Study on Microstructure and In Situ Tensile Deformation Behavior of Fe-25Mn-xAl-8Ni-C Alloy Prepared by Vacuum Arc Melting
Abstract
1. Introduction
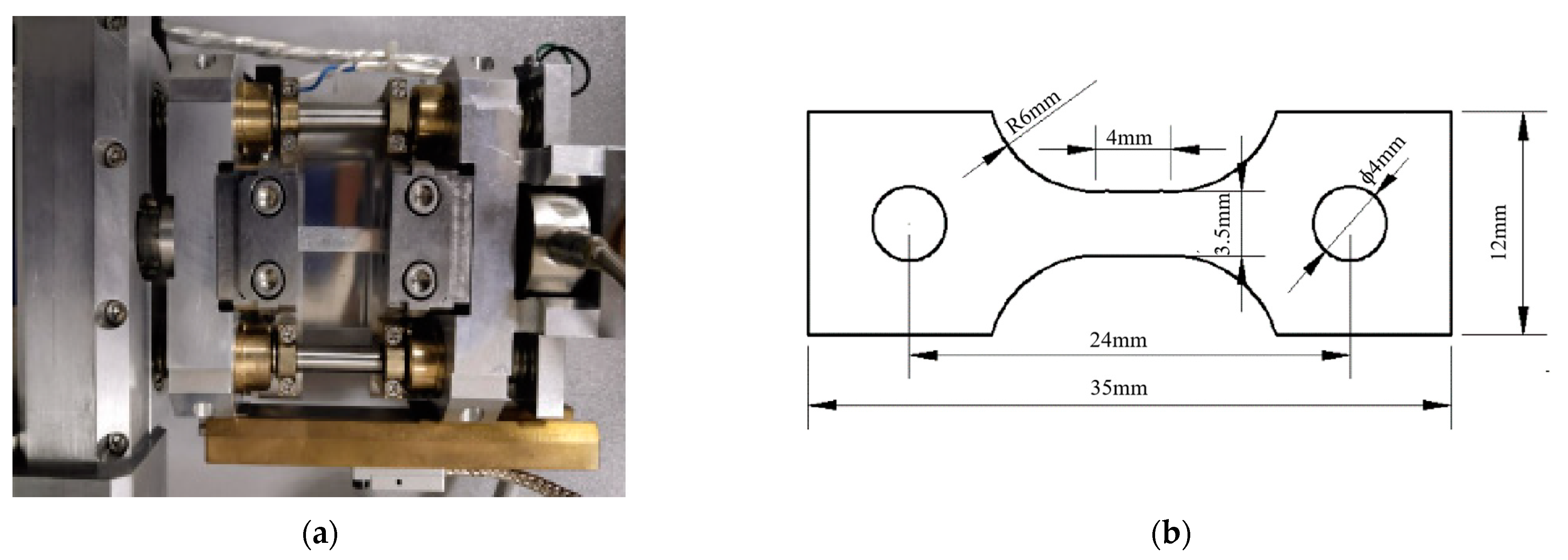
2. Experimental Procedures
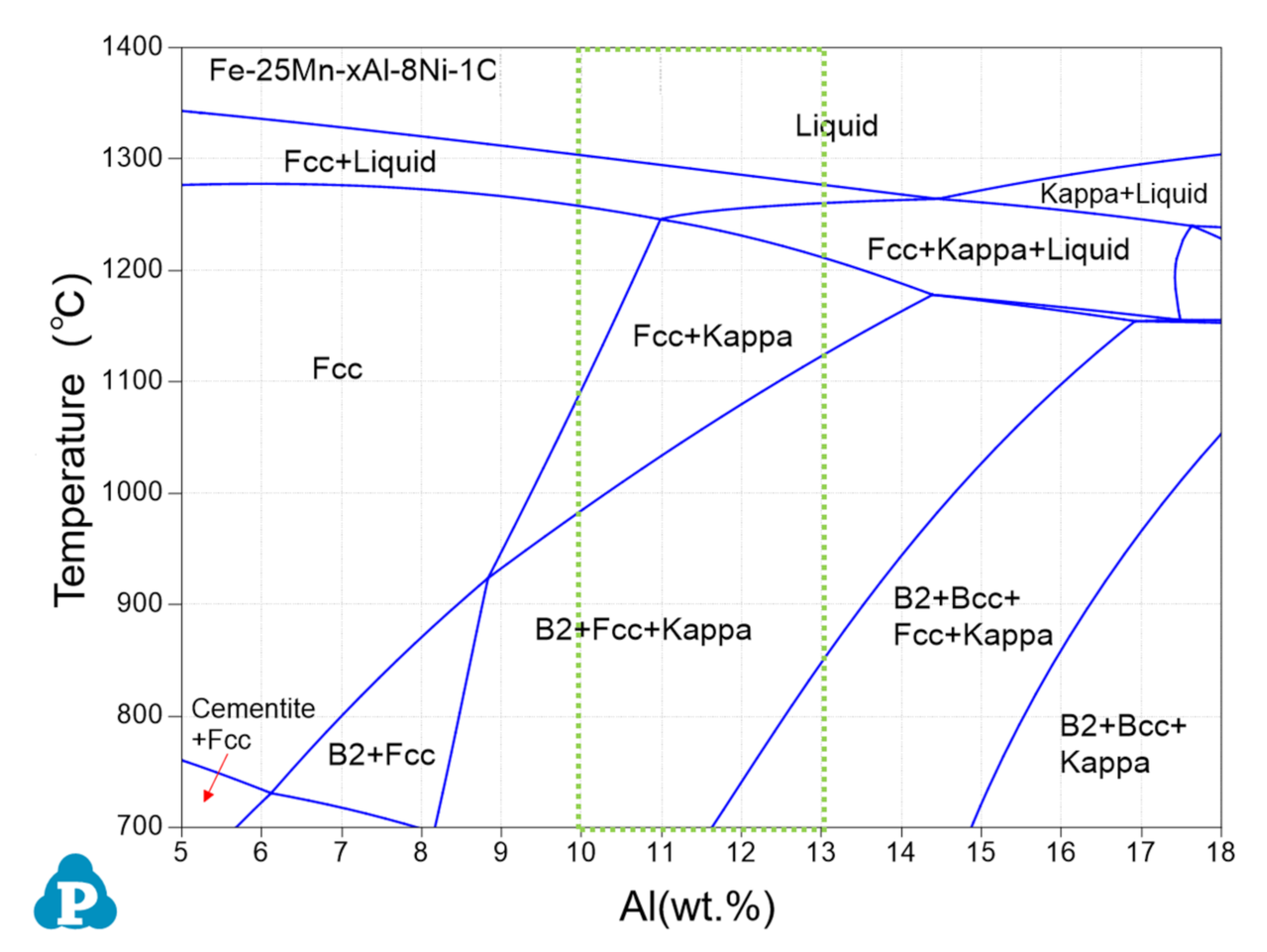
