Synthesis of Ti–Al Bimodal Powder for High Flowability Feedstock by Electrical Explosion of Wires
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Powders
2.2. Characterization of Powders
2.3. Preparation of the Powder–Polymer Feedstock
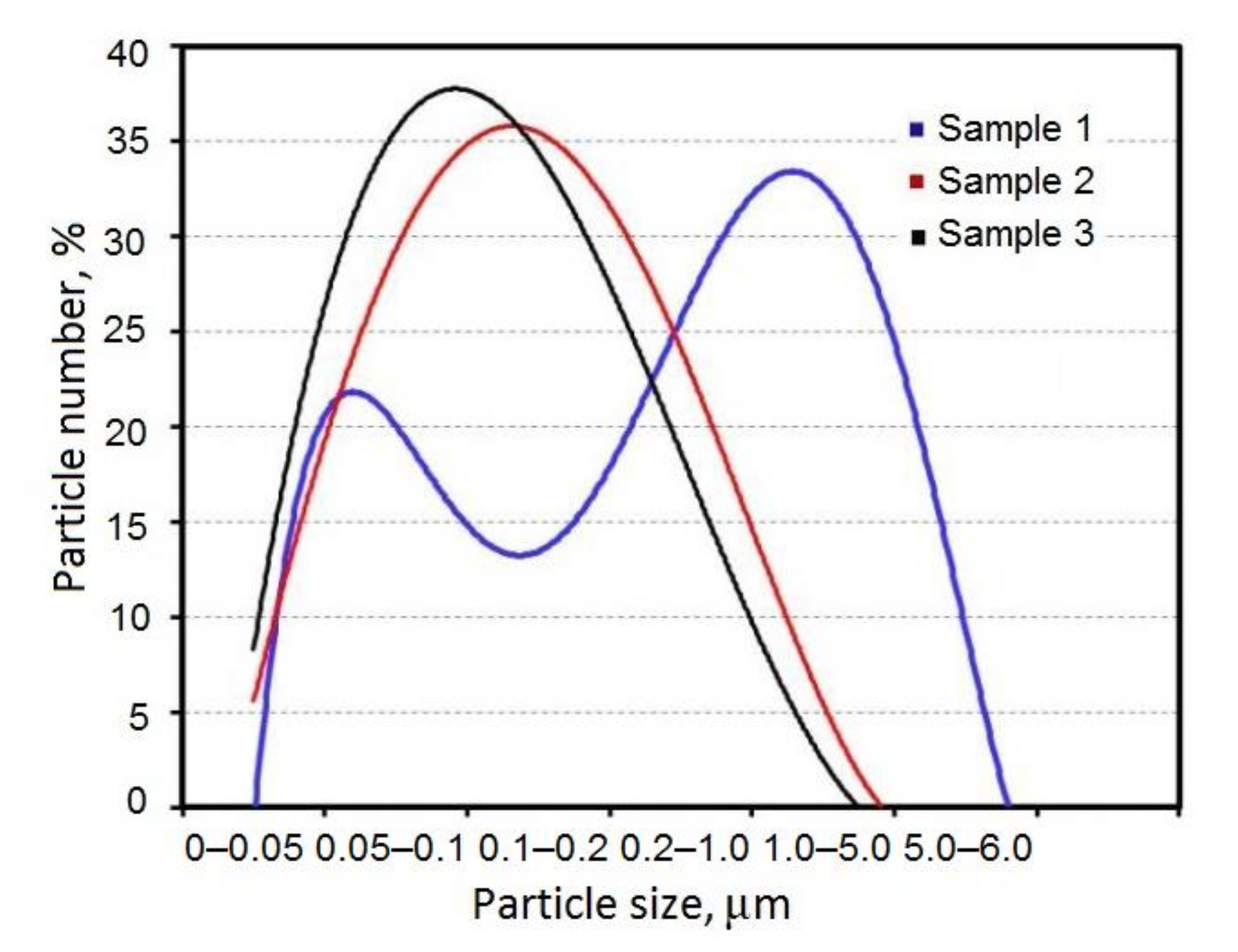
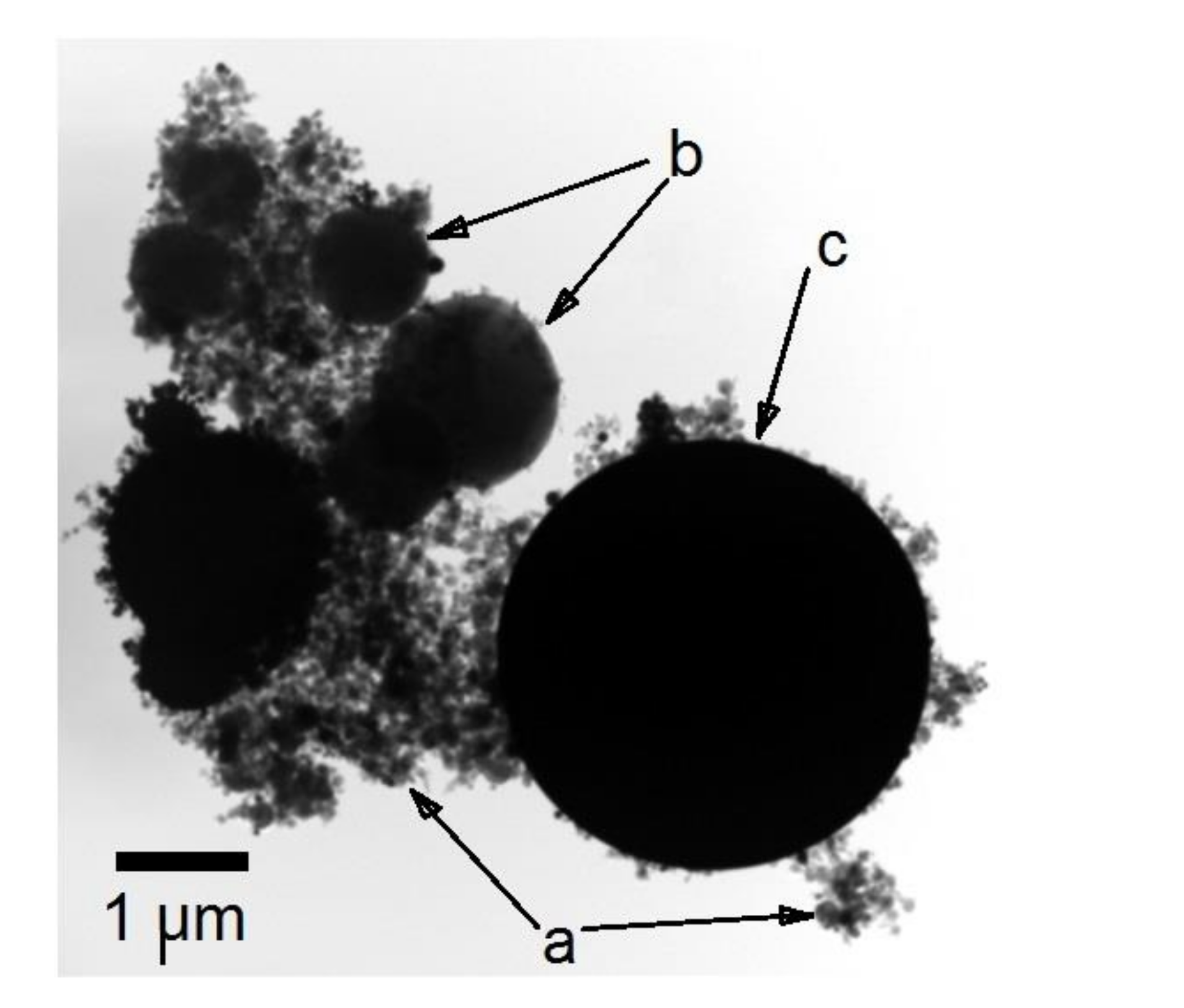
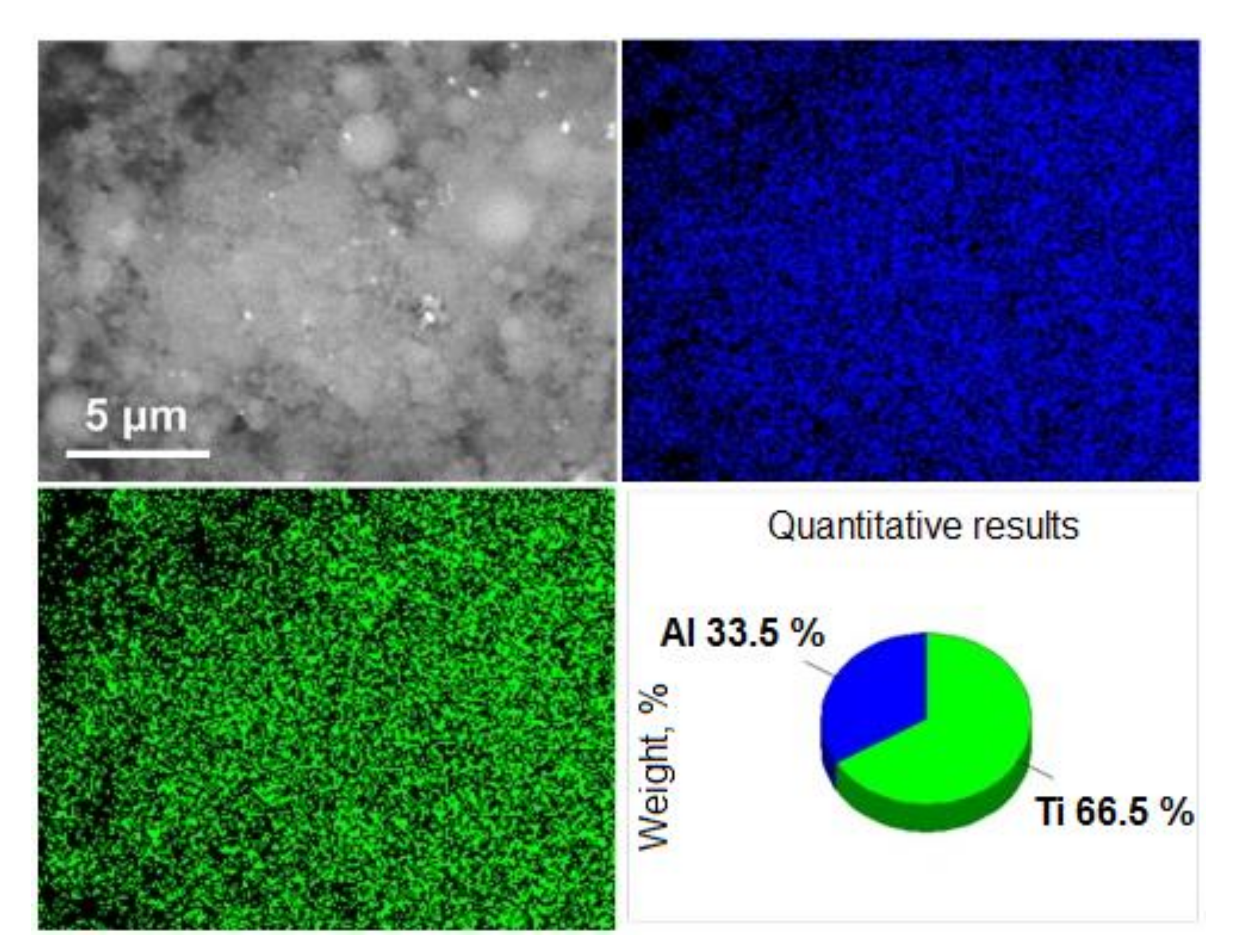
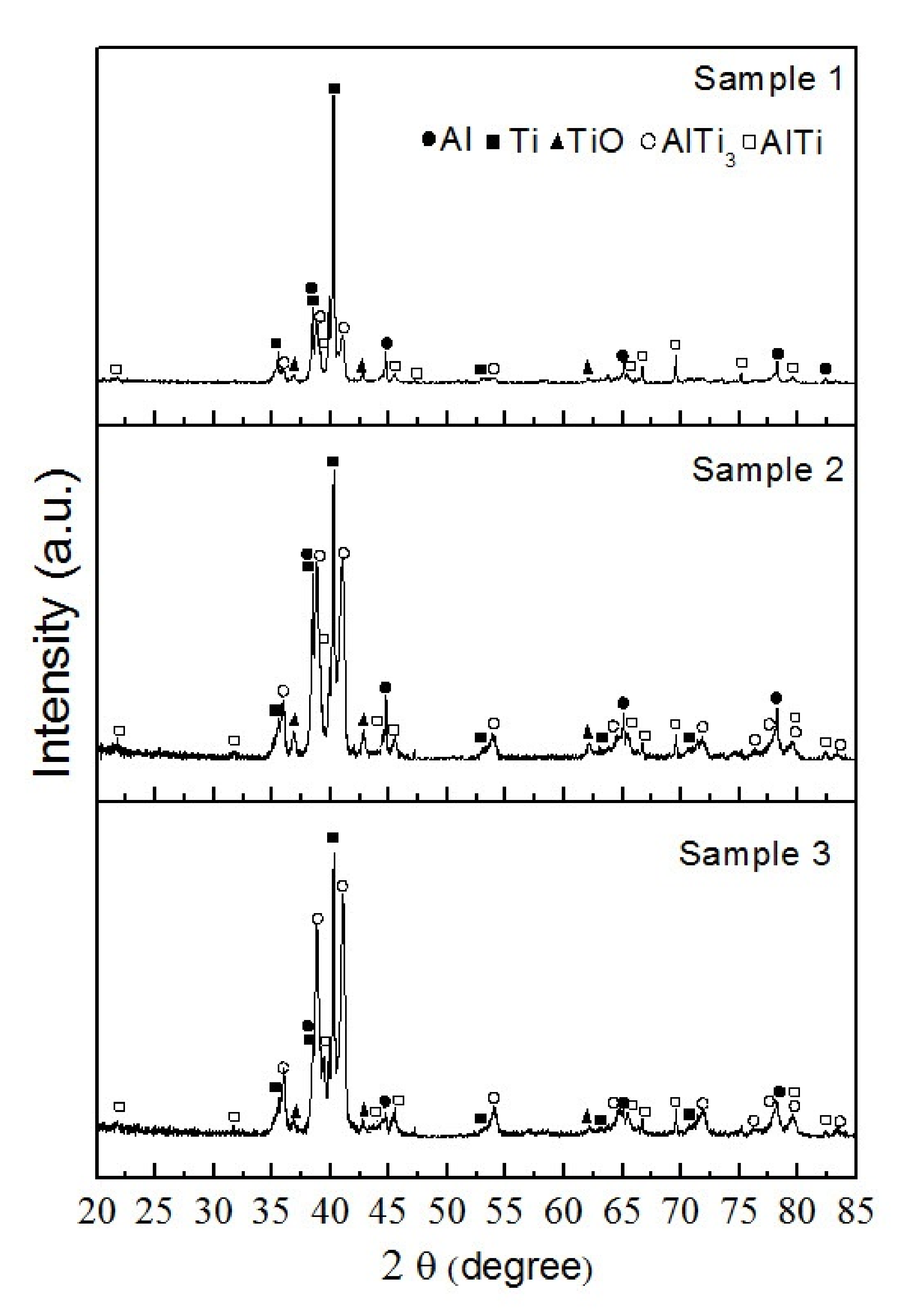
3. Results and Discussion
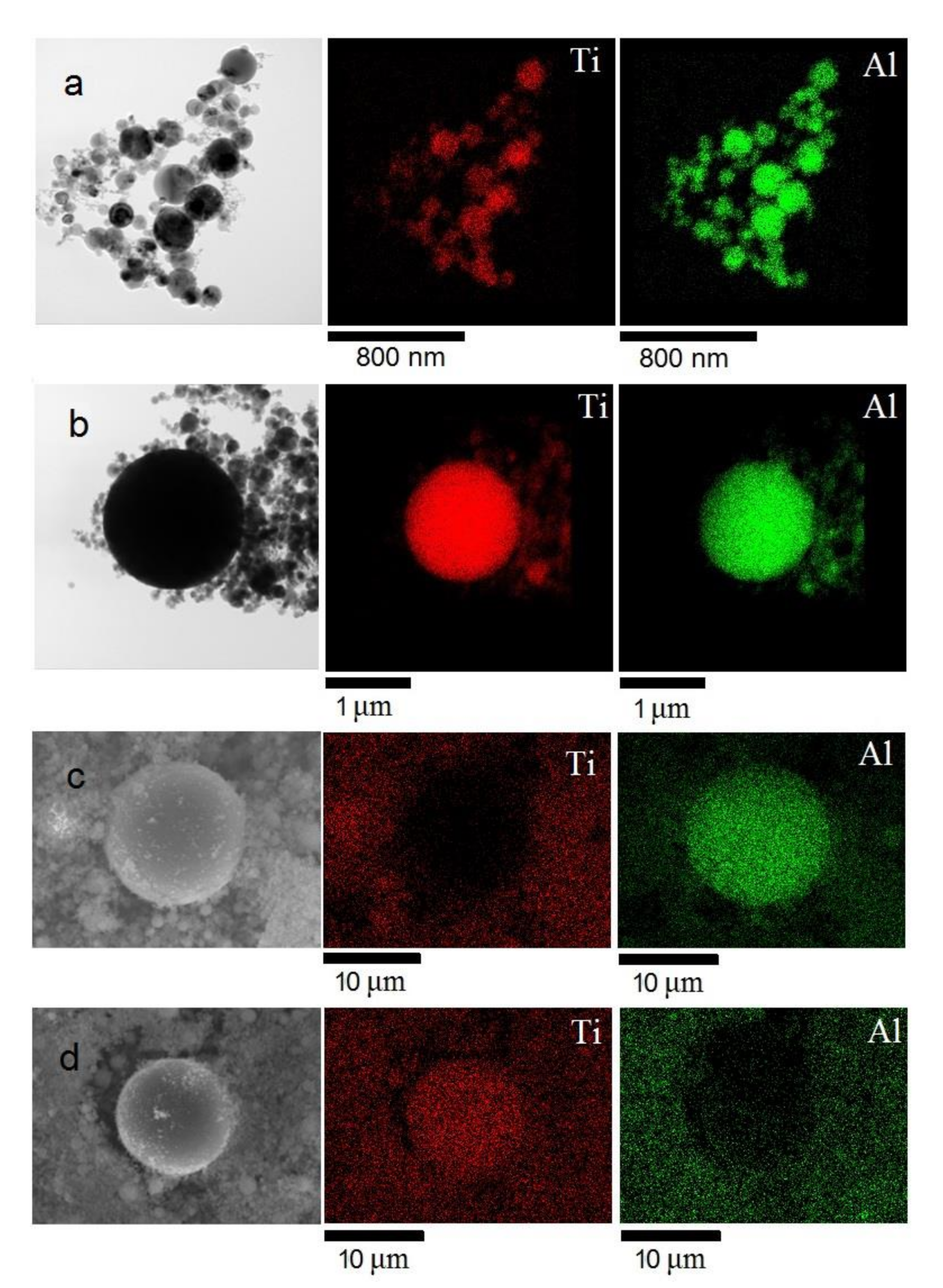
3.1. Preparation and Characterization of Ti–Al Powders
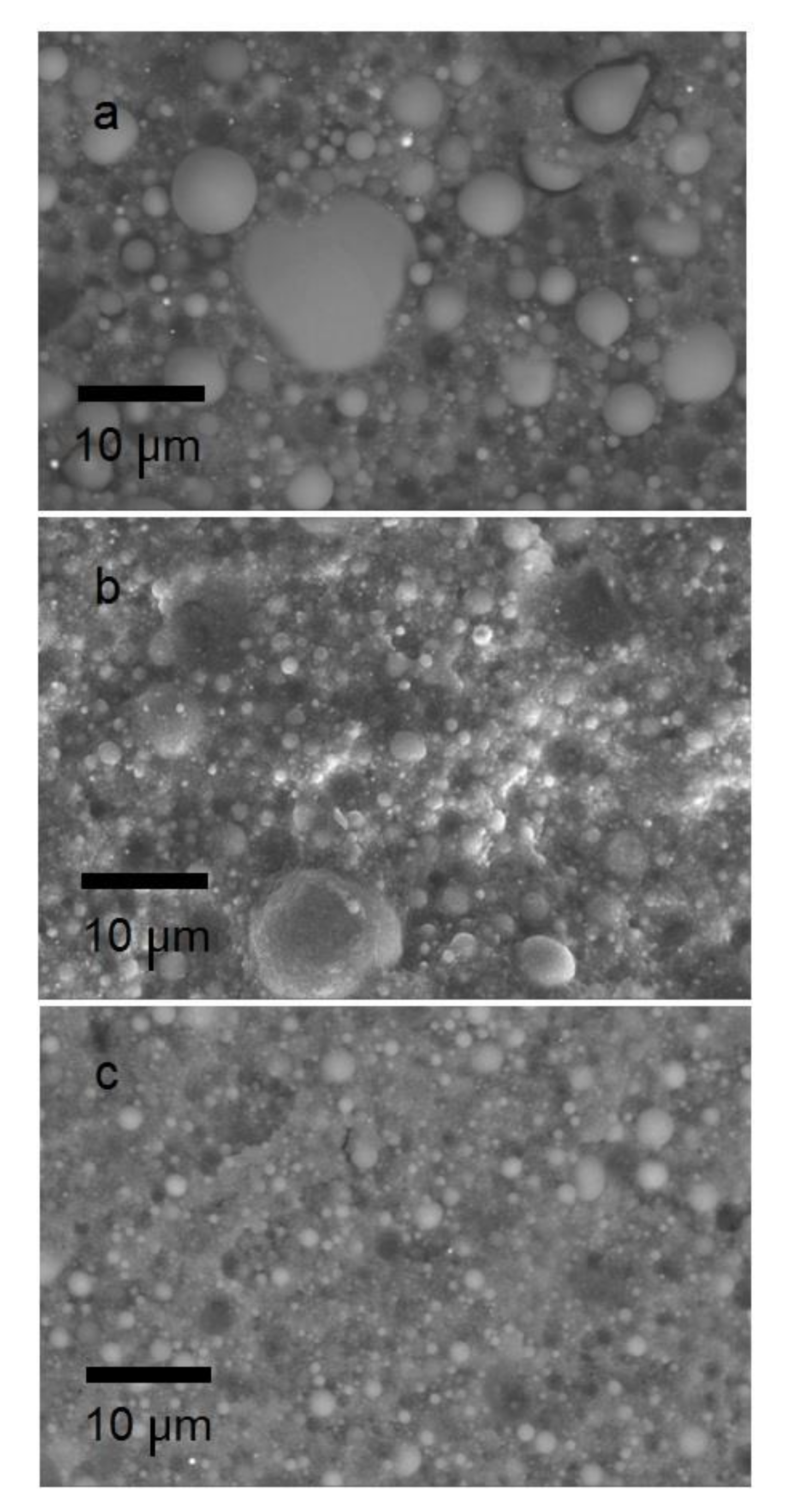
3.2. The Powder–Polymer Feedstock Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hegab, H.A. Design for additive manufacturing of composite materials and potential alloys: A review. Manuf. Rev. 2016, 3, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Kaplas, M. Comparative Study of 3D Printing Technologies for Rapid Casting of Aluminium Alloy. Mater. Manuf. Process. 2009, 24, 1405–1411. [Google Scholar] [CrossRef]
- Kristiawan, R.B.; Imaduddin, F.; Ariawan, D.; Ubaidillah; Arifin, Z. A review on the fused deposition modeling (FDM) 3D printing: Filament processing, materials, and printing parameters. Open Eng. 2021, 11, 639–649. [Google Scholar] [CrossRef]
- Zhang, J.; Amini, N.; Morton, D.A.; Hapgood, K.P. 3D printing with particles as feedstock materials. Adv. Powder Technol. 2021, 32, 3324–3345. [Google Scholar] [CrossRef]
