A Review on Adhesively Bonded Aluminium Joints in the Automotive Industry
Abstract
:1. Introduction
2. Adhesive Joints in Automotive
2.1. Advantages
2.2. Disadvantages
3. Epoxy Resin
3.1. Chemical Properties
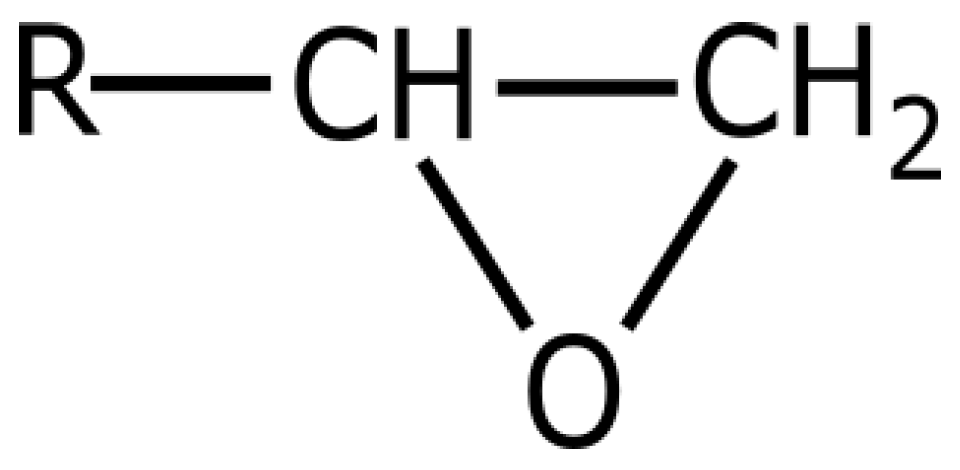
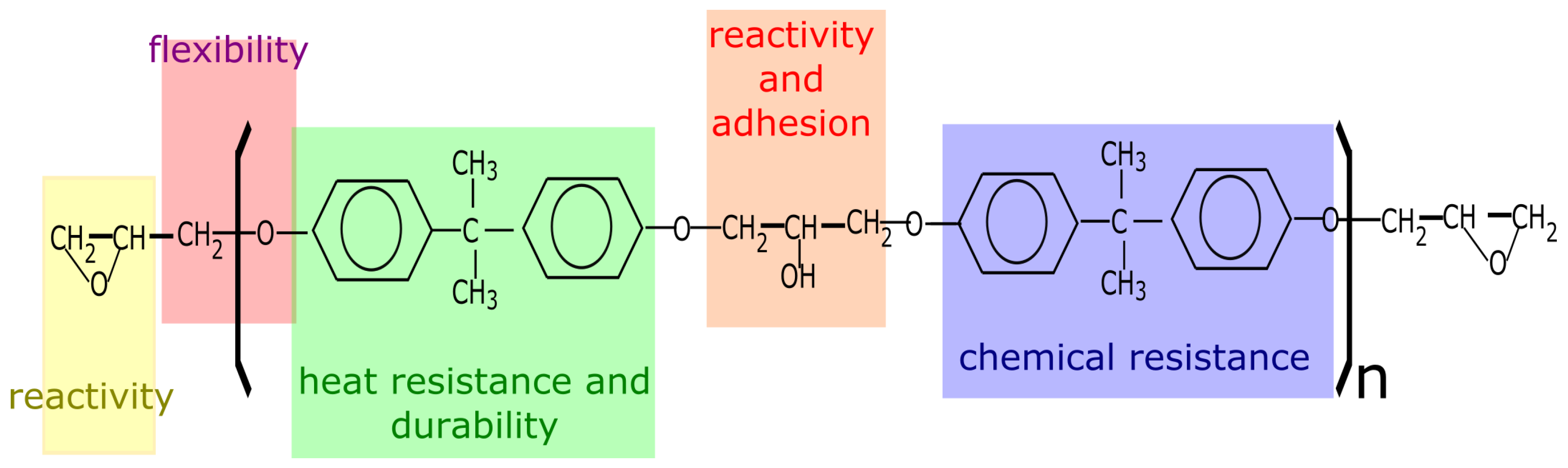
- the epoxy groups at both terminals of the molecule and the hydroxyl groups at the midpoint of the molecule are highly reactive
- the outstanding adhesion of epoxy resin is largely due to the secondary hydroxyl groups located along the molecular chain, the epoxy groups are generally consumed during cure
- the large part of the epoxy resin backbone contains aromatic rings, which provide a high heat and chemical resistance
- the aliphatic sequence between epoxy linkages confer chemical resistance and flexibility
- the epoxy molecule can be of different molecular weight and chemistry. Resins can be low viscosity liquids or hard solids.
- a large variety of polymeric structures can be obtained depending on the polymerization reaction and the curing agents involved. This can lead to versatile resins that can cure slowly or very quickly at room or at elevated temperatures.
3.2. Curing Mechanisms
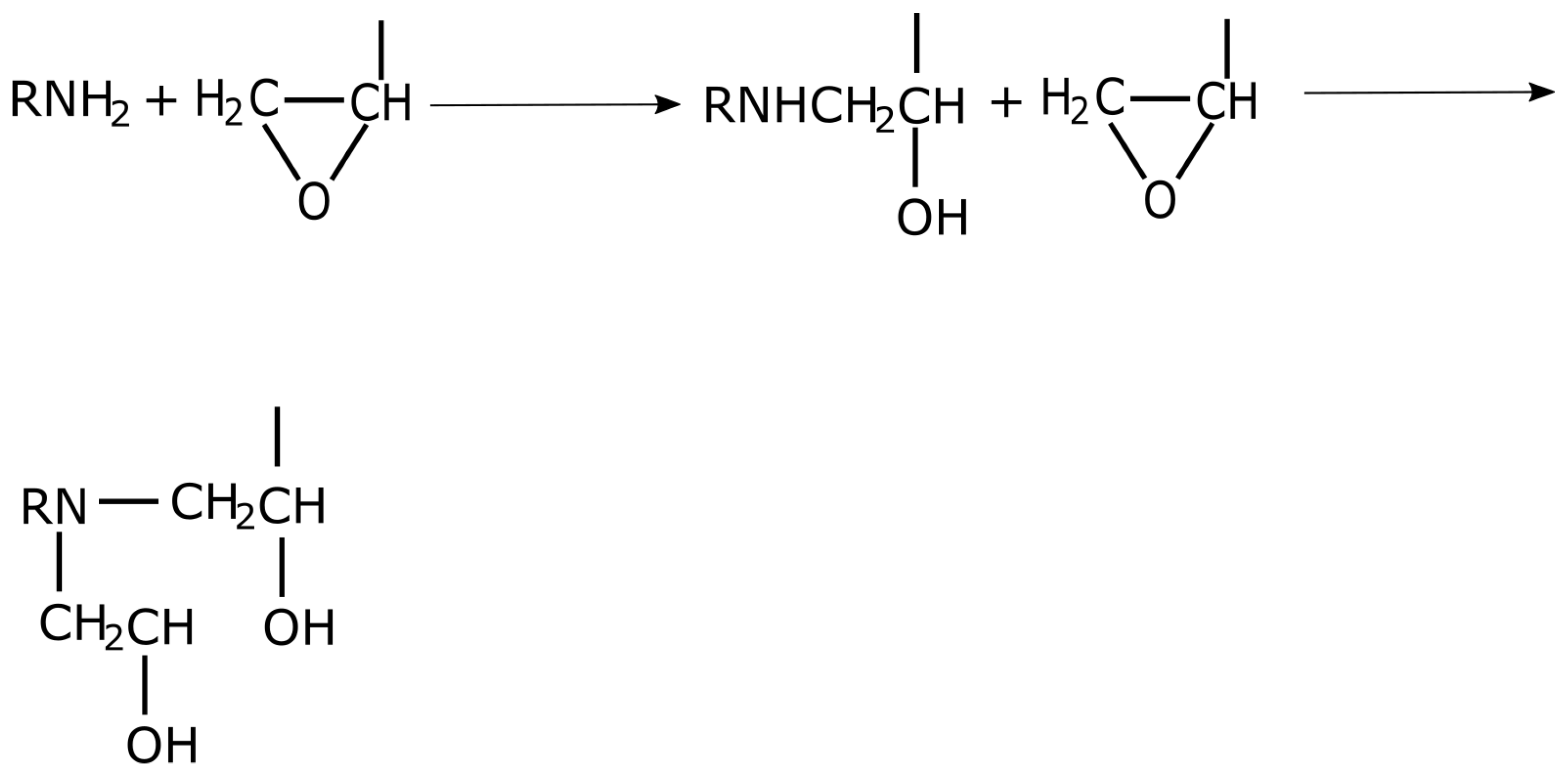
3.3. Amine Curing Agents
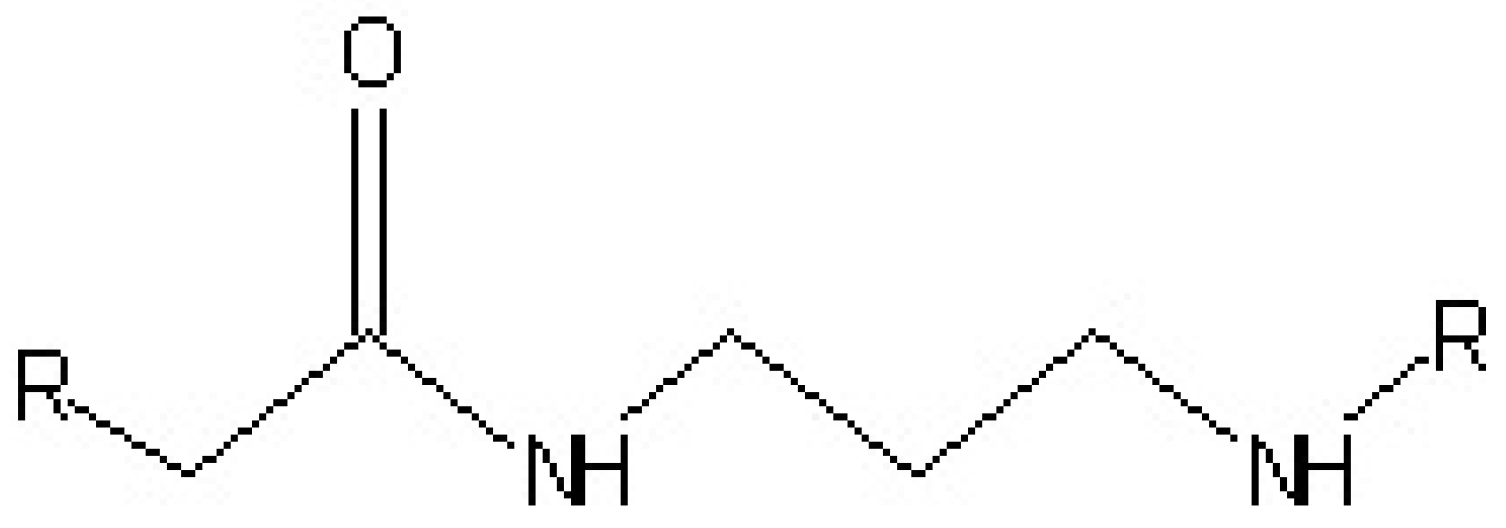
3.4. Polyamide Resins Curing Agents
3.5. Imidazoles Curing Agents
3.6. Anhydrides Curing Agents
3.7. Latent Curing Agents
3.8. Epoxy Additives
- Diluents They are used to reduce viscosity for both the ease of processability and allowing a greater incorporation of formulatory ingredients. Diluents are used as well to improve wettability [14]. Examples of diluents for epoxy resins include: phenylglycidyl ether, butylglycidyl ether, allylglycidyl ether, butanediol diglycidyl ether and glycerol-based epoxy resins [21].
- Fillers They are the most common ingredient used in the majority of the epoxy formulations. Hundreds of different fillers can be used to modify specific properties of the epoxy. Even if fillers are considered beneficial for most of the applications, the disadvantage is the increase of density (and therefore weight) and viscosity which can influence the way in which the formulation behaves. Table 2 shows a non exhaustive list of fillers which have been used in epoxy formulations [14].
