An Overview of Molecular Dynamic Simulation for Corrosion Inhibition of Ferrous Metals
Abstract
:1. Introduction
2. Corrosion of Ferrous Metals in Acidic Solution
2.1. Corrosion Mechanism
2.2. Corrosion Inhibition of Ferrous Metal
3. Molecular Dynamics Simulation of Ferrous Metal Corrosion Inhibition
3.1. Basics of Molecular Dynamics Simulation
3.2. Steps in Performing a Molecular Dynamics Simulation for Corrosion Studies
3.2.1. Construction of a Corrosion System
3.2.2. Specifying Boundary Condition
3.2.3. Simulation Tool
3.2.4. Selection of Ensembles
3.2.5. Choosing Time Step
3.2.6. Selection of Force Field
3.3. Parameters Derived from Molecular Dynamics Simulation of Corrosion Inhibition
3.3.1. Adsorption Energy
3.3.2. Binding Energy
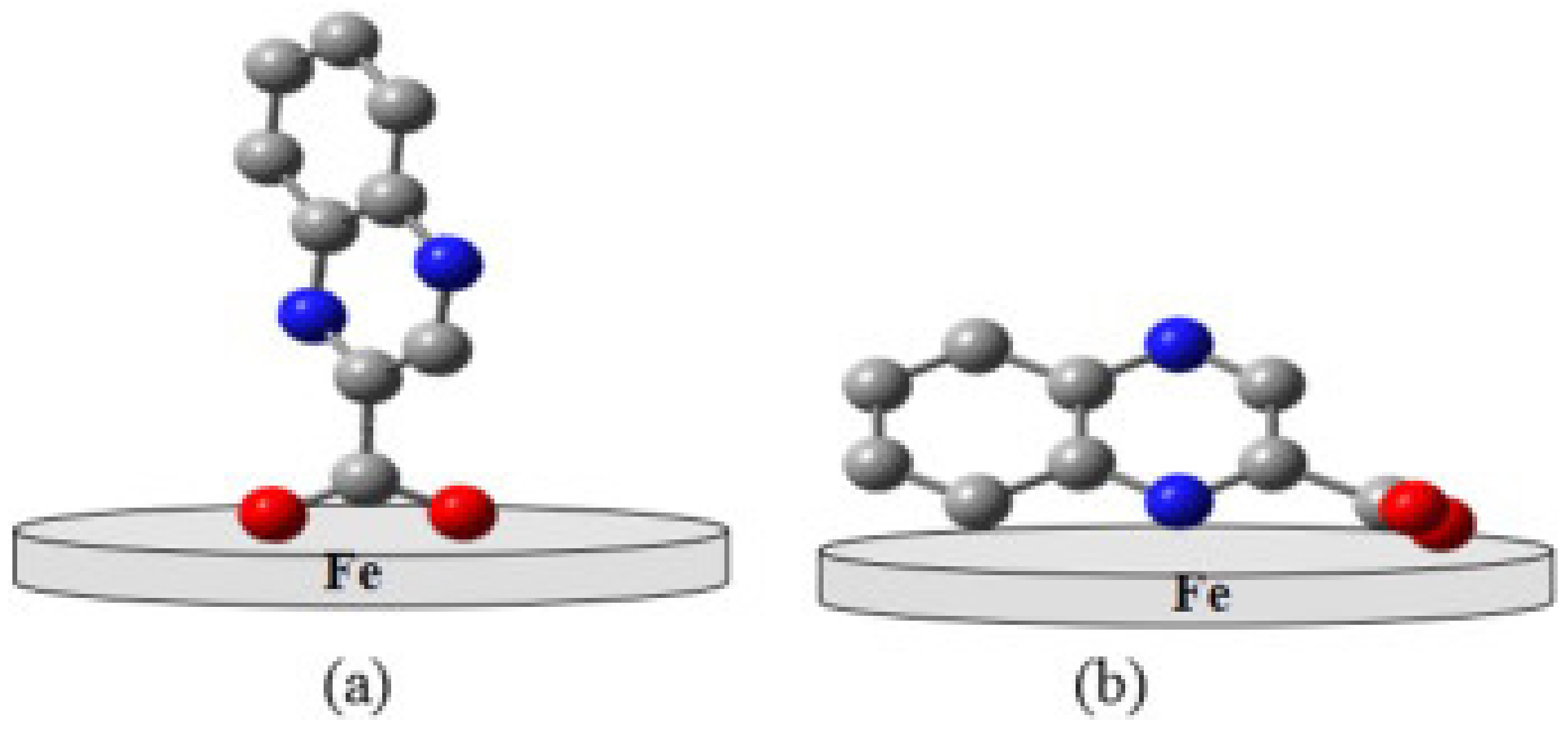
3.3.3. Inhibitor Molecule Orientation
3.4. Application of Molecular Dynamics Simulation for Corrosion Inhibition Studies of Ferrous Metal in Acidic Solution
4. Future Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bartley, J.; Huynh, N.; Bottle, S.E.; Flitt, H.; Notoya, T.; Schweinsberg, D.P. Computer Simulation of the Corrosion Inhibition of Copper in Acidic Solution by Alkyl Esters of 5-Carboxybenzotriazole. Corros. Sci. 2003, 45, 81–96. [Google Scholar] [CrossRef]
- Battimelli, G.; Ciccotti, G. Berni Alder and the Pioneering Times of Molecular Simulation. Eur. Phys. J. H 2018, 43, 303–335. [Google Scholar] [CrossRef]
- Alder, B.; Wainwright, T.E. Molecular Dynamics by Electronic Computers. In International Symposium on Transport Processes in Statistical Mechanics; Prigogine, I., Ed.; John Wiley International: New York, NY, USA, 1957. [Google Scholar]
- Fourati, N.; Blel, N.; Lattach, Y.; Ktari, N.; Zerrouki, C. Chemical and Biological Sensors from Conducting and Semiconducting Polymers. Ref. Modul. Mater. Sci. Mater. Eng. 2016. [Google Scholar] [CrossRef]
- Ewis, D.; Talkhan, A.G.; Benamor, A.; Qiblawey, H.; Nasser, M.; Ba-Abbad, M.M.; El-Naas, M. Corrosion Behavior of API-X120 Carbon Steel Alloy in a GTL F-T Process Water Environment at Low COD Concentration. Metals 2020, 10, 707. [Google Scholar] [CrossRef]
- Ron, T.; Levy, G.K.; Dolev, O.; Leon, A.; Shirizly, A.; Aghion, E. Environmental Behavior of Low Carbon Steel Produced by a Wire Arc Additive Manufacturing Process. Metals 2019, 9, 888. [Google Scholar] [CrossRef] [Green Version]
- Ha, H.Y.; Lee, T.H.; Kim, S.D.; Jang, J.H.; Moon, J. Improvement of the Corrosion Resistance by Addition of Ni in Lean Duplex Stainless Steels. Metals 2020, 10, 891. [Google Scholar] [CrossRef]
- MEPS International Ltd. Global Composite Steel Price and Index; MEPS International Ltd.: Sheffield, UK, 2020. [Google Scholar]
- Alar, V.; Stojanović, I.; Mezdić, D. A Comparative Study of Green Inhibitors for Galvanized Steel in Aqueous Solutions. Metals 2020, 10, 448. [Google Scholar] [CrossRef] [Green Version]
- Lgaz, H.; Masroor, S.; Chafiq, M.; Damej, M.; Brahmia, A.; Salghi, R.; Benmessaoud, M.; Ali, I.H.; Alghamdi, M.M.; Chaouiki, A.; et al. Evaluation of 2-Mercaptobenzimidazole Derivatives as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid. Metals 2020, 10, 357. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Ansari, K.R.; Chauhan, D.S.; Quraishi, M.A.; Lgaz, H.; Chung, I.M. Comprehensive Investigation of Steel Corrosion Inhibition at Macro/Micro Level by Ecofriendly Green Corrosion Inhibitor in 15% HCl Medium. J. Colloid Interface Sci. 2020, 560, 225–236. [Google Scholar] [CrossRef]
- Ye, Y.; Yang, D.; Chen, H.; Guo, S.; Yang, Q.; Chen, L.; Zhao, H.; Wang, L. A High-Efficiency Corrosion Inhibitor of N-Doped Citric Acid-Based Carbon Dots for Mild Steel in Hydrochloric Acid Environment. J. Hazard. Mater. 2020, 381, 121019. [Google Scholar] [CrossRef] [PubMed]
- Mouaden, K.E.; Quraishi, M.A.; Quraishi, M.A.; Bazzi, L. Thiocarbohydrazide-crosslinked chitosan as a bioinspired corrosion inhibitor for protection of stainless steel in 3.5% NaCl. Sustain. Chem. Pharm. 2020, 15, 100213. [Google Scholar] [CrossRef]
- Verma, C.; Lgaz, H.; Verma, D.K.; Ebenso, E.E.; Bahadur, I.; Quraishi, M.A. Molecular Dynamics and Monte Carlo Simulations as Powerful Tools for Study of Interfacial Adsorption Behavior of Corrosion Inhibitors in Aqueous Phase: A Review. J. Mol. Liq. 2018, 260, 99–120. [Google Scholar] [CrossRef]
- Ni, H.; Wu, J.; Sun, Z.; Lu, G.; Yu, J. Molecular Simulation of the Structure and Physical Properties of Alkali Nitrate Salts for Thermal Energy Storage. Renew. Energy 2019, 136, 955–967. [Google Scholar] [CrossRef]
- Ni, M.; Pan, J.; Hu, X.; Gong, D.; Zhang, G. Inhibitory Effect of Corosolic Acid on α-Glucosidase: Kinetics, Interaction Mechanism, and Molecular Simulation. J. Sci. Food Agric. 2019, 99, 5881–5889. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhang, H.; Bian, X.; Li, J.; Li, J.; Zhang, H. Insight into the Binding of ACE-Inhibitory Peptides to Angiotensin-Converting Enzyme: A Molecular Simulation. Mol. Simul. 2019, 45, 215–222. [Google Scholar] [CrossRef]
