Modelling the Elements Reaction–Diffusion Behavior on Interface of Ti/Al2O3 Composite Prepared by Hot Pressing Sintering
Abstract
:1. Introduction
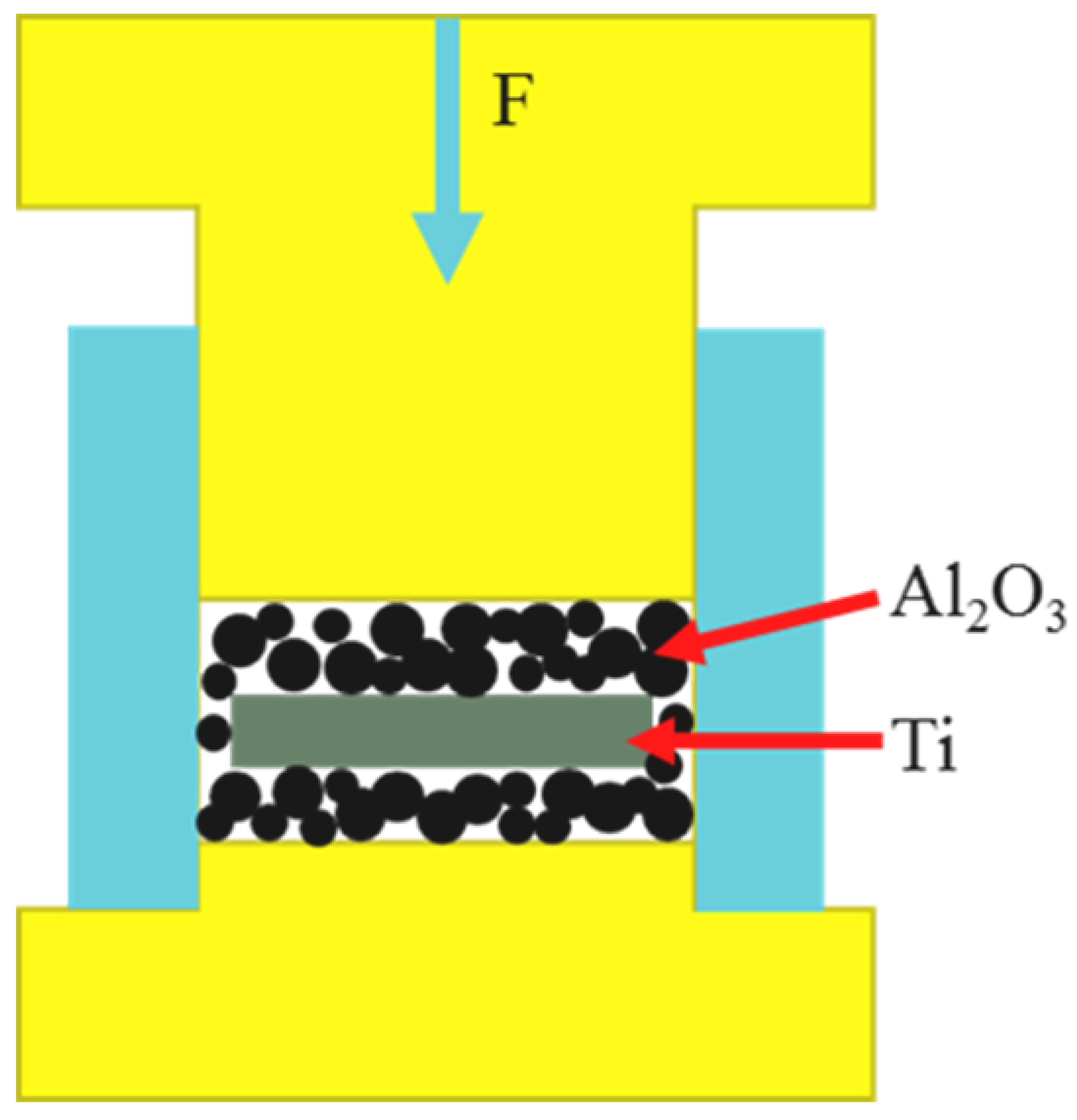
2. Experimental Methods
3. Results and Discussion
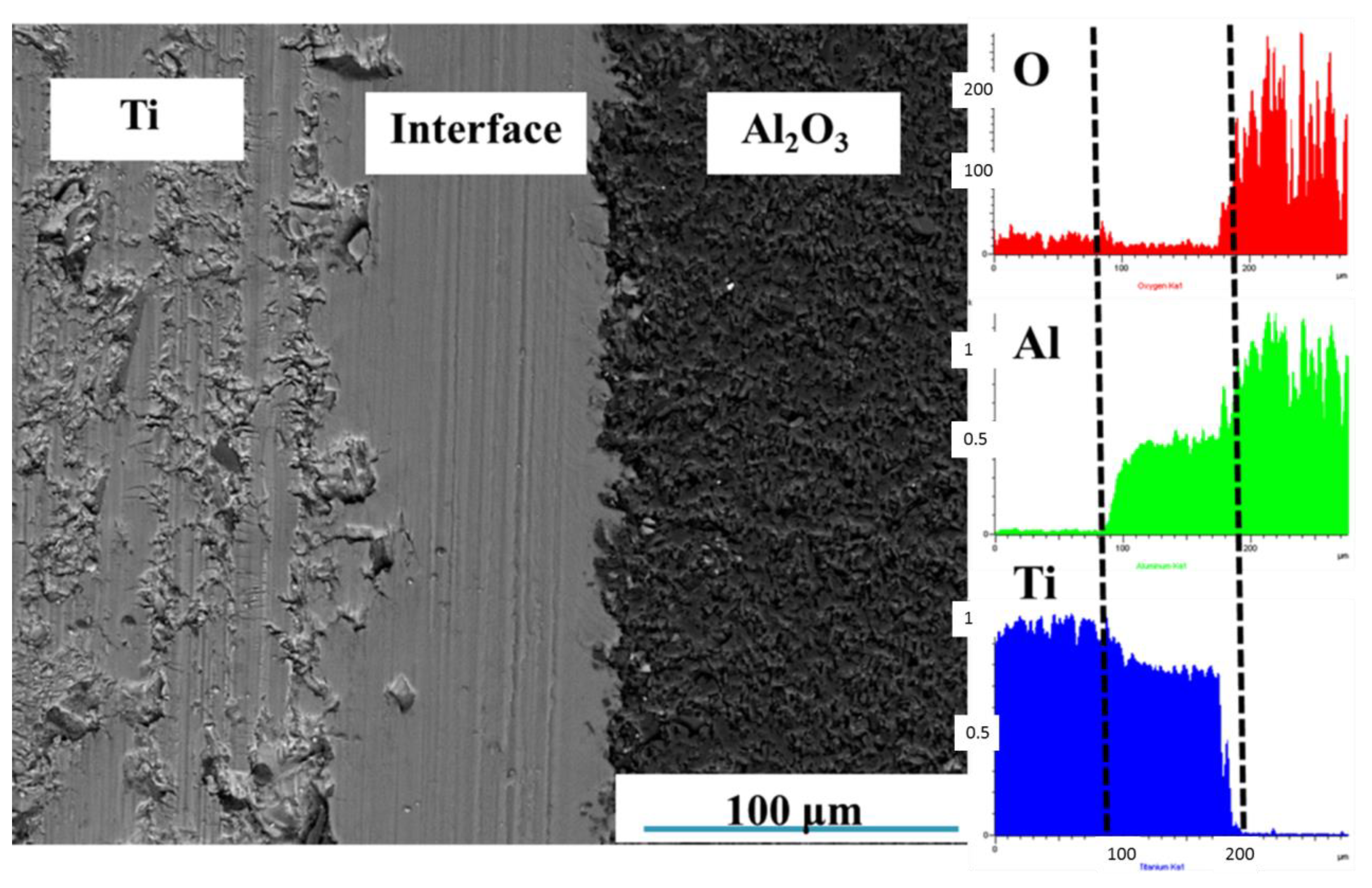
3.1. Effects of Sintering Schedule on Microstructure of Ti-Al2O3 Interface Reaction Layer
3.1.1. Effects of Sintering Temperature on Microstructure of Ti-Al2O3 Interface Reaction Layer
3.1.2. Effects of Holding Time on Microstructure of Ti-Al2O3 Interface Reaction Layer
3.2. Research on the Interface Reaction Kinetic of Ti/Al2O3 Composites
3.2.1. Calculation Method of Diffusion Coefficient
3.2.2. Calculation of the Diffusion Coefficient of Every Element on the Interface Layer
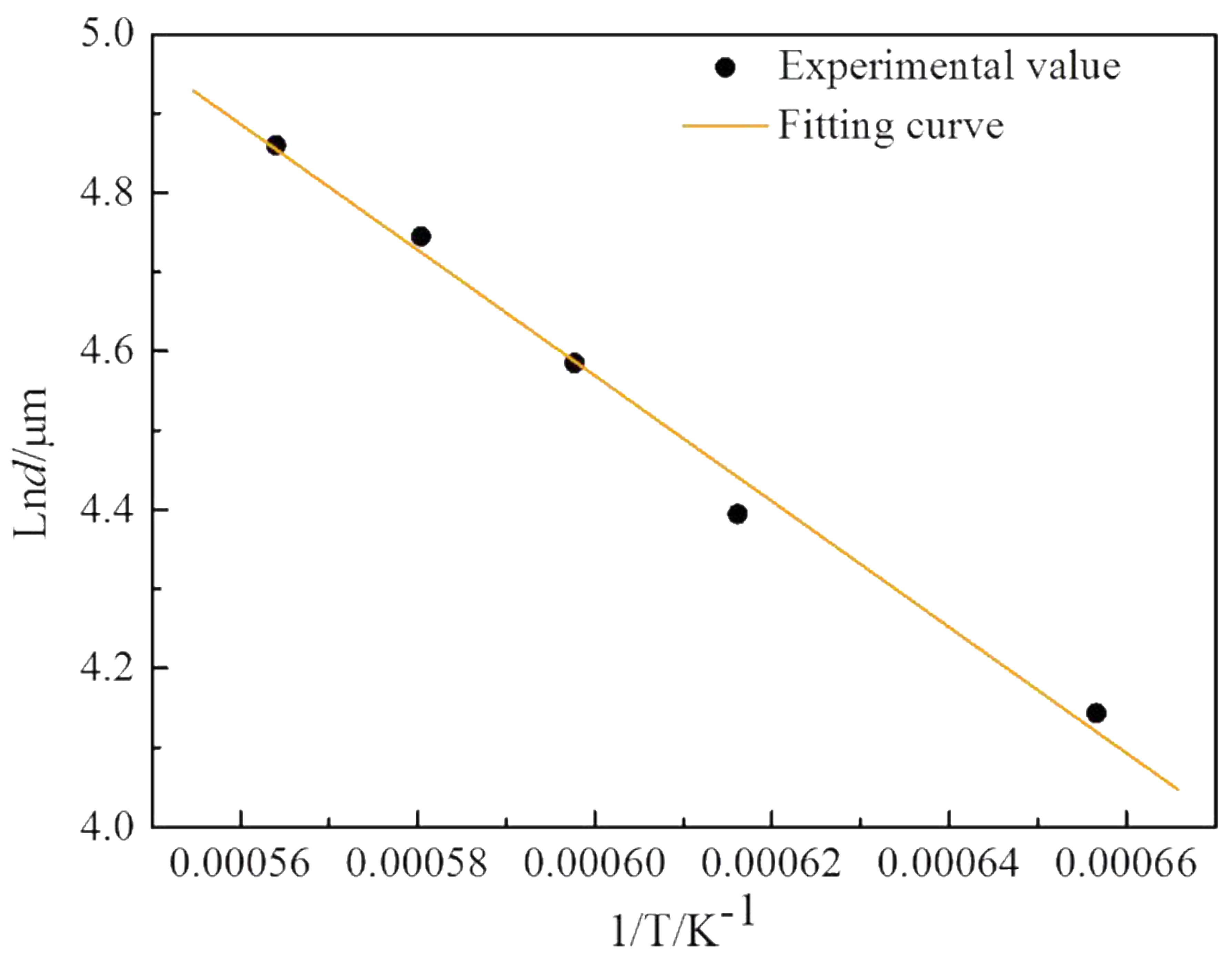
3.2.3. Kinetic Equations of Interface Layer Growth
