Sustainable Production of Powder Metallurgy Aluminum Foams Sintered by Concentrated Solar Energy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
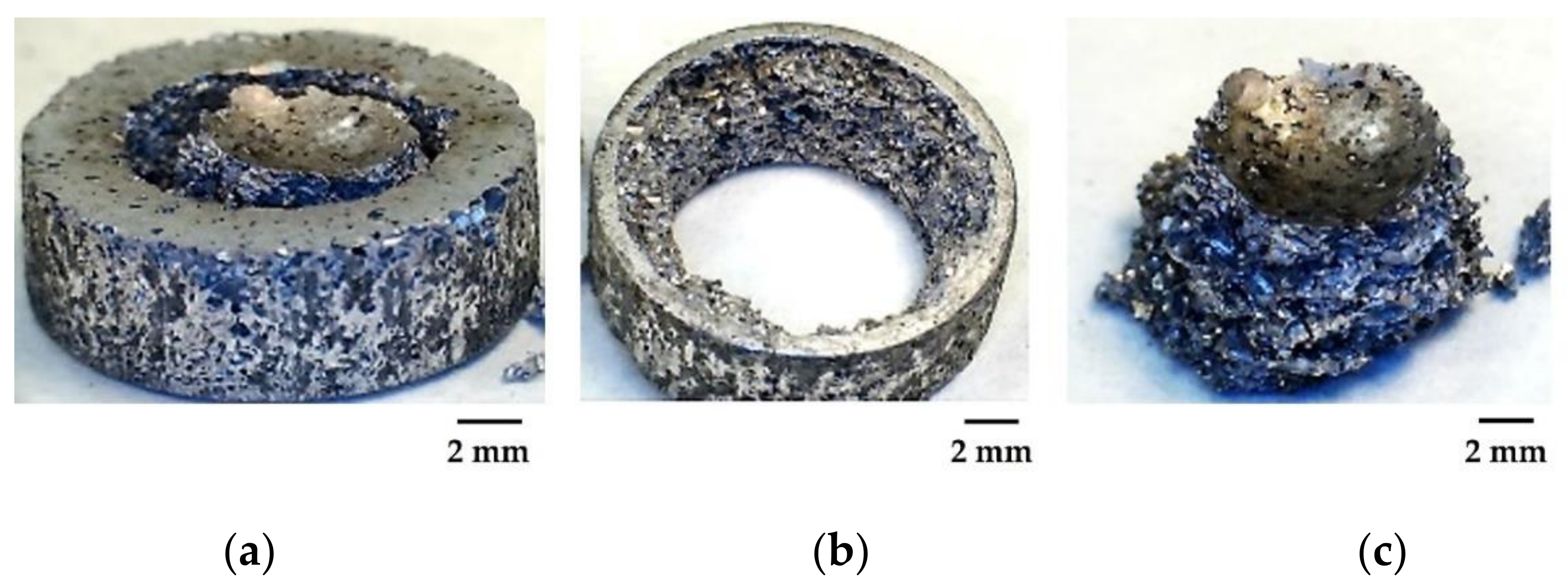
3.1. Foam Manufacturing
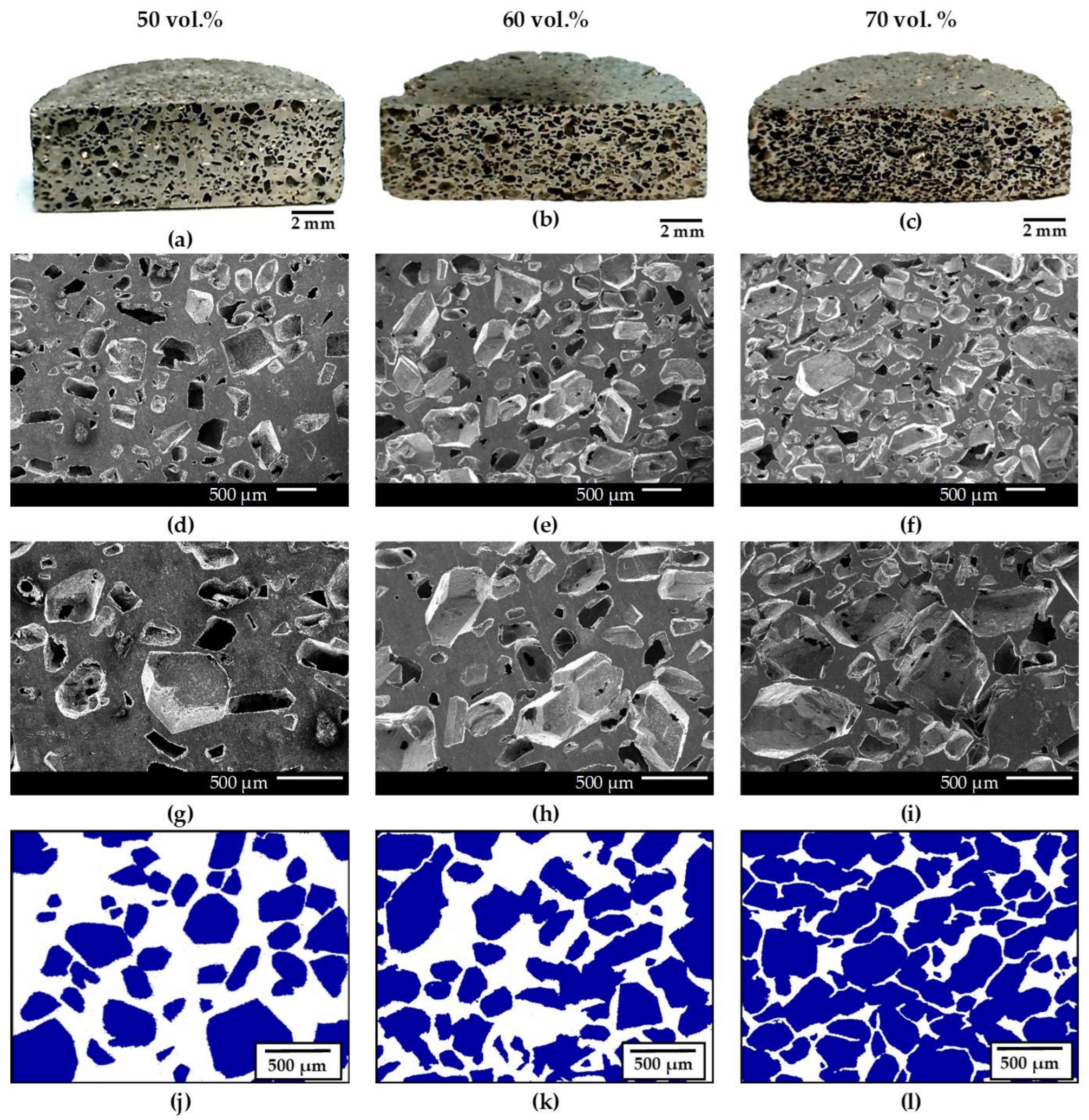
3.2. Porosity Analysis
3.3. Mechanical Properties Evaluation
4. Conclusions
- Saccharose is an excellent spacer for the fabrication of aluminum foams which allowed the obtention of high-quality Al foams without damage after the manufacturing process. In addition, it has allowed the attainment of foams with final porosities very close to the design porosity, with maximum differences of 2.1%. Increasing the amount of saccharose particles increases the foam porosity, including open porosity, permitting control of the size and geometry of the porosity of the resulting foams, which always showed a homogeneous pore distribution.
- The space-holder debinding stage through water dissolution is effective, since no remaining saccharose particles are detected and the mass remains stable after the last wash cycle, and its duration depends on the nominal design porosity. Aluminum foams with higher porosity percentage (60 and 70 vol.%), have shorter dissolution processes, e.g., as low as 8 h in the case of the foams with 70 vol.% of design porosity.
