Effect of DC Currents and Strain on Corrosion of X80 Steel in a Near-Neutral Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Simulated Soil Solution
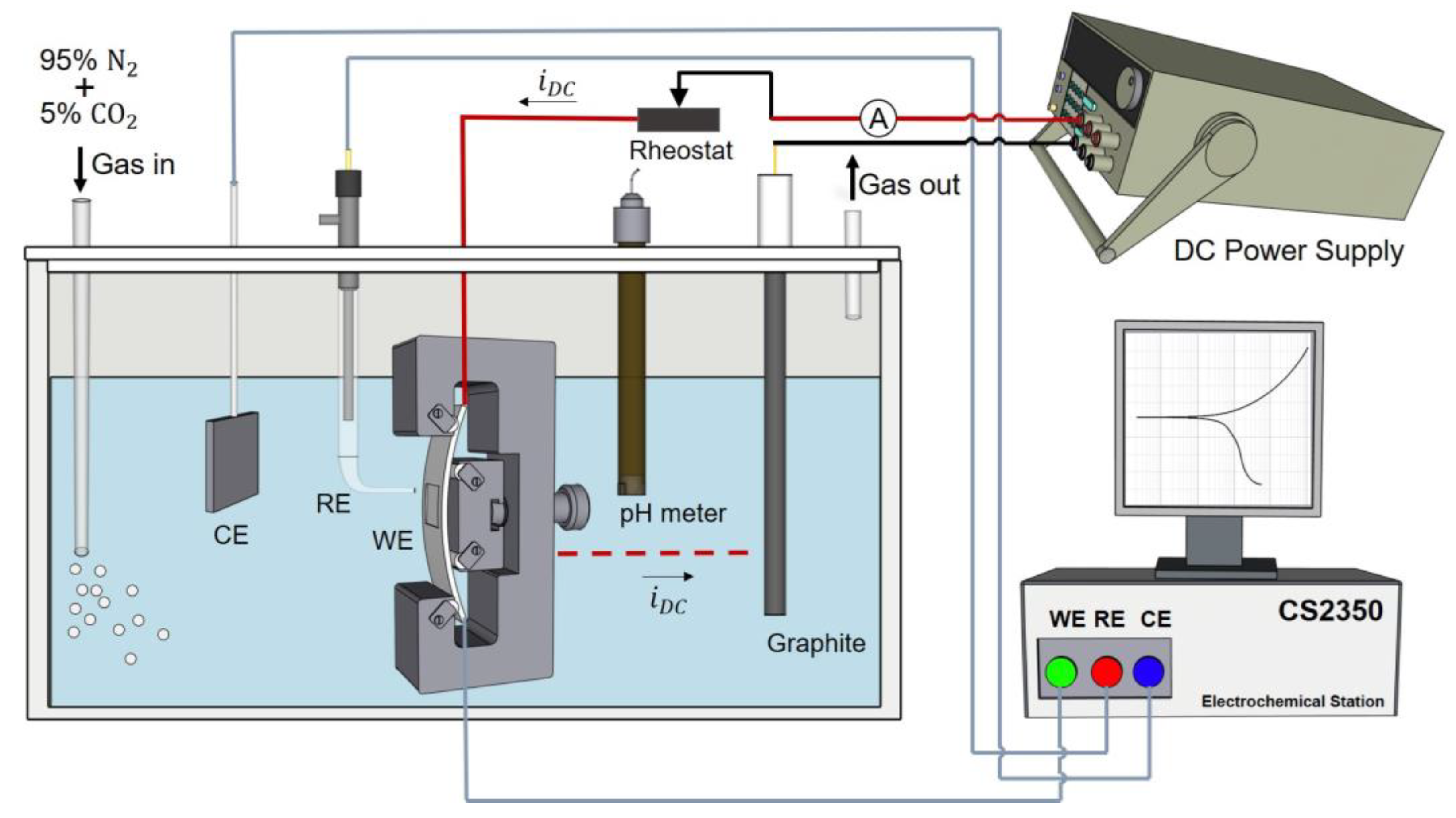
2.3. Experimental Set-Up
2.4. Electrochemical Measurement
2.5. Morphologies Characterization Analysis
3. Results
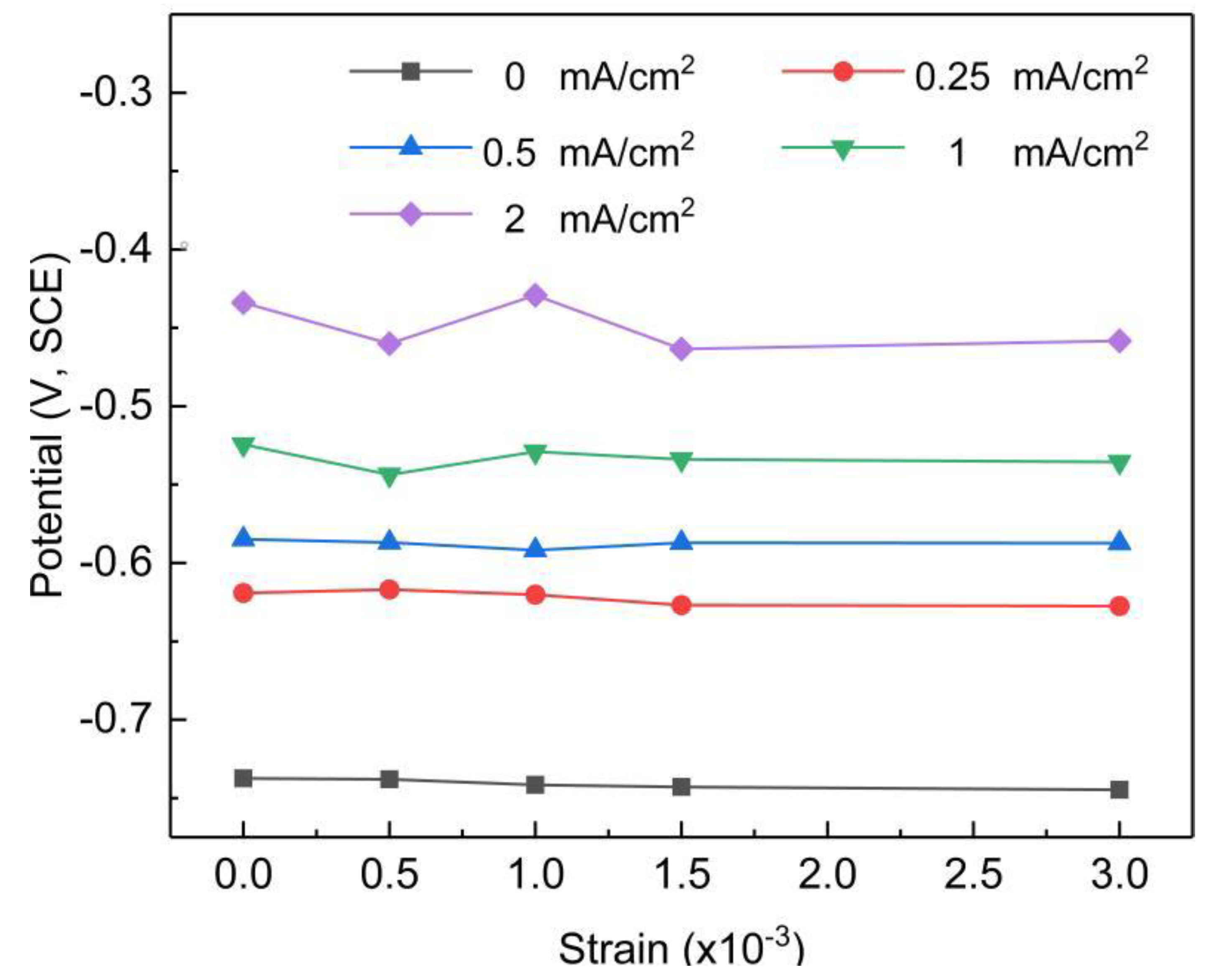
3.1. Potential Analysis
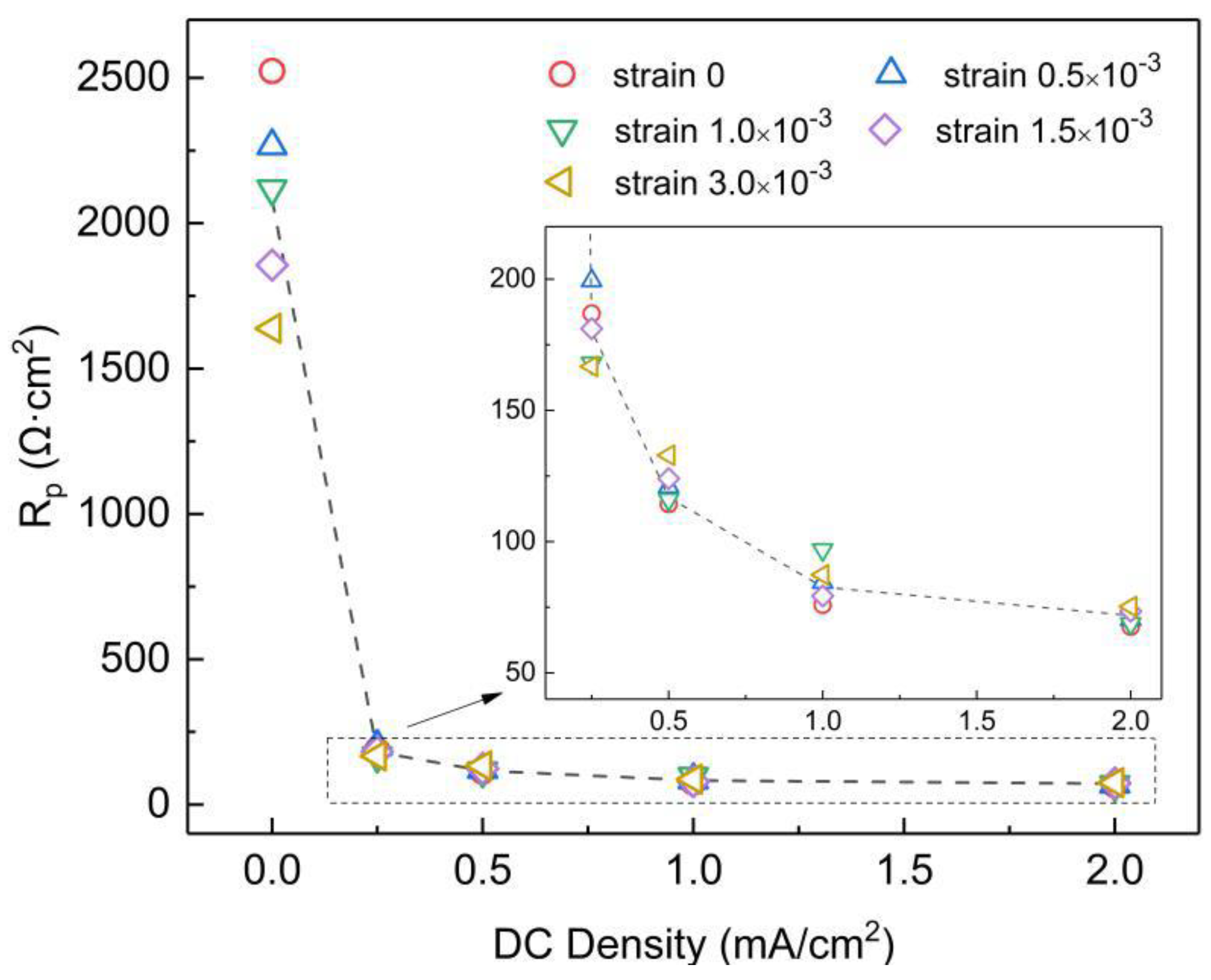
3.2. Linear Potential Resistance
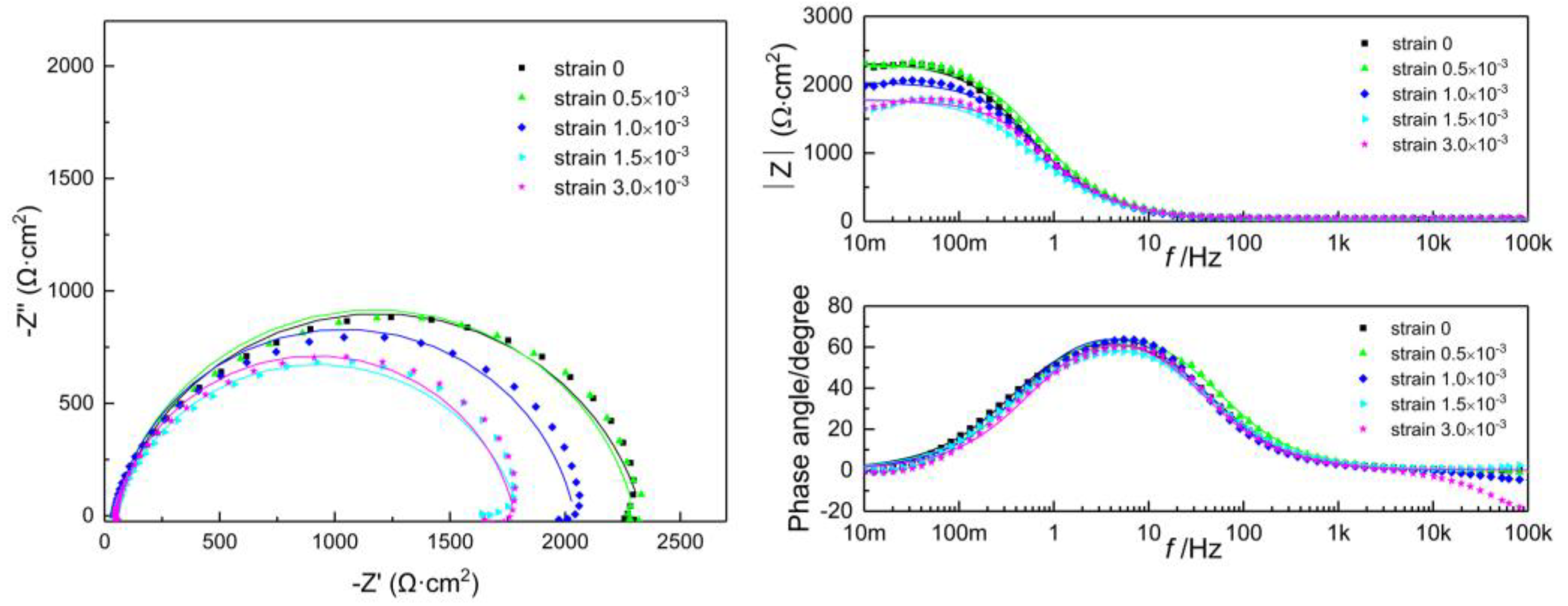
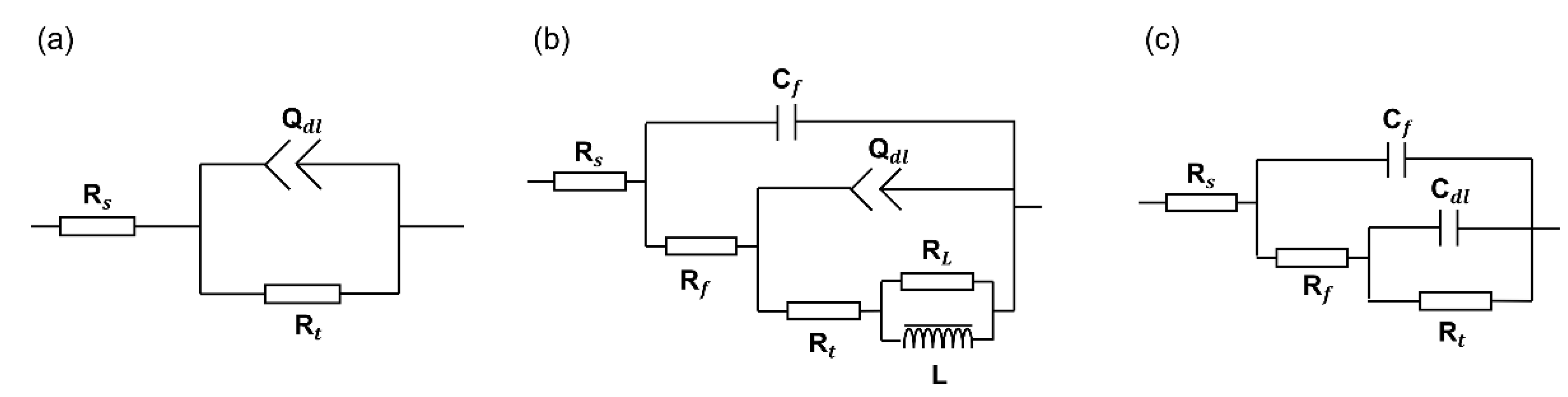
3.3. Electrochemical Impedance Spectroscopy
3.4. Microscopic Characterization of Corrosion Products and Morphology
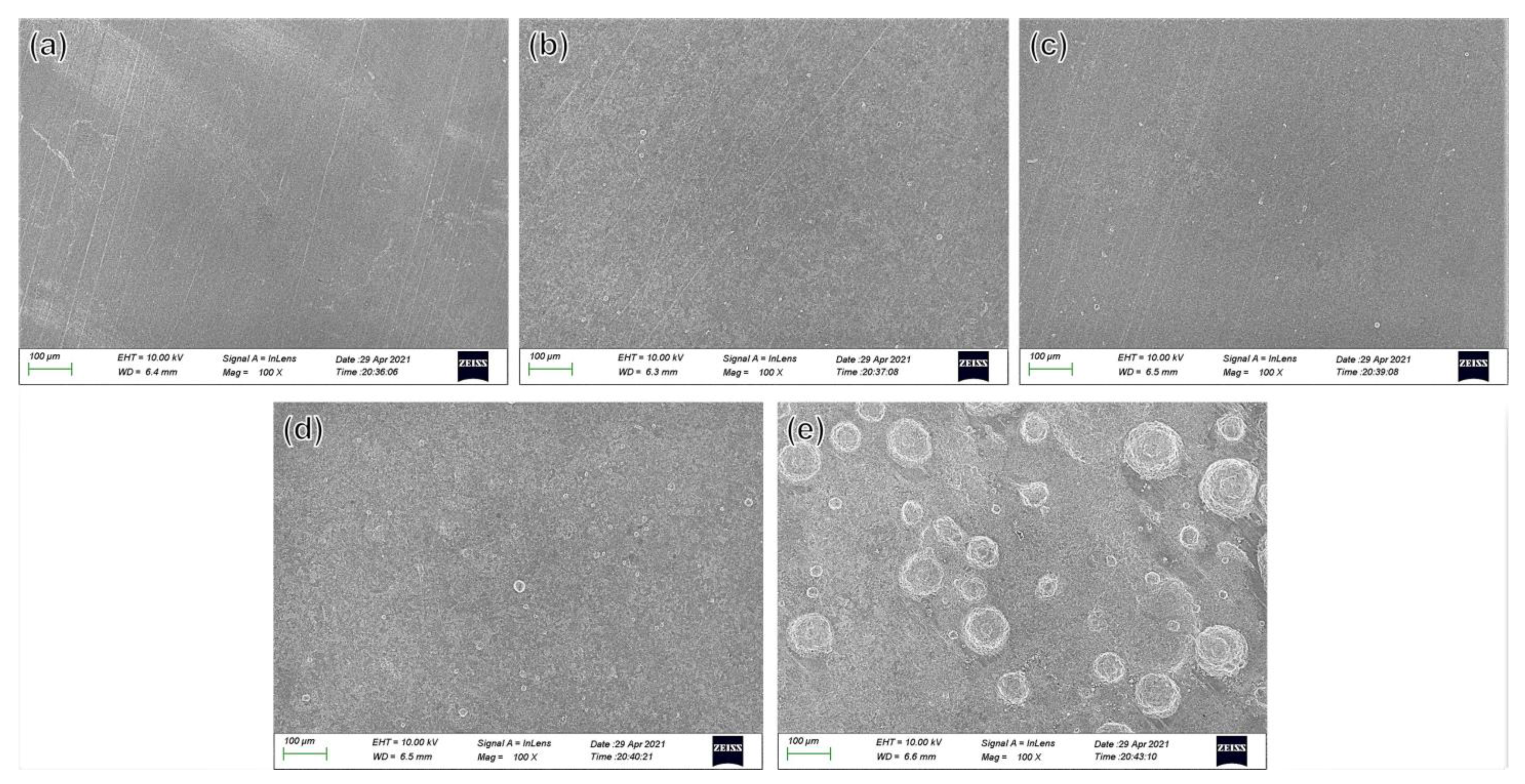
3.4.1. Scanning Electron Microscopy Morphology of the Corrosion Products
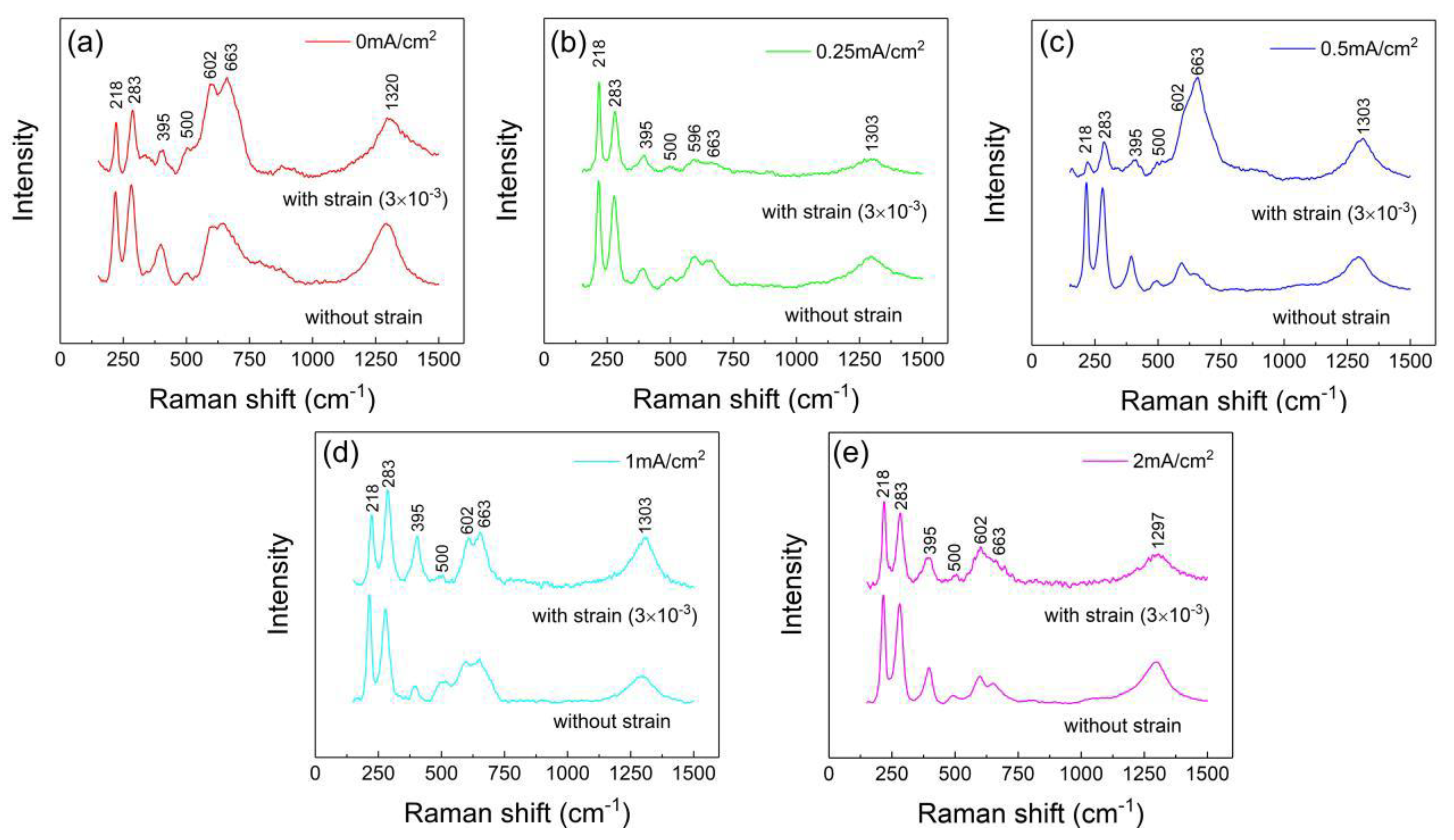
3.4.2. Raman Spectrum Analysis of the Corrosion Products
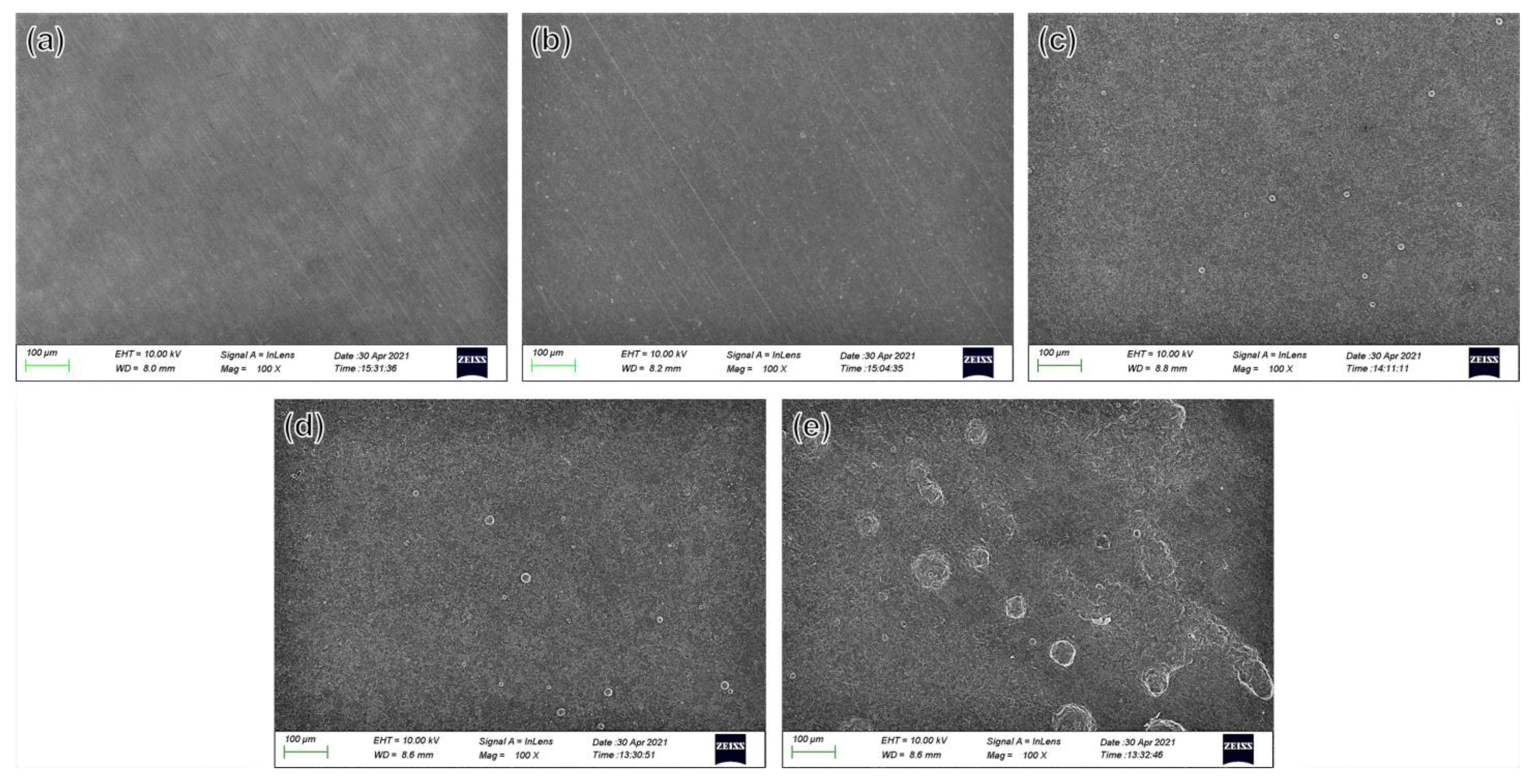
3.4.3. Analysis of Corrosion Morphology
4. Discussion
4.1. Formatting of Mathematical Components
4.2. Formatting of Mathematical Components
4.3. Corrosion Process of X80 Steel under DC Currents and Strain
4.4. Proposed Mechanism of X80 Pipeline Steel Corrosion with DC Currents and Strain Applied
5. Conclusions
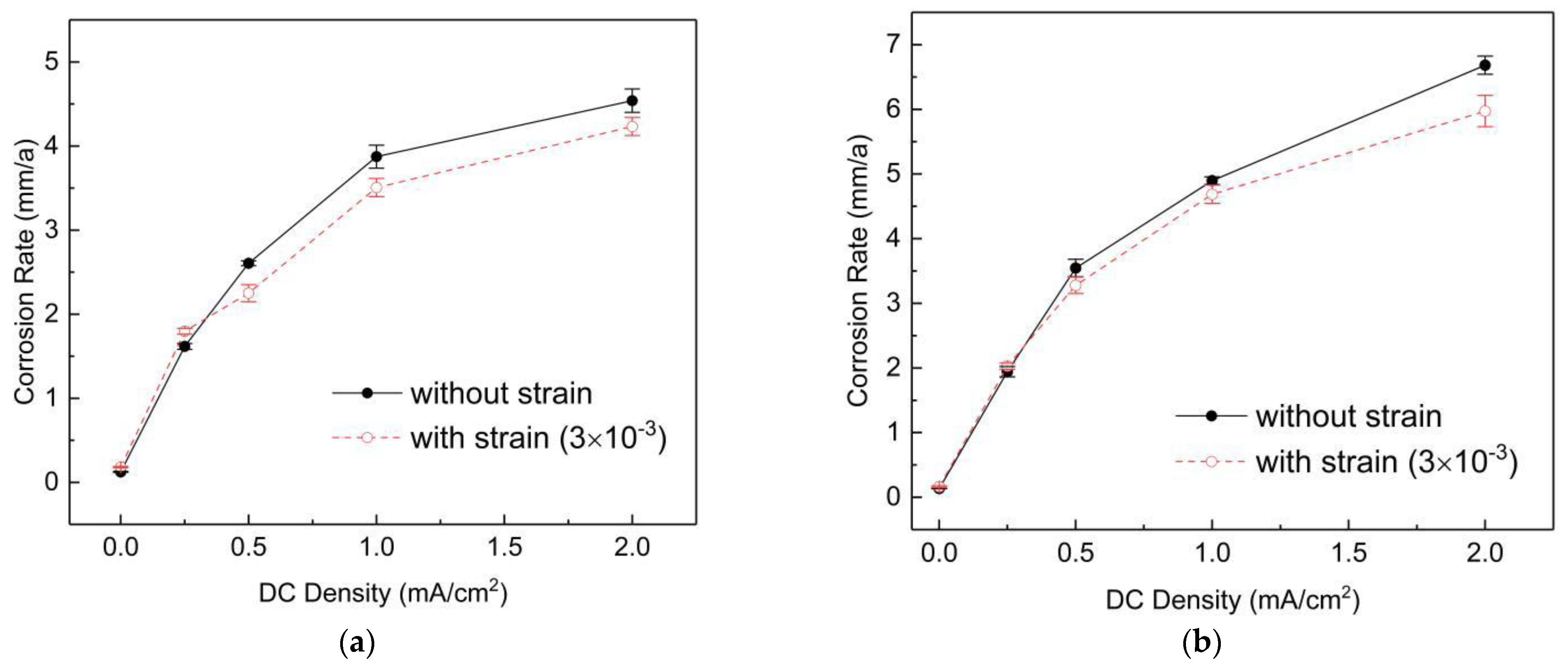
- Both DC currents and strain can promote the corrosion of X80 in a near-neutral soil simulation solution, but the influence of DC currents is much more significant than strain.
- When both DC currents and strain are applied, they have a synergistic effect on the corrosion of the steel sample if the . However, if , the strain will slightly reduce the corrosion rate of the steel.
- DC currents and strain can change the composition and structure of the corrosion product layer of X80 steel. The generated under applied DC currents can increase the porosity of the product layer and can promote corrosion. However, with the addition of strain, the corrosion scales become denser, which indirectly inhibits corrosion.
- When strain or DC currents are applied to X80 steel, the pitting resistance of the steel will decrease. Without DC currents, the strain makes the steel more vulnerable to pitting. Once DC currents are applied, the number and size of pitting holes increase with current density.