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yan, S.; Song, G.L.; Li, Z.; Wang, H.; Zheng, D.; Cao, F.; Horynova, M.; Dargusch, M.S.; Zhou, L. A state-of-the-art review on passivation and biofouling of Ti and its alloys in marine environments. J. Mater. Sci. Technol. 2018, 34, 421–435. [Google Scholar] [CrossRef]
- Han, G.; Lu, X.; Xia, Q.; Lei, B.; Yan, Y.; Shang, C.J. Face-centered-cubic titanium-A new crystal structure of Ti in a Ti- 8Mo-6Fe alloy. J. Alloys Compd. 2018, 748, 943–952. [Google Scholar] [CrossRef]
- Wu, B.T.; Pan, Z.X.; Li, S.Y.; Cuiuri, D.; Ding, D.H.; Li, H.J. The anisotropic corrosion behaviour of wire arc additive manufactured Ti-6Al-4V alloy in 3.5% NaCl solution. Corros. Sci. 2018, 137, 176–183. [Google Scholar] [CrossRef]
- Çomaklı, O.; Yazıcı, M.; Yetim, T.; Yetim, A.F.; Çelik, A. Effect of Ti amount on wear and corrosion properties of Ti-doped Al2O3 nanocomposite ceramic coated CP titanium implant material. Ceram. Int. 2018, 44, 7421–7428. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Wu, J.Y.; Shi, G.P.; Xu, H.Q. Comparison of the homemade and commercial graphene in heightening mechanical properties of Al2O3 ceramic. Ceram. Int. 2017, 43, 2143–2149. [Google Scholar] [CrossRef]
- Shi, G.P.; Zhang, L.; Wang, Z.; Li, L.; Zhai, P.; Li, Q.G. Effects of Nb doping on the mechanical properties and interfacial reactions of Ti/Al2O3 composites. Ceram. Int. 2018, 44, 14913–14919. [Google Scholar] [CrossRef]
- Guo, X.Y.; Zhang, Y.; Jung, Y.G.; Li, L.; Knapp, J.; Zhang, J. Ideal tensile strength and shear strength of ZrO2(111)/Ni(111) ceramic-metal interface: A first principle study. Mater. Des. 2016, 112, 254–262. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Knowles, K.M.; Mallinson, P.M.; Fernie, J.A. Microstructural evolution and characterization of interfacial phases in Al2O3/Ag-Cu-Ti/Al2O3 braze joints. Acta Mater. 2015, 96, 143–158. [Google Scholar] [CrossRef]
- Feng, S.; Wang, Z.; Shi, G.P.; Ma, Y.; Liu, L.H. Phase Analysis and Mechanical Properties of Ti/Al2O3 Composite Prepared by Hot Pressing. Rare Met. Mater. Eng. 2013, 42, 392–395. [Google Scholar]
- Shi, G.P.; Wang, Z.; Liu, H.H.; Wang, C.G. Influence of Y2O3 addition on the Ti/Al2O3 cermets by hot pressing sintering. J. Alloys Compd. 2014, 628, 413–415. [Google Scholar] [CrossRef]
- Lu, H.; Bao, C.L.; Shen, D.H.; Zhang, X.J. Study of the Ti/Al2O3 interface. J. Mater. Sci. 1995, 30, 339–346. [Google Scholar] [CrossRef]
- Ji, H.X.; Jones, S.; Marquis, P.M. Characterization of the interaction between molten titanium alloy and Al2O3. J. Mater. Sci. 1995, 30, 5617–5620. [Google Scholar] [CrossRef]
- Chaug, Y.S.; Chou, N.J.; Kim, H. Interaction of Ti with fused silica and sapphire during metallization. J. Vac. Sci. Technol. 1987, 5, 1288–1292. [Google Scholar] [CrossRef]