- Ganesan, P.; Ramalingam, R. Investigation of Structural Stability and Functionality of Homodimeric Gramicidin towards Peptide-Based Drug: A Molecular Simulation Approach. J. Cell. Biochem. 2019, 120, 4903–4911. [Google Scholar] [CrossRef]
- Hossain, S.; Kabedev, A.; Parrow, A.; Bergström, C.A.S.; Larsson, P. Molecular Simulation as a Computational Pharmaceutics Tool to Predict Drug Solubility, Solubilization Processes and Partitioning. Eur. J. Pharm. Biopharm. 2019, 137, 46–55. [Google Scholar] [CrossRef]
- Ganazzoli, F.; Raffaini, G. Dendrimer Dynamics: A Review of Analytical Theories and Molecular Simulation Methods. Polymers 2020, 12, 1387. [Google Scholar] [CrossRef]
- Hu, J.-P.; Wu, Z.-X.; Xie, T.; Liu, X.-Y.; Yan, X.; Sun, X.; Liu, W.; Liang, L.; He, G.; Gan, Y.; et al. Applications of Molecular Simulation in the Discovery of Antituberculosis Drugs: A Review. Protein Pept. Lett. 2019, 26, 648–663. [Google Scholar] [CrossRef]
- Feng, D.; Feng, Y.; Qiu, L.; Li, P.; Zang, Y.; Zou, H.; Yu, Z.; Zhang, X. Review on Nanoporous Composite Phase Change Materials: Fabrication, Characterization, Enhancement and Molecular Simulation. Renew. Sustain. Energy Rev. 2019, 109, 578–605. [Google Scholar] [CrossRef]
- Pedeferri, P. Uniform Corrosion in Acidic and Aerated Solutions. Transp. Metal-Oxide-Semicond. Struct. 2018, 145–167. [Google Scholar] [CrossRef]
- Lazzari, L. Basic Principles. In European Federation of Corrosion (EFC) Series; Woodhead Publishing: Cambridge, UK, 2017; pp. 1–23. [Google Scholar] [CrossRef]
- Popoola, L.T. Organic Green Corrosion Inhibitors (OGCIs): A Critical Review. Corros. Rev. 2019, 37, 71–102. [Google Scholar] [CrossRef]
- Rassouli, L.; Naderi, R.; Mahdavian, M. Study of the Active Corrosion Protection Properties of Epoxy Ester Coating with Zeolite Nanoparticles Doped with Organic and Inorganic Inhibitors. J. Taiwan Inst. Chem. Eng. 2018, 85, 207–220. [Google Scholar] [CrossRef]
- Noorbakhsh Nezhad, A.H.; Davoodi, A.; Mohammadi Zahrani, E.; Arefinia, R. The Effects of an Inorganic Corrosion Inhibitor on the Electrochemical Behavior of Superhydrophobic Micro-Nano Structured Ni Films in 3.5% NaCl Solution. Surf. Coat. Technol. 2020, 395, 125946. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Mazumder, M.A.J.; Quraishi, M.A.; Ansari, K.R. Chitosan-Cinnamaldehyde Schiff Base: A Bioinspired Macromolecule as Corrosion Inhibitor for Oil and Gas Industry. Int. J. Biol. Macromol. 2020, 158, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Popov, B.N. Chapter 14—Corrosion Inhibitors. In Corrosion Engineering: Principles and Solved Problems; Elsevier: Oxford, UK, 2015. [Google Scholar]
- Feng, L.; Zhang, S.; Qiang, Y.; Xu, Y.; Guo, L.; Madkour, L.H.; Chen, S. Experimental and Theoretical Investigation of Thiazolyl Blue as a Corrosion Inhibitor for Copper in Neutral Sodium Chloride Solution. Materials 2018, 11, 1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monticelli, C. Corrosion Inhibitors. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Elsevier: Oxford, UK, 2018; pp. 164–171. [Google Scholar] [CrossRef]
- Paquet, E.; Viktor, H.L. Molecular Dynamics, Monte Carlo Simulations, and Langevin Dynamics: A Computational Review. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Allen, M.P. Introduction to Molecular Dynamics Simulation Introduction. Comput. Soft Matter Synth. Polym. Proteins 2004, 23, 1–28. [Google Scholar]
- Fouda, A.S.; Ismail, M.A.; Elewady, G.Y.; Abousalem, A.S. Evaluation of 4-Amidinophenyl-2,2′-Bithiophene and Its Aza-Analogue as Novel Corrosion Inhibitors for CS in Acidic Media: Experimental and Theoretical Study. J. Mol. Liq. 2017, 240, 372–388. [Google Scholar] [CrossRef]
- Salarvand, Z.; Amirnasr, M.; Talebian, M.; Raeissi, K.; Meghdadi, S. Enhanced Corrosion Resistance of Mild Steel in 1 M HCl Solution by Trace Amount of 2-Phenyl-Benzothiazole Derivatives: Experimental, Quantum Chemical Calculations and Molecular Dynamics (MD) Simulation Studies. Corros. Sci. 2017, 114, 133–145. [Google Scholar] [CrossRef]
- Hsissou, R.; Abbout, S.; Seghiri, R.; Rehioui, M.; Berisha, A.; Erramli, H.; Assouag, M.; Elharfi, A. Evaluation of Corrosion Inhibition Performance of Phosphorus Polymer for Carbon Steel in [1 M] HCl: Computational Studies (DFT, MC and MD Simulations). J. Mater. Res. Technol. 2020, 9, 2691–2703. [Google Scholar] [CrossRef]
- Thanh, L.T.; Vu, N.S.H.; Binh, P.M.Q.; Dao, V.A.; Thu, V.T.H.; Van Hien, P.; Panaitescu, C.; Nam, N.D. Combined Experimental and Computational Studies on Corrosion Inhibition of Houttuynia Cordata Leaf Extract for Steel in HCl Medium. J. Mol. Liq. 2020, 315, 113787. [Google Scholar] [CrossRef]
- Asfia, M.P.; Rezaei, M.; Bahlakeh, G. Corrosion Prevention of AISI 304 Stainless Steel in Hydrochloric Acid Medium Using Garlic Extract as a Green Corrosion Inhibitor: Electrochemical and Theoretical Studies. J. Mol. Liq. 2020, 315, 113679. [Google Scholar] [CrossRef]
