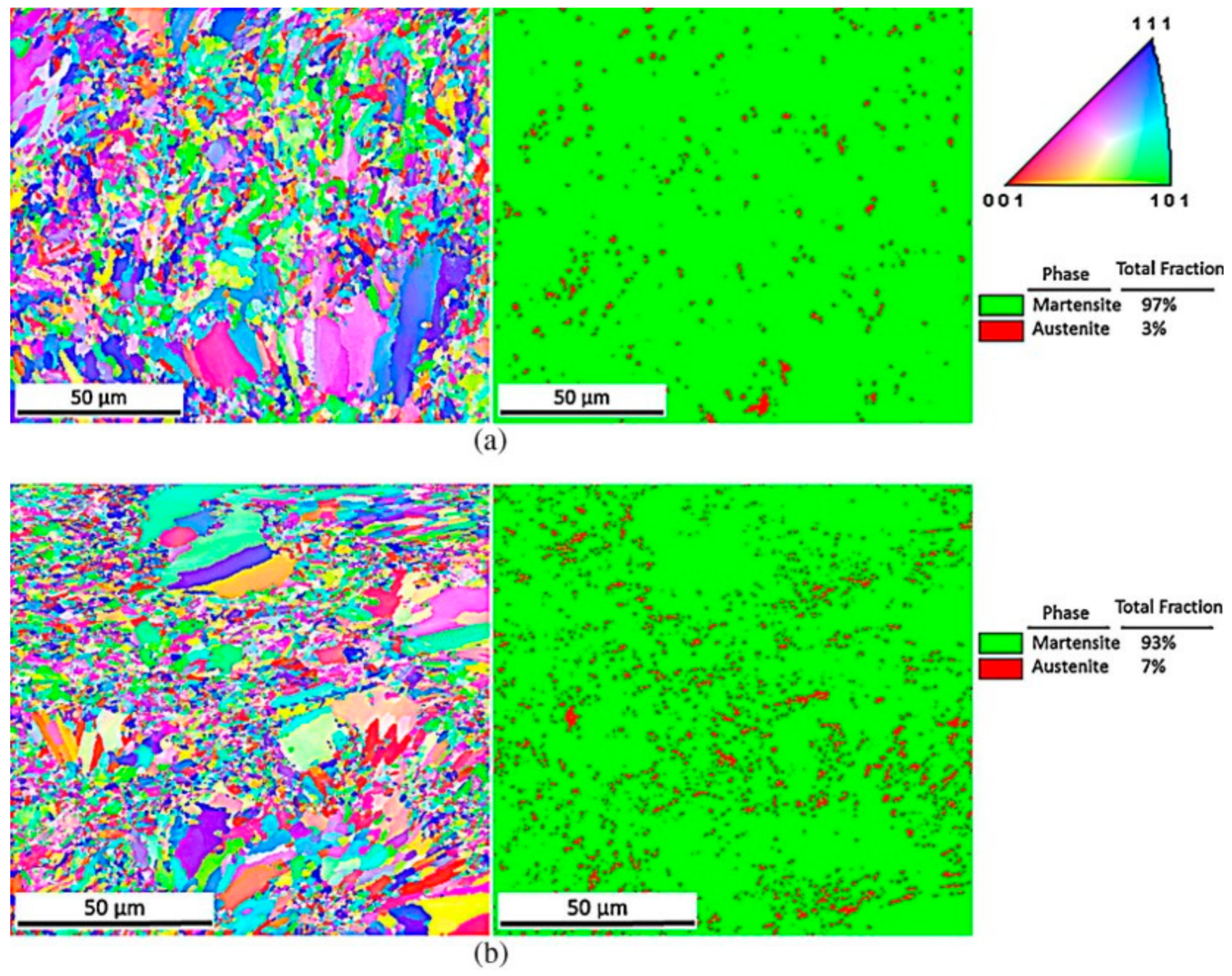
2.2. Microstructure after Heat Treatments
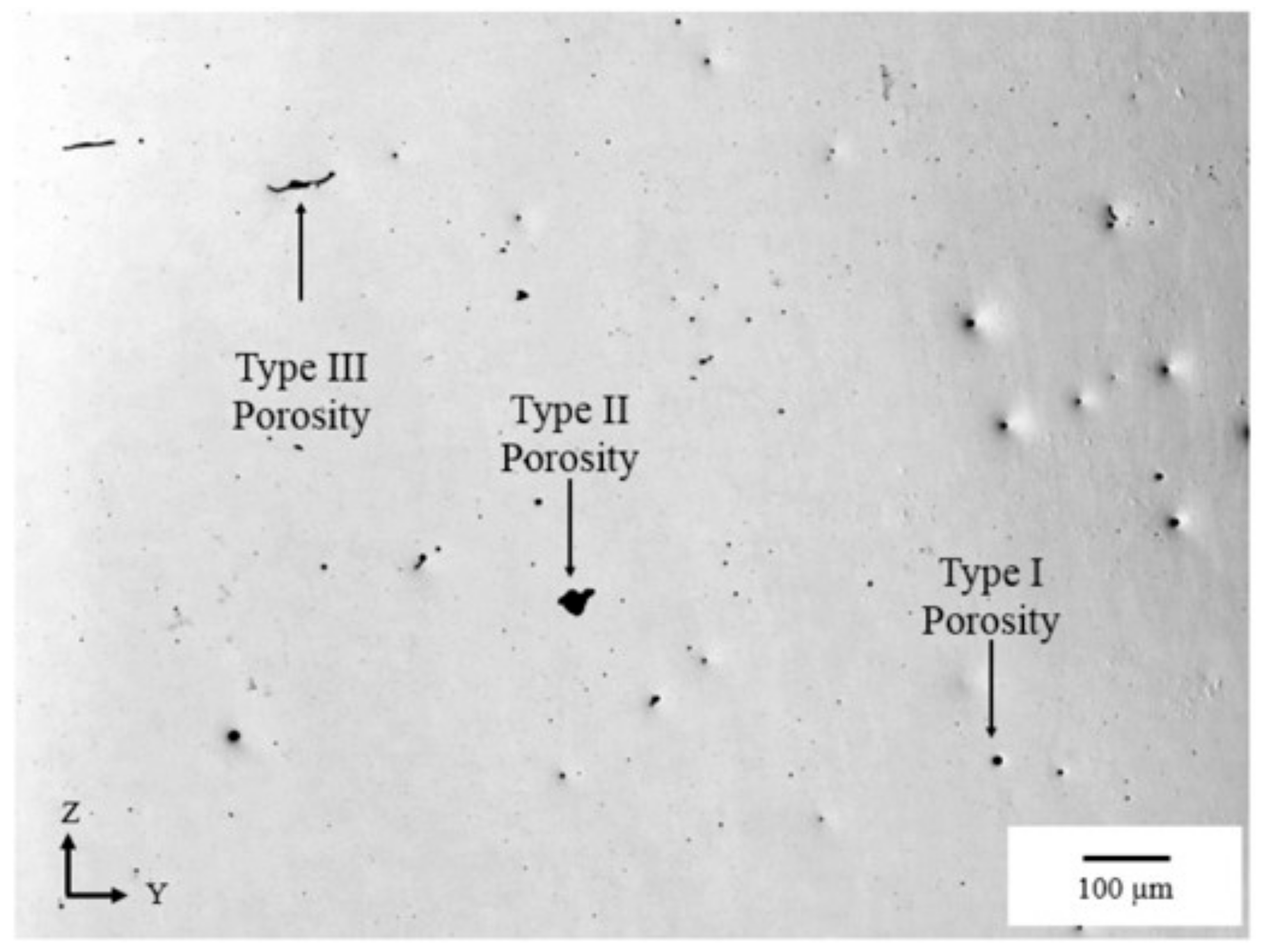
3. Defects
4. Mechanical Properties
4.1. Hardness Distribution
4.1.1. Effect of Process Parameters on Hardness
4.1.2. Effect of Heat Treatment on Hardness
4.2. Tensile Properties
4.3. Fatigue Properties
5. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Bahrami Balajaddeh, M.; Naffakh-Moosavy, H. Pulsed Nd:YAG laser welding of 17–4 PH stainless steel: Microstructure, mechanical properties, and weldability investigation. Opt. Laser Technol. 2019, 119, 105651. [Google Scholar] [CrossRef]
- Wang, D.; Chi, C.T.; Wang, W.Q.; Li, Y.L.; Wang, M.S.; Chen, X.G.; Chen, Z.H.; Cheng, X.P.; Xie, Y.J. The effects of fabrication atmosphere condition on the microstructural and mechanical properties of laser direct manufactured stainless steel 17–4 PH. J Mater. Sci. Technol. 2019, 35, 1315–1322. [Google Scholar] [CrossRef]
- Jan Kazior, A.S.-N.; Tadeusz, P.; Marek, H.; Marek, N. Properties of Precipitation Hardening 17–4 PH Stainless Steel Manufactured by Powder Metallurgy Technology. Adv. Mater. Res. 2013, 811, 87–92. [Google Scholar] [CrossRef]
- Wang, J.; Zou, H.; Li, C.; Qiu, S.; Shen, B. The spinodal decomposition in 17–4PH stainless steel subjected to long-term aging at 350 °C. Mater. Charact. 2008, 59, 587–591. [Google Scholar] [CrossRef]
- Murayama, M.; Hono, K.; Katayama, Y. Microstructural evolution in a 17–4 PH stainless steel after aging at 400 °C. Metall. Mater. Trans. A 1999, 30, 345–353. [Google Scholar] [CrossRef]
- Hsiao, C.-N.; Chiou, C.S.; Yang, J.-R. Aging reactions in a 17–4 PH stainless steel. Mater. Chem. Phys. 2002, 74, 134–142. [Google Scholar] [CrossRef]
- Viswanathan, U.K. Effects of Aging on the Microstructure of 17–4 PH Stainless Steel. Mater. Sci. Eng. A 1988, 104, 181–189. [Google Scholar] [CrossRef]
- Bayode, A.; Pityana, S.; Akinlabi, E.T.; Shongwe, M.B. Effect of scanning speed on laser deposited 17–4PH stainless steel. In Proceedings of the 2017 8th International Conference on Mechanical and Intelligent Manufacturing Technologies (ICMIMT), Cape Town, South Africa, 3–6 February 2017; IEEE: Piscataway, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Deng, D.; Chen, R.; Sun, Q.; Li, X. Microstructural Study of 17–4PH Stainless Steel after Plasma-Transferred Arc Welding. Materials 2015, 8, 424–434. [Google Scholar] [CrossRef] [Green Version]
- Bhambroo, R.; Roychowdhury, S.; Kain, V.; Raja, V.S. Effect of reverted austenite on mechanical properties of precipitation hardenable 17–4 stainlesssteel. Mater. Sci. Eng. A 2013, 568, 127–133. [Google Scholar] [CrossRef]
- Chien, W.-T.; Tsai, C.-S. The investigation on the prediction of tool wear and the determination of optimum cutting conditions in machining 17–4PH stainless steel. J. Mater. Process. Technol. 2003, 140, 340–345. [Google Scholar] [CrossRef]
- Raj, S.V.; Ghosn, L.J.; Lerch, B.A.; Hebsur, M.; Cosgriff, L.M.; Fedor, J. Mechanical properties of 17–4PH stainless steel foam panels. Mater. Sci. Eng. A 2007, 456, 305–316. [Google Scholar] [CrossRef] [Green Version]
- Arisoy, C.F.; Başman, G.; Şeşen, M.K. Failure of a 17–4 PH stainless steel sailboat propeller shaft. Eng. Fail. Anal. 2003, 10, 711–717. [Google Scholar] [CrossRef]
- Bhaduri, A.K.; Gill, T.P.S.; Srinivasan, G.; Sujith, S. Optimised post-weld heat treatment procedures and heat input for welding 17–4PH stainless steel. Sci. Technology Weld. Join. 2013, 4, 295–301. [Google Scholar] [CrossRef]
- Lin, X.; Cao, Y.; Wu, X.; Yang, H.; Chen, J.; Huang, W. Microstructure and mechanical properties of laser forming repaired 17–4PH stainless steel. Mater. Sci. Eng. A 2012, 553, 80–88. [Google Scholar] [CrossRef]
- Jui-hung Wu, C.-k.L. Tensile and fatigue properties of 17–4 PH stainless steel at high temperatures. Metall. Mater. Trans. A 2002, 33, 1715–1724. [Google Scholar]
- Akita, M.; Uematsu, Y.; Kakiuchi, T.; Nakajima, M.; Kawaguchi, R. Defect-dominated fatigue behavior in type 630 stainless steel fabricated by selective laser melting. Mater. Sci. Eng. A 2016, 666, 19–26. [Google Scholar] [CrossRef]
- Pham, M.-S.; Dovgyy, B.; Hooper, P.A.; Gourlay, C.M.; Piglione, A. The role of side-branching in microstructure development in laser powder-bed fusion. Nat. Communications 2020, 11, 749. [Google Scholar] [CrossRef]
- Hou, H.; Simsek, E.; Ma, T.; Johnson, N.S.; Qian, S.; Cissé, C.; Stasak, D.; Hasan, N.A.; Zhou, L.; Hwang, Y.; et al. Fatigue-resistant high-performance elastocaloric materials made by additive manufacturing. Science 2019, 366, 1116–1121. [Google Scholar] [CrossRef] [Green Version]
- Todaro, C.J.; Easton, M.A.; Qiu, D.; Zhang, D.; Bermingham, M.J.; Lui, E.W.; Brandt, M.; StJohn, D.H.; Qian, M. Grain structure control during metal 3D printing by high-intensity ultrasound. Nat. Commun. 2020, 11, 142. [Google Scholar] [CrossRef]