- Solar sintering is a sustainable, clean, and highly efficient energy process that has allowed for the reduction of conventional sintering times of aluminum foams (habitually higher than 9 h) to just 30 min. The use of an argon protective atmosphere was sufficient in preventing the oxidation and contamination of the material during the sintering cycle. The use of more expensive reducing atmospheres such as N2-H2, which are habitual in conventional procedures, was unnecessary.
- Aluminum foams sintered by CSE exhibited similar or even higher mechanical properties than Al foams fabricated by conventional methods. The compressive stress– strain curves obtained were very stable and reflected that the compressive strength decreases when the foam porosity increases. Aluminum foams with a design porosity of 50 vol.% presented the highest energy absorption values in the impact test and a strong aluminum matrix structure which lends them high mechanical properties, specifically high compressive strength and low weight, which makes them very appealing and potentially applicable.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbasi, M.; Reddy, S.; Ghafari-Nazari, A.; Fard, M. Multiobjective crashworthiness optimization of multi-cornered thin-walled sheet metal members. Thin-Walled Struct. 2015, 89, 31–41. [Google Scholar] [CrossRef]
- Sun, G.; Chen, D.; Huo, X.; Zheng, G.; Li, Q. Experimental and numerical studies on indentation and perforation characteristics of honeycomb sandwich panels. Compos. Struct. 2018, 184, 110–124. [Google Scholar] [CrossRef]
- Rong, Y.; Liu, J.; Luo, W.; He, W. Effects of geometric configurations of corrugated cores on the local impact and planar compression of sandwich panels. Compos. B Eng. 2018, 152, 324–335. [Google Scholar] [CrossRef]
- Tiwari, G.; Iqbal, M.A.; Gupta, P.K. Energy absorption characteristics of thin aluminium plate against hemispherical nosed projectile impact. Thin-Walled Struct. 2018, 126, 246–257. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, B.; Zhang, K.; Yang, B.; Yu, X. Ballistic performance and energy absorption characteristics of thin nickel-based alloy plates at elevated temperatures. Int. J. Impact Eng. 2019, 126, 160–171. [Google Scholar] [CrossRef]
- Banhart, J. Manufacture, characterisation and application of cellular metals and metal foams. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Rajak, D.K.; Kumaraswamidhas, L.A.; Das, S. An energy absorption behaviour of foam filled structures. Proc. Mater. Sci. 2014, 5, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Simancik, F.; Jerz, J. Aluminium foam: A new light weight structural material. Metall. Mater. 1997, 35, 187–194. [Google Scholar] [CrossRef]
- Fuganti, A.; Lorenzi, L.; Gronsund, A.; Langseth, M. Aluminium foam for automotive applications. Adv. Eng. Mater. 2000, 2, 200–204. [Google Scholar] [CrossRef]
- Chen, C.; Fleck, N.A.; Lu, T.J. The mode I crack growth resistance of metallic foams. J. Mech. Phys. Solids 2001, 49, 231–259. [Google Scholar] [CrossRef]
- Gutiérrez-Vázquez, J.A.; Oñoro, J. Espumas de aluminio. Fabricación, propiedades y aplicaciones. Rev. Metal. 2008, 44, 457–476. [Google Scholar] [CrossRef]