- Qiang, Y.; Guo, L.; Li, H.; Lan, X. Fabrication of Environmentally Friendly Losartan Potassium Film for Corrosion Inhibition of Mild Steel in HCl Medium. Chem. Eng. J. 2021, 406, 126863. [Google Scholar] [CrossRef]
- Chaouiki, A.; Chafiq, M.; Rbaa, M.; Salghi, R.; Lakhrissi, B.; Ali, I.H.; Bashir, S.; Chung, I.M. Comprehensive Assessment of Corrosion Inhibition Mechanisms of Novel Benzimidazole Compounds for Mild Steel in HCl: An Experimental and Theoretical Investigation. J. Mol. Liq. 2020, 114383. [Google Scholar] [CrossRef]
- El Arrouji, S.; Karrouchi, K.; Berisha, A.; Ismaily, K.; Warad, I.; Rais, Z.; Radi, S.; Taleb, M.; Ansar, M.; Zarrouk, A. New Pyrazole Derivatives as Effective Corrosion Inhibitors on Steel-Electrolyte Interface in 1 M HCl: Electrochemical, Surface Morphological (SEM) and Computational Analysis. Colloids Surf. A 2020, 604, 125325. [Google Scholar] [CrossRef]
- El Faydy, M.; Benhiba, F.; Berisha, A.; Kerroum, Y.; Jama, C.; Lakhrissi, B.; Guenbour, A.; Warad, I.; Zarrouk, A. An Experimental-Coupled Empirical Investigation on the Corrosion Inhibitory Action of 7-Alkyl-8-Hydroxyquinolines on C35E Steel in HCl Electrolyte. J. Mol. Liq. 2020, 317, 113973. [Google Scholar] [CrossRef]
- El Aoufir, Y.; Zehra, S.; Lgaz, H.; Chaouiki, A.; Serrar, H.; Kaya, S.; Salghi, R.; Abdelraheem, S.K.; Boukhris, S.; Guenbour, A.; et al. Evaluation of Inhibitive and Adsorption Behavior of Thiazole-4-Carboxylates on Mild Steel Corrosion in HCl. Colloids Surf. A Physicochem. Eng. Asp. 2020, 606, 125351. [Google Scholar] [CrossRef]
- Bedair, M.A.; Soliman, S.A.; Bakr, M.F.; Gad, E.S.; Lgaz, H.; Chung, I.M.; Salama, M.; Alqahtany, F.Z. Benzidine-Based Schiff Base Compounds for Employing as Corrosion Inhibitors for Carbon Steel in 1.0 M HCl Aqueous Media by Chemical, Electrochemical and Computational Methods. J. Mol. Liq. 2020, 317, 114015. [Google Scholar] [CrossRef]
- Ech-chihbi, E.; Nahle, A.; Salim, R.; Benhiba, F.; Moussaif, A.; El-hajjaji, F.; Oudda, H.; Guenbour, A.; Taleb, M.; Warad, I. Zarro. Computational, MD Simulation, SEM/EDX and Experimental Studies for Understanding Adsorption of Benzimidazole Derivatives as Corrosion Inhibitors in 1.0 M HCl Solution. 2020, 844, 155842. [Google Scholar] [CrossRef]
- Ituen, E.; Mkpenie, V.; Ekemini, E.; Eduok, S.; Yuanhua, L.; Akaranta, O. Inhibition of Acid and Bio-Corrosion of Pipeline Steel Using Tabersonine: Experimental, DFT and Molecular Dynamics Simulations Approaches. J. Bio-Tribo-Corrosion 2020, 6, 1–16. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Chen, B.; Zhang, L. Novel Surfactants as Green Corrosion Inhibitors for Mild Steel in 15% HCl: Experimental and Theoretical Studies. Chem. Eng. J. 2020, 402, 126219. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Ituen, E.; Guo, L.; Abdul Wahab, M.; Quraishi, M.A.; Kong, X.; Lin, Y. A New Series of Synthesized Compounds as Corrosion Mitigator for Storage Tanks: Detailed Electrochemical and Theoretical Investigations. Constr. Build. Mater. 2020, 259, 120421. [Google Scholar] [CrossRef]
- Guo, L.; Tan, J.; Kaya, S.; Leng, S.; Li, Q.; Zhang, F. Multidimensional Insights into the Corrosion Inhibition of 3,3-Dithiodipropionic Acid on Q235 Steel in H2SO4 Medium: A Combined Experimental and in Silico Investigation. J. Colloid Interface Sci. 2020, 570, 116–124. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, R.; Tan, B.; Li, W.; Liu, H.; Wu, S. Locust Bean Gum as a Green and Novel Corrosion Inhibitor for Q235 Steel in 0.5 M H2SO4 Medium. J. Mol. Liq. 2020, 310, 113239. [Google Scholar] [CrossRef]
- Saranya, J.; Benhiba, F.; Anusuya, N.; Subbiah, R.; Zarrouk, A.; Chitra, S. Experimental and Computational Approaches on the Pyran Derivatives for Acid Corrosion. Colloids Surf. A 2020, 603, 125231. [Google Scholar] [CrossRef]
- Berisha, A. Experimental, Monte Carlo and Molecular Dynamic Study on Corrosion Inhibition of Mild Steel by Pyridine Derivatives in Aqueous Perchloric Acid. Electrochem 2020, 1, 13. [Google Scholar] [CrossRef]
- Błoński, P.; Kiejna, A. Structural, Electronic, and Magnetic Properties of Bcc Iron Surfaces. Surf. Sci. 2007. [Google Scholar] [CrossRef]
- Haque, J.; Srivastava, V.; Quraishi, M.A.; Singh, D.; Lgaz, H.; Chung, I. Polar Group Substituted Imidazolium Zwitterions as Eco-Friendly Corrosion Inhibitors for Mild Steel in Acid Solution. Corros. Sci. 2020, 172, 108665. [Google Scholar] [CrossRef]
- Bajpai, D.; Murmu, M.; Banerjee, P.; Ahmad, M. Palmitic Acid Based Environmentally Benign Corrosion Inhibiting Formulation Useful during Acid Cleansing Process in MSF Desalination Plants. Desalination 2019, 472, 114128. [Google Scholar] [CrossRef]
- Sulaiman, K.O.; Onawole, A.T.; Faye, O.; Shuaib, D.T. Understanding the Corrosion Inhibition of Mild Steel by Selected Green Compounds Using Chemical Quantum Based Assessments and Molecular Dynamics Simulations. J. Mol. Liq. 2019, 279, 342–350. [Google Scholar] [CrossRef]