- Martin, A.A.; Calta, N.P.; Khairallah, S.A.; Wang, J.; Depond, P.J.; Fong, A.Y.; Thampy, V.; Guss, G.M.; Kiss, A.M.; Stone, K.H.; et al. Dynamics of pore formation during laser powder bed fusion additive manufacturing. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.-C.; Cao, C.; Sokoluk, M.; Jiang, L.; Wang, X.; Schoenung, J.M.; Lavernia, E.J.; Li, X. Aluminum with dispersed nanoparticles by laser additive manufacturing. Nat. Commun. 2019, 10, 4124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DebRoy, T.; Mukherjee, T.; Milewski, J.O.; Elmer, J.W.; Ribic, B.; Blecher, J.J.; Zhang, W. Scientific, technological and economic issues in metal printing and their solutions. Nat. Mater. 2019, 18, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.H.; Yahata, B.D.; Hundley, J.M.; Mayer, J.A.; Schaedler, T.A.; Pollock, T.M. 3D printing of high-strength aluminium alloys. Nature 2017, 549, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Huang, S.; Zhang, G.; Qin, R.; Liu, W.; Xiong, H.; Shi, G.; Blackburn, J. Progress in additive manufacturing on new materials: A review. J Mater. Sci. Technol. 2019, 35, 242–269. [Google Scholar] [CrossRef]
- Yadollahi, A.; Shamsaei, N.; Thompson, S.M.; Elwany, A.; Bian, L. Effects of building orientation and heat treatment on fatigue behavior of selective laser melted 17–4 PH stainless steel. Int. J. Fatigue 2017, 94, 218–235. [Google Scholar] [CrossRef]
- LeBrun, T.; Nakamoto, T.; Horikawa, K.; Kobayashi, H. Effect of retained austenite on subsequent thermal processing and resultant mechanical properties of selective laser melted 17–4 PH stainless steel. Mater. Des. 2015, 81, 44–53. [Google Scholar] [CrossRef]
- Caballero, A.; Ding, J.; Ganguly, S.; Williams, S. Wire + Arc Additive Manufacture of 17–4 PH stainless steel: Effect of different processing conditions on microstructure, hardness, and tensile strength. J. Mater. Process. Technol. 2019, 268, 54–62. [Google Scholar] [CrossRef]
- Williams, S.W.; Martina, F.; Addison, A.C.; Ding, J.; Pardal, G.; Colegrove, P. Wire + Arc Additive Manufacturing. Mater. Sci. Technol. 2016, 32, 641–647. [Google Scholar] [CrossRef] [Green Version]
- Martina, F.; Ding, J.; Williams, S.; Caballero, A.; Pardal, G.; Quintino, L. Tandem metal inert gas process for high productivity wire arc additive manufacturing in stainless steel. Addit. Manuf. 2019, 25, 545–550. [Google Scholar] [CrossRef] [Green Version]
- Jin, W.; Zhang, C.; Jin, S.; Tian, Y.; Wellmann, D.; Liu, W. Wire arc additive manufacturing of stainless steels: a review. Appl. Sci. 2020. [Google Scholar]
- Gu, D.D.; Meiners, W.; Wissenbach, K.; Poprawe, R. Laser additive manufacturing of metallic components: materials, processes and mechanisms. Int. Mater. Rev. 2013, 57, 133–164. [Google Scholar] [CrossRef]
- Frazier, W.E. Metal Additive Manufacturing: A Review. J. Mater. Eng. Perform. 2014, 23, 1917–1928. [Google Scholar] [CrossRef]
- Sames, W.J.; List, F.A.; Pannala, S.; Dehoff, R.R.; Babu, S.S. The metallurgy and processing science of metal additive manufacturing. Int. Mater. Rev. 2016, 61, 315–360. [Google Scholar] [CrossRef]
- Wood, P.; Libura, T.; Kowalewski, L.Z.; Williams, G.; Serjouei, A. Influences of Horizontal and Vertical Build Orientations and Post-Fabrication Processes on the Fatigue Behavior of Stainless Steel 316L Produced by Selective Laser Melting. Materials 2019, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strakosova, A.; Kubásek, J.; Michalcová, A.; Průša, F.; Vojtěch, D.; Dvorský, D. High Strength X3NiCoMoTi 18-9-5 Maraging Steel Prepared by Selective Laser Melting from Atomized Powder. Materials 2019, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Kashouty, F.M.; Rennie, E.W.A.; Ghazy, M. Tool Life Performance of Injection Mould Tooling Fabricated by Selective Laser Melting for High-Volume Production. Materials 2019, 12. [Google Scholar] [CrossRef] [Green Version]
- Natali, S.; Brotzu, A.; Pilone, D. Comparison between Mechanical Properties and Structures of a Rolled and a 3D-Printed Stainless Steel. Materials 2019, 12. [Google Scholar] [CrossRef] [Green Version]