- Resinous modifiers Resinous materials are sometimes used together with epoxy to reduce the cost or to impart property modifications. Adding resinous materials such as nylon to epoxy has shown to increase the toughness enough to be used as structural adhesives. However, due to the presence of hygroscopic constituents, the use of these systems is limited as they lead to durability problems in presence of moisture [14].
- Flexibilising/plasticising additives Another way to overcome brittle behaviour from adhesives, besides using elastomers or fillers, is by incorporation of plasticising or flexibilising additives. The difference between them is that while plasticisers are long-chain non-reactive molecules, which are not incorporated into the epoxy network, flexibilisers react with the epoxy system during cure [22]. Examples of plasticizers are phthalates or bisphenol A diglycidyl ether, while among flexibilizers there are thermoplastic polymers such as polyvinyl ethers or polyurethanes [23].
- Miscellaneous additives In addition to the additives described above, there are other additives which can be added to epoxy systems. Here two examples are shown. It is a common practice for some epoxy manufacturers to add in the epoxy some coupling agents (such as organosilanes). By adding the coupling agent to the epoxy and not on the substrate, a step in the preparation of the substrate can be skipped. Another example is the use of “expanding monomers” which help reduce the shrinkage occuring during cure [24].
4. Aluminium Surface Preparation Prior to Bonding
- Remove the weak boundary layers, including the weak oxide layers formed by heat treatment or exposure to humid atmosphere, air-borne contamination and protective oils and greases
- Enhance the molecular contact between the adhesive and the substrate to promote the formation of intrinsic adhesion