- Vengatesh, G.; Sundaravadivelu, M. Non-Toxic Bisacodyl as an Effective Corrosion Inhibitor for Mild Steel in 1 M HCl: Thermodynamic, Electrochemical, SEM, EDX, AFM, FT-IR, DFT and Molecular Dynamics Simulation Studies. J. Mol. Liq. 2019, 287, 110906. [Google Scholar] [CrossRef]
- Xu, L.; Kirvassilis, D.; Bai, Y.; Mavrikakis, M. Atomic and Molecular Adsorption on Fe(110). Surf. Sci. 2018, 667, 54–65. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, X.; Li, X.; Wang, J.; Hu, Z.; Ma, X. 2-Aminobenzimidazole Derivative with Surface Activity as Corrosion Inhibitor of Carbon Steel in HCl: Experimental and Theoretical Study. J. Mol. Liq. 2020, 297, 111720. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Quraishi, M.A.; Lin, Y. Investigation of Corrosion Inhibitors Adsorption on Metals Using Density Functional Theory and Molecular Dynamics Simulation. In Corrosion Inhibitors; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Douche, D.; Elmsellem, H.; Anouar, E.H.; Guo, L.; Hafez, B.; Tüzün, B.; El Louzi, A.; Bougrin, K.; Karrouchi, K.; Himmi, B. Anti-Corrosion Performance of 8-Hydroxyquinoline Derivatives for Mild Steel in Acidic Medium: Gravimetric, Electrochemical, DFT and Molecular Dynamics Simulation Investigations. J. Mol. Liq. 2020, 308, 113042. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, B.; Liang, X. Corrosion Inhibition Performance of Coconut Leaf Extract as a Green Corrosion Inhibitor for X65 Steel in Hydrochloric Acid Solution. Int. J. Electrochem. Sci. 2020. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Y.; Zhou, C.; Lu, H. 1-Phenyl-1H-Tetrazole-5-Thiol as Corrosion Inhibitor for Q235 Steel in 1 M HCl Medium: Combined Experimental and Theoretical Researches. Int. J. Electrochem. Sci. 2020, 2499–2510. [Google Scholar] [CrossRef]
- Pal, S.; Ji, G.; Lgaz, H.; Chung, I.; Prakash, R. Lemon Seeds as Green Coating Material for Mitigation of Mild Steel Corrosion in Acid Media: Molecular Dynamics Simulations, Quantum Chemical Calculations and Electrochemical Studies. J. Mol. Liq. 2020, 316, 113797. [Google Scholar] [CrossRef]
- Farhadian, A.; Rahimi, A.; Safaei, N.; Shaabani, A.; Abdouss, M. A Theoretical and Experimental Study of Castor Oil-Based Inhibitor for Corrosion Inhibition of Mild Steel in Acidic Medium at Elevated Temperatures. Corros. Sci. 2020, 175, 108871. [Google Scholar] [CrossRef]
- Khamaysa, O.M.A.; Selatnia, I.; Zeghache, H.; Lgaz, H.; Sid, A.; Chung, I.M.; Benahmed, M.; Gherraf, N.; Mosset, P. Enhanced Corrosion Inhibition of Carbon Steel in HCl Solution by a Newly Synthesized Hydrazone Derivative: Mechanism Exploration from Electrochemical, XPS, and Computational Studies. J. Mol. Liq. 2020, 315, 113805. [Google Scholar] [CrossRef]
- Ouksel, L.; Bourzami, R.; Chafaa, S.; Chafai, N. Solvent and Catalyst-Free Synthesis, Corrosion Protection, Thermodynamic, MDS and DFT Calculation of Two Environmentally Friendly Inhibitors: Bis-Phosphonic Acids. J. Mol. Struct. 2020, 1222, 128813. [Google Scholar] [CrossRef]
- Gao, C.; Zhao, X.; Liu, K.; Dong, X.; Wang, S.; Kong, F. Construction of Eco-Friendly Corrosion Inhibitor Lignin Derivative with Excellent Corrosion-Resistant Behavior in Hydrochloric Acid Solution. Mater. Corros. 2020, 71, 1903–1912. [Google Scholar] [CrossRef]
- Kurapati, Y. A Molecular Dynamics Study to Understand Behavior of Corrosion Inhibitors in Bulk Aqueous Phase and Near Metal-Water Interface. Ph.D. Thesis, Ohio University, Athens, OH, USA, 2018. [Google Scholar]
- John, S.; Joseph, A.; Sajini, T.; James Jose, A. Corrosion Inhibition Properties of 1,2,4-Hetrocyclic Systems: Electrochemical, Theoretical and Monte Carlo Simulation Studies. Egypt. J. Pet. 2017, 26, 721–732. [Google Scholar] [CrossRef] [Green Version]
- El Ibrahimi, B.; Jmiai, A.; Bazzi, L.; El Issami, S. Amino Acids and Their Derivatives as Corrosion Inhibitors for Metals and Alloys. Arab. J. Chem. 2020, 13, 740–771. [Google Scholar] [CrossRef]
- Nishikawa, K.; Akiyama, H.; Yagishita, K.; Washizu, H. Molecular Dynamics Analysis of Adsorption Process of Anti-Copper-Corrosion Additives to the Copper Surface. arXiv 2018, arXiv:1812.10647. [Google Scholar]
- Yan, T.; Zhang, S.; Feng, L.; Qiang, Y.; Lu, L.; Fu, D.; Wen, Y.; Chen, J.; Li, W.; Tan, B. Investigation of Imidazole Derivatives as Corrosion Inhibitors of Copper in Sulfuric Acid: Combination of Experimental and Theoretical Researches. J. Taiwan Inst. Chem. Eng. 2020, 106, 118–129. [Google Scholar] [CrossRef]
- Tan, J.; Guo, L.; Wu, D.; Duan, X.; Leng, S.; Obot, I.B.; Kaya, S. Electrochemical and Computational Investigations on the Corrosion Inhibition of X65 Steel by 2-Phenylbenzimidazole in H2SO4 Solution. Int. J. Electrochem. Sci. 2020, 8837–8848. [Google Scholar] [CrossRef]
- Materials Studio Forcite Plus Datasheet; Dassault Systèmes, 2014.