- Antunes, F.; Santos, L.; Capela, C.; Ferreira, J.; Costa, J.; Jesus, J.; Prates, P. Fatigue Crack Growth in Maraging Steel Obtained by Selective Laser Melting. Appl. Sciences 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Mugwagwa, L.; Yadroitsev, I.; Matope, S. Effect of Process Parameters on Residual Stresses, Distortions, and Porosity in Selective Laser Melting of Maraging Steel 300. Metals 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Abolhasani, D.; Seyedkashi, M.H.S.; Kang, N.; Kim, J.Y.; Woo, Y.Y.; Moon, H.Y. Analysis of Melt-Pool Behaviors during Selective Laser Melting of AISI 304 Stainless-Steel Composites. Metals 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Papula, S.; Song, M.; Pateras, A.; Chen, X.-B.; Brandt, M.; Easton, M.; Yagodzinskyy, Y.; Virkkunen, I.; Hänninen, H. Selective Laser Melting of Duplex Stainless Steel 2205: Effect of Post-Processing Heat Treatment on Microstructure, Mechanical Properties, and Corrosion Resistance. Materials 2019, 12. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.-S.; Park, H.-S.; Lee, C.-M. Applying Selective Laser Melting to Join Al and Fe: An Investigation of Dissimilar Materials. Appl. Sci. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.Y.; Casati, R.; Coduri, M.; Vedani, M.; Lee, S.C. Hydrogen Embrittlement Behavior of 18Ni 300 Maraging Steel Produced by Selective Laser Melting. Materials 2019, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esarte, J.; Blanco, M.J.; Bernardini, A.; Sancibrián, R. Performance Assessment of a Three-Dimensional Printed Porous Media Produced by Selective Laser Melting Technology for the Optimization of Loop Heat Pipe Wicks. Appl. Sci. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.; Wang, P.; Liu, Y.; Han, G. Experiment of Process Strategy of Selective Laser Melting Forming Metal Nonhorizontal Overhanging Structure. Metals 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Li, J.; Bai, P.; Qu, H.; Liang, M.; Liao, H.; Wu, L.; Huo, P.; Liu, H.; Zhang, J. Microstructure and Mechanical Properties of TiC-Reinforced 316L Stainless Steel Composites Fabricated Using Selective Laser Melting. Metals 2019, 9, 267. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Zhou, Y.; Gu, R.; Zhang, X.; Quach, W.-M.; Yan, M. A Comprehensive Study of Steel Powders (316L, H13, P20 and 18Ni300) for Their Selective Laser Melting Additive Manufacturing. Metals 2019, 9, 86. [Google Scholar] [CrossRef] [Green Version]
- Kurzynowski, T.; Stopyra, W.; Gruber, K.; Ziółkowski, G.; Kuźnicka, B.; Chlebus, E. Effect of Scanning and Support Strategies on Relative Density of SLM-ed H13 Steel in Relation to Specimen Size. Materials 2019, 12, 239. [Google Scholar] [CrossRef] [Green Version]
- Siddique, S.; Awd, M.; Wiegold, T.; Klinge, S.; Walther, F. Simulation of Cyclic Deformation Behavior of Selective Laser Melted and Hybrid-Manufactured Aluminum Alloys Using the Phase-Field Method. Appl. Sci. 2018, 8, 948. [Google Scholar] [CrossRef] [Green Version]
- Santos, M.S.L.; De Jesus, J.; Ferreira, M.J.; Costa, D.J.; Capela, C. Fracture Toughness of Hybrid Components with Selective Laser Melting 18Ni300 Steel Parts. Appl. Sci. 2018, 8, 879. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Yin, G.; Feng, Z.; Liao, X. Effect of Powder Feedstock on Microstructure and Mechanical Properties of the 316L Stainless Steel Fabricated by Selective Laser Melting. Metals 2018, 8, 729. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.V.; Kim, E.-a.; Lee, S.-R.; Yun, J.; Choe, J.; Yang, D.-y.; Lee, H.-s.; Lee, C.-w.; Yu, J.-H. Evaluation of Strain-Rate Sensitivity of Selective Laser Melted H13 Tool Steel Using Nanoindentation Tests. Metals 2018, 8, 589. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Jia, X.; Gu, R.; Zhou, P.; Huang, Y.; Sun, J.; Yan, M. 316L Stainless Steel Manufactured by Selective Laser Melting and Its Biocompatibility with or without Hydroxyapatite Coating. Metals 2018, 8, 548. [Google Scholar] [CrossRef] [Green Version]
- Branco, R.; Costa, D.M.J.; Berto, F.; Razavi, M.S.; Ferreira, A.M.J.; Capela, C.; Santos, L.; Antunes, F. Low-Cycle Fatigue Behaviour of AISI 18Ni300 Maraging Steel Produced by Selective Laser Melting. Metals 2018, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Segura-Cardenas, E.; Ramirez-Cedillo, G.E.; Sandoval-Robles, A.J.; Ruiz-Huerta, L.; Caballero-Ruiz, A.; Siller, R.H. Permeability Study of Austenitic Stainless Steel Surfaces Produced by Selective Laser Melting. Metals 2017, 7, 521. [Google Scholar] [CrossRef] [Green Version]
- Hitzler, L.; Hirsch, J.; Heine, B.; Merkel, M.; Hall, W.; Öchsner, A. On the Anisotropic Mechanical Properties of Selective Laser-Melted Stainless Steel. Materials 2017, 10, 136. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, M.S.; Chen, Y.; Boardman, R.; Yang, S.; Gao, N. Investigation on Porosity and Microhardness of 316L Stainless Steel Fabricated by Selective Laser Melting. Metals 2017, 7, 64. [Google Scholar] [CrossRef] [Green Version]
- Prashanth, G.K.; Löber, L.; Klauss, H.-J.; Kühn, U.; Eckert, J. Characterization of 316L Steel Cellular Dodecahedron Structures Produced by Selective Laser Melting. Technologies 2016, 4, 34. [Google Scholar] [CrossRef] [Green Version]
- Casati, R.; Lemke, N.J.; Tuissi, A.; Vedani, M. Aging Behaviour and Mechanical Performance of 18-Ni 300 Steel Processed by Selective Laser Melting. Metals 2016, 6, 218. [Google Scholar] [CrossRef]