- Create a continuous film on the oxide layer which has a high stability over a wide pH range, protects against hydration, create a barrier against corrosion.Adhesive bonding is a technology applicable for various product forms such as sheets, extrusion and casting. For different aluminium products the preparation procedure or application products may somehow differ but the essential steps are still the same [5]. In the following sections the case of aluminium sheets is considered.
4.1. The Need for Surface Preparation and the Weak Boundary Layer Theory
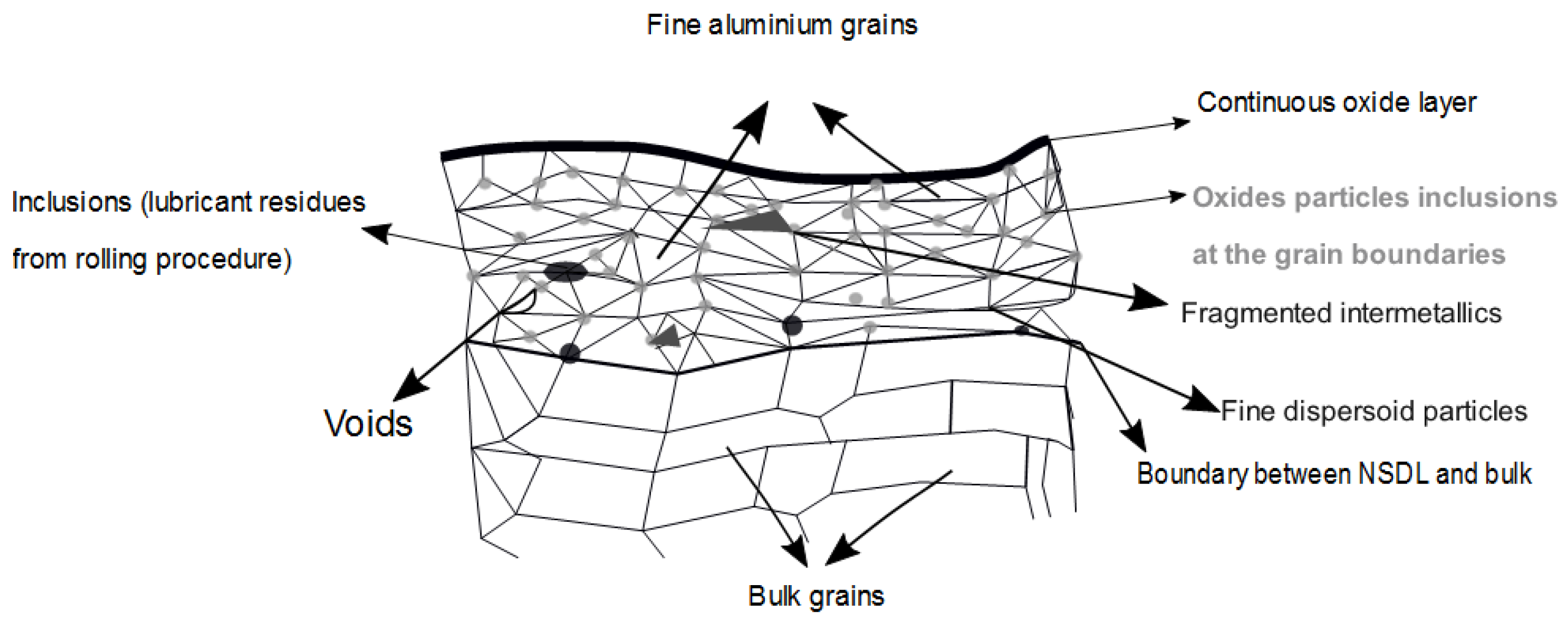
4.2. The Aluminium Substrate after the Rolling Process
4.3. Cleaning Step
4.4. Chemical Surface Pre-Treatments Options in Automotive
- Metal ions and inorganic molecules which react with or precipitate on the oxidized Al to form a mixed oxide
- Coupling agents which promote adhesion
- Anodised film, which modify the aluminium oxide
4.5. The Effect of the Stamping Lubricant
5. Adhesion Theory
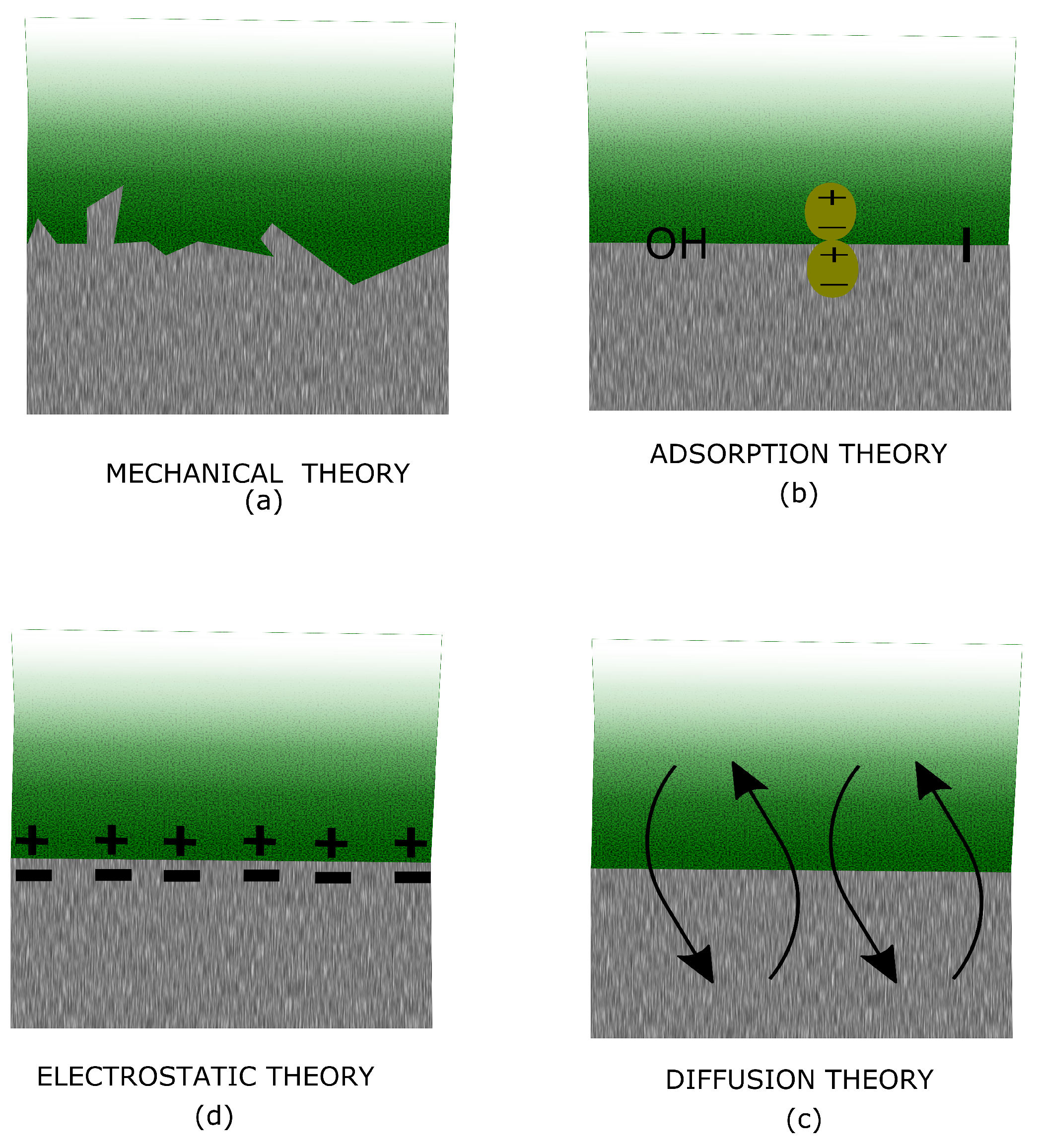
5.1. Mechanical Theory
5.2. Adsorption Theory
5.2.1. Van der Waals Interactions
5.2.2. Chemical Bonds
5.2.3. Acid-Base Theory
- Unhydroxylated metal ions, M
- Protonated surface hydroxyls,
- Surface hydroxyls, −
- Unhydroxylated oxygen anions,
- Dissociated surface hydroxyls,
- Surface hydroxyls,
5.3. Diffusion Theory
5.4. Electrostatic Theory
6. Environmental Degradation of Adhesive Joints
6.1. The Effect of Water
6.1.1. The Effect of Water on The Adhesive
- causing plasticization by altering the properties of the adhesive in a reversible way
- causing the adhesive to crack, craze or hydrolize, in this case the properties of the adhesive are altered in an irreversible manner
- attacking the adhesive-adherent interface
- causing stress due to swelling
6.1.2. The Effect of Water at the Adhesive Metal Oxide Interface
6.1.3. Adhesive Joint Strength in Humid Environment
6.2. The Effect of Corrosion on the Durability of the Adhesive Joints
6.2.1. Filiform Corrosion
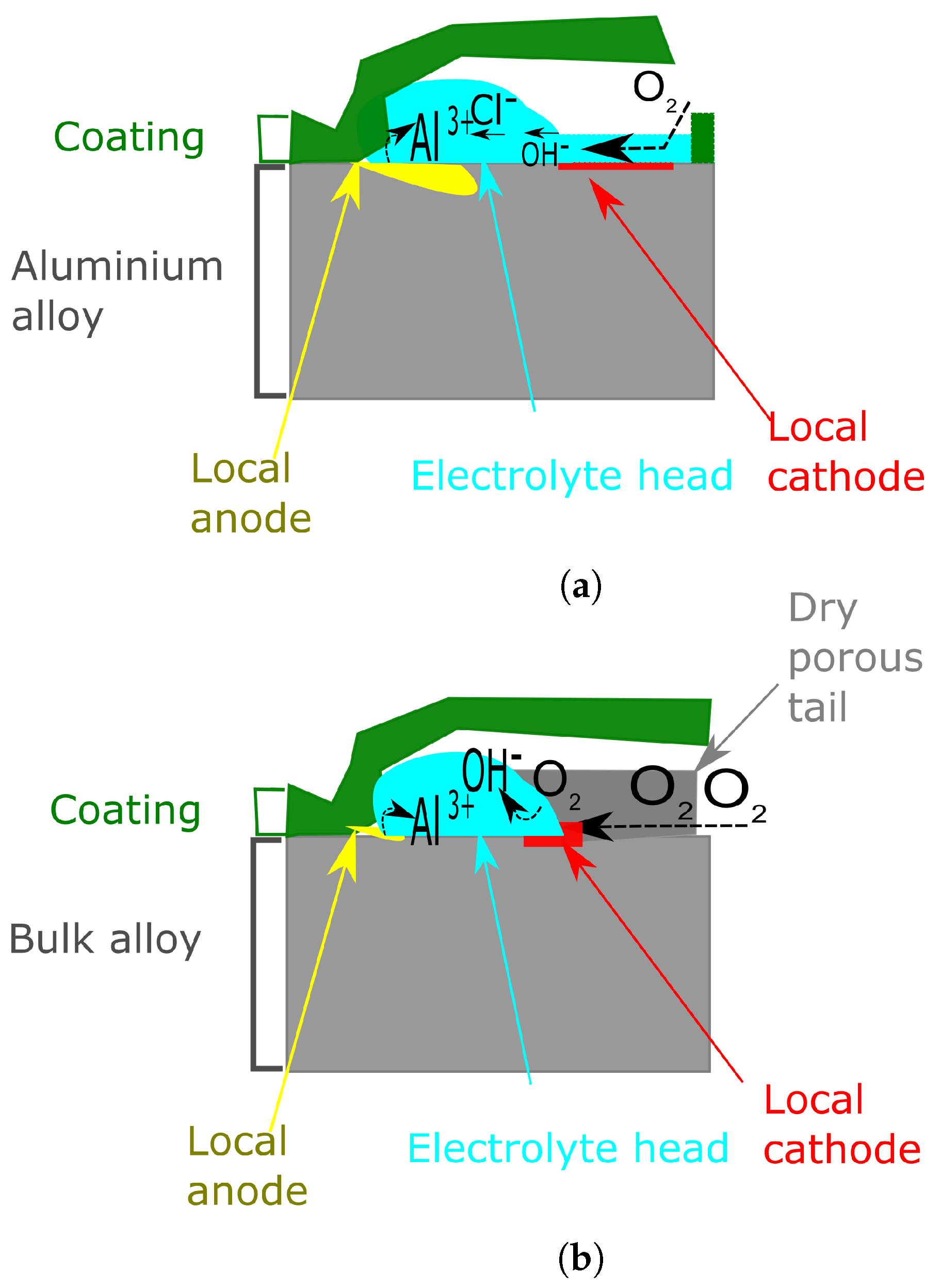
6.2.2. The Effect of the Near-Surface Deformed Layer on Filiform Corrosion
- Area 1, not corroded with NSDL still present
- Area 2, anodic site with dissolution of the NSDL
- Area 3, in which the NSDL is com ely dissolved and the bulk aluminium acts as cathodic O2 reduction site
- Area 4, comprising a dry porous tail as described in the previous section.