- Selverian, J.H.; Ohuchi, F.S.; Bortz, M.; Notis, M.R. Interface reactions between titanium thin films and (112) sapphire substrates. J. Mater. Sci. 1991, 26, 6300–6308. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Z.; Li, Q.G.; Shi, G.P.; Ma, G.P.Y.; Liu, L.H. Fabrication of laminated Ti/Al2O3 composite by vacuum hot-pressing sintering. J. Ceram. Soc. Jpn. 2014, 122, 222–225. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.J.; Wang, Z.; Wu, J.Y.; Li, Q.G. Ti/Al2O3 interfacial diffusion: Kinetic equation for growth of reaction layer and formation mechanism. J. Alloys Compd. 2015, 652, 260–265. [Google Scholar] [CrossRef]
- Liu, M.J.; Wang, Z.; Luan, X.W.; Wu, J.Y.; Li, Q.G. Effects of CeO2 and Y2O3 on the interfacial diffusion of Ti/Al2O3 composites. J. Alloys Compd. 2016, 656, 929–935. [Google Scholar] [CrossRef]
- Wu, B.H. Research on Reaction Mechanism and Diffusion Reaction Kinetics of Ti/Al. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 5 June 2013. [Google Scholar]
- Cui, Y.W.; Xu, G.L.; Chen, Y.; Tang, B.; Li, J.S.; Zhou, L. Computational diffusion kinetics and its applications in study and design of rare metallic materials. Chin. Sci. Bull. 2013, 58, 3680–3691. [Google Scholar] [CrossRef]
- Zeng, Y.W. Basic Science of Inorganic Materials; Wuhan University of Technology Press: Wuhan, China, 2011. [Google Scholar]
- Zhou, P.Y. Theoretical Mechanics; Science Press: Beijing, China, 2012. [Google Scholar]
- Helmut, M. Diffusion in Solids; World Book Publishing House: Beijing, China, 2014. [Google Scholar]
- Luo, G.L.; Acoff, V.L. Using cold roll bonding and annealing to process Ti/Al multi-layered composites from elemental foils. Mater. Sci. Eng. A 2004, 379, 164–172. [Google Scholar] [CrossRef]
- Yao, W.; Wu, A.P.; Zou, G.S.; Ren, J.L. Formation process of the bonding joint in Ti/Al diffusion bonding. Mater. Sci. Eng. A 2008, 480, 456–463. [Google Scholar]