- Boegelein, T.; Dryepondt, S.N.; Pandey, A.; Dawson, K.; Tatlock, G.J. Mechanical response and deformation mechanisms of ferritic oxide dispersion strengthened steel structures produced by selective laser melting. Acta Mater. 2015, 87, 201–215. [Google Scholar] [CrossRef] [Green Version]
- Guan, K.; Wang, Z.; Gao, M.; Li, X.; Zeng, X. Effects of processing parameters on tensile properties of selective laser melted 304 stainless steel. Mater. Des. 2013, 50, 581–586. [Google Scholar] [CrossRef]
- Nguyen, Q.B.; Zhu, Z.; Ng, F.L.; Chua, B.W.; Nai, S.M.L.; Wei, J. High mechanical strengths and ductility of stainless steel 304L fabricated using selective laser melting. J. Mater. Science Technol. 2019, 35, 388–394. [Google Scholar] [CrossRef]
- Tilita, G.A.; Chen, W.; Kwan, C.C.F.; Yuen, M.M.F. The effect of ultrasonic excitation on the microstructure of selective laser melted 304 L stainless steel. Mater. und Werkst. 2017, 48, 342–348. [Google Scholar] [CrossRef]
- Abd-Elghany, K.; Bourell, D.L. Property evaluation of 304L stainless steel fabricated by selective laser melting. Rapid Prototyp. J. 2012, 18, 420–428. [Google Scholar] [CrossRef]
- Kong, D.; Ni, X.; Dong, C.; Zhang, L.; Man, C.; Yao, J.; Xiao, K.; Li, X. Heat treatment effect on the microstructure and corrosion behavior of 316L stainless steel fabricated by selective laser melting for proton exchange membrane fuel cells. Electrochim. Acta 2018, 276, 293–303. [Google Scholar] [CrossRef]
- Ma, M.; Wang, Z.; Zeng, X. A comparison on metallurgical behaviors of 316L stainless steel by selective laser melting and laser cladding deposition. Mater. Sci. Eng. A 2017, 685, 265–273. [Google Scholar] [CrossRef]
- Ahmadi, A.; Mirzaeifar, R.; Moghaddam, N.S.; Turabi, A.S.; Karaca, H.E.; Elahinia, M. Effect of manufacturing parameters on mechanical properties of 316L stainless steel parts fabricated by selective laser melting: A computational framework. Mater. Des. 2016, 112, 328–338. [Google Scholar] [CrossRef]
- Zhang, B.; Dembinski, L.; Coddet, C. The study of the laser parameters and environment variables effect on mechanical properties of high compact parts elaborated by selective laser melting 316L powder. Mater. Sci. Eng. A 2013, 584, 21–31. [Google Scholar] [CrossRef]
- Suryawanshi, J.; Prashanth, K.G.; Ramamurty, U. Mechanical behavior of selective laser melted 316L stainless steel. Mater. Sci. Eng. A 2017, 696, 113–121. [Google Scholar] [CrossRef]
- Harun, W.S.W.; Asri, R.I.M.; Romlay, F.R.M.; Sharif, S.; Jan, N.H.M.; Tsumori, F. Surface characterisation and corrosion behaviour of oxide layer for SLMed-316L stainless steel. J. Alloy. Compd. 2018, 748, 1044–1052. [Google Scholar] [CrossRef]
- Casati, R.; Lemke, J.; Vedani, M. Microstructure and Fracture Behavior of 316L Austenitic Stainless Steel Produced by Selective Laser Melting. J. Mater. Sci. Technol. 2016, 32, 738–744. [Google Scholar] [CrossRef]
- Li, H.; Ramezani, M.; Li, M.; Ma, C.; Wang, J. Effect of process parameters on tribological performance of 316L stainless steel parts fabricated by selective laser melting. Manuf. Lett. 2018, 16, 36–39. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Buciumeanu, M.; Pinto, E.; Alves, N.; Carvalho, O.; Silva, F.S.; Miranda, G. 316L stainless steel mechanical and tribological behavior—A comparison between selective laser melting, hot pressing and conventional casting. Addit. Manufacturing 2017, 16, 81–89. [Google Scholar] [CrossRef]
- Aggarwal, A.; Patel, S.; Kumar, A. Selective Laser Melting of 316L Stainless Steel: Physics of Melting Mode Transition and Its Influence on Microstructural and Mechanical Behavior. JOM 2018, 71, 1105–1116. [Google Scholar] [CrossRef]
- Alrbaey, K.; Wimpenny, D.; Tosi, R.; Manning, W.; Moroz, A. On Optimization of Surface Roughness of Selective Laser Melted Stainless Steel Parts: A Statistical Study. J. Mater. Eng. Perform. 2014, 23, 2139–2148. [Google Scholar] [CrossRef]
- Kong, D.; Ni, X.; Dong, C.; Lei, X.; Zhang, L.; Man, C.; Yao, J.; Cheng, X.; Li, X. Bio-functional and anti-corrosive 3D printing 316L stainless steel fabricated by selective laser melting. Mater. Des. 2018, 152, 88–101. [Google Scholar] [CrossRef]
- Kuznetsov, P.A.; Krasikov, A.V.; Staritsyn, M.V.; Mushnikova, S.Y.; Parmenova, O.N. Features of Local Corrosion of AISI316L Steel Manufactured by Selective Laser Melting. Prot. Met. Phys. Chem. Surf. 2018, 54, 484–489. [Google Scholar] [CrossRef]
- Mertens, A.; Reginster, S.; Paydas, H.; Contrepois, Q.; Dormal, T.; Lemaire, O.; Lecomte-Beckers, J. Mechanical properties of alloy Ti–6Al–4V and of stainless steel 316L processed by selective laser melting: influence of out-of-equilibrium microstructures. Powder Metall. 2014, 57, 184–189. [Google Scholar] [CrossRef]
- Shang, Y.; Yuan, Y.; Li, D.; Li, Y.; Chen, J. Effects of scanning speed on in vitro biocompatibility of 316L stainless steel parts elaborated by selective laser melting. Int. J. Adv. Manuf. Technol. 2017, 92, 4379–4385. [Google Scholar] [CrossRef]