- Gonzalez-Gutierrez, J.; Cano, S.; Schuschnigg, S.; Kukla, C.; Sapkota, J.; Holzer, C. Additive Manufacturing of Metallic and Ceramic Components by the Material Extrusion of Highly-Filled Polymers: A Review and Future Perspectives. Materials 2018, 11, 840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subaşı, M.; Safarian, A.; Karataş, Ç. An investigation on characteristics and rheological behaviour of titanium injection moulding feedstocks with thermoplastic-based binders. Powder Met. 2019, 62, 229–239. [Google Scholar] [CrossRef]
- Giberti, H.; Strano, M.; Annoni, M. An innovative machine for Fused Deposition Modeling of metals and advanced ceramics. In Proceedings of the MATEC Web of Conferences; EDP Sciences: New York, NY, USA, 2016; Volume 43, p. 3003. [Google Scholar]
- Choi, J.-P.; Lyu, H.-G.; Lee, W.-S.; Lee, J.-S. Investigation of the rheological behavior of 316L stainless steel micro-nano powder feedstock for micro powder injection molding. Powder Technol. 2014, 261, 201–209. [Google Scholar] [CrossRef]
- Bricout, J.; Gelin, J.-C.; Ablitzer, C.; Matheron, P.; Brothier, M. Influence of powder characteristics on the behaviour of PIM feedstock. Chem. Eng. Res. Des. 2013, 91, 2484–2490. [Google Scholar] [CrossRef]
- Oh, J.W.; Ryu, S.K.; Lee, W.S.; Park, S.J. Analysis of compaction and sintering behavior of 316L stainless steel nano/micro bimodal powder. Powder Technol. 2017, 322, 1–8. [Google Scholar] [CrossRef]