6.3. The Effect of Static and Dynamic Stresses on the Durability of the Adhesive Joints
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Njuguna, J. Lightweight Composite Structures in Transport: Design, Manufacturing, Analysis and Performance; Elsevier Science: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Hirsch, J. Recent development in aluminium for automotive applications. Trans. Nonferr. Met. Soc. China 2014. [Google Scholar] [CrossRef]
- Crolla, D.; Ribbens, W.; Heisler, H.; Blundell, M.; Harty, D.; Brown, J.; Serpento, S.; Robertson, A.; Garrett, T.; Fenton, J.; et al. Automotive Engineering e-Mega Reference; Elsevier Science: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Barnes, T.; Pashby, I. Joining techniques for aluminium spaceframes used in automobiles. J. Mater. Process. Technol. 2002, 99, 72–79. [Google Scholar] [CrossRef]
- European Aluminium Association. EAA Aluminium Automotive Manual—Joining. In The Aluminium Automotive Manual; European Aluminium Association: Ljubljana, Slovenia, 2015; pp. 1–5. [Google Scholar]
- Adams, R. Adhesive Bonding: Science, Technology and Applications; Woodhead Publishing Series in Welding and Other Joining Technologies; Elsevier Science: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Schwartz, M. Adhesive Bonding; CRC Press: Boca Raton, FL, USA, 2010; pp. 11–52. [Google Scholar] [CrossRef]
- Brockmann, W.; Geiß, P.; Klingen, J.; Mikhail, B.; Schröder, K. Adhesive Bonding: Materials, Applications and Technology; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Omar, M. The Automotive Body Manufacturing Systems and Processes; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Dunn, D. Update on Engineering and Structural Adhesives; Smithers Rapra Technology: Akron, OH, USA, 2010; Volume 15. [Google Scholar]
- Ozer, H. Applied Adhesive Bonding in Science and Technology; IntechOpen: London, UK, 2018. [Google Scholar]
- Adams, R.; Adams, R.; Comyn, J.; Wake, W.; Wake, W. Structural Adhesive Joints in Engineering; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- May, C. Epoxy Resins: Chemistry and Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Ellis, B. Chemistry and Technology of Epoxy Resins; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Dodiuk, H.; Goodman, S.H. Handbook of Thermoset Plastics; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–768. [Google Scholar] [CrossRef]
- Petrie, E. Epoxy Adhesive Formulations; McGraw-Hill Education: New York, NY, USA, 2005. [Google Scholar]
- Hara, O. Curing agents for epoxy resins. In Chemistry and Technology of Epoxy Resins; Springer: Berlin/Heidelberg, Germany, 1993; pp. 37–71. [Google Scholar] [CrossRef]
- Kent, J. Riegel’s Handbook of Industrial Chemistry; Springer: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Kulshreshtha, A.; Vasile, C. Handbook of Polymer Blends and Composites; Number vol. 1 Handbook of Polymer Blends and Composites; Rapra Technology: Akron, OH, USA, 2002. [Google Scholar]
- Fink, J. Reactive Polymers: Fundamentals and Applications: A Concise Guide to Industrial Polymers; Plastics Design Library; Elsevier Science: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Licari, J.; Swanson, D. Adhesives Technology for Electronic Applications: Materials, Processing, Reliability; Materials and Processes for Electronic Applications; Elsevier Science: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Hamerton, I. Recent Developments in Epoxy Resins; Smithers Rapra Technology: Akron, OH, USA, 1996. [Google Scholar]
- Minges, M.; Committee, A. Electronic Materials Handbook: Packaging; Electronic Materials Handbook; Taylor & Francis: Abingdon, UK, 1989. [Google Scholar]
- Meister, J. Polymer Modification: Principles, Techniques, and Applications; Plastics Engineering; Taylor & Francis: Abingdon, UK, 2000. [Google Scholar]
- Lunder, O. Chromate-Free Pre-Treatment of Aluminium for Adhesive Bonding. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2003. [Google Scholar]
- Da Silva, L.; Öchsner, A.; Adams, R. Handbook of Adhesion Technology; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Messler, R. Joining of Materials and Structures: From Pragmatic Process to Enabling Technology; Elsevier Science: Amsterdam, The Netherlands, 2004. [Google Scholar]
- King, R. Surface Treatment & Finishing of Aluminium; Pergamon Materials Engineering Practice Series; Elsevier Science: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Totten, G.; MacKenzie, D. Handbook of Aluminum: Volume 2: Alloy Production and Materials Manufacturing; Number vol. 2 in Handbook of Aluminum; Taylor & Francis: Abingdon, UK, 2003. [Google Scholar]
- Esposto, F.J.; Zhang, C.S.; Norton, P.R.; Timsit, R.S. Segregation of Mg to the surface of an AlMg single crystal alloy and its influence on the initial oxidation at room temperature. Surf. Sci. 1994, 302, 109–120. [Google Scholar] [CrossRef]
- Textor, M.; Amstutz, M. Surface analysis of thin films and interfaces in commercial aluminium products. Anal. Chim. Acta 1994, 297, 15–26. [Google Scholar] [CrossRef]
- Dunlop, H.; Benmalek, M. Role and Characterization of Surfaces in the Aluminium Industry. J. Phys. IV 1997, 7, C6–C174. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.K.; Prasanna, S.; Subramanian, B.; Jayakumar, S.; Rao, G.M. A transmission electron microscopy and X-ray photoelectron spectroscopy study of annealing induced γ-phase nucleation, clustering, and interfacial dynamics in reactively sputtered amorphous alumina thin films. J. Appl. Phys. 2015, 117. [Google Scholar] [CrossRef]
- Frolish, M.F.; Walker, J.C.; Jiao, C.; Rainforth, W.M.; Beynon, J.H. Formation and structure of a subsurface layer in hot rolled aluminium alloy AA3104 transfer bar. Tribol. Int. 2005, 38, 1050–1058. [Google Scholar] [CrossRef]
- Fishkis, M.; Lin, J.C. Formation and evolution of a subsurfacenext term layer in a metalworking process. Wear 1997, 206, 156–170. [Google Scholar] [CrossRef]
- Keuong, Y.W.; Nordlien, J.H.; Nisancioglu, K. Characterization of Electrochemically Active Surface Layers on Rolled Commercial Alloys AA8006 and AA5182 Aluminum. J. Electrochem. Soc. 2002, 148, B497. [Google Scholar] [CrossRef]
- Buytaert, G.; Terryn, H.; Van Gils, S.; Kernig, B.; Grzemba, B.; Mertens, M. Study of the near-surface of hot- and cold-rolled AlMg0.5 aluminium alloy. Surf. Interface Anal. 2005, 37, 534–543. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, X.; Thompson, G.E.; Hashimoto, T.; Scamans, G.M.; Afseth, A. Precipitation and corrosion behaviour of nano-structured near-surface layers on an AA6111 aluminium alloy. J. Phys. Conf. Ser. 2006, 26, 103–106. [Google Scholar] [CrossRef]
- Liu, Y.; Hashimoto, T.; Zhou, X.; Thompson, G.E.; Scamans, G.M.; Rainforth, W.M.; Hunter, J.A. Influence of near-surface deformed layers on filiform corrosion of AA3104 aluminium alloy. Surf. Interface Anal. 2013, 45, 1553–1557. [Google Scholar] [CrossRef]