- Djefaflia, R.; Lerari, D.; Bachari, K. Covid-19 and Nanoform Zinc Oxide Interaction: A Forcite Module of Materials Studio Software Study. Clay Res. 2019, 38, 43. [Google Scholar] [CrossRef]
- Hadi Al Hasan, N. Molecular Dynamic Simulation of the Density and Mechanical Properties of Polyvinyl Chloride(PVC)/High Density Polyethylene (HDPE) Composites Based on Materials Studio. J. Phys. Conf. Ser. 2019, 1294, 052062. [Google Scholar] [CrossRef]
- Eshaghi Malekshah, R.; Fahimirad, B.; Aallaei, M.; Khaleghian, A. Synthesis and Toxicity Assessment of Fe3O4 NPs Grafted by ∼ NH2-Schiff Base as Anticancer Drug: Modeling and Proposed Molecular Mechanism through Docking and Molecular Dynamic Simulation. Drug Deliv. 2020, 27, 1201–1217. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, Q.; Wang, Y.; Tang, J.; Wang, Y. A Study on Inhibition Performance of Mercaptoalcohols As Corrosion Inhibitors by First Principle and Molecular Dynamics Simulation. Russ. J. Phys. Chem. A 2020, 94, 1877–1886. [Google Scholar] [CrossRef]
- Rbaa, M.; Benhiba, F.; Dohare, P.; Lakhrissi, L.; Touir, R.; Lakhrissi, B.; Zarrouk, A.; Lakhrissi, Y. Synthesis of New Epoxy Glucose Derivatives as a Non-Toxic Corrosion Inhibitors for Carbon Steel in Molar HCl: Experimental, DFT and MD Simulation. Chem. Data Collect. 2020, 27, 100394. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Quraishi, M.A.; Kaya, S.; Guo, L. Aminoantipyrine Derivatives as a Novel Eco-Friendly Corrosion Inhibitors for P110 Steel in Simulating Acidizing Environment: Experimental and Computational Studies. J. Nat. Gas Sci. Eng. 2020, 83, 103547. [Google Scholar] [CrossRef]
- Rbaa, M.; Dohare, P.; Berisha, A.; Dagdag, O.; Lakhrissi, L.; Galai, M.; Lakhrissi, B.; Touhami, M.E.; Warad, I.; Zarrouk, A. New Epoxy Sugar Based Glucose Derivatives as Eco Friendly Corrosion Inhibitors for the Carbon Steel in 1.0 M HCl: Experimental and Theoretical Investigations. J. Alloys Compd. 2020, 833, 154949. [Google Scholar] [CrossRef]
- El-Hajjaji, F.; Merimi, I.; El Ouasif, L.; El Ghoul, M.; Achour, R.; Hammouti, B.; Belghiti, M.E.; Chauhan, D.S.; Quraishi, M.A. 1-Octyl-2-(Octylthio)-1H-Benzimidazole as a New and Effective Corrosion Inhibitor for Carbon Steel in 1 M HCl. Port. Electrochim. Acta 2019, 37, 131–145. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.A.; Sorour, A.A.; Saha, S.K.; Banerjee, P. Triazole-Modified Chitosan: A Biomacromolecule as a New Environmentally Benign Corrosion Inhibitor for Carbon Steel in a Hydrochloric Acid Solution. RSC Adv. 2019, 9, 14990–15003. [Google Scholar] [CrossRef] [Green Version]
- Farahati, R.; Ghaffarinejad, A.; Mousavi-Khoshdel, S.M.; Rezania, J.; Behzadi, H.; Shockravi, A. Synthesis and Potential Applications of Some Thiazoles as Corrosion Inhibitor of Copper in 1 M HCl: Experimental and Theoretical Studies. Prog. Org. Coat. 2019, 132, 417–428. [Google Scholar] [CrossRef]
- Kim, S. Issues on the Choice of a Proper Time Step in Molecular Dynamics. Phys. Procedia 2014, 53, 60–62. [Google Scholar] [CrossRef] [Green Version]
- Schneider, R.; Sharma, A.R.; Rai, A. Introduction to Molecular Dynamics. In Computational Many-Particle Physics; Springer: Berlin/Heidelberg, Germany, 2008; pp. 3–40. [Google Scholar] [CrossRef] [Green Version]
- Farahati, R.; Mousavi-khoshdel, S.M.; Ghaffarinejad, A.; Behzadi, H. Progress in Organic Coatings Experimental and Computational Study of Penicillamine Drug and Cysteine as Water-Soluble Green Corrosion Inhibitors of Mild Steel. Prog. Org. Coatings 2020, 142, 105567. [Google Scholar] [CrossRef]
- Wang, X.; Tang, C.; Wang, Q.; Li, X.; Hao, J. Selection of Optimal Polymerization Degree and Force Field in the Molecular Dynamics Simulation of Insulating Paper Cellulose. Energies 2017, 10, 1377. [Google Scholar] [CrossRef] [Green Version]
- Manssouri, M.; Znini, M.; Lakbaibi, Z.; Ansari, A.; El Ouadi, Y. Experimental and Computational Studies of Perillaldehyde Isolated from Ammodaucus Leucotrichus Essential Oil as a Green Corrosion Inhibitor for Mild Steel in 1.0 M HCl. Chem. Pap. 2020. [Google Scholar] [CrossRef]
- Ituen, E.; Mkpenie, V.; Dan, E. Surface Protection of Steel in Oil Well Acidizing Fluids Using L-Theanine-Based Corrosion Inhibitor Formulations: Experimental and Theoretical Evaluation. Surf. Interfaces 2019, 16, 29–42. [Google Scholar] [CrossRef]
- Obot, I.B.; Haruna, K.; Saleh, T.A. Atomistic Simulation: A Unique and Powerful Computational Tool for Corrosion Inhibition Research. Arab. J. Sci. Eng. 2019, 44, 1–32. [Google Scholar] [CrossRef]
- Akkermans, R.L.C.; Spenley, N.A.; Robertson, S.H. COMPASS III: Automated Fitting Workflows and Extension to Ionic Liquids. Mol. Simul. 2020. [Google Scholar] [CrossRef]
- Gao, W.; Chen, Y.; Li, B.; Liu, S.P.; Liu, X.; Jiang, Q. Determining the Adsorption Energies of Small Molecules with the Intrinsic Properties of Adsorbates and Substrates. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Han, P.; He, Y.; Chen, C.; Yu, H.; Liu, F.; Yang, H.; Ma, Y.; Zheng, Y. Study on Synergistic Mechanism of Inhibitor Mixture Based on Electron Transfer Behavior. Sci. Rep. 2016, 6, 33252. [Google Scholar] [CrossRef] [Green Version]