- Wu, Z.; He, D.P.; Song, Z.L.; Ma, L.Q. Effects of viscosity on cellular structure of foamed aluminum in foaming process. Sci. Bull. 2000, 45, 348–352. [Google Scholar] [CrossRef]
- Singh, S.; Bhatnagar, N. A survey of fabrication and application of metallic foams (1925–2017). J. Porous Mater. 2018, 25, 537–554. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Sun, D.X. A novel sintering-dissolution process for manufacturing Al foams. Scr. Mater. 2001, 44, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Zhao, N.Q.; Shi, C.S.; Li, J.J. Processing of open cell aluminum foams with tailored porous morphology. Scr. Mater. 2005, 53, 781–785. [Google Scholar] [CrossRef]
- Hussain, Z.; Suffin, N.S.A. Microstructure and mechanical behaviour of aluminium foam produced by sintering dissolution process using NaCl space holder. J. Eng. Sci. 2011, 7, 37–49. [Google Scholar]
- Kovacik, J.; Emmer, S.; Rodriguez, J.; Cañadas, I.S. Solar furnace: Thermal shock behavior of TiB2 coating on steel. In Proceedings of the International Conference on Metallurgy and Materials, Brno, Czech Republic, 21–23 May 2014; pp. 863–868. [Google Scholar]
- Van den Abeelen, L. Spaceplane HERMES: Europe’s Dream of Independent Manned Spaceflight, 1st ed.; Springer Praxis Books; Springer: Hilversum, The Netherlands, 2017. [Google Scholar]
- Pantelis, D.I.; Griniari, A.; Sarafoglou, C. Surface alloying of pre-deposited molybdenum-based powder on 304L stainless steel using concentrated solar energy. Sol. Energy Mater. Sol. Cells 2005, 89, 1–11. [Google Scholar] [CrossRef]
- Sánchez Bautista, C.; Ferriere, A.; Rodríguez, G.P.; López-Almodóvar, M.; Barba, A.; Sierra, C.; Vázquez, A.C. NiAl intermetallic coatings elaborated by a solar assisted SHS process. Intermetallics 2006, 14, 1270–1275. [Google Scholar] [CrossRef]
- Rodríguez, G.P.; Herranz, G.; Romero, A. Solar gas nitriding of Ti6Al4V alloy. App. Surf. Sci. 2013, 283, 445–452. [Google Scholar] [CrossRef]
- Romero, A.; García, I.; Arenas, M.A.; López, V.; Vázquez, A. High melting point metals welding by concentrated solar energy. J. Sol. Energy 2013, 95, 131–143. [Google Scholar] [CrossRef] [Green Version]
- Romero, A.; García, I.; Arenas, M.A.; López, V.; Vázquez, A. Ti6Al4V titanium alloy welded using concentrated solar energy. J. Mater. Process. Technol. 2015, 223, 284–291. [Google Scholar] [CrossRef]
- Karalis, D.G.; Pantelis, D.I.; Daniolos, N.M.; Bougiouri, V.D.; Rodríguez, J.; Karakizis, P.N.; Kazasidis, M.E. An attempt of 5083–H111 aluminum alloy welding using variable concentrated solar energy. In Proceedings of the 6th ICMEN International Conference, Tessaloniki, Greece, 5–6 October 2017; pp. 1–10. [Google Scholar]
- Herranz, G.; Romero, A.; de Castro, V.; Rodríguez, G.P. Development of high speed steel sintered using concentrated solar energy. J. Mater. Process. Technol. 2013, 213, 2065–2073. [Google Scholar] [CrossRef]
- Herranz, G.; Romero, A.; de Castro, V.; Rodríguez, G.P. Processing of AISI M2 high speed steel reinforced with vanadium carbide by solar sintering. Mater. Des. 2014, 54, 934–946. [Google Scholar] [CrossRef]
- Romero, A.; Rodríguez, G.P.; Barea, R. Sinter-hardening of chromium PM steels with concentrated solar energy. J. Mater. Process. Technol. 2020, 280, 116–616. [Google Scholar] [CrossRef]