- Suri, P.; Atre, S.V.; German, R.M.; de Souza, J.P. Effect of mixing on the rheology and particle characteristics of tungsten-based powder injection molding feedstock. Mater. Sci. Eng. A 2003, 356, 337–344. [Google Scholar] [CrossRef]
- Li, J.; Pan, Y.; Qiu, F.; Huang, L.; Guo, J. Alumina ceramics fabricated from bimodal alumina with additives. Mater. Sci. Eng. A 2006, 435–436, 611–619. [Google Scholar] [CrossRef]
- Prabhu, T.R. Effect of bimodal size particles reinforcement on the wear, friction and mechanical properties of brake composites. Tribol. Mater. Surf. Interfaces 2016, 10, 163–171. [Google Scholar] [CrossRef]
- Rajabi, J.; Muhamad, N.; Sulong, A.B.; Fayyaz, A.; Raza, M.R. The effect of nano-sized stainless steel powder addition on mechanical and physical properties of micropowder injection molded part. Mater. Des. 2014, 63, 223–232. [Google Scholar] [CrossRef]
- Crane, N.B.; Wilkes, J.; Sachs, E.; Allen, S.M. Improving accuracy of powder-based SFF processes by metal deposition from a nanoparticle dispersion. Rapid Prototyp. J. 2006, 12, 266–274. [Google Scholar] [CrossRef]
- Du, W.; Singh, M.; Singh, D. Binder jetting additive manufacturing of silicon carbide ceramics: Development of bimodal powder feedstocks by modeling and experimental methods. Ceram. Int. 2020, 46, 19701–19707. [Google Scholar] [CrossRef]