Author Contributions
Funding
Conflicts of Interest
References
- Thakur, A.K.; Arya, A.K.; Sharma, P. The science of alternating current-induced corrosion: A review of literature on pipeline corrosion induced due to high-voltage alternating current transmission pipelines. Corros. Rev. 2020, 38, 463–472. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Cui, G.; Liu, J.; Kong, C.; Wang, L.; Gao, G.; Guo, J. Corrosion behaviors of X70 steel under direct current interference. Anti-Corros. Methods Mater. 2019, 66, 307–316. [Google Scholar] [CrossRef]
- Chen, Z.; Koleva, D.; Van Breugel, K. A review on stray current-induced steel corrosion in infrastructure. Corros. Rev. 2017, 35, 397–423. [Google Scholar] [CrossRef]
- Niazi, H.; Eadie, R.; Chen, W.; Zhang, H. High pH stress corrosion cracking initiation and crack evolution in buried steel pipelines: A review. Eng. Fail. Anal. 2021, 120, 105013. [Google Scholar] [CrossRef]
- Wasim, M.; Shoaib, S.; Mubarak, N.M.; Inamuddin; Asiri, A.M. Factors influencing corrosion of metal pipes in soils. Environ. Chem. Lett. 2018, 16, 861–879. [Google Scholar] [CrossRef]
- Wasim, M.; Djukic, M.B. Long-term external microbiologically influenced corrosion of buried cast iron pipes in the presence of sulfate-reducing bacteria (SRB). Eng. Fail. Anal. 2020, 115, 104657. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Zhang, B.; Shi, W.; Li, C.-Q. Failure prediction of buried pipe network with multiple failure modes and spatial randomness of corrosion. Int. J. Press. Vessel. Pip. 2021, 191, 104367. [Google Scholar] [CrossRef]
- Wasim, M.; Li, C.-Q.; Mahmoodian, M.; Robert, D. Mechanical and Microstructural Evaluation of Corrosion and Hydrogen-Induced Degradation of Steel. J. Mater. Civ. Eng. 2019, 31, 04018349. [Google Scholar] [CrossRef]
- Cotton, I.I.; Charalambous, C.A.; Aylott, P.; Ernst, P. Stray Current Control in DC Mass Transit Systems. IEEE Trans. Veh. Technol. 2005, 54, 722–730. [Google Scholar] [CrossRef]
- Bertolini, L.; Carsana, M.; Pedeferri, P. Corrosion behaviour of steel in concrete in the presence of stray current. Corros. Sci. 2007, 49, 1056–1068. [Google Scholar] [CrossRef]