- Kovacik, J.; Emmer, S.; Rodríguez, J.; Cañadas, I. Sintering of HDH Ti powder in a solar furnace at Plataforma Solar de Almería. J. Alloy. Compd. 2017, 695, 52–59. [Google Scholar] [CrossRef]
- García, I.; Gracia-Escosa, E.; Bayod, M.; Conde, A.; Arenas, M.A.; Damborenea, J.; Romero, A.; Rodríguez, G. Sustainable production of titanium foams for biomedical applications by concentrated solar energy. Mater. Lett. 2016, 185, 420–423. [Google Scholar] [CrossRef]
- García-Cambronero, L.E.; Cañadas, I. Foaming of aluminium-silicon alloy using concentrated solar energy. Sol. Energy 2010, 84, 879–887. [Google Scholar] [CrossRef]
- Cambronero, L.E.G.; Cañadas, I.; Diaz, J.J.; Ruiz Román, J.M.; Martinez, D. Properties of aluminium nodules foamed with concentrated solar energy. In Proceedings of the Euro International Powder Metallurgy Congress and Exhibition (Euro PM 2011), Barcelona, Spain, 9–12 October 2011; Volume 2, pp. 339–344. [Google Scholar]
- Ferriere, A.; Rodríguez, G.P.; Sobrino, J.A. Flux distribution delivered by a Fresnel lens used for concentrating solar energy. J. Sol. Energy. Eng. 2004, 126, 654–660. [Google Scholar] [CrossRef]
- Arnold, M.; Kroner, C.; Singer, R.F. PM aluminium foams: Stabilizing mechanisms and optimisation. In Proceedings of the 3rd International Conference on Cellular Metals and Metal Foaming Technology, Berlin, Germany, 23–25 June 2003; Volume 25, pp. 371–376. [Google Scholar]
- Romero, A.; Rodríguez, G.P. Sinterización de espumas de titanio en un horno solar parabólico. Rev. Soc. Esp. Mater. 2018, 2, 18–21. [Google Scholar]
- UNE-EN ISO 2738:2000, Materiales Metálicos Sinterizados, Excepto Metal Duro. Determinación de la Densidad, Contenido de Aceite Y Porosidad. 2000. Available online: https://tienda.aenor.com/norma-une-en-iso-2738-2000-n0023097 (accessed on 27 September 2021).
- UNE-EN ISO 4506:2018, Materiales Metálicos Sinterizados, Excepto Metal Duro. Ensayo de Compresión. 2018. Available online: https://www.en.aenor.com/normas-y-libros/buscador-de-normas/une/?c=N0060628 (accessed on 27 September 2021).
- UNE-EN ISO 5754:2017, Materiales Metálicos Sinterizados, Excepto Metal Duro. Probeta Sin Entalla Para Ensayo de Impacto. 2017. Available online: https://tienda.aenor.com/norma-une-en-iso-5754-2018-n0059736 (accessed on 27 September 2021).
- Michailidis, N.; Stergioudi, F. Establishment of process parameters for producing Al-foam by dissolution and powder sintering method. Mater. Des. 2011, 32, 1559–1564. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, F.; Fung, T. Optimization of compaction and liquid-state sintering in sintering and dissolution process for manufacturing Al-foams. Mater. Sci. Eng. 2004, 364, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Idris, M.I.; Ehsan, I.I.; Mohamed, S.H. Effect of organic space holder in fabrication of closed-cell aluminium foam. Trans. Tech. Publ. 2014, 594, 780–785. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, A. Porous metals and metal foams made from powders. Powder Metall. 2012, 124, 33060. [Google Scholar] [CrossRef] [Green Version]
- Torres, Y.; Pavón, J.; Rodríguez, J.A. Processing and characterization of porous titanium for implants by using NaCl as space holder. J. Mater. Process. Technol. 2012, 212, 1061–1069. [Google Scholar] [CrossRef]