- Rajabi, J.; Fayyaz, A. Effects of Nanopowder Addition on Rheological Properties of Feedstock for Micropowder Injection Moulding Process. J. Kejuruteraan. 2019, 31, 229–241. [Google Scholar] [CrossRef]
- Pervikov, A.; Rodkevich, N.; Glazkova, E.; Lerner, M. Bimodal metal micro-nanopowders for powder injection molding. AIP Conf. Proc. 2017, 1915, 040045. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.W.; Seong, Y.; Shin, D.S.; Park, S.J. Investigation and two-stage modeling of sintering behavior of nano/micro-bimodal powders. Powder Technol. 2019, 352, 42–52. [Google Scholar] [CrossRef]
- Bai, Y.; Wagner, G.; Williams, C.B. Effect of Particle Size Distribution on Powder Packing and Sintering in Binder Jetting Additive Manufacturing of Metals. J. Manuf. Sci. Eng. 2017, 139, 081019. [Google Scholar] [CrossRef] [Green Version]
- Song, J.-L.; Lee, G.-Y.; Hong, E.-J.; Lee, C.S.; Lee, J.-S. Sintering behavior of bimodal iron nanopowder agglomerates. J. Am. Ceram. Soc. 2019, 102, 3791–3801. [Google Scholar] [CrossRef]
- Macak, J.; Syrovy, T.; Wagner, T.; Kubac, L.; Josefik, F. Method for Preparation of a Bimodal Mixture of Copper Nanoparticles and Microparticles with a Polymeric Protective Layer, a Bimodal Mixture of Copper Nanoparticles and Microparticles with a Polymeric Protective Layer Prepared by This Method and a Printing Formula Containing This Bimodal Mixture. WO Patent No. WO2016045648, 31 March 2016. [Google Scholar]