3. Results
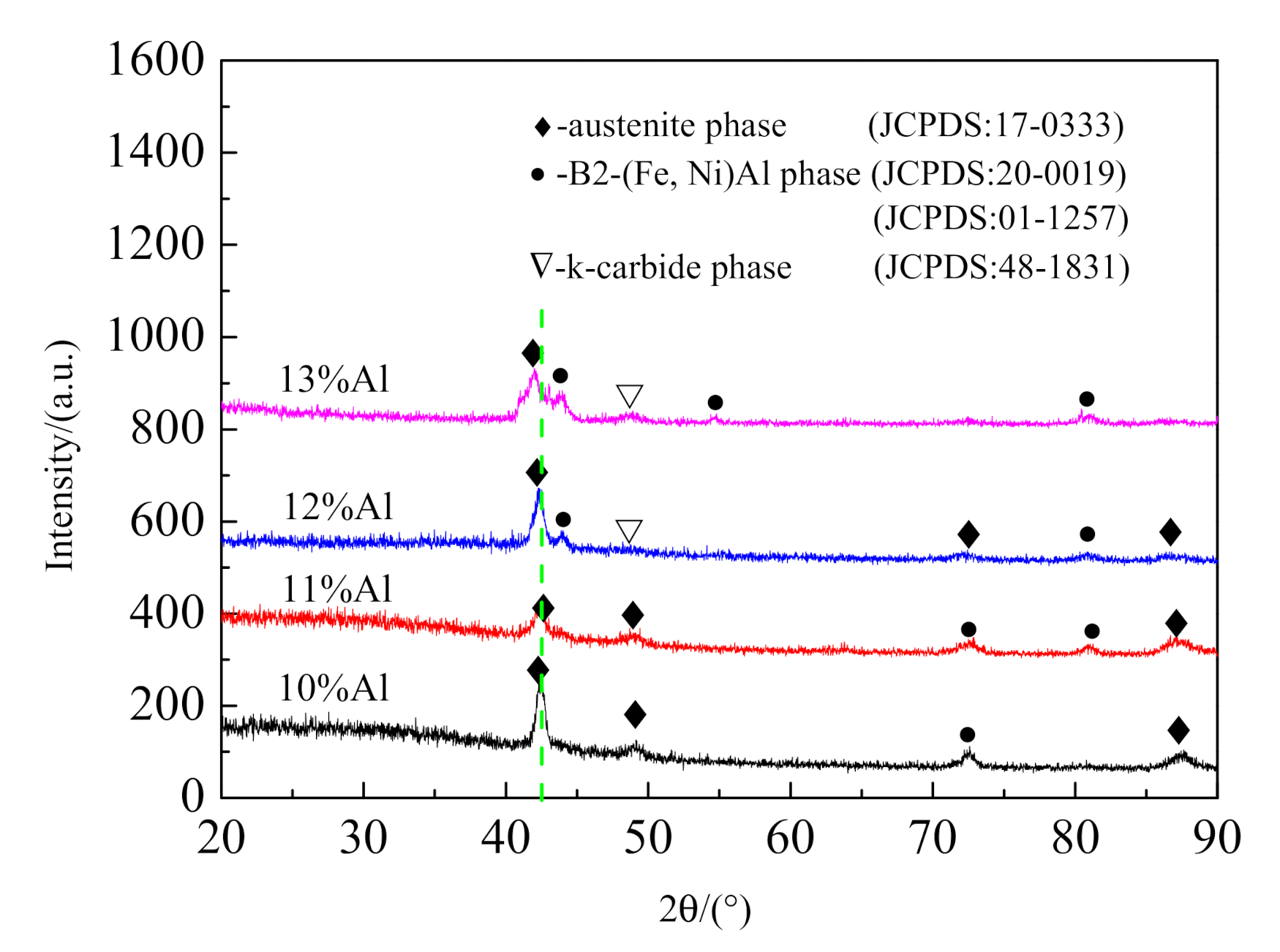
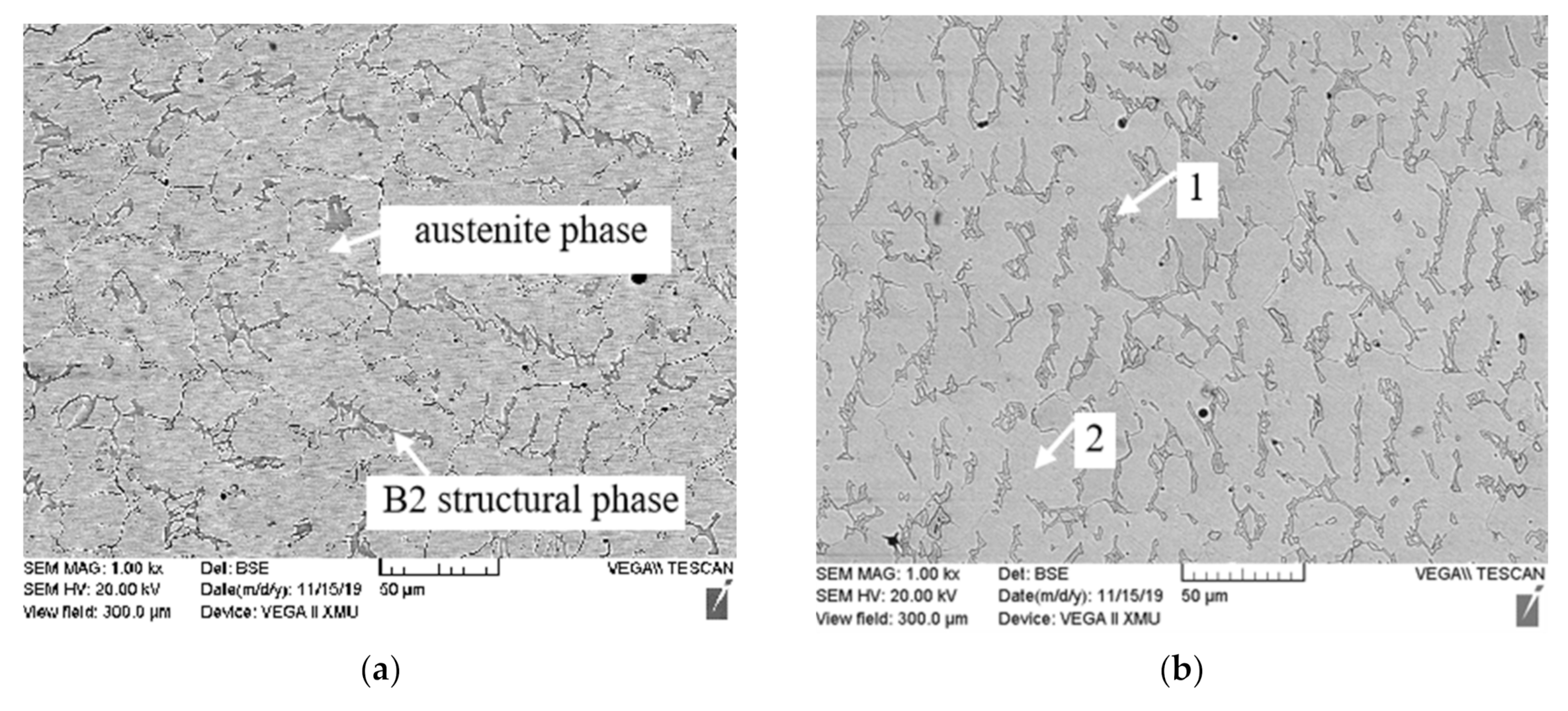
3.1. Microstructure