- Li, K.; Zhou, X.R.; Thompson, G.E.; Hunter, J.A.; Yuan, Y.D. Evolution of Near-Surface Deformed Layers on AA3104 Aluminium Alloy. Mater. Sci. Forum 2013, 765, 358–362. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, X.; Thompson, G.E.; Hunter, J.A.; Yuan, Y. Delamination of near-surface layer on cold rolled AlFeSi alloy during sheet forming. Mater. Charact. 2015, 99, 109–117. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Thompson, G.E.; Scamans, G.M.; Skeldon, P.; Hunter, J.A. Near-surface deformed layers on rolled aluminum alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2011, 42, 1373–1385. [Google Scholar] [CrossRef]
- Afseth, A.; Nordlien, J.H.; Scamans, G.M.; Nisancioglu, K. Influence of heat treatment and surface conditioning on filiform corrosion of aluminium alloys AA3005 and AA5754. Corros. Sci. 2001, 43, 2359–2377. [Google Scholar] [CrossRef]
- Afseth, A.; Nordlien, J.H.; Scamans, G.M.; Nisancioglu, K. Effect of thermo-mechanical processing on filiform corrosion of aluminium alloy AA3005. Corros. Sci. 2002, 44, 2491–2506. [Google Scholar] [CrossRef]
- Afseth, A.; Nordlien, J.H.; Scamans, G.M.; Nisancioglu, K. Effect of heat treatment on filiform corrosion of aluminium alloy AA3005. Corros. Sci. 2001, 43, 2093–2109. [Google Scholar] [CrossRef]
- Kinloch, A.J.; Bishop, H.E.; Smart, N.R. Surface Analysis and Bonding of Aluminium-Magnesium Alloys. J. Adhes. 1982, 14, 105–118. [Google Scholar] [CrossRef]
- Kozma, L.; Olefjord, I. Basic processes of surface preparation and bond formation of adhesively joined aluminium. Mater. Sci. Technol. 1987, 3, 860–874. [Google Scholar] [CrossRef]
- Scamans, G.M.; Afseth, A.; Thompson, G.E.; Liu, Y.; Zhou, X.R. Corrosion of Painted Aluminium Sheet. Mater. Sci. Forum 2009, 519–521, 647–654. [Google Scholar] [CrossRef]
- Buchheit, R. Critical Factors for the Transition from Chromate to Chromate-Free Corrosion Protection; Technical Report; The Ohio State University: Columbus, OH, USA, 2005. [Google Scholar]
- Milošev, I.; Frankel, G.S. Review—Conversion Coatings Based on Zirconium and/or Titanium. J. Electrochem. Soc. 2018, 165, C127–C144. [Google Scholar] [CrossRef]
- Andreatta, F.; Turco, A.; de Graeve, I.; Terryn, H.; de Wit, J.H.; Fedrizzi, L. SKPFM and SEM study of the deposition mechanism of Zr/Ti based pre-treatment on AA6016 aluminum alloy. Surf. Coat. Technol. 2007, 201, 7668–7685. [Google Scholar] [CrossRef]
- Karmaschek, U.; Roland, A.; Vennshott, H.; Wennemann, E. Chromium-Free Conversion Coating Treatment of Aluminum. U.S. Patent 55,849,46A, 17 May 1994. [Google Scholar]
- Deck, P.D.; Moon, M.; Sujdak, R.J. Investigation of fluoacid based conversion coatings on aluminum. Prog. Org. Coat. 1997, 34, 39–48. [Google Scholar] [CrossRef]
- Hubert, H.; Puderbach, H.; Pulm, H.; Roland, W.A. Investigations on zirconium-containing conversion layers on aluminium. Fresenius’ Z. Anal. Chem. 1989, 333, 304–307. [Google Scholar] [CrossRef]
- Posner, R.; Fink, N.; Wolpers, M.; Grundmeier, G. Electrochemical electrolyte spreading studies of the protective properties of ultra-thin films on zinc galvanized steel. Surf. Coat. Technol. 2013, 228, 286–295. [Google Scholar] [CrossRef]
- Andreatta, F.; Lanzutti, A.; Paussa, L.; Fedrizzi, L. Addition of phosphates or copper nitrate in a fluotitanate conversion coating containing a silane coupling agent for aluminium alloy AA6014. Prog. Org. Coat. 2014, 77, 2107–2115. [Google Scholar] [CrossRef]
- Paloumpa, I.; Yfantis, A.; Hoffmann, P.; Burkov, Y.; Yfantis, D.; Schmeißer, D. Mechanisms to inhibit corrosion of Al alloys by polymeric conversion coatings. Surf. Coat. Technol. 2004, 180–181, 308–312. [Google Scholar] [CrossRef]
- Wang, L.; Peng, B.; Guo, X.; Ding, W.; Chen, Y. Ferric molybdate nanotubes synthesized based on the Kirkendall effect and their catalytic property for propene epoxidation by air. Chem. Commun. 2009, 1565–1567. [Google Scholar] [CrossRef]
- Smit, M.; Hunter, J.; Sharman, J.; Scamans, G.; Sykes, J. Effect of organic additives on the performance of titanium-based conversion coatings. Corros. Sci. 2003, 45, 1903–1920. [Google Scholar] [CrossRef]
- Zakir, M.; Ashraf, U.; Tian, T.; Han, A.; Qiao, W.; Jin, X.; Zhang, M.; Tsoi, J.K.H.; Matinlinna, J.P. The Role of Silane Coupling Agents and Universal Primers in Durable Adhesion to Dental Restorative Materials—A Review. Curr. Oral Health Rep. 2016, 3, 244–253. [Google Scholar] [CrossRef]
- Comyn, J. Adhesion Science; RSC Paperbacks; Royal Society of Chemistry: London, UK, 1997. [Google Scholar]
- Van Ooij, W.J.; Zhu, D.; Stacy, M.; Seth, A.; Mugada, T.; Gandhi, J.; Puomi, P. Corrosion protection properties of organofunctional silanes—An overview. Tsinghua Sci. Technol. 2005, 10, 639–664. [Google Scholar] [CrossRef]
- Hauffman, T.; Hubin, A.; Terryn, H. Study of the self-assembling of n-octylphosphonic acid layers on aluminum oxide from ethanolic solutions. Surf. Interface Anal. 2013, 45, 1435–1440. [Google Scholar] [CrossRef]
- Bulusu, A.; Paniagua, S.A.; Macleod, B.A.; Sigdel, A.K.; Berry, J.J.; Olson, D.C.; Marder, S.R.; Graham, S. Efficient modification of metal oxide surfaces with phosphonic acids by spray coating. Langmuir 2013, 29, 3935–3942. [Google Scholar] [CrossRef] [PubMed]
- Luschtinetz, R.; Oliveira, A.F.; Frenzel, J.; Joswig, J.O.; Seifert, G.; Duarte, H.A. Adsorption of phosphonic and ethylphosphonic acid on aluminum oxide surfaces. Surf. Sci. 2008, 602, 1347–1359. [Google Scholar] [CrossRef]
- Raman, A.; Dubey, M.; Gouzman, I.; Gawalt, E.S. Formation of self-assembled monolayers of alkylphosphonic acid on the native oxide surface of SS316L. Langmuir 2006, 22, 6469–6472. [Google Scholar] [CrossRef]
- Textor, M.; Ruiz, L.; Hofer, R.; Rossi, A.; Feldman, K.; Hähner, G.; Spencer, N.D. Structural chemistry of self-assembled monolayers of octadecylphosphoric acid on tantalum oxide surfaces. Langmuir 2000, 16, 3257–3271. [Google Scholar] [CrossRef]
- Grundmeier, G.; Schmidt, W.; Stratmann, M. Corrosion protection by organic coatings: Electrochemical mechanism and novel methods of investigation. Electrochim. Acta 2000, 45, 2515–2533. [Google Scholar] [CrossRef]
- Thissen, P.; Valtiner, M.; Grundmeier, G. Stability of phosphonic acid self-assembled monolayers on amorphous and Single-crystalline aluminum oxide surfaces in aqueous solution. Langmuir 2010, 26, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Rupper, P.; Gaan, S. Recent Development in Phosphonic Acid-Based Organic Coatings on Aluminum. Coatings 2017, 7, 133. [Google Scholar] [CrossRef] [Green Version]