- Badrossamay, M.; Childs, T.H.C. Further studies in selective laser melting of stainless and tool steel powders. Int. J. Mach. Tools Manufacture 2007, 47, 779–784. [Google Scholar] [CrossRef]
- Strano, G.; Hao, L.; Everson, R.M.; Evans, K.E. Surface roughness analysis, modelling and prediction in selective laser melting. J. Mater. Process. Technol. 2013, 213, 589–597. [Google Scholar] [CrossRef]
- Morgan, R.; Sutcliffe, C.J.; O’Neill, W. Density analysis of direct metal laser re-melted 316L stainless steel cubic primitives. J. Mater. Science 2004, 39, 1195–1205. [Google Scholar] [CrossRef]
- Riemer, A.; Leuders, S.; Thöne, M.; Richard, H.A.; Tröster, T.; Niendorf, T. On the fatigue crack growth behavior in 316L stainless steel manufactured by selective laser melting. Eng. Fracture Mech. 2014, 120, 15–25. [Google Scholar] [CrossRef]
- Tolosa, I.; Garciandía, F.; Zubiri, F.; Zapirain, F.; Esnaola, A. Study of mechanical properties of AISI 316 stainless steel processed by “selective laser melting”, following different manufacturing strategies. Int. J. Adv. Manuf. Technol. 2010, 51, 639–647. [Google Scholar] [CrossRef]
- Pinomaa, T.; Lindroos, M.; Walbrühl, M.; Provatas, N.; Laukkanen, A. The significance of spatial length scales and solute segregation in strengthening rapid solidification microstructures of 316L stainless steel. Acta Mater. 2020, 184, 1–16. [Google Scholar] [CrossRef]
- Hengsbach, F.; Koppa, P.; Duschik, K.; Holzweissig, M.J.; Burns, M.; Nellesen, J.; Tillmann, W.; Tröster, T.; Hoyer, K.-P.; Schaper, M. Duplex stainless steel fabricated by selective laser melting - Microstructural and mechanical properties. Mater. Des. 2017, 133, 136–142. [Google Scholar] [CrossRef]
- Saeidi, K.; Kevetkova, L.; Lofaj, F.; Shen, Z. Novel ferritic stainless steel formed by laser melting from duplex stainless steel powder with advanced mechanical properties and high ductility. Mater. Sci. Eng. A 2016, 665, 59–65. [Google Scholar] [CrossRef]
- Davidson, K.; Singamneni, S. Selective Laser Melting of Duplex Stainless Steel Powders: An Investigation. Mater. Manuf. Process. 2015, 31, 1543–1555. [Google Scholar] [CrossRef]
- Davidson, K.P.; Singamneni, S.B. Metallographic evaluation of duplex stainless steel powders processed by selective laser melting. Rapid Prototyp. J. 2017, 23, 1146–1163. [Google Scholar] [CrossRef]
- Vunnam, S.; Saboo, A.; Sudbrack, C.; Starr, T.L. Effect of powder chemical composition on the as-built microstructure of 17–4 PH stainless steel processed by selective laser melting. Addit. Manuf. 2019, 30, 100876. [Google Scholar] [CrossRef]
- Sun, Y.; Hebert, R.J.; Aindow, M. Non-metallic inclusions in 17–4PH stainless steel parts produced by selective laser melting. Mater. Des. 2018, 140, 153–162. [Google Scholar] [CrossRef]
- Pasebani, S.; Ghayoor, M.; Badwe, S.; Irrinki, H.; Atre, S.V. Effects of atomizing media and post processing on mechanical properties of 17–4 PH stainless steel manufactured via selective laser melting. Addit. Manuf. 2018, 22, 127–137. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Elwany, A.; Yadollahi, A.; Thompson, S.M.; Bian, L.; Shamsaei, N. Mechanical properties and microstructural characterization of selective laser melted 17–4 PH stainless steel. Rapid Prototyp. J. 2017, 23, 280–294. [Google Scholar] [CrossRef] [Green Version]
- Rafi, H.K.; Pal, D.; Patil, N.; Starr, T.L.; Stucker, B.E. Microstructure and Mechanical Behavior of 17–4 Precipitation Hardenable Steel Processed by Selective Laser Melting. J. Mater. Eng. Perform. 2014, 23, 4421–4428. [Google Scholar] [CrossRef]
- Murr, L.E.; Martinez, E.; Hernandez, J.; Collins, S.; Amato, K.N.; Gaytan, S.M.; Shindo, P.W. Microstructures and Properties of 17–4 PH Stainless Steel Fabricated by Selective Laser Melting. J. Mater. Res. Technol. 2012, 1, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Averyanova, M.; Cicala, E.; Bertrand, P.; Grevey, D. Experimental design approach to optimize selective laser melting of martensitic 17–4 PH powder: part I-single laser tracks and first layer. Rapid Prototyp. J. 2012, 18, 28–37. [Google Scholar] [CrossRef]
- Facchini, L.; Vicente, N.; Lonardelli, I.; Magalini, E.; Robotti, P.; Molinari, A. Metastable Austenite in 17–4 Precipitation-Hardening Stainless Steel Produced by Selective Laser Melting. Adv. Eng. Mater. 2010, 12, 184–188. [Google Scholar] [CrossRef]