- Huo, S.J.; He, J.M.; Chen, L.H.; Fang, J.H. Adsorption Configuration of Sodium 2-Quinoxalinecarboxylate on Iron Substrate: Investigation by in Situ SERS, XPS and Theoretical Calculation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 156, 123–130. [Google Scholar] [CrossRef]
- Oyekunle, D.T.; Agboola, O.; Ayeni, A.O. Corrosion Inhibitors as Building Evidence for Mild Steel: A Review. J. Phys. Conf. Ser. 2019, 1378. [Google Scholar] [CrossRef]
- Berrissoul, A.; Ouarhach, A.; Benhiba, F.; Romane, A.; Zarrouk, A.; Guenbour, A.; Dikici, B.; Dafali, A. Evaluation of Lavandula Mairei Extract as Green Inhibitor for Mild Steel Corrosion in 1 M HCl Solution. Experimental and Theoretical Approach. J. Mol. Liq. 2020, 313, 113493. [Google Scholar] [CrossRef]
- Boughoues, Y.; Benamira, M. RSC Advances Experimental and Theoretical Investigations of Four Amine Derivatives as e Ff Ective Corrosion Inhibitors for Mild Steel in HCl Medium. RSC Adv. 2020, 10, 24145–24158. [Google Scholar] [CrossRef]
- Satpati, S.; Saha, S.K.; Suhasaria, A.; Banerjee, P.; Sukul, D. Adsorption and Anti-Corrosion Characteristics of Vanillin Schiff Bases on Mild Steel in 1 M HCl: Experimental and Theoretical Study. RSC Adv. 2020, 10, 9258–9273. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Deng, S.; Li, N.; Xie, X. Inhibition Effect of Bamboo Leaves Extract on Cold Rolled Steel in Cl3CCOOH Solution. J. Mater. Res. Technol. 2017, 6, 158–170. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Deng, S.; Lin, T.; Xie, X.; Du, G. Cassava Starch Ternary Graft Copolymer as a Corrosion Inhibitor for Steel in HCl Solution. J. Mater. Res. Technol. 2020, 9, 2196–2207. [Google Scholar] [CrossRef]
- Laabaissi, T.; Benhiba, F.; Missioui, M.; Roui, Z.; Rbaa, M.; Oudda, H.; Ramli, Y.; Guenbour, A.; Warad, I.; Zarrouk, A. Coupling of Chemical, Electrochemical and Theoretical Approach to Study the Corrosion Inhibition of Mild Steel by New Quinoxaline Compounds in 1 M HCl. Heliyon 2020, 6, e03939. [Google Scholar] [CrossRef] [PubMed]
- Chaouiki, A.; Lgaz, H.; Salghi, R.; Chafiq, M.; Gaonkar, S.L.; Bhat, K.S.; Oudda, H.; Ali, I.H.; Chung, I. Inhibitory Effect of a New Isoniazid Derivative as an Effective Inhibitor for Mild Steel Corrosion in 1.0 M HCl: Combined Experimental and Computational Study. Res. Chem. Intermed. 2020, 46, 2919–2950. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Zhang, Y.; Wang, L.; Wu, Y.; Li, H. 9-Substituted Acridines as Effective Corrosion Inhibitors for Mild Steel: Electrochemical, Surface Morphology, and Computational Studies. New J. Chem. 2020, 44, 6464–6474. [Google Scholar] [CrossRef]
- Suhasaria, A.; Murmu, M.; Satpati, S.; Banerjee, P.; Sukul, D. Bis-Benzothiazoles as Ef Fi Cient Corrosion Inhibitors for Mild Steel in Aqueous HCl: Molecular Structure-Reactivity Correlation Study. J. Mol. Liq. 2020, 313, 113537. [Google Scholar] [CrossRef]
- Chugh, B.; Singh, A.K.; Thakur, S.; Pani, B.; Lgaz, H.; Chung, I.; Jha, R.; Ebenso, E.E. Comparative Investigation of Corrosion-Mitigating Behavior of Thiadiazole-Derived Bis-Schi Ff Bases for Mild Steel in Acid Medium: Experimental, Theoretical, and Surface Study. ACS Omega 2020, 5, 13503–13520. [Google Scholar] [CrossRef]
- Hrimla, M.; Bahsis, L.; Boutouil, A.; Laamari, M.R.; Stiriba, S. A Combined Computational and Experimental Study on the Mild Steel Corrosion Inhibition in Hydrochloric Acid by New Multifunctional Phosphonic Acid Containing 1, 2, 3-Triazoles. J. Adhes. Sci. Technol. 2020, 34, 1741–1773. [Google Scholar] [CrossRef]
- El-hajjaji, F.; Ech-chihbi, E.; Rezki, N.; Benhiba, F.; Taleb, M.; Singh, D.; Quraishi, M.A. Electrochemical and Theoretical Insights on the Adsorption and Corrosion Inhibition of Novel Pyridinium-Derived Ionic Liquids for Mild Steel in 1 M HCl. J. Mol. Liq. 2020, 314, 113737. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, Y.; Chen, L.; Wang, L.; Wu, Y.; Li, H. Aloe Polysaccharide as an Eco-Friendly Corrosion Inhibitor for Mild Steel in Simulated Acidic Oil Fi Eld Water: Experimental and Theoretical Approaches. J. Mol. Liq. 2020, 307, 112950. [Google Scholar] [CrossRef]
- Chaouiki, A.; Chafiq, M.; Rbaa, M.; Lgaz, H.; Salghi, R.; Lakhrissi, B.; Ali, I.H.; Masroor, S.; Cho, Y. New 8-Hydroxyquinoline-Bearing Quinoxaline Derivatives as Effective Corrosion Inhibitors for Mild Steel in HCl: Electrochemical and Computational Investigations. Coatings 2020, 10, 811. [Google Scholar] [CrossRef]
- Boughoues, Y.; Benamira, M.; Messaadia, L.; Ribouh, N. Adsorption and Corrosion Inhibition Performance of Some Environmental Friendly Organic Inhibitors for Mild Steel in HCl Solution via Experimental and Theoretical Study. Colloids Surf. A 2020, 593, 124610. [Google Scholar] [CrossRef]