- Kim, K.-H.; Choi, H.; Han, C. Tungsten Micropowder/Copper Nanoparticle Core/Shell-Structured Composite Powder Synthesized by Inductively Coupled Thermal Plasma Process. Met. Mater. Trans. A 2017, 48, 439–445. [Google Scholar] [CrossRef]
- Lerner, M.I.; Svarovskaya, N.V.; Psakhie, S.G.; Bakina, O.V. Production technology, characteristics, and some applications of electric-explosion nanopowders of metals. Nanotechnol. Russ. 2009, 4, 741–757. [Google Scholar] [CrossRef]
- Sedoi, V.S.; Ivanov, Y.F. Particles and crystallites under electrical explosion of wires. Nanotechnology 2008, 19, 145710. [Google Scholar] [CrossRef] [PubMed]
- Pervikov, A.; Glazkova, E.; Lerner, M. Energy characteristics of the electrical explosion of two intertwined wires made of dissimilar metals. Phys. Plasmas 2018, 25, 070701. [Google Scholar] [CrossRef]
- Lerner, M.; Pervikov, A.; Glazkova, E.; Rodkevich, N.; Toropkov, N. Electrical Explosion Synthesis, Oxidation and Sintering Behavior of Ti-Al Intermetallide Powders. Metals 2021, 11, 760. [Google Scholar] [CrossRef]
- Pervikov, A.; Toropkov, N.; Kazantsev, S.; Bakina, O.V.; Glazkova, E.; Lerner, M. Preparation of Nano/Micro Bimodal Aluminum Powder by Electrical Explosion of Wires. Materials 2021, 14, 6602. [Google Scholar] [CrossRef]