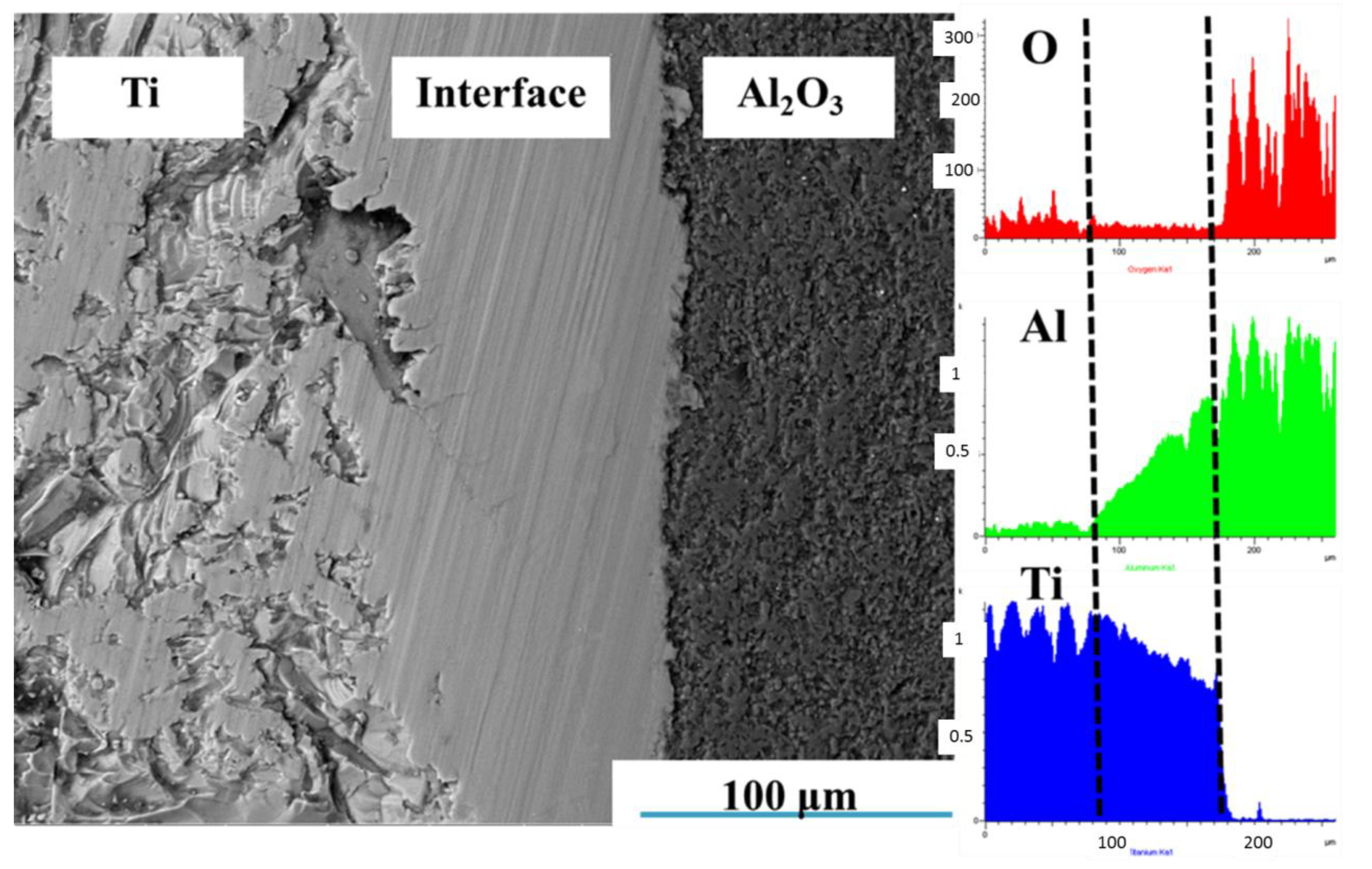

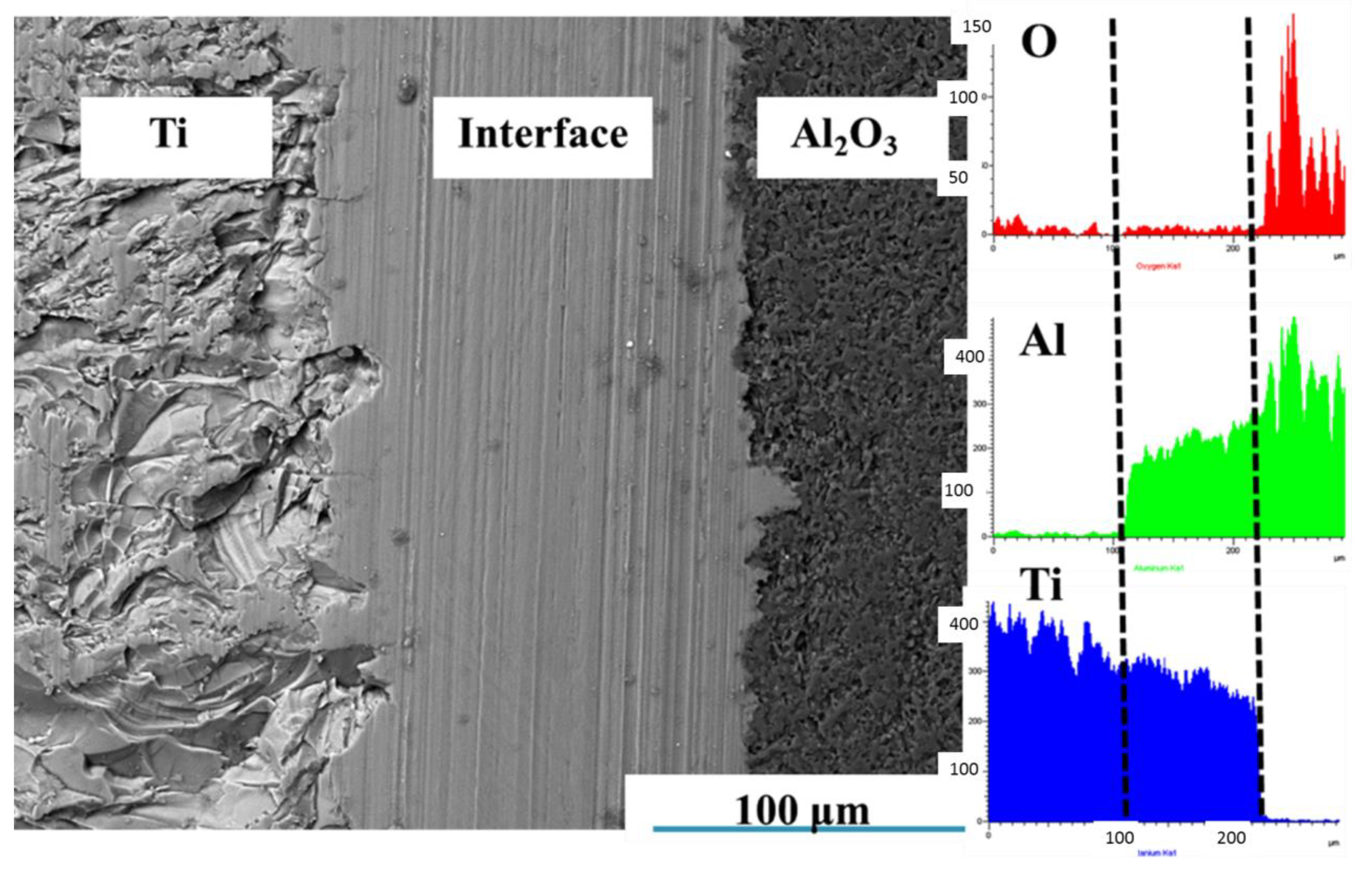
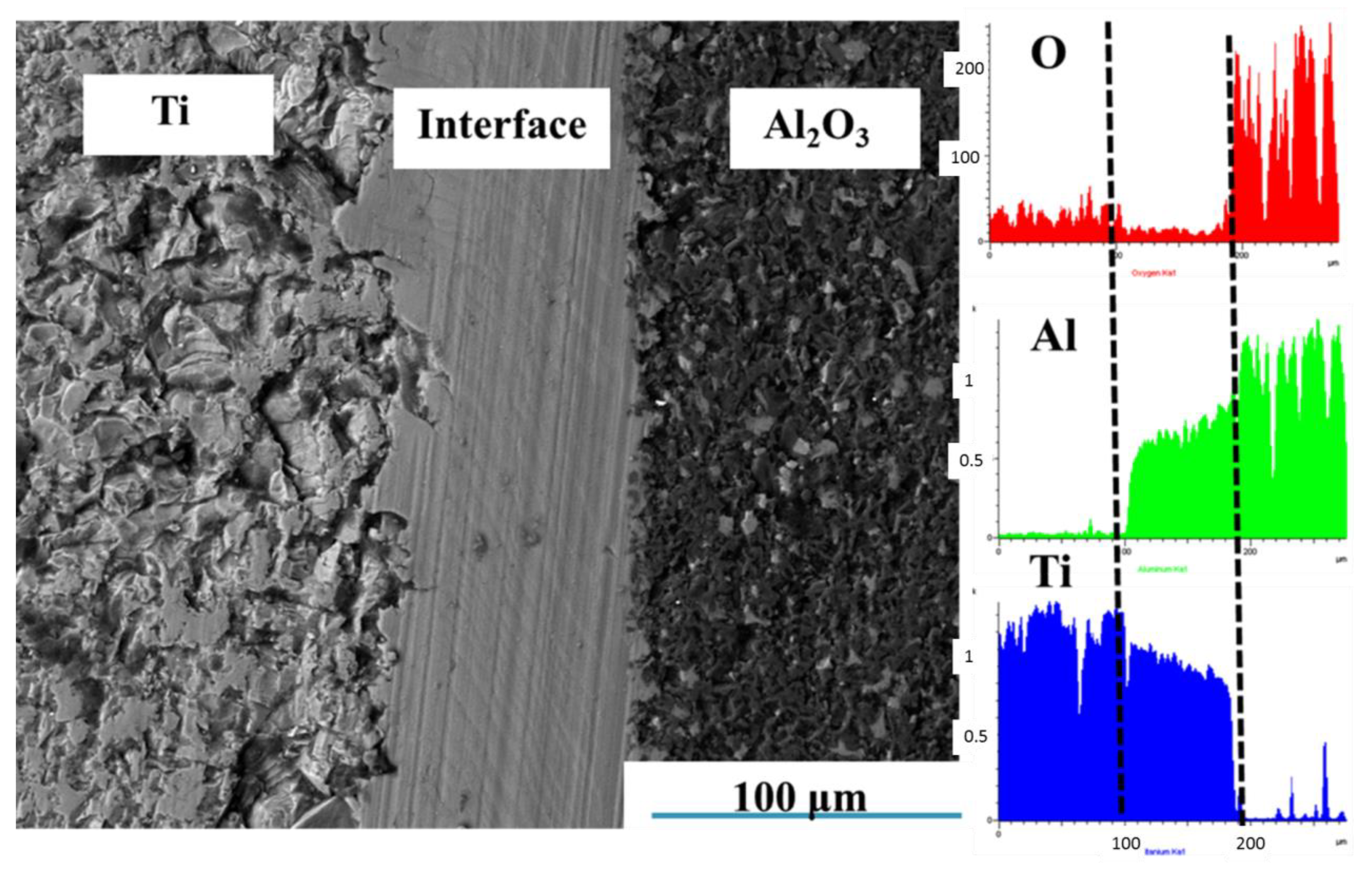


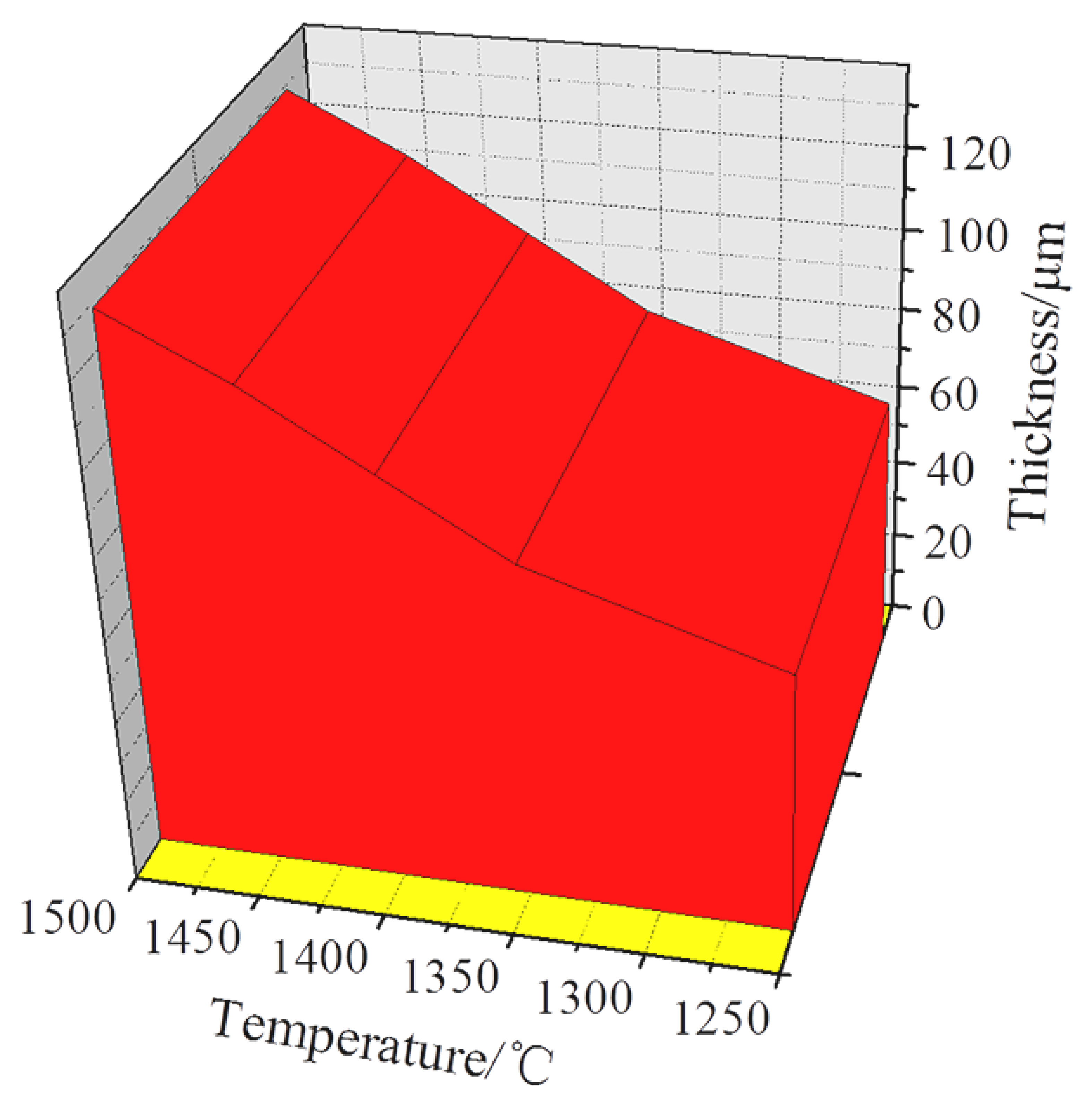
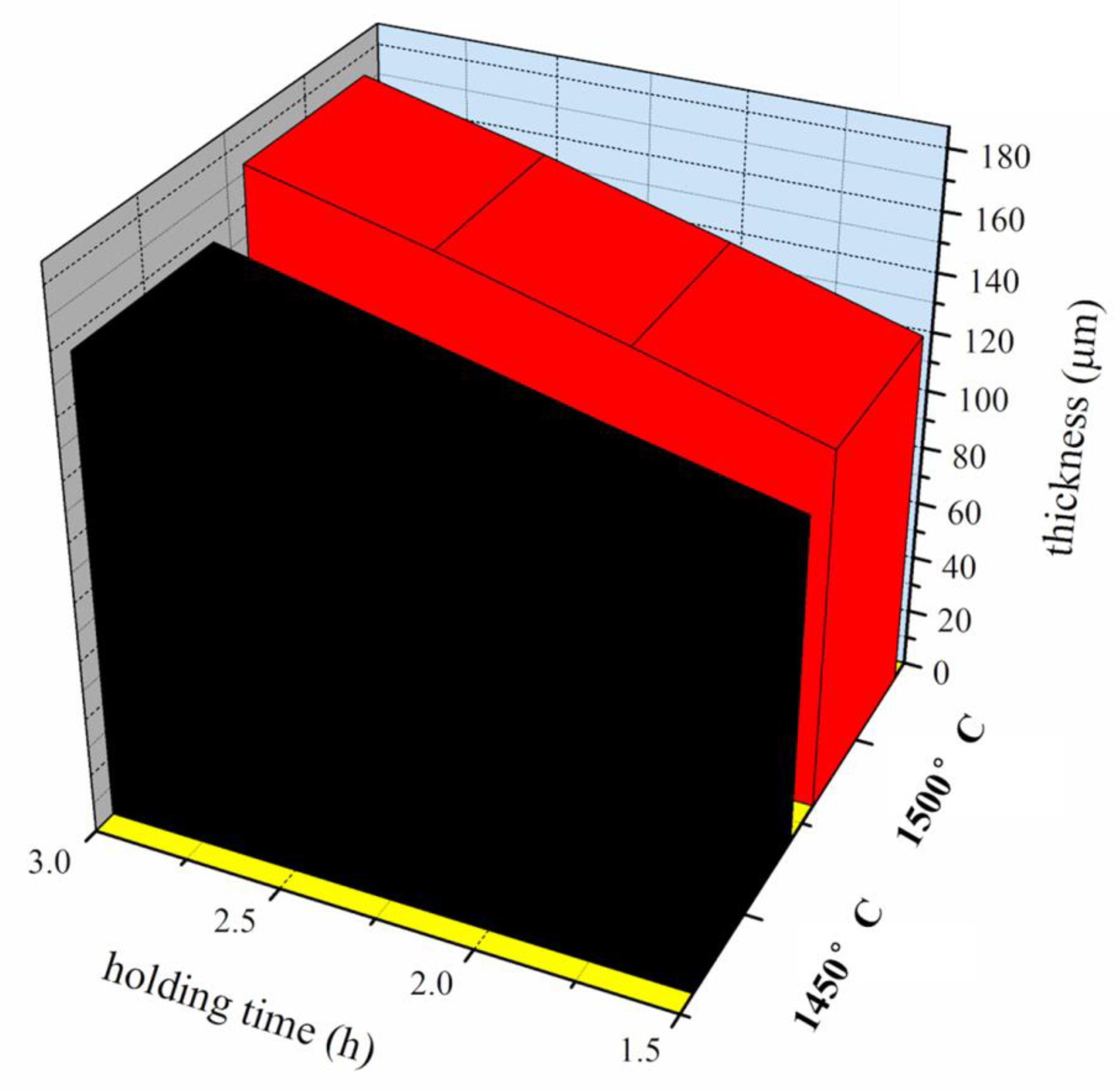
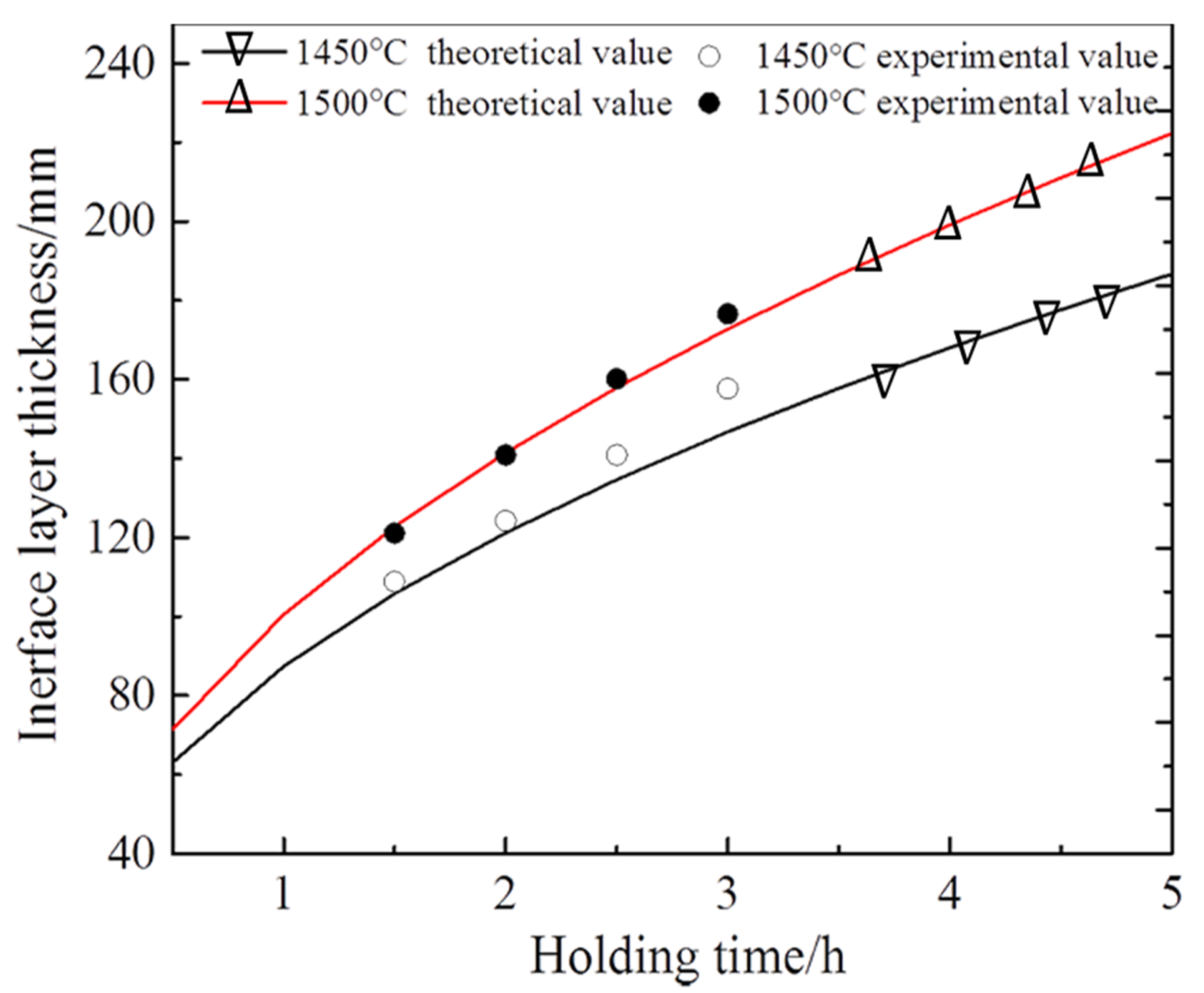
| Sintering Temperature (°C) | Holding Time (h) | Average Thickness (μm) |
|---|---|---|
| 1250 | 1.5 | 63.1 |
| 1350 | 1.5 | 81.4 |
| 1400 | 1.5 | 98.6 |
| 1450 | 1.5 | 112.3 |
| 1500 | 1.5 | 123.4 |
| Sintering Temperature (°C) | Holding Time (h) | Average Thickness (μm) |
|---|---|---|
| 1450 | 1.5 | 112.3 |
| 2.0 | 126.2 | |
| 2.5 | 141.3 | |
| 3.0 | 156.5 | |
| 1500 | 1.5 | 123.4 |
| 2.0 | 141.3 | |
| 2.5 | 158.7 | |
| 3.0 | 173.6 |
| Sintering Temperature (°C) | DAl (cm2∙s−1) | DO (cm2∙s−1) | DTi (cm2∙s−1) |
|---|---|---|---|
| 1250 | 7.37 × 10−9 | 4.71 × 10−11 | - |