- Sun, D.X.; Zhaoc, Y.Y. Static and dynamic energy absorption of Al foams produced by the sintering and dissolution process. Metall. Mater. Trans. B 2003, 34, 69–74. [Google Scholar] [CrossRef]
- Banhart, J.; Baumeister, J. Deformation characteristics of metal foams. J. Mater. Sci. 1998, 33, 1431–1440. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure & Properties; Pergamon Press: Oxford, UK, 1988. [Google Scholar] [CrossRef]
- Szlancsik, A.; Katona, B.; Dombóvári, Z.; Orbulov, I.N. On the effective young’s modulus of metal matrix syntactic foams. Mater. Sci. Technol. 2017, 33, 2283–2289. [Google Scholar] [CrossRef]
- Orbulov, I.N.; Szlancsik, A. On the mechanical properties of aluminum matrix syntactic foams. Adv. Eng. Mater. 2018, 20, 1700980. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Hu, Q.; Du, J.; Song, H.; Zou, T.; Sha, J.; He, C.; Zhao, N. Compression fatigue properties of open-cell aluminium foams fabricated by space-holder method. Int. J. Fatigue 2019, 121, 272–280. [Google Scholar] [CrossRef]
- Cao, X.Q.; Wang, Z.H.; Ma, H.W.; Zhao, L.M.; Yang, G.T. Effects of cell size on compressive properties of aluminium foam. Trans. Nonferr. Met. Soc. China 2006, 16, 351–356. [Google Scholar] [CrossRef]
- Miyoshi, T.; Elnasri, I.; Pattofatto, S.; Zhao, H.; Tsitsiris, H.; Hild, F.; Girard, Y. Shock enhancement of cellular structures under impact loading: Part I experiments. Scr. Mater. 1999, 41, 1055–1060. [Google Scholar] [CrossRef]










| Design Porosity | Green State | Sintered State | %Var. | ||||
|---|---|---|---|---|---|---|---|
| (vol.%) | Diameter (mm) | H (mm) | Vol. (cm3) | Diameter (mm) | H (mm) | Vol. (cm3) | Vol. (cm3) |
| 50 | 16.00 ± 0.10 | 5.00 ± 0.10 | 1.00 ± 0.01 | 15.97 ± 0.09 | 5.14 ± 0.10 | 1.03 ± 0.02 | 0.03 |
| 60 | 15.93 ± 0.10 | 5.12 ± 0.11 | 1.02 ± 0.02 | 0.02 | |||
| 70 | 15.96 ± 0.04 | 5.16 ± 0.02 | 1.03 ± 0.01 | 0.03 | |||
| Al Foam | 50 vol.% | 60 vol.% | 70 vol.% | |
|---|---|---|---|---|
| Porosity (%) | Total | 52.1 ± 1.6 | 60.8 ± 1.6 | 70.1 ± 0.9 |
| Open | 17.2 ± 0.9 | 26.3 ± 0.8 | 37.9 ± 0.8 | |
| Design Porosity | 50 vol.% | 60 vol.% | 70 vol.% |
|---|---|---|---|
| σcomp (MPa) | 27.4 ± 0.7 | 16.5 ± 0.9 | 7.5 ± 1.5 |
| Mass (g) | 1.33 ± 0.03 | 1.05 ± 0.03 | 0.79 ± 0.02 |
| σ’comp (MPa/g) | 20.6 ± 0.9 | 15.7 ± 1.2 | 9.5 ± 1.9 |
| Effective E (MPa) | 313.2 ± 10.7 | 281.6 ± 10.2 | 116.7 ± 16.8 |
| Design Porosity | 50 vol.% | 60 vol.% | 70 vol.% |
|---|---|---|---|
| Eabs (MJ/m3) | 24.42 ± 0.44 | 16.42 ± 0.88 | 10.69 ± 1.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cañadilla, A.; Romero, A.; Rodríguez, G.P. Sustainable Production of Powder Metallurgy Aluminum Foams Sintered by Concentrated Solar Energy. Metals 2021, 11, 1544. https://doi.org/10.3390/met11101544
Cañadilla A, Romero A, Rodríguez GP. Sustainable Production of Powder Metallurgy Aluminum Foams Sintered by Concentrated Solar Energy. Metals. 2021; 11(10):1544. https://doi.org/10.3390/met11101544
Chicago/Turabian StyleCañadilla, Antonio, Ana Romero, and Gloria P. Rodríguez. 2021. "Sustainable Production of Powder Metallurgy Aluminum Foams Sintered by Concentrated Solar Energy" Metals 11, no. 10: 1544. https://doi.org/10.3390/met11101544