- Shenoy, A.V.; Saini, D.R.; Nadkarni, V.M. Melt Rheology of Polymer Blends from Melt Flow Index. Int. J. Polym. Mater. Polym. Biomater. 1984, 10, 213–235. [Google Scholar] [CrossRef]
- Schuster, J.C.; Palm, M. Reassessment of the binary Aluminum-Titanium phase diagram. J. Phase Equilibria Diffus. 2006, 27, 255–277. [Google Scholar] [CrossRef]
- Lerner, M.I.; Pervikov, A.V.; Glazkova, E.A.; Svarovskaya, N.V.; Lozhkomoev, A.; Psakhie, S.G. Structures of binary metallic nanoparticles produced by electrical explosion of two wires from immiscible elements. Powder Technol. 2016, 288, 371–378. [Google Scholar] [CrossRef]
- Choi, J.-P.; Park, J.-S.; Hong, E.-J.; Lee, W.-S.; Lee, J.-S. Analysis of the rheological behavior of Fe trimodal micro-nano powder feedstock in micro powder injection molding. Powder Technol. 2017, 319, 253–260. [Google Scholar] [CrossRef]
- You, W.-K.; Choi, J.-P.; Yoon, S.-M.; Lee, J.-S. Low temperature powder injection molding of iron micro-nano powder mixture. Powder Technol. 2012, 228, 199–205. [Google Scholar] [CrossRef]
- Oh, J.W.; Bollina, R.; Lee, W.S.; Park, S.J. Effect of nanopowder ratio in bimodal powder mixture on powder injection molding. Powder Technol. 2016, 302, 168–176. [Google Scholar] [CrossRef]
- Krinitcyn, M.; Toropkov, N.; Pervikov, A.; Glazkova, E.; Lerner, M. Characterization of nano/micro bimodal 316L SS powder obtained by electrical explosion of wire for feedstock application in powder injection molding. Powder Technol. 2021, 394, 225–233. [Google Scholar] [CrossRef]