- Qian, S.; Cheng, Y.F. Accelerated corrosion of pipeline steel and reduced cathodic protection effectiveness under direct current interference. Constr. Build. Mater. 2017, 148, 675–685. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Chen, Y.; Song, X.; Yang, Y. Effect of a DC Stray Current on the Corrosion of X80 Pipeline Steel and the Cathodic Disbondment Behavior of the Protective 3PE Coating in 3.5% NaCl Solution. Coatings 2019, 9, 29. [Google Scholar] [CrossRef] [Green Version]
- Qian, S.; Cheng, Y.F. Corrosion of pipelines under dynamic direct current interference. Constr. Build. Mater. 2020, 261, 120550. [Google Scholar] [CrossRef]
- Allahkaram, S.R.; Isakhani-Zakaria, M.; Derakhshani, M.; Samadian, M.; Sharifi-Rasaey, H.; Razmjoo, A. Investigation on corrosion rate and a novel corrosion criterion for gas pipelines affected by dynamic stray current. J. Nat. Gas Sci. Eng. 2015, 26, 453–460. [Google Scholar] [CrossRef]
- Ormellese, M.; Beretta, S.; Brugnetti, F.; Brenna, A. Effects of non-stationary stray current on carbon steel buried pipelines under cathodic protection. Constr. Build. Mater. 2021, 281, 122645. [Google Scholar] [CrossRef]
- Qin, H.; Du, Y.; Lu, M.; Liu, J.; Zhu, X. Accelerated corrosion of pipeline steel under dynamic DC stray current interference. Corros. Eng. Sci. Technol. 2020, 55, 670–680. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Wang, Y.; Xu, S.; Yang, X. Chloride-induced stray current corrosion of Q235A steel and prediction model. Constr. Build. Mater. 2019, 219, 164–175. [Google Scholar] [CrossRef]
- Shi, J.; Zou, Y.; Ming, J.; Wu, M. Effect of DC stray current on electrochemical behavior of low-carbon steel and 10%Cr steel in saturated Ca(OH)2 solution. Corros. Sci. 2020, 169, 108610. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, Y. Corrosion of X100 pipeline steel under plastic strain in a neutral pH bicarbonate solution. Corros. Sci. 2012, 64, 145–152. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, Y. An experimental investigation of corrosion of X100 pipeline steel under uniaxial elastic stress in a near-neutral pH solution. Corros. Sci. 2012, 59, 103–109. [Google Scholar] [CrossRef]