- Qiu, C.; Kindi, M.A.; Aladawi, A.S.; Hatmi, I.A. A comprehensive study on microstructure and tensile behaviour of a selectively laser melted stainless steel. Scientific reports 2018, 8, 7785. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, C.; Wang, D.; Liu, W.; Fang, X.; Wellmann, D.; Zhao, Y.; Tian, Y. A Review on Laser Powder Bed Fusion of Inconel 625 Nickel-Based Alloy. Appl. Sci. 2019, 10, 81. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhang, C.; Jia, D.; Wellmann, D.; Liu, W. Corrosion Behaviors of Selective Laser Melted Aluminum Alloys: A Review. Metals 2020, 10, 102. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Tan, X.; Tor, S.B.; Yeong, W.Y. Selective laser melting of stainless steel 316L with low porosity and high build rates. Mater. Des. 2016, 104, 197–204. [Google Scholar] [CrossRef]
- Li, X.P.; Van Humbeeck, J.; Kruth, J.P. Selective laser melting of weak-textured commercially pure titanium with high strength and ductility: A study from laser power perspective. Mater. Des. 2017, 116, 352–358. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Y.; Yi, Z.; Su, X. Research on the fabricating quality optimization of the overhanging surface in SLM process. Int. J. Adv. Manuf. Technol. 2012, 65, 1471–1484. [Google Scholar] [CrossRef]
- Yadroitsev, I.; Smurov, I. Selective laser melting technology: From the single laser melted track stability to 3D parts of complex shape. Phys. Procedia 2010, 5, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Hanzl, P.; Zetek, M.; Bakša, T.; Kroupa, T. The Influence of Processing Parameters on the Mechanical Properties of SLM Parts. Procedia Eng. 2015, 100, 1405–1413. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Zhou, J.; Li, H.; Lin, F. A study of the microstructures and mechanical properties of Ti6Al4V fabricated by SLM under vacuum. Mater. Sci. Eng. A 2018, 724, 1–10. [Google Scholar] [CrossRef]
- Hunt, J.; Derguti, F.; Todd, I. Selection of steels suitable for additive layer manufacturing. Ironmak. Steelmak. 2014, 41, 254–256. [Google Scholar] [CrossRef]
- Xiebin Wang, S.K.; Van Humbeeck, J. A Short Review on the Microstructure, Transformation Behavior and Functional Properties of NiTi Shape Memory Alloys Fabricated by Selective Laser Melting. Materials 2018, 11, 1683. [Google Scholar] [CrossRef] [Green Version]
- Qi, L.-h.; Chao, Y.-p.; Luo, J.; Zhou, J.-m.; Hou, X.-h.; Li, H.-j. A novel selection method of scanning step for fabricating metal components based on micro-droplet deposition manufacture. Int. J. Mach. Tools Manuf. 2012, 56, 50–58. [Google Scholar] [CrossRef]
- Pinkerton, A.J. [INVITED] Lasers in additive manufacturing. Opt. Laser Technol. 2016, 78, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Santos, E.C.; Shiomi, M.; Osakada, K.; Laoui, T. Rapid manufacturing of metal components by laser forming. Int. J. Mach. Tools Manuf. 2006, 46, 1459–1468. [Google Scholar] [CrossRef]
- Zinovieva, O.; Zinoviev, A.; Ploshikhin, V. Three-dimensional modeling of the microstructure evolution during metal additive manufacturing. Comput. Mater. Sci. 2018, 141, 207–220. [Google Scholar] [CrossRef]
- Körner, C. Additive manufacturing of metallic components by selective electron beam melting — a review. Int. Mater. Rev. 2016, 61, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Irrinki, H.; Dexter, M.; Barmore, B.; Enneti, R.; Pasebani, S.; Badwe, S.; Stitzel, J.; Malhotra, R.; Atre, S.V. Effects of Powder Attributes and Laser Powder Bed Fusion (L-PBF) Process Conditions on the Densification and Mechanical Properties of 17–4 PH Stainless Steel. JOM 2016, 68, 860–868. [Google Scholar] [CrossRef]
- Gu, H.G.H.; Pal, D.; Rafi, K.; Starr, T.; Stucker, B. Influences of Energy Density on Porosity and Microstructure of Selective Laser Melted 17–4 PH Stainless Steel. Solid Free. Fabr. Symp. 2013, 474–489. [Google Scholar]
- Alnajjar, M.; Christien, F.; Wolski, K.; Bosch, C. Evidence of austenite by-passing in a stainless steel obtained from laser melting additive manufacturing. Addit. Manuf. 2019, 25, 187–195. [Google Scholar] [CrossRef]
- Ouyang, D.; Li, N.; Xing, W.; Zhang, J.; Liu, L. 3D printing of crack-free high strength Zr-based bulk metallic glass composite by selective laser melting. Intermetallics 2017, 90, 128–134. [Google Scholar] [CrossRef]
- Lebrun, T.; Tanigaki, K.; Horikawa, K.; Kobayashi, H. Strain rate sensitivity and mechanical anisotropy of selective laser melted 17–4 PH stainless steel. Mech. Eng. J. 2014, 1, SMM0049. [Google Scholar] [CrossRef] [Green Version]
- Jerrard, P.G.E.; Hao, L.; Evans, K.E. Experimental investigation into selective laser melting of austenitic and martensitic stainless steel powder mixtures. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2009, 223, 1409–1416. [Google Scholar] [CrossRef]
- Kokosza, A.; Pacyna, J. Evaluation of retained austenite stability in heat treated cold work tool steel. J. Mater. Process. Technol. 2005, 162–163, 327–331. [Google Scholar] [CrossRef]
- Hsu, T.-H.; Chang, Y.-J.; Huang, C.-Y.; Yen, H.-W.; Chen, C.-P.; Jen, K.-K.; Yeh, A.-C. Microstructure and property of a selective laser melting process induced oxide dispersion strengthened 17–4 PH stainless steel. J. Alloy Compd. 2019, 803, 30–41. [Google Scholar] [CrossRef]