- Oh, J.W.; Seong, Y.; Park, S.J. Effect of nanoparticles in bimodal powder on physical and mechanical properties of powder injection molded parts. J. Mater. Process. Technol. 2018, 262, 503–510. [Google Scholar] [CrossRef]
- Oh, J.W.; Park, J.M.; Shin, D.S.; Noh, J.; Park, S.J. Comparative study of nanoparticle effects on feedstock behavior for injection molding. Mater. Manuf. Process. 2019, 34, 414–421. [Google Scholar] [CrossRef]
- Oh, J.W.; Lee, W.S.; Park, S.J. Nanopowder Effect on Fe Nano/Micro-Bimodal Powder Injection Molding. Met. Mater. Trans. A 2018, 49, 5535–5545. [Google Scholar] [CrossRef]
- Rueda, M.M.; Auscher, M.-C.; Fulchiron, R.; Périé, T.; Martin, G.; Sonntag, P.; Cassagnau, P. Rheology and applications of highly filled polymers: A review of current understanding. Prog. Polym. Sci. 2017, 66, 22–53. [Google Scholar] [CrossRef]




| Sample | U0 (kV) | C (μF) | Wire Dimensions | |||
|---|---|---|---|---|---|---|
| Diameter (mm) | Length (mm) | |||||
| Ti | Al | Ti | Al | |||
| 1 | 15 | 1.6 | 0.31 | 0.25 | 70 | 70 |
| 2 | 21 | |||||
| 3 | 25 | |||||
| Sample | I(t) 107 (A/cm2) | E (J) | E(t)/t (J/μs) | Specific Surface Area (m2/g) | Size Distribution Maximum |
|---|---|---|---|---|---|
| 1 | 1.2 | 147 | 56.5 | 9.4 | 59 nm, 2 μm |
| 2 | 1.6 | 292 | 127 | 10.5 | 132 nm |