- Wu, W.; Yin, H.; Zhang, H.; Kang, J.; Li, Y.; Dan, Y. Electrochemical Investigation of Corrosion of X80 Steel under Elastic and Plastic Tensile Stress in CO2 Environment. Metals 2018, 8, 949. [Google Scholar] [CrossRef] [Green Version]
- Romaniv, O.M.; Heneha, B.Y.; Huta, O.M.; Vasylechko, V. Effect of stresses on the electrochemical corrosion of steel in aqueous media. Mater. Sci. 1996, 32, 760–763. [Google Scholar] [CrossRef]
- Behera, P.K.; Katiyar, P.K.; Misra, S.; Mondal, K. Effect of Pre-induced Plastic Strains on the Corrosion Behavior of Reinforcing Bar in 3.5 pct NaCl Solution. Met. Mater. Trans. A 2021, 52, 605–626. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, Y. Development of a finite element model for simulation and prediction of mechanoelectrochemical effect of pipeline corrosion. Corros. Sci. 2013, 73, 150–160. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, W.; Ai, H.; Zhou, X.; Zhang, T. Effects of strain on the corrosion behaviour of X80 steel. Corros. Sci. 2011, 53, 2761–2766. [Google Scholar] [CrossRef]
- Yang, H.-Q.; Zhang, Q.; Tu, S.-S.; Wang, Y.; Li, Y.-M.; Huang, Y. Effects of inhomogeneous elastic stress on corrosion behaviour of Q235 steel in 3.5% NaCl solution using a novel multi-channel electrode technique. Corros. Sci. 2016, 110, 2124–2128. [Google Scholar] [CrossRef]
- Xu, J.; Bai, Y.; Wu, T.; Yan, M.; Yu, C.; Sun, C. Effect of elastic stress and alternating current on corrosion of X80 pipeline steel in simulated soil solution. Eng. Fail. Anal. 2019, 100, 192–205. [Google Scholar] [CrossRef]
- Wang, X.; Tang, X.; Wang, L.; Wang, C.; Zhou, W. Synergistic effect of stray current and stress on corrosion of API X65 steel. J. Nat. Gas Sci. Eng. 2014, 21, 474–480. [Google Scholar] [CrossRef]
- Wasim, M.; Mahmoodian, M.; Robert, D.; Li, C.-Q. Correlation Model for the Corrosion Rates of Buried Cast Iron Pipes. J. Mater. Civ. Eng. 2020, 32, 04020353. [Google Scholar] [CrossRef]
- Goidanich, S.; Lazzari, L.; Ormellese, M. AC corrosion. Part 2: Parameters influencing corrosion rate. Corros. Sci. 2010, 52, 916–922. [Google Scholar] [CrossRef]