| 1350 | 1.23 × 10−8 | 1.85 × 10−10 | 1.67 × 10−11 |
| 1400 | 1.80 × 10−8 | 3.63 × 10−10 | 4.17 × 10−11 |
| 1450 | 2.46 × 10−8 | 5.22 × 10−10 | 4.62 × 10−11 |
| 1500 | 3.09 × 10−8 | 7.07 × 10−10 | 4.81 × 10−11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, G.; Zhang, L.; Wang, Z. Modelling the Elements Reaction–Diffusion Behavior on Interface of Ti/Al2O3 Composite Prepared by Hot Pressing Sintering. Metals 2020, 10, 259. https://doi.org/10.3390/met10020259
Shi G, Zhang L, Wang Z. Modelling the Elements Reaction–Diffusion Behavior on Interface of Ti/Al2O3 Composite Prepared by Hot Pressing Sintering. Metals. 2020; 10(2):259. https://doi.org/10.3390/met10020259
Chicago/Turabian StyleShi, Guopu, Liu Zhang, and Zhi Wang. 2020. "Modelling the Elements Reaction–Diffusion Behavior on Interface of Ti/Al2O3 Composite Prepared by Hot Pressing Sintering" Metals 10, no. 2: 259. https://doi.org/10.3390/met10020259