- Rowe, J. Advanced Materials in Automotive Engineering; Woodhead Publishing in Materials; Elsevier Science: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Correia, S.; Anes, V.; Reis, L. Effect of surface treatment on adhesively bonded aluminium-aluminium joints regarding aeronautical structures. Eng. Fail. Anal. 2018, 84, 34–45. [Google Scholar] [CrossRef]
- Meiler, M.; Jaschke, H. Lubrication of aluminium sheet metal within the automotive industry. Adv. Mater. Res. 2005, 6–8, 551–558. [Google Scholar] [CrossRef]
- Debski, M.; Shanahan, M.; Schultz, J. Mechanisms of contaminant elimination by oil-accommodating adhesives Part 1: Displacement and absorption. Int. J. Adhes. Adhes. 1986, 6, 145–149. [Google Scholar] [CrossRef]
- Debski, M.; Shanahan, M.E.R.; Schultz, J. Mechanisms of contaminant elimination by oil-accommodating adhesives Part 2: A model of the processes involved. Int. J. Adhes. Adhes. 1986, 6, 150–152. [Google Scholar] [CrossRef]
- Ogawa, T.; Ochiai, K.; Masuichi, M. Simulation Analyses on the Diffusion of a Rust-Preventing Oil Into an Oil-Accommodating Adhesive. J. Adhes. 1995, 49, 83–96. [Google Scholar] [CrossRef]
- Greiveldinger, M.; Shanahan, M.E.; Jacquet, D.; Verchère, D. Oil-covered substrates: A model study of the evolution in the interphase during cure of an epoxy adhesive. J. Adhes. 2000, 73, 179–195. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, H.P.; Hicks, C.; Yang, X.; Carlson, B.E.; Zhou, Q. Experimental study of initial strengths and hygrothermal degradation of adhesive joints between thin aluminum and steel substrates. Int. J. Adhes. Adhes. 2013, 43, 14–25. [Google Scholar] [CrossRef]
- Zheng, R.; Lin, J.; Wang, P.C.; Wu, Q.; Wu, Y. Effects of a sheet metal stamping lubricant on static strength of adhesive-bonded aluminum alloys. J. Adhes. Sci. Technol. 2015, 29, 1382–1402. [Google Scholar] [CrossRef]
- Packham, D.E.; Van Ooij, W.J.; Anderson, H.R.; Mittel, K.L. The mechanical theory of adhesion—A seventy year perspective and its current status. In First International Congress on Adhesion Science and Technology; CRC Press: Boca Raton, FL, USA, 1998; pp. 81–108. [Google Scholar]
- Brockmann, W.; Hennemann, O.D.; Kollek, H.; Matz, C. Adhesion in bonded aluminium joints for aircraft construction. Int. J. Adhes. Adhes. 1986, 6, 115–143. [Google Scholar] [CrossRef]
- Venables, J.; McNamara, D.; Chen, J.; Sun, T.; Hopping, R. Oxide morphologies on aluminum prepared for adhesive bonding. Appl. Surf. Sci. 1979, 3, 88–98. [Google Scholar] [CrossRef]
- Yendall, K.A.; Critchlow, G.W. Novel methods, incorporating pre- and post-anodising steps, for the replacement of the Bengough–Stuart chromic acid anodising process in structural bonding applications. Int. J. Adhes. Adhes. 2009, 29, 503–508. [Google Scholar] [CrossRef] [Green Version]
- Pizzi, A.; Mittal, K. Handbook of Adhesive Technology, Revised and Expanded; Taylor & Francis: Abingdon, UK, 2003. [Google Scholar]
- Boutar, Y.; Naïmi, S.; Mezlini, S.; Ali, M.B.S. Effect of surface treatment on the shear strength of aluminium adhesive single-lap joints for automotive applications. Int. J. Adhes. Adhes. 2016, 67, 38–43. [Google Scholar] [CrossRef]
- Ishida, H.; Koenig, J.L. Molecular Organization of the Coupling Agent Interphase of Fiber-Glass Reinforced Plastics. J. Polym. Sci. Polym. Phys. Ed. 1979, 17, 1807–1813. [Google Scholar] [CrossRef]
- Plueddemann, E.P. Adhesion Through Silane Coupling Agents. J. Adhes. 1970, 2, 184–201. [Google Scholar] [CrossRef]
- Mohseni, M.; Mirabedini, M.; Hashemi, M.; Thompson, G. Adhesion performance of an epoxy clear coat on aluminum alloy in the presence of vinyl and amino-silane primers. Prog. Org. Coat. 2006, 57, 307–313. [Google Scholar] [CrossRef]
- Jang, J.; Kim, E.K. Corrosion Protection of Epoxy-Coated Steel Using Different Silane Coupling Agents. J. Appl. Polym. Sci. 1999, 71, 585–593. [Google Scholar] [CrossRef]
- Romero, M.A.; Chabert, B.; Claude, U.; Lyon, B. IR Spectroscopy Approach for the Study of Interactions Between an Oxidized Aluminium Surface and a Poly(Propylene-g-Acrylic Acid) Film. J. Appl. Polym. Sci. 1993, 47, 543–554. [Google Scholar] [CrossRef]
- Konstadinidis, K.; Thakkar, B.; Chakraborty, A.; Potts, L.W.; Tannenbaum, R.; Tirrell, M.; Evans, J.F. Segment Level Chemistry and Chain Conformation in the Reactive Adsorption of Poly ( methy1 methacrylate ) on Aluminum Oxide Surfaces. Langmuir 1992, 8, 1307–1317. [Google Scholar] [CrossRef]
- Ulren, L.; Hjertberg, T.; Ishida, H. An FT-IR Study on Interfacial Interactions in Ethylene Copolymers/Aluminium Laminates in Relation to Adhesion Properties. J. Adhes. 1990, 31, 117–136. [Google Scholar] [CrossRef]
- Vermohlen, K.; Lewandowski, H.; Narres, H.; Koglin, E. Adsorption of polyacrylic acid on aluminium oxide: DRIFT spectroscopy and ab initio calculations. Colloids Surf. A 2000, 170, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Tannenbaum, R.; King, S.; Lecy, J.; Tirrell, M.; Potts, L. Infrared Study of the Kinetics and Mechanism of Adsorption of Acrylic Polymers on Alumina Surfaces. Langmuir 2004, 20, 4507–4514. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.R.; Payan, S.; Duc, T.M. Interfacial Interactions of Plasma-polymerized Acrylic Acid and an Oxidized Aluminium Surface Investigated using XPS, FTIR and Poly(acrylic acid) as a Model Compound. Surf. Interface Anal. 1998, 26, 961–973. [Google Scholar] [CrossRef]
- Pletincx, S.; Trotochaud, L.; Fockaert, L.L.; Mol, J.M.; Head, A.R.; Karslloǧlu, O.; Bluhm, H.; Terryn, H.; Hauffman, T. In situ characterization of the initial effect of water on molecular interactions at the interface of organic/inorganic hybrid systems. Sci. Rep. 2017, 7, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Brand, J.V.D.; Blajiev, O.; Beentjes, P.C.J.; Terryn, H.; Wit, J.H.W.D. Interaction of Anhydride and Carboxylic Acid Compounds with Aluminum Oxide Surfaces Studied Using Infrared Reflection Absorption Spectroscopy. Langmuir 2004, 20, 6308–6317. [Google Scholar] [CrossRef]
- Fowkes, F.M. Acid-Base Interactions in Polymer Adhesion. Tribol. Ser. 1981, 7, 119–137. [Google Scholar] [CrossRef]
- Lee, L. Fundamentals of Adhesion; Language of Science; Springer: New York, NY, USA, 2013. [Google Scholar]
- Lewis, G.N. Valence and the Structure of Atoms and Molecules; The Chemical Catalog Company: New York, NY, USA, 1923. [Google Scholar]
- Van Den Brand, J. On the Adhesion Between Aluminium and Polymers. Ph.D. Thesis, Technische Universiteit Delft, Delft, The Netherlands, 6 October 2004. [Google Scholar]
- McCafferty, E. Lewis acid/Lewis base effects in corrosion and polymer adhesion at aluminum surfaces. J. Electrochem. Soc. 2003, 150, 342–347. [Google Scholar] [CrossRef]
- Prolongo, S.; Ureña, A. Effect of surface pre-treatment on the adhesive strength of epoxy–aluminium joints. Int. J. Adhes. Adhes. 2009, 29, 23–31. [Google Scholar] [CrossRef]