- López, D.A.; Simison, S.; de Sánchez, S. The influence of steel microstructure on CO2 corrosion. EIS studies on the inhibition efficiency of benzimidazole. Electrochim. Acta 2003, 48, 845–854. [Google Scholar] [CrossRef]
- Choudhary, S.; Garg, A.; Mondal, K. Relation Between Open Circuit Potential and Polarization Resistance with Rust and Corrosion Monitoring of Mild Steel. J. Mater. Eng. Perform. 2016, 25, 2969–2976. [Google Scholar] [CrossRef]
- Li, M.C.; Lin, H.C.; Cao, C.N. Study on soil corrosion of carbon steel by electrochemical impedance spectroscopy (EIS). J. Chin. Soc. Corros. Prot. 2000, 2, 47–53. [Google Scholar]
- Yan, M.C.; Yang, S.; Xu, J.; Sun, C.; Wu, T.Q.; Yu, C.K.; Ke, W. Stress corrosion cracking of ×80 pipeline steel at coating defect in acidic soil. Acta Metall. Sin. 2016, 52, 1133–1141. [Google Scholar] [CrossRef]
- Zhu, Q.Z.; Xue, W.B.; Lu, L.; Du, J.C.; Liu, G.J.; Li, W.F. Preparation of microarc oxidation coating on (al2o3-sio2)sf/az91d magnesium matrix composite and its electrochemical impedance spectroscopic analysis. Acta Metall. Sin. 2011, 47, 74–80. [Google Scholar] [CrossRef]
- King, A.; Birbilis, N.; Scully, J. Accurate Electrochemical Measurement of Magnesium Corrosion Rates; a Combined Impedance, Mass-Loss and Hydrogen Collection Study. Electrochim. Acta 2014, 121, 394–406. [Google Scholar] [CrossRef]
- Schwertmann, U.; Cornell, R.M. Iron oxides in the laboratory. Soil Sci. 1991, 156, 281–282. [Google Scholar] [CrossRef]
- Alcántara, J.; Chico, B.; Simancas, J.; Díaz, I.; de la Fuente, D.; Morcillo, M. An attempt to classify the morphologies presented by different rust phases formed during the exposure of carbon steel to marine atmospheres. Mater. Charact. 2016, 118, 65–78. [Google Scholar] [CrossRef]
- Dai, N.-W.; Zhang, J.-X.; Chen, Q.-M.; Zhang, X.; Cao, F.-H.; Zhang, J.-Q. Influence of Direct Current Electric Field on the Formation, Composition and Microstructure of Corrosion Products Formed on the Steel in Simulated Marine Atmospheric Environment. Acta Met. Sin. Eng. Lett. 2016, 29, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; Cherifi, S.; Despert, G. Raman identification of corrosion products on automotive galvanized steel sheets. J. Raman Spectrosc. 2008, 39, 881–886. [Google Scholar] [CrossRef]