- Sun, Y.; Hebert, R.J.; Aindow, M. Effect of heat treatments on microstructural evolution of additively manufactured and wrought 17–4PH stainless steel. Mater. Des. 2018, 156, 429–440. [Google Scholar] [CrossRef]
- Wu, J.; Wray, P.J.; Garcia, C.I.; Hua, M.; Deardo, A.J. Image Quality Analysis: A New Method of Characterizing Microstructures. ISIJ Int. 2005, 45, 254–262. [Google Scholar] [CrossRef] [Green Version]
- Irrinki, H.; Jangam, J.S.D.; Pasebani, S.; Badwe, S.; Stitzel, J.; Kate, K.; Gulsoy, O.; Atre, S.V. Effects of particle characteristics on the microstructure and mechanical properties of 17–4 PH stainless steel fabricated by laser-powder bed fusion. Powder Technol. 2018, 331, 192–203. [Google Scholar] [CrossRef]
- Hoeges, S.; Zwiren, A.; Schade, C. Additive manufacturing using water atomized steel powders. Met. Powder Rep. 2017, 72, 111–117. [Google Scholar] [CrossRef]
- Cheruvathur, S.; Lass, E.A.; Campbell, C.E. Additive Manufacturing of 17–4 PH Stainless Steel: Post-processing Heat Treatment to Achieve Uniform Reproducible Microstructure. JOM 2015, 68, 930–942. [Google Scholar] [CrossRef]
- Yadollahi, A.; Shamsaei, N.; Thompson, S.M.; Seely, D.W. Effects of process time interval and heat treatment on the mechanical and microstructural properties of direct laser deposited 316L stainless steel. Mater. Sci. Eng. A 2015, 644, 171–183. [Google Scholar] [CrossRef]
- Kudzal, A.; McWilliams, B.; Hofmeister, C.; Kellogg, F.; Yu, J.; Taggart-Scarff, J.; Liang, J. Effect of scan pattern on the microstructure and mechanical properties of Powder Bed Fusion additive manufactured 17–4 stainless steel. Mater. Des. 2017, 133, 205–215. [Google Scholar] [CrossRef]
- Krantz, M.; Zhang, H.; Zhu, J. Characterization of powder flow: Static and dynamic testing. Powder Technol. 2009, 194, 239–245. [Google Scholar] [CrossRef]
- Hu, Z.; Zhu, H.; Zhang, H.; Zeng, X. Experimental investigation on selective laser melting of 17–4PH stainless steel. Opt. Laser Technol. 2017, 87, 17–25. [Google Scholar] [CrossRef]
- Li, R.; Shi, Y.; Wang, Z.; Wang, L.; Liu, J.; Jiang, W. Densification behavior of gas and water atomized 316L stainless steel powder during selective laser melting. Appl. Surf. Sci. 2010, 256, 4350–4356. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, J.; Lin, X.; Huang, W. Study on microstructure and mechanical properties of laser rapid forming Inconel 718. Mater. Sci. Eng. A 2008, 478, 119–124. [Google Scholar] [CrossRef]
- King, W.E.; Barth, H.D.; Castillo, V.M.; Gallegos, G.F.; Gibbs, J.W.; Hahn, D.E.; Kamath, C.; Rubenchik, A.M. Observation of keyhole-mode laser melting in laser powder-bed fusion additive manufacturing. J. Mater. Process. Technol. 2014, 214, 2915–2925. [Google Scholar] [CrossRef]
- Ponnusamy, P.; Masood, S.H.; Palanisamy, S.; Rahman Rashid, R.A.; Ruan, D. Characterization of 17–4PH alloy processed by selective laser melting. Mater. Today Proc. 2017, 4, 8498–8506. [Google Scholar] [CrossRef]
- Meredith, S.D.; Zuback, J.S.; Keist, J.S.; Palmer, T.A. Impact of composition on the heat treatment response of additively manufactured 17–4 PH grade stainless steel. Mater. Sci. Eng. A 2018, 738, 44–56. [Google Scholar] [CrossRef]
- Stoudt, M.R.; Ricker, R.E.; Lass, E.A.; Levine, L.E. The Influence of Post-Build Microstructure on the Electrochemical Behavior of Additively Manufactured 17–4 PH Stainless Steel. JOM 2017, 69, 506–515. [Google Scholar] [CrossRef] [Green Version]









| Powder Type | Argon Gas Fabrication | Nitrogen Gas Fabrication | Reference |
|---|---|---|---|
| Argon-atomized (α) | α 1 | α | [96] |
| Nitrogen-atomized α (6%) + γ (94%) | α | α (15%) + γ (85%) | [96] |
| Nitrogen-atomized (α + γ) | α (92%) + γ (8%) | α + γ (more than 50%) | [95] |
| Nitrogen-atomized α (dominate) + γ | α | - | [93] |
| Water-atomized α + γ (dominate) | α + γ 1 | - | [93] |
| Gas-atomized | α | - | [94] |
| Gas-atomized α (70%) + γ (30%) | α | - | [125] |
| Water-atomized α (20%) + γ (80%) | α (more than 20%) + γ | - | [125] |
| Energy Density(J/mm3) | Gas-Atomized Powder | Water-Atomized Powder | Chamber Atmosphere | Reference |
|---|---|---|---|---|
| 64 | α (coarse) | α + γ | Argon | [93] |
| 104 | α (fine) | α (fraction of phase increased) + γ | Argon | [93] |
| 64 | α | α (70 ± 5%) + γ (30 ± 5%) | Argon | [125] |
| 80 | α | α (75 ± 5%) + γ (25 ± 5%) | Argon | [125] |
| 84 | α | α (80 ± 5%) + γ (20 ± 5%) | Argon | [125] |
| 104 | α | α (90 ± 5%) + γ (10 ± 5%) | Argon | [125] |
| Scanning Strategies | Average Austenite (Volume fraction, %) | Reference |
|---|---|---|
| 17–4 PH powder | 62.9 | [129] |
| Hexagon pattern 1 | ~58.3 | |
| Concentric middle 2 | ~82.4 | |
| Concentric edges 2 | ~50.3 | |
| 90-BF-F 3 | ~27.2 | |
| 90-BF-T 4 | ~25.2 | |
| 0-BF-F 5 | ~43.6 | |
| 0-BF-T 6 | ~69.9 |