| 3 | 1.8 | 430 | 215 | 11.5 | 95 nm |
| Sample | MFI (g/10 min) | ν (Pa·s) |
|---|---|---|
| bimodal feedstock 1 | 310 ± 14 | 41.4 ± 0.6 |
| monomodal feedstock 2 | 230 ± 16 | 55.9 ± 0.6 |
| monomodal feedstock 3 | 170 ± 19 | 75.6 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lerner, M.; Pervikov, A.; Glazkova, E.; Rodkevich, N.; Suliz, K.; Kazantsev, S.; Toropkov, N.; Bakina, O. Synthesis of Ti–Al Bimodal Powder for High Flowability Feedstock by Electrical Explosion of Wires. Metals 2022, 12, 478. https://doi.org/10.3390/met12030478
Lerner M, Pervikov A, Glazkova E, Rodkevich N, Suliz K, Kazantsev S, Toropkov N, Bakina O. Synthesis of Ti–Al Bimodal Powder for High Flowability Feedstock by Electrical Explosion of Wires. Metals. 2022; 12(3):478. https://doi.org/10.3390/met12030478
Chicago/Turabian StyleLerner, Marat, Alexander Pervikov, Elena Glazkova, Nikolay Rodkevich, Konstantin Suliz, Sergey Kazantsev, Nikita Toropkov, and Olga Bakina. 2022. "Synthesis of Ti–Al Bimodal Powder for High Flowability Feedstock by Electrical Explosion of Wires" Metals 12, no. 3: 478. https://doi.org/10.3390/met12030478
APA StyleLerner, M., Pervikov, A., Glazkova, E., Rodkevich, N., Suliz, K., Kazantsev, S., Toropkov, N., & Bakina, O. (2022). Synthesis of Ti–Al Bimodal Powder for High Flowability Feedstock by Electrical Explosion of Wires. Metals, 12(3), 478. https://doi.org/10.3390/met12030478