- Yamashita, M.; Miyuki, H.; Matsuda, Y.; Nagano, H.; Misawa, T. The long term growth of the protective rust layer formed on weathering steel by atmospheric corrosion during a quarter of a century. Corros. Sci. 1994, 36, 283–299. [Google Scholar] [CrossRef]
- Cook, D.; Oh, S.; Balasubramanian, R.; Yamashita, M. The role of goethite in the formation of the protective corrosion layer on steels. Hyperfine Interact. 1999, 122, 59–70. [Google Scholar] [CrossRef]
- Kamimura, T.; Hara, S.; Miyuki, H.; Yamashita, M.; Uchida, H. Composition and protective ability of rust layer formed on weathering steel exposed to various environments. Corros. Sci. 2006, 48, 2799–2812. [Google Scholar] [CrossRef]
- Sato, N. A theory for breakdown of anodic oxide films on metals. Electrochim. Acta 1971, 16, 1683–1692. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, Z.; Wang, L.; Li, X.; Du, C.; Wang, X. Effect of plastic deformation on the electrochemical and stress corrosion cracking behavior of X70 steel in near-neutral pH environment. Mater. Sci. Eng. A 2016, 677, 259–273. [Google Scholar] [CrossRef]
- Feng, Y.; Teo, W.-K.; Siow, K.-S.; Tan, K.-L.; Hsieh, A.-K. The corrosion behaviour of copper in neutral tap water. Part I: Corrosion mechanisms. Corros. Sci. 1996, 38, 369–385. [Google Scholar] [CrossRef]
- Cao, C.N. Principles of Electrochemical of Corrosion, 3rd ed.; Chemical Industry Press: Beijing, China, 2008; ISBN 7-5025-5187-5. [Google Scholar]
- Stern, M.; Geary, A.L. Electrochemical polarization: A theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Gadala, I.M.; Alfantazi, A. Electrochemical behavior of API-X100 pipeline steel in NS4, near-neutral, and mildly alkaline pH simulated soil solutions. Corros. Sci. 2014, 82, 45–57. [Google Scholar] [CrossRef]
- Han, J.; Young, D.; Colijn, H.; Tripathi, A.; Nešić, S. Chemistry and Structure of the Passive Film on Mild Steel in CO2 Corrosion Environments. Ind. Eng. Chem. Res. 2009, 48, 6296–6302. [Google Scholar] [CrossRef]