- Aslam, R.; Mobin, M.; Aslam, J.; Lgaz, H.; Chung, I.M. Inhibitory Effect of Sodium Carboxymethylcellulose and Synergistic Biodegradable Gemini Surfactants as Effective Inhibitors for MS Corrosion in 1 M HCl. J. Mater. Res. Technol. 2019, 8, 4521–4533. [Google Scholar] [CrossRef]
- Basik, M.; Mobin, M. Chondroitin Sulfate as Potent Green Corrosion Inhibitor for Mild Steel in 1 M HCl. J. Mol. Struct. 2020, 1214, 128231. [Google Scholar] [CrossRef]
- Shahmoradi, A.R.; Talebibahmanbigloo, N.; Javidparvar, A.A.; Bahlakeh, G.; Ramezanzadeh, B. Studying the Adsorption/ Inhibition Impact of the Cellulose and Lignin Compounds Extracted from Agricultural Waste on the Mild Steel Corrosion in HCl Solution. J. Mol. Liq. 2020, 304, 112751. [Google Scholar] [CrossRef]
- Berrissoul, A.; Loukili, E.; Mechbal, N.; Benhiba, F.; Guenbour, A.; Dikici, B.; Zarrouk, A.; Dafali, A. Anticorrosion Effect of a Green Sustainable Inhibitor on Mild Steel in Hydrochloric Acid. J. Colloid Interface Sci. 2020, 580, 740–752. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Applying Detailed Molecular/ Atomic Level Simulation Studies and Electrochemical Explorations of the Green Inhibiting Molecules Adsorption at the Interface of the Acid Solution-Steel Substrate. J. Mol. Liq. 2020, 299, 112220. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Potential Role of a Novel Green Eco-Friendly Inhibitor in Corrosion Inhibition of Mild Steel in HCl Solution: Detailed Macro/Micro-Scale Experimental and Computational Explorations. Constr. Build. Mater. 2020, 245, 118464. [Google Scholar] [CrossRef]
- Goyal, M.; Vashist, H.; Kumar, S.; Bahadur, I. Acid Corrosion Inhibition of Ferrous and Non-Ferrous Metal by Nature Friendly Ethoxycarbonylmethyltriphenylphosphonium Bromide (ECMTPB): Experimental and MD Simulation Evaluation. J. Mol. Liq. 2020, 315, 113705. [Google Scholar] [CrossRef]






| Acid Solution | Aqueous Phase | Reference |
|---|---|---|
| HCl | ||
| 0.1 M | 556 H2O, 1 H3O+ and 1 Cl− | [37] |
| 0.5 M | 491 H2O, 9 H3O+ and 9 Cl− | [38] |
| 1.0 M | 500 H2O | [39] |
| H2O, H3O+ and Cl− | [40] | |
| 233 H2O, 15 H3O+ and 15 Cl− | [41] | |
| 350 H2O, 10 H3O+ and 10 Cl− | [42] | |
| 491 H2O, 9 H3O+ and 9 Cl− | [43,44] | |
| 500 H2O, 5 H3O+ and 5 Cl− | [45] | |
| 533 H2O, 5 H3O+ and 5 Cl− | [46] | |
| H2O | [47] | |
| 15% (4.86 M) | 500 H2O | [48] |
| H2SO4 | ||
| 0.5 M | H2O | [49] |
| 400 H2O | [50] | |
| 1.0 M | 500 H2O, 20 H3O+ and 10 SO42− | [51] |
| HClO4 | ||
| 0.1 M | 400 H2O, 15 H3O+ and 15 ClO4− | [52] |
| Ferrous Metal | Fe Lattice | Reference |
|---|---|---|
| Fe (110) | Mild steel | [40,41,43,45,51,52,64,65] |
| Carbon steel | [44,66] | |
| C35E steel | [42] | |
| N80 steel | [48] | |
| Q235 steel | [39,49,50] | |
| XC48 steel | [67] | |
| X80 steel | [46] | |
| AISI 304 stainless steel | [38] | |
| Fe (100) | Mild steel | [47] |
| Carbon steel | [59] | |
| Fe (001) | Q235 steel | [68] |
| Name and Chemical Structure of Corrosion Inhibitor | Metal and Acid Solution | MD Parameters
| MD Result Orientation and Binding Energy (kJ/moL) | Ref. |
|---|---|---|---|---|
Bis-phosphonic acid | XC48 steel 1 M HCl | Fe (110) 17.20 Å × 22.93 Å × 22.93 Å H2O 0.1 fs, 50 ps COMPASS, NVT | Horizontal to Fe surface Ebinding = 5027.03 | [67] |
Surfactant | Mild steel 15% HCl | Fe (110) 23.00 Å × 23.00 Å × 10.03 Å 500 H2O 1 fs Forcite | Parallel to Fe surface Ebinding = 1971.50 | [47] |
Amine derivative | Mild steel 1 M HCl | Fe (110) 24.82 Å × 24.82 Å × 38.10 Å 100 H2O, 1 Cl−, 1 H3O+ 1.0 fs, 500 ps COMPASS | Flat position Ebinding = 1562.3 | [99] |
Losartan potassium drug  | Q235 steel 1 M HCl | Fe (110) 24.3 Å × 17.2 Å × 67.1 Å 500 H2O 1.0 fs, 500 fs COMPASS, NVT | Horizontal orientation Ebinding = 1120.89 | [39] |
Vanillin Schiff base | Mild steel 1 M HCl | Fe (110) 40.11 Å × 40.11 Å × 78.00 Å 150 H2O, 15 Cl−, 15 H3O+ 1.0 fs, 100 fs COMPASS, NVT | Close to Fe surface Ebinding = 1199.05 | [100] |
Bamboo leaves extract | Cold rolled steel 0.1 M Cl3CCOOH | Fe (001) 31.53 Å × 31.53 Å × 15.30 Å 1.0 fs, 1000 ps COMPASS, NVT | Flat orientation Ebinding = 1104.35 | [101] |
Cassava starch ternary graft copolymer | Steel 1 M HCl | Fe (001) 31.53 Å × 31.53 Å × 15.30 Å 500 H2O 1.0 fs, 1000 ps COMPASS, NVT | Flat orientation Ebinding = 1072.90 | [102] |
Quinoxaline compound | Mild steel 1 M HCl | Fe (110) 24.82 Å × 24.82 Å × 25.28 Å 491 H2O 1.0 fs, 200 fs COMPASS, NVT | Distributed over Fe surface Ebinding = 903.70 kJ/mol | [103] |
Amino acid derivatives  | Mild steel 1 M HCl | Fe (110), α-Fe2O3 (110) 24.82 Å × 24.82 Å × 35.69 Å 491 H2O, 9 Cl−, 9 H3O+ 1.0 fs, 2000 ps Forcite, COMPASS, NVT, | Planar orientation Ebinding = 829.17 | [54] |
Isoniazid derivative | Mild steel 1 M HCl | Fe (110) 24.82 Å × 24.82 Å × 25.14 Å 491 H2O, 9 Cl−, 9 H3O+ 1.0 fs, 5000 ps COMPASS, NVT | Close to Fe surface Ebinding = 804.20 | [104] |
Substituted acridines | Mild steel 15% HCl | Fe (110) 24.82 Å × 24.82 Å × 43.21 Å 500 H2O, 5 Cl−, 5 H3O+ 1.0 fs, 500 ps COMPASSII, NVT | Parallel to Fe surface Ebinding = 791.40 | [105] |