- Abrahami, S.; Hauffman, T.; Kok, J.; Terryn, H.; Mol, J. The role of acid-base properties in the interactions across the oxide-primer interface in aerospace applications. Surf. Interface Anal. 2015. [Google Scholar] [CrossRef]
- Okanat, O.; Wit, F.; Dewit, J.; Terryn, H.; Mol, J.M.C. Influence of pretreatments and aging on the adhesion performance of epoxy-coated aluminum. Surf. Coat. Technol. 2013, 215, 260–265. [Google Scholar] [CrossRef]
- Lopez, S.; Petit, J.P.; Dunlop, H.M.; Butruille, J.R.; Tourillon, G. Acid-base properties of passive films on aluminum: I. A photoelectrochemical study. J. Electrochem. Soc. 1998, 145, 823–829. [Google Scholar] [CrossRef]
- Lopez, S.; Petit, J.P.; Dunlop, H.M.; Butruille, J.R.; Tourillon, G. Acid-base properties of passive films on aluminum: II. An X-ray Photoelectron Spectroscopy and X-ray Absorption Near Edge Structure Study. J. Electrochem. Soc. 1998, 145, 829–834. [Google Scholar] [CrossRef]
- Nakamae, K.; Nishino, T.; Airu, X.; Asaoka, S. Localization of the curing agent at an epoxy resin/oxidized aluminium interface. Int. J. Adhes. Adhes. 1995, 15, 15–20. [Google Scholar] [CrossRef]
- Hong, S.G.; Tsai, J.S. Adsorption and curing behaviors of the epoxy/aminoamine system in the presence of metal oxides. J. Therm. Anal. Calorim. 2001, 63, 31–46. [Google Scholar] [CrossRef]
- Abel, M.L.; Rattana, A.; Watts, J.F. Interaction of epoxy analogue molecules with organosilane-treated aluminum: A study by XPS and ToF-SIMS. Langmuir 2000, 16, 6510–6518. [Google Scholar] [CrossRef]
- Schreiber, H.P.; Ouhlal, A. Polymer diffusion and the evolution of adhesive bond strength. J. Adhes. 2003, 79, 141–153. [Google Scholar] [CrossRef]
- Landrock, A.; Ebnesajjad, S. Adhesives Technology Handbook; Plastics Design Library; Elsevier Science: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Lee, L. Adhesive Bonding; Springer: New York, NY, USA, 2013. [Google Scholar]
- Randow, C.L.; Williams, C.A.; Ward, T.C.; Dillard, D.A.; Dillard, J.G.; Wightman, J.P. An Investigation of the Cling of Thin Polymeric Films. J. Adhes. 1997, 63, 285–307. [Google Scholar] [CrossRef]
- Weitsman, Y. Stress assisted diffusion in elastic and viscoelastic materials. J. Mech. Phys. Solids 1987, 35, 73–93. [Google Scholar] [CrossRef]
- Morsch, S.; Lyon, S.; Greensmith, P.; Smith, S.D.; Gibbon, S.R. Mapping water uptake in organic coatings using AFM-IR. Faraday Discuss. 2015, 180, 527–542. [Google Scholar] [CrossRef]
- Xu, S.; Dillard, D.A.; Dillard, J. Environmental aging effects on the durability of electrically conductive adhesive joints. Int. J. Adhes. Adhes. 2003, 23, 235–250. [Google Scholar] [CrossRef]
- Viana, G.; Costa, M.; Banea, M.D.; da Silva, L.F. Water diffusion in double cantilever beam adhesive joints. Lat. Am. J. Solids Struct. 2017, 14, 188–201. [Google Scholar] [CrossRef]
- Gledhill, R.A.; Kinloch, A.J. Environmental Failure of Structural Adhesive Joints. J. Adhes. 1974, 6, 315–330. [Google Scholar] [CrossRef]
- Da Silva, L.; Sato, C. Design of Adhesive Joints Under Humid Conditions; Advanced Structured Materials; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Pletincx, S.; Marcoen, K.; Trotochaud, L.; Fockaert, L.L.; Mol, J.M.; Head, A.R.; Karslioǧlu, O.; Bluhm, H.; Terryn, H.; Hauffman, T. Unravelling the chemical influence of water on the PMMA/Aluminum oxide hybrid interface in situ. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pletincx, S.; Mol, J.M.; Terryn, H.; Hubin, A.; Hauffman, T. An in situ spectro-electrochemical monitoring of aqueous effects on polymer/metal oxide interfaces. J. Electroanal. Chem. 2019, 848, 113311. [Google Scholar] [CrossRef]
- Abrahami, S.T.; Hauffman, T.; De Kok, J.M.; Mol, J.M.; Terryn, H. Effect of Anodic Aluminum Oxide Chemistry on Adhesive Bonding of Epoxy. J. Phys. Chem. C 2016, 120, 19670–19677. [Google Scholar] [CrossRef]
- Brewis, D.M.; Comyn, J.; Tegg, J.L. The uptake of water vapour by an epoxide adhesive formed from the diglycidyl ether of bisphenol-A and di-(1-aminopropyl-3-ethoxy) ether. Polymer 1980, 21, 134–138. [Google Scholar] [CrossRef]
- Brewis, D.M.; Comyn, J.; Tegg, J.L. The durability of some epoxide adhesive-bonded joints on exposure to moist warm air. Int. J. Adhes. Adhes. 1980, 1, 35–39. [Google Scholar] [CrossRef]
- Brewis, D.M.; Comyn, J.; Cope, B.C.; Moloney, A.C. Effect of carriers on the performance of aluminum alloy joints bonded with a structural film adhesive. Polym. Eng. Sci. 1981, 21, 797–803. [Google Scholar] [CrossRef]
- Mubashar, A.; Ashcroft, I.A.; Critchlow, G.W.; Crocombe, A.D. Moisture absorption-desorption effects in adhesive joints. Int. J. Adhes. Adhes. 2009, 29, 751–760. [Google Scholar] [CrossRef] [Green Version]
- Gledhill, R.A.; Kinloch, A.J.; Shaw, S.J. A Model for Predicting Joint Durability. J. Adhes. 1980, 11, 3–15. [Google Scholar] [CrossRef]
- Wang, S.; Min, J.; Lin, J.; Wu, Y. Effect of neutral salt spray (NSS) exposure on the lap-shear strength of adhesive-bonded 5052 aluminum alloy (AA5052) joints. J. Adhes. Sci. Technol. 2019, 33, 549–560. [Google Scholar] [CrossRef]
- Critchlow, G.W.; Brewis, D.M. A comparison of chromate-phosphate and chromate-free conversion coatings for adhesive bonding. J. Adhes. 1997, 61, 213–230. [Google Scholar] [CrossRef]
- Lunder, O.; Lapique, F.; Johnsen, B.; Nisancioglu, K. Effect of pre-treatment on the durability of epoxy-bonded AA6060 aluminium joints. Int. J. Adhes. Adhes. 2004, 24, 107–117. [Google Scholar] [CrossRef]
- Hua, D.; Lin, J.; Zhang, B. Effects of salt spray on the mechanical properties of aluminum-epoxy adhesive joints. J. Adhes. Sci. Technol. 2013, 27, 1580–1589. [Google Scholar] [CrossRef]
- Wang, C.; Huang, Y.D.; Xv, H.Y.; Liu, W.B. The durability of adhesive/carbon-carbon composites joints in salt water. Int. J. Adhes. Adhes. 2004, 24, 471–477. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, J.; Wang, P.C.; Zheng, R.; Wu, Q. Effect of long-term neutral salt spray exposure on durability of adhesive-bonded Zr-Ti coated aluminum joint. Int. J. Adhes. Adhes. 2016, 64, 97–108. [Google Scholar] [CrossRef]
- McCafferty, E. Introduction to Corrosion Science; Springer: New York, NY, USA, 2010. [Google Scholar]
- Shaw, B.; Buchheit, R.; Moran, J.; Division, E.S.C.; Meeting, E.S. Corrosion and Corrosion Prevention of Low Density Metals and Alloys: Proceedings of the International Symposium; Proceedings (Electrochemical Society); Electrochemical Society: Pennington, NJ, USA, 2001. [Google Scholar]
- Vargel, C. Corrosion of Aluminium; Elsevier Science: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Williams, G.; McMurray, H.N.; Hayman, D.; Morgan, P.C. Time-lapse potentiometric imaging of active filiform corrosion using a scanning Kelvin probe technique. PhysChemComm 2001, 4, 1–6. [Google Scholar] [CrossRef]