- Lu, Z.; Huang, C.; Huang, D.; Yang, W. Effects of a magnetic field on the anodic dissolution, passivation and transpassivation behaviour of iron in weakly alkaline solutions with or without halides. Corros. Sci. 2006, 48, 3049–3077. [Google Scholar] [CrossRef]
- Dai, N.; Chen, Q.; Zhang, J.; Zhang, X.; Ni, Q.; Jiang, Y.; Li, J. The corrosion behavior of steel exposed to a DC electric field in the simulated wet-dry cyclic environment. Mater. Chem. Phys. 2017, 192, 190–197. [Google Scholar] [CrossRef]
- Gutman, E.M. Mechanochemistry of Materials; Cambridge International Science Publishing: Cambridge, UK, 1998; ISBN 1-898326-32-0. [Google Scholar]
- Lu, B.; Luo, J.; Norton, P.; Ma, H. Effects of dissolved hydrogen and elastic and plastic deformation on active dissolution of pipeline steel in anaerobic groundwater of near-neutral pH. Acta Mater. 2009, 57, 41–49. [Google Scholar] [CrossRef]
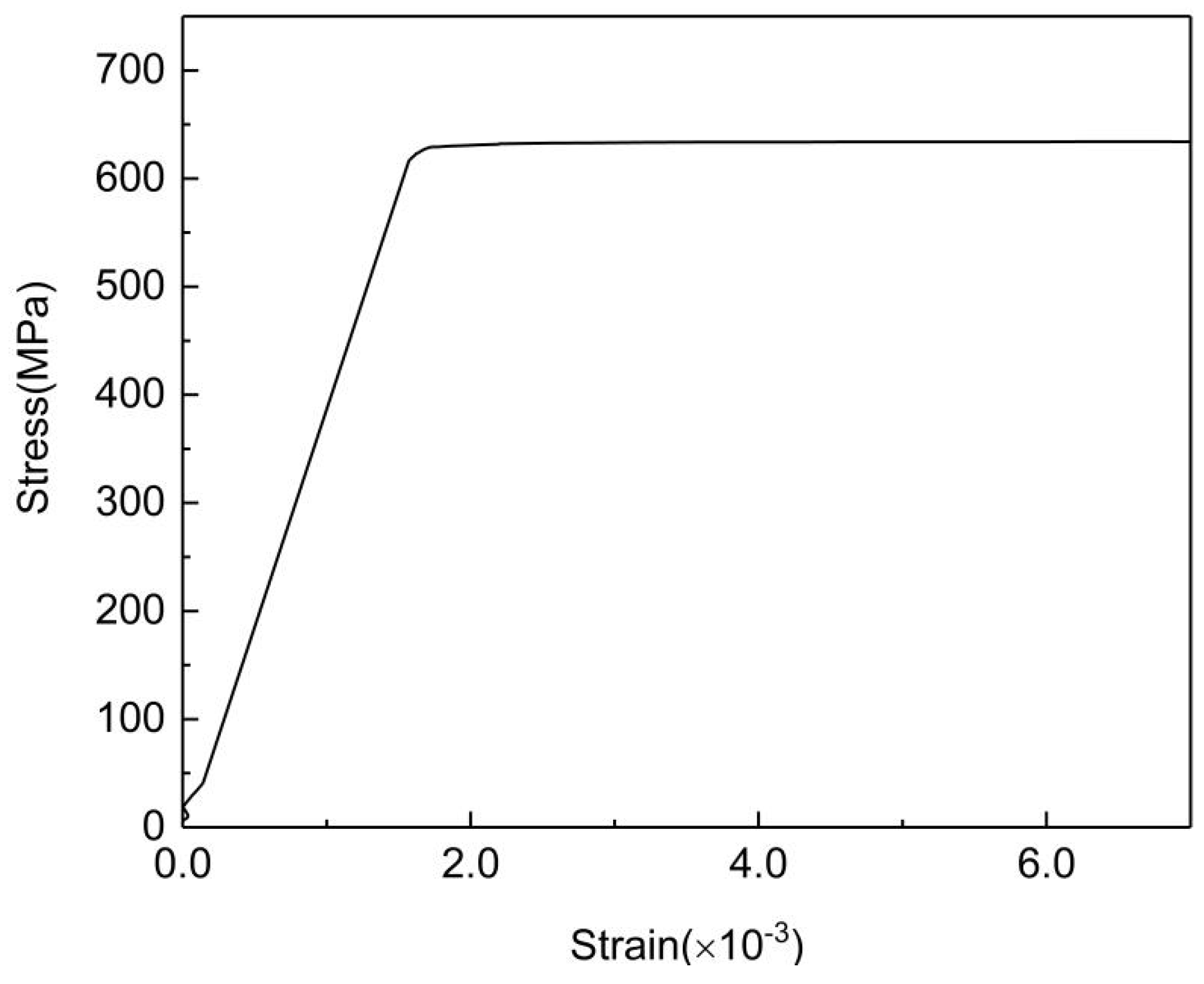






| Element | C | Si | Mn | P | S | V | Nb | Ti | Cr | Mo | Al | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 0.090 | 0.350 | 1.900 | 0.030 | 0.005 | 0.060 | 0.065 | 0.016 | 0.030 | 0.210 | 0.060 | balance |
| Composition | ||||
|---|---|---|---|---|
| Content | 0.483 | 0.122 | 0.131 | 0.181 |
| DC Density | Strain | L | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Q-n | (H) | ||||||||
| 0 | 0 | 43.13 | - | - | 2.10 | 0.84 | 2293 | - | - |
| 0.5 | 38.14 | - | - | 1.71 | 0.86 | 2266 | - | - | |
| 1 | 41.33 | - | - | 1.96 | 0.88 | 2000 | - | - | |
| 1.5 | 48.51 | - | - | 2.86 | 0.87 | 1733 | - | - | |
| 3 | 42.39 | - | - | 2.40 | 0.83 | 1764 | - | - | |
| 0.25 | 0 | 17.20 | 9.84 | 33.38 | 2.35 | 0.84 | 86.31 | 22.34 | 33.21 |
| 0.5 | 16.75 | 7.21 | 40.07 | 2.02 | 0.83 | 90.77 | 29.03 | 40.79 | |
| 1 | 16.78 | 8.87 | 37.33 | 1.98 | 0.86 | 77.89 | 34.44 | 40.76 | |
| 1.5 | 16.83 | 11.79 | 28.38 | 2.24 | 0.86 | 83.17 | 24.29 | 35.69 | |
| 3 | 18.46 | 8.04 | 30.53 | 2.50 | 0.83 | 81.33 | 28.69 | 37.81 | |
| 0.5 | 0 | 18.11 | 10.00 | 32.28 | 2.49 | 0.89 | 40.64 | 7.69 | 14.08 |
| 0.5 | 17.47 | 11.45 | 27.69 | 2.93 | 0.85 | 44.66 | 7.67 | 16.41 | |