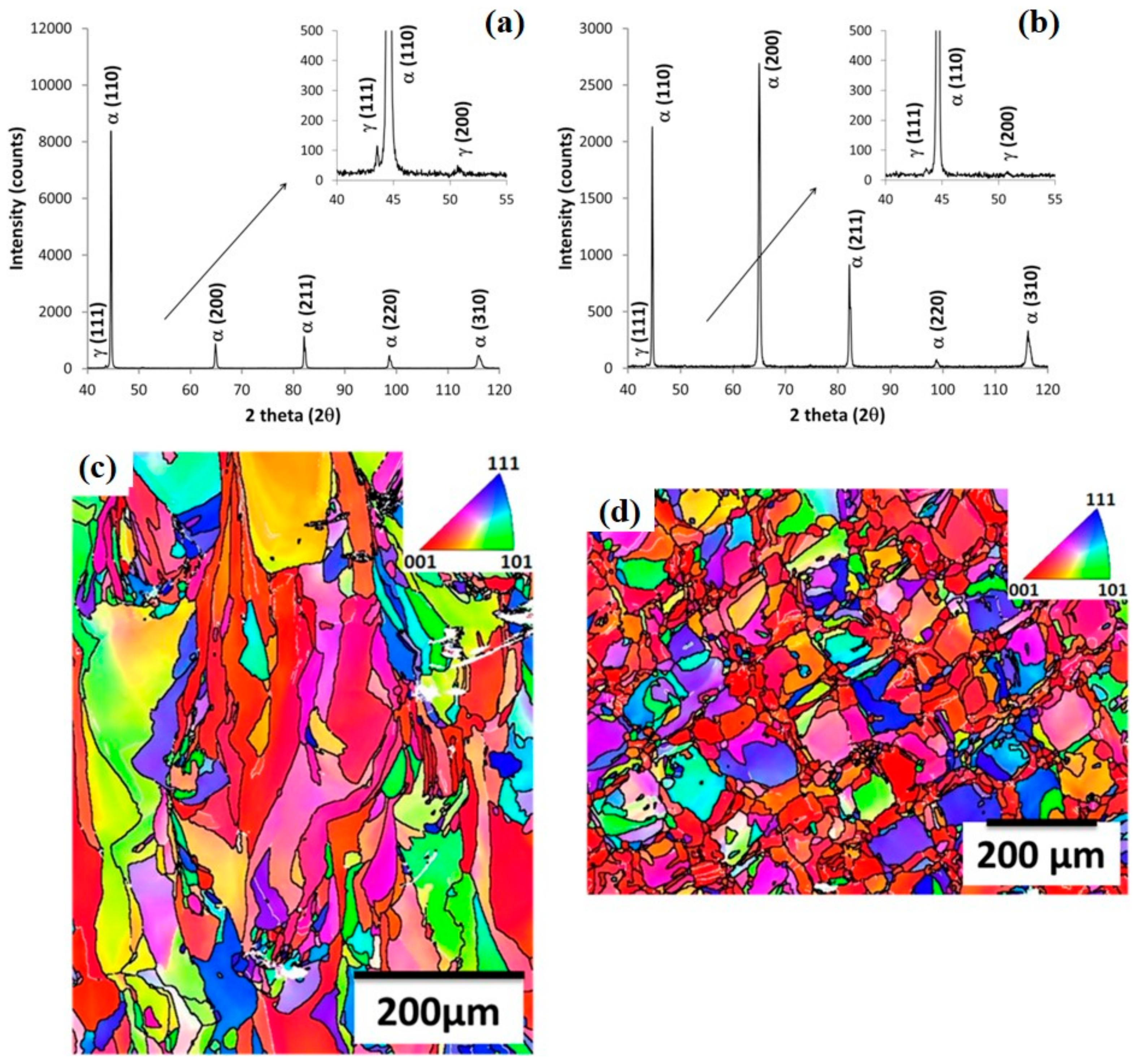
| Condition | Martensite (bcc, α), % | Austenite (fcc, γ), % | Reference |
|---|---|---|---|
| As-fabricated | 64.0 | 36.0 | [27] |
| H900 1 | 59.5 | 40.5 | [27] |
| H1025 | 89.6 | 10.4 | [27] |
| H1150 | 94.4 | 5.6 | [27] |
| Condition A 2 | 100 | 0 | [27] |
| CA-H900 | 96.7 | 3.3 | [27] |
| CA-H1025 | 95.3 | 4.7 | [27] |
| CA-H1150 | 79.6 | 20.4 | [27] |
| As-L-PBF | 93.8 | 6.2 | [122] |
| L-PBF + SHT 3 | 98.5 | 1.5 | [122] |
| L-PBF + SHT + Aging 4 | 95 | 5 | [122] |
| L-PBF + Direct Aging | 82.1 | 17.9 | [122] |
| L-PBF Parts from | Scanning Strategy | Energy Density (J/mm3) | Density (%) | Reference |
|---|---|---|---|---|
| Gas-atomized powder D50 = 13 μm Water-atomized powder D50 = 17 μm Water-atomized powder D50 = 24 μm Water-atomized powder D50 = 43 μm | N/A | 64 | 96.6 ± 0.5/96 ± 0.8/ 87 ± 0.3/89.7 ± 0.3 | [125] |
| N/A | 80 | 97.4 ± 0.5/97.1 ± 0.6/ 91.4 ± 0.6/94.5 ± 0.7 | [125] | |
| N/A | 84 | 97.6 ± 0.5/97 ± 0.8/ 96.3 ± 0.5/97 ± 0.5 | [125] | |
| N/A | 104 | 97.5 ± 0.5/97 ± 0.5/ 96.8 ± 0.1/97 ± 0.5 | [125] | |
| Average particle size 14.5 μm, D10 = 3.28 μm, D90 = 30.14 μm | Hexagon | 62.5 | 98.9 | [129] |
| Concentric | 62.5 | 98.2 | [129] | |
| 90-BF-F | 62.5 | 98.5 | [129] | |
| 90-BF-T | 62.5 | 98.8 | [129] | |
| 0-BF-F | 62.5 | 98.7 | [129] | |
| 0-BF-T | 62.5 | 98.7 | [129] |
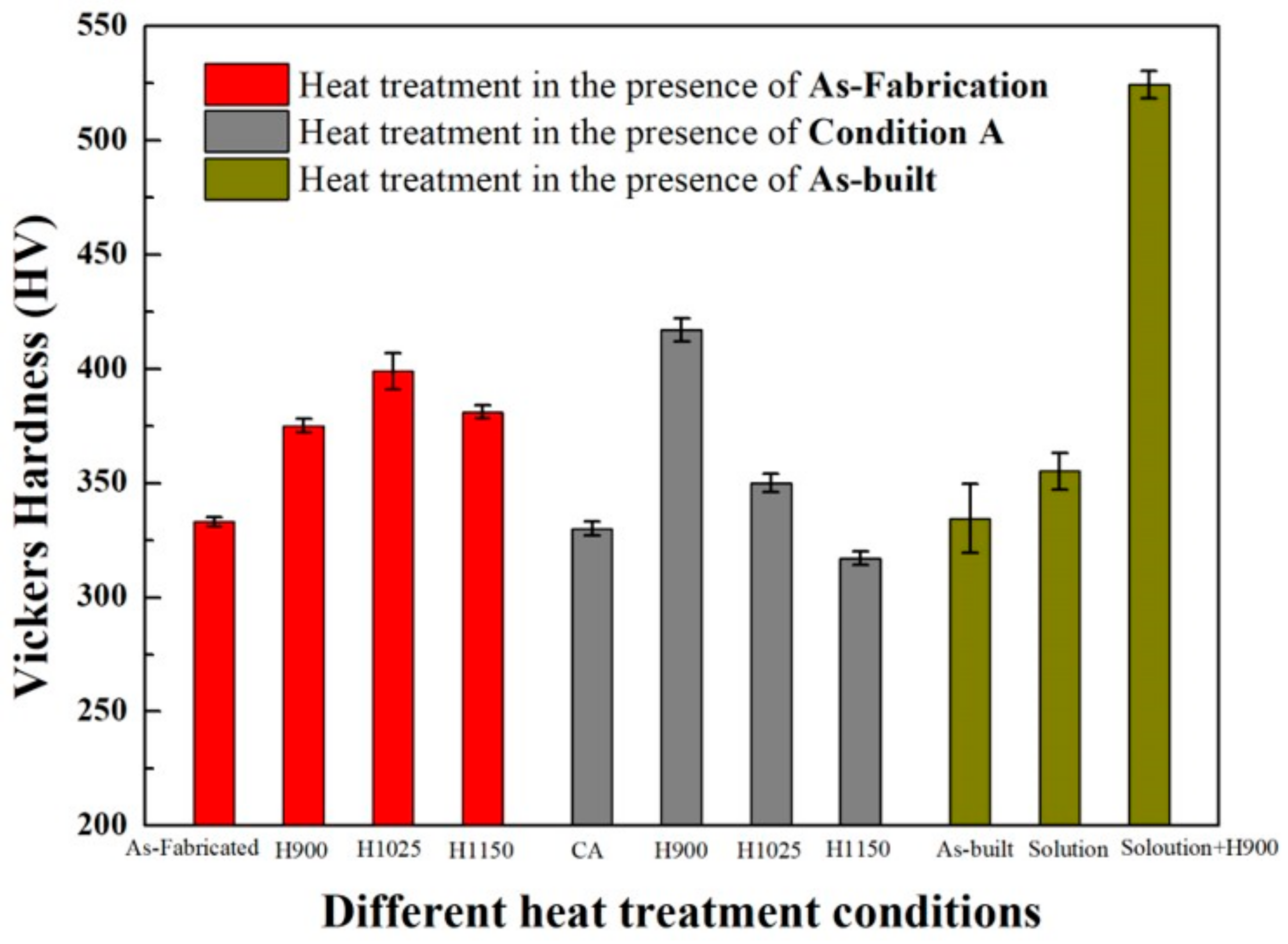
| Condition | Hardness (HV) | Martensite (bcc, α), % | Austenite (fcc, γ), % | Reference |
|---|---|---|---|---|
| As-fabricated | 333 ± 2 | 64.0 | 36.0 | [27] |
| H900 1 | 375 ± 3 | 59.5 | 40.5 | [27] |
| H1025 | 399 ± 8 | 89.6 | 10.4 | [27] |
| H1150 | 381 ± 3 | 94.4 | 5.6 | [27] |
| Condition A 2 | 330 ± 3 | 100 | 0 | [27] |
| CA-H900 | 417 ± 5 | 96.7 | 3.3 | [27] |
| CA-H1025 | 350 ± 4 | 95.3 | 4.7 | [27] |
| CA-H1150 | 317 ± 3 | 79.6 | 20.4 | [27] |
| As-built | 334.5 ± 15 | N/A | N/A | [123] |
| Solution | 355.2 ± 8 | N/A | N/A | [123] |
| Solution + H900 | 524.5 ± 6 | N/A | N/A | [123] |
| Samples | Yield Strength (MPa) | Ultimate Tensile Strength (MPa) | Elongation (%) | Reference | |
|---|---|---|---|---|---|
| As-built samples | Vertical | 580 | 940 | 5.8 | [26] |
| Horizontal | 650 | 1060 | 14.5 | ||
| CA-H900 samples | Vertical | 1020 | 1150 | 2.8 | |
| Horizontal | 1250 | 1410 | 11 | ||
| Condition | Yield Strength (MPa) | Ultimate Tensile Strength (MPa) | Elongation (%) | Reference | |
|---|---|---|---|---|---|
| As-built samples, vertical | 580 | 940 | 5.8 | [26] | |
| CA-H900 samples, vertical | 1020 | 1150 | 2.8 | [26] | |
| As-built samples, horizontal | 650 | 1060 | 14.5 | [26] | |
| CA-H900 samples, horizontal | 1250 | 1410 | 11 | [26] | |
| As-fabricated | 661 ± 24 | 1255 ± 3 | 16.2 ± 2.5 | [27] | |
| H900 1 | 945 ± 12 | 1417 ± 6 | 15.5 ± 1.3 | [27] | |
| H1025 | 870 ± 25 | 1358 ± 8 | 13.3 ± 1.5 | [27] | |
| H1150 | 1005 ± 15 | 1319 ± 2 | 11.1 ± 0.4 | [27] | |
| Condition A 2 | 939 ± 9 | 1188 ± 6 | 9.0 ± 1.5 | [27] | |
| CA-H900 | 1352 ± 18 | 1444 ± 2 | 4.6 ± 0.4 | [27] | |
| CA-H1025 | 1121 ± 9 | 1172 ± 2 | 9.6 ± 1.7 | [27] | |
| CA-H1150 | 859 ± 11 | 1017 ± 15 | 16.6 ± 1.2 | [27] | |
| As-L-PBFed | 803 | 1228 | 12.7 | [122] | |
| L-PBF + SHT 3 | 966 | 1268 | 8.8 | [122] | |
| L-PBF + SHT + Aging 4 | 1276 | 1381 | 13.6 | [122] | |
| L-PBF + Direct Aging | 1173 | 1478 | 9.8 | [122] | |
| Solutionized at 1015 °C and aged at 482 °C | Gas-atomized (energy density y1 = 64 J/mm3, y2 = 104 J /mm3 | 1116/1200 | 1358/1368 | 5.2/2.6 | [93] |
| Water-atomized (energy density y1 = 64 J/mm3, y2 = 104 J/mm3 | 365/500 | 510/990 | 1/3.3 | [93] | |
| Solutionized at 1315 °C and aged at 482 °C | Gas-atomized (energy density y1 = 64 J/mm3, y2 = 104 J /mm3 | 1186/1255 | 1308/1300 | 2.6/2 | [93] |
| Water-atomized (energy density y1 = 64 J/mm3, y2 = 104 J/mm3 | 650/1000 | 780/1261 | 0.7/5.5 | [93] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zai, L.; Zhang, C.; Wang, Y.; Guo, W.; Wellmann, D.; Tong, X.; Tian, Y. Laser Powder Bed Fusion of Precipitation-Hardened Martensitic Stainless Steels: A Review. Metals 2020, 10, 255. https://doi.org/10.3390/met10020255
Zai L, Zhang C, Wang Y, Guo W, Wellmann D, Tong X, Tian Y. Laser Powder Bed Fusion of Precipitation-Hardened Martensitic Stainless Steels: A Review. Metals. 2020; 10(2):255. https://doi.org/10.3390/met10020255
Chicago/Turabian StyleZai, Le, Chaoqun Zhang, Yiqiang Wang, Wei Guo, Daniel Wellmann, Xin Tong, and Yingtao Tian. 2020. "Laser Powder Bed Fusion of Precipitation-Hardened Martensitic Stainless Steels: A Review" Metals 10, no. 2: 255. https://doi.org/10.3390/met10020255
APA StyleZai, L., Zhang, C., Wang, Y., Guo, W., Wellmann, D., Tong, X., & Tian, Y. (2020). Laser Powder Bed Fusion of Precipitation-Hardened Martensitic Stainless Steels: A Review. Metals, 10(2), 255. https://doi.org/10.3390/met10020255