- Coleman, A.; McMurray, H.; Williams, G.; Afseth, A.; Scamans, G.M. Filiform Corrosion on 6000 Series Aluminium: Kinetics and Inhibition Strategies. Mater. Sci. Forum 2006, 519–521, 629–634. [Google Scholar] [CrossRef]
- Coleman, A.J.; McMurray, H.N.; Williams, G.; Afseth, A.; Scamans, G.M. Inhibition of Filiform Corrosion on AA6111-T4 Using In-Coating Phenylphosphonic Acid. Electrochem. Solid-State Lett. 2007, 10, C35. [Google Scholar] [CrossRef]
- Liu, Y.; Laurino, A.; Hashimoto, T.; Zhou, X.; Skeldon, P.; Thompson, G.E.; Scamans, G.M.; Blanc, C.; Rainforth, W.M.; Frolish, M.F. Corrosion behaviour of mechanically polished AA7075-T6 aluminium alloy. Surf. Interface Anal. 2010, 42, 185–188. [Google Scholar] [CrossRef]
- McMurray, H.N.; Holder, A.; Williams, G.; Scamans, G.M.; Coleman, A.J. The kinetics and mechanisms of filiform corrosion on aluminium alloy AA6111. Electrochim. Acta 2010, 55, 7843–7852. [Google Scholar] [CrossRef]
- Institute, W.; Cho, G. The Automotive Industry: Core Research from TWI; Core Research from TWI; Elsevier Science: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Marceau, J.A.; McMillan, J. Exploratory Development on Durability of Adhesive Bonded Joints; Technical Report; Boeing Commercial Airplane Company: Seattle, WA, USA, 1976. [Google Scholar]
- Wahab, M.; Ashcroft, I.; Crocombe, A.; Hughes, D.; Shaw, S. The effect of environment on the fatigue of bonded composite joints. Part 2: Fatigue threshold prediction. Compos. Part A Appl. Sci. Manuf. 2001, 32, 59–69. [Google Scholar] [CrossRef]
- So, H.W.; Chen, N.N.; Niem, P.I. Fatigue performance of adhesive joints immersed in different solutions. J. Adhes. 1994, 44, 245–256. [Google Scholar] [CrossRef]
- Small, G.; Fay, P.A. Creep of adhesive lap-joints in dry and high humidity environments. In Proceedings of the Adhesion ’90, Plastic and Rubber Inst. Meetings, Cambridge, UK, 10–12 September 1990. [Google Scholar]
- Croccolo, D.; De Agostinis, M.; Mauri, P. Influence of the assembly process on the shear strength of shaft-hub hybrid joints. Int. J. Adhes. Adhes. 2013, 44, 174–179. [Google Scholar] [CrossRef]
- Croccolo, D.; De Agostinis, M.; Fini, S.; Olmi, G. Influence of the engagement ratio on the shear strength of an epoxy adhesive by push-out tests on pin-and-collar joints: Part II: Campaign at different temperature levels. Int. J. Adhes. Adhes. 2016, 67, 76–85. [Google Scholar] [CrossRef]
- Da Silva, L. Modeling of Adhesively Bonded Joints; Modeling of Adhesively Bonded Joints; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]






| Curing Agent | Advantages | Disadvantages |
|---|---|---|
| Polyamides | Room temperature cure Low toxicity Good bond strength and flexibility High peel and impact strength | High formulation cost Long curing times at room temperature High viscosity Low heat and chemical resistance |
| Aliphatic amines | Room temperature cure, fast elevated temperature cure Low viscosity Low formulation cost Moderate chemical resistance | Critical mix ratios Strong skin irritant High vapor pressure Short working life, exothermic Poor bond strength above 80 C Rigid, poor peel and impact properties |
| Amidoamines | Reduced volatility Good toughness | Poor elevated temperature performance Some incompatibility with certain epoxy resins |
| Aromatic amines | Moderate heat and chemical resistance | Solid at room temperature Rigid Long elevated-temperature cures |
| Tertiary amines (catalytic curing agent) | Long pot life High heat resistance Can be used as an accelerator or as the sole curative | Long elevated-temperature cure Poor moisture resistance Rigid |
| Filler | Property Modification |
|---|---|
| Aluminium | Machinability, impact resistance, thermal conductivity, mechanical properties dimensional stability |
| Aluminia | Abrasion resistance, electrical resistivity, dimensional stability, toughness, thermal conductivity |
| Aluminium silicate | Extender, pigmentation, dimensional stability, chemical resistance |
| Aluminium trioxide | Flame retardation |
| Arsenic pentoxide | Thermal resistance |
| Barium sulphate | Extender |
| Beryllium oxide | Thermal conductivity |
| Calcium carbonate | Extender, pigmentation, dimensional stability, machinability, mechanical properties |
| Calcium sulphate | Extender, dimensional stability |
| Calcium silicate | Mechanical properties |
| Carbon black | Reinforcement, pigmentation, thermal conductivity, electrical conductivity, thermal resistance |
| Copper | Electrical conductivity, thermal conductivity, mechanical properties |
| Colloidal silica | Thixotropy |
| Fibrous glass | Impact strength |
| Graphite | Lubricity, pigmentation, thermal conductivity, electrical conductivity, abrasion resistance |
| Glass microballoons | Density reduction |
| Kaolinclay | Extender |
| Lithium aluminium silicate | Thermal expansion coefficient |
| Mica | Electrical resistance, dielectric properties, chemical resistance, toughness, moisture resistance, lubricity |
| Molybdenum disulphide | Lubricity |
| Quartz | Electrical properties, dimensional stability, extender |
| Sand | Abrasion, thermal conductivity |
| Silica | Abrasion resistance, electrical properties, extender, dimensional stability, thermal conductivity, moisture resistance |
| Silver | Electrical conductivity, thermal conductivity |
| Titanium dioxide | Pigmentation, dielectric properties, extender |
| Talc | Extender |
| Zirconium silicate | Arc resistance |
| Type of Interaction | Energy kJmol |
|---|---|
| Ionic | |
| NaCl | 503 |
| Ti O | 5340 |
| Covalent | |
| C-C | 368 |
| C-O | 377 |
| Si-O | 368 |
| C-N | 297 |
| Hydrogen bond | |
| -OH—OH (methanol) | 30 |
| -OH—N (Phenol-trimethylamine) | 35 |
| F—HF | 163 |
| Lewis acid-base | |
| BF3 + C2H5OC2H5 | 64 |
| C6H5OH + NH3 | 33 |
| van der Waals forces | |
| dipole-dipole | ≥2 |
| dipole-induced dipole | 0.05 |
| dispersion | ≥2 |
| Interface | Work of Adhesion (mJ/m2) | |
|---|---|---|
| Air | Water | |
| Epoxide\steel | 291 | −255 |
| Epoxy\aluminium | 232 | −137 |
| Epoxy\silica | 178 | −57 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavezza, F.; Boehm, M.; Terryn, H.; Hauffman, T. A Review on Adhesively Bonded Aluminium Joints in the Automotive Industry. Metals 2020, 10, 730. https://doi.org/10.3390/met10060730
Cavezza F, Boehm M, Terryn H, Hauffman T. A Review on Adhesively Bonded Aluminium Joints in the Automotive Industry. Metals. 2020; 10(6):730. https://doi.org/10.3390/met10060730
Chicago/Turabian StyleCavezza, Francesca, Matthieu Boehm, Herman Terryn, and Tom Hauffman. 2020. "A Review on Adhesively Bonded Aluminium Joints in the Automotive Industry" Metals 10, no. 6: 730. https://doi.org/10.3390/met10060730
APA StyleCavezza, F., Boehm, M., Terryn, H., & Hauffman, T. (2020). A Review on Adhesively Bonded Aluminium Joints in the Automotive Industry. Metals, 10(6), 730. https://doi.org/10.3390/met10060730