| 1 | 19.32 | 11.69 | 24.95 | 3.16 | 0.84 | 43.89 | 6.94 | 16.13 | |
| 1.5 | 17.97 | 8.10 | 37.23 | 2.38 | 0.88 | 42.62 | 8.15 | 16.81 | |
| 3 | 16.94 | 9.73 | 27.79 | 2.88 | 0.82 | 52.6 | 6.74 | 16.00 | |
| 1 | 0 | 16.5 | 9.84 | 27.40 | 3.53 | 0.79 | 26.26 | 1.67 | 8.50 |
| 0.5 | 16.08 | 10.47 | 29.30 | 3.34 | 0.85 | 23.53 | 2.02 | 7.30 | |
| 1 | 17.42 | 7.55 | 36.49 | 3.02 | 0.84 | 24.35 | 1.59 | 7.41 | |
| 1.5 | 16.63 | 8.86 | 33.97 | 2.71 | 0.88 | 21.99 | 1.61 | 7.05 | |
| 3 | 17.59 | 9.11 | 34.87 | 2.87 | 0.86 | 23.00 | 1.26 | 6.83 | |
| DC Density | Strain | |||||
|---|---|---|---|---|---|---|
| 2 | 0 | 17.59 | 9.64 | 33.11 | 2.17 | 12.39 |
| 0.5 | 17.60 | 8.78 | 35.23 | 1.55 | 12.94 | |
| 1 | 16.80 | 7.90 | 38.77 | 1.48 | 13.53 | |
| 1.5 | 16.52 | 7.67 | 35.76 | 1.70 | 12.05 | |
| 3 | 17.67 | 8.28 | 38.79 | 2.01 | 14.04 |
| Element | DC Density | C | O | Mg | Cl | Ca | Fe |
|---|---|---|---|---|---|---|---|
| without strain | 0 mA/cm2 | 10.20 | 14.27 | 0.12 | 0.57 | 0.27 | 74.58 |
| 0.25 mA/cm2 | 12.22 | 37.01 | 0.04 | 0.12 | - | 50.61 | |
| 0.5 mA/cm2 | 20.51 | 27.40 | - | 0.23 | 0.06 | 51.80 | |
| 1 mA/cm2 | 30.62 | 17.65 | - | 0.23 | 0.13 | 51.37 | |
| 2 mA/cm2 | 32.81 | 26.33 | - | 0.14 | 0.27 | 40.45 | |
| with strain (3) | 0 mA/cm2 | 17.23 | 8.42 | 0.01 | - | 0.02 | 74.32 |
| 0.25 mA/cm2 | 19.53 | 31.67 | 0.12 | 0.04 | 0.11 | 48.52 | |
| 0.5 mA/cm2 | 27.38 | 14.38 | - | - | - | 58.24 | |
| 1 mA/cm2 | 29.61 | 28.95 | 0.28 | 0.08 | 0.09 | 41.00 | |
| 2 mA/cm2 | 26.57 | 43.15 | 0.37 | 0.09 | 0.08 | 29.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Wu, W.; Zhao, P.; Dan, Y. Effect of DC Currents and Strain on Corrosion of X80 Steel in a Near-Neutral Environment. Metals 2021, 11, 1601. https://doi.org/10.3390/met11101601
Ma Z, Wu W, Zhao P, Dan Y. Effect of DC Currents and Strain on Corrosion of X80 Steel in a Near-Neutral Environment. Metals. 2021; 11(10):1601. https://doi.org/10.3390/met11101601
Chicago/Turabian StyleMa, Zeyu, Wei Wu, Pengxiong Zhao, and Yong Dan. 2021. "Effect of DC Currents and Strain on Corrosion of X80 Steel in a Near-Neutral Environment" Metals 11, no. 10: 1601. https://doi.org/10.3390/met11101601