Bisbenzothiazole derivative | Mild steel 1 M HCl | Fe (110) 39.85 Å × 39.85 Å × 76.79 Å 150 H2O, 15 Cl−, 15 H3O+ 1.0 fs, 200 ps COMPASS, NVT | Distributed over Fe surface Ebinding = 789.20 | [106] |
| Thiadiazole-Derived Bis-Schiff Base | Mild steel 1 M HCl | Fe (110) 24.82 Å × 24.82 Å × 35.69 Å 491 H2O, 9 Cl−, 9 H3O+ 1.0 fs, 2000 ps COMPASS, NVT | Parallel to Fe surface Ebinding = 758.79 | [107] |
Thiazole carboxylates  | Mild steel 1 M HCl | Fe (110) 25.02 Å × 25.02 Å × 38.32 Å 491 H2O, 9 Cl−, 9 H3O+ 0.1 fs, 5000 ps Forcite, COMPASS, NVT | Planar arrangement Ebinding = 753.20 | [43] |
Phosphonic acid | Mild steel 1 M HCl | Fe (110) 9.78 Å × 29.78 Å × 60.13 Å 500 H2O, 10 Cl−, 10 H3O+ 1 fs, 100,000 steps Forcite, NVT | Parallel to Fe surface Ebinding = 722.00 | [108] |
Pyridinium-derived ionic liquid | Mild steel 1 M HCl | Fe (110) 32.27 Å × 32.27 Å × 34.13 Å 500 H2O, 5 Cl−, 5 H3O+ 1.0 fs, 400 ps Forcite, COMPASS, NVT | Parallel to Fe surface Ebinding = 689.27 | [109] |
Benzimidazole derivative | Mild steel 1 M HCl | Fe (110) 32.27 Å × 32.27 Å × 31.13 Å 500 H2O, 5 Cl−, 5 H3O+ 1 fs, 400 ps Forcite, COMPASS, NVT | Parallel to Fe surface Ebinding = 641.94 | [45] |
Aloe polysaccharide | Mild steel 15% HCl | Fe (110) 24.82 Å × 24.82 Å × 43.21 Å 500 H2O, 5 Cl−, 5 H3O+ 1.0 fs, 500 ps COMPASSII, NVT | Parallel to Fe surface Ebinding = 598.75 | [110] |
Quinoxaline derivative | Mild steel 1 M HCl | Fe (110) 500 H2O, 5 Cl−, 5 H3O+ NVT Forcite | Parallel to Fe surface Ebinding = 583.12 | [111] |
Amine derivative | Mild steel 1 M HCl | Fe (110) 24.82 Å × 24.82 Å × 38.10 Å 100 H2O, 1 Cl−, 1 H3O+ 1.0 fs, 500 fs COMPASS | Parallel to Fe surface Ebinding = 541.55 | [112] |
Mixture of cellulose derivative and Gemini surfactant | Mild steel 1 M HCl | Fe (110) 24.82 Å × 24.82 Å × 35.69 Å 491 H2O, 9 Cl−, 9 H3O+ 0.1 fs, 2000 ps COMPASS, NVT | Parallel to Fe surface Ebinding = 507.48 | [113] |
Pyrazole derivatives | Mild steel 1 M HCl | Fe (110) 14.89 Å × 14.89 Å × 6.45 Å 230 H2O, 15 Cl−, 15 H3O+ 1 fs, 500 ps COMPASSII, NVT | CI molecule oriented towards Fe atoms Ebinding = 456.81 | [41] |
Pyran derivatives | Mild steel 1 M H2SO4 | Fe (110) 27.45 Å × 27.45 Å × 29.14 Å 500 H2O, 5 10 SO42−, 20 H3O+ Forcite, COMPASS, NVT | Flat configuration Ebinding = 438.47 | [51] |
Lemon seeds extract | Mild steel 1 M HCl | Fe (110) 25.22 Å × 25.22 Å × 39.62 Å 1.0 fs, 2000 ps Forcite, COMPASS, NVT | Parallel to Fe surface Ebinding = 375.65 | [64] |
Chondroitin sulfate | Mild steel 1 M HCl | Fe (110) 24.82 Å × 24.82 Å × 25.14 Å 491 H2O, 9 Cl−, 9 H3O+ 1.0 fs, 5000 ps COMPASS, NVT | Parallel to Fe surface Ebinding = 334.24 | [114] |
Pistachio nut extract | Mild steel 1 M HCl | Fe (110) H2O, Cl−, H3O+ (and vacuum) 1.0 fs, 50,000 steps COMPASS, Forcite | Distributed over Fe surface Ebinding = 317.25 | [115] |
Penicillamine drug | Mild steel 1 M HCl | Fe (110) H2O, Cl−, H3O+ 1.0 fs, 1000 fs COMPASS, NVT | Close to Fe surface Ebinding = 268.00 | [88] |
Artemisia herba alba Extract | Mild steel 1 M HCl | Fe (110) 27.30 Å × 27.30 Å × 37.13 Å 500 H2O, 5 Cl−, 5 H3O+ 1 ns, 200 ps Forcite, COMPASS, NVT | Parallel to Fe surface Ebinding = 256.00 | [116] |
Rosa damascena flower extract | Mild steel 1 M HCl | Fe (110) 491 H2O, 9 Cl−, 9 H3O+ 1.0 fs, 20,000 steps COMPASS, NVT | Planar orientation Ebinding = 237.69 | [117] |
Ziziphora leaves extract | Steel 1 M HCl | Fe (110) 24.82 Å × 24.82 Å × 38.10 Å 491 H2O, 9 Cl−, 9 H3O+ 1.0 fs, 1000 ps COMPASS, NVT | Parallel to Fe surface Ebinding = 216.68 | [118] |
Phosphonium compound | Mild steel 0.5 M H2SO4 | Fe (110) 37.22 Å × 37.22 Å × 38.69 Å 5 Cl−, 5 H3O+ 1.0 ns, 600 ps Forcite, COMPASS, NVT | CI molecule located on Fe atoms Ebinding = 212.75 | [119] |
Lavandula mairei extract | Mild steel 1 M HCl | Fe (001) 27.30 Å × 27.30 Å × 33.13 Å 500 H2O, 5 Cl−, 5 H3O+ 1.0 fs, 400 ps Forcite, COMPASS, NVT | Distributed over Fe surface Ebinding = 197.75 | [98] |
Phosphorus polymer | Carbon Steel 1 M HCl | Fe (110) 39.72 Å × 39.72 Å × 56.08 Å 500 H2O, 50 Cl−, 50 H3O+ 1.0 ps, 300 ps COMPASSII, NVT | CI molecule positioned above Fe atoms | [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haris, N.I.N.; Sobri, S.; Yusof, Y.A.; Kassim, N.K. An Overview of Molecular Dynamic Simulation for Corrosion Inhibition of Ferrous Metals. Metals 2021, 11, 46. https://doi.org/10.3390/met11010046
Haris NIN, Sobri S, Yusof YA, Kassim NK. An Overview of Molecular Dynamic Simulation for Corrosion Inhibition of Ferrous Metals. Metals. 2021; 11(1):46. https://doi.org/10.3390/met11010046
Chicago/Turabian StyleHaris, Nur Izzah Nabilah, Shafreeza Sobri, Yus Aniza Yusof, and Nur Kartinee Kassim. 2021. "An Overview of Molecular Dynamic Simulation for Corrosion Inhibition of Ferrous Metals" Metals 11, no. 1: 46. https://doi.org/10.3390/met11010046
APA StyleHaris, N. I. N., Sobri, S., Yusof, Y. A., & Kassim, N. K. (2021). An Overview of Molecular Dynamic Simulation for Corrosion Inhibition of Ferrous Metals. Metals, 11(1), 46. https://doi.org/10.3390/met11010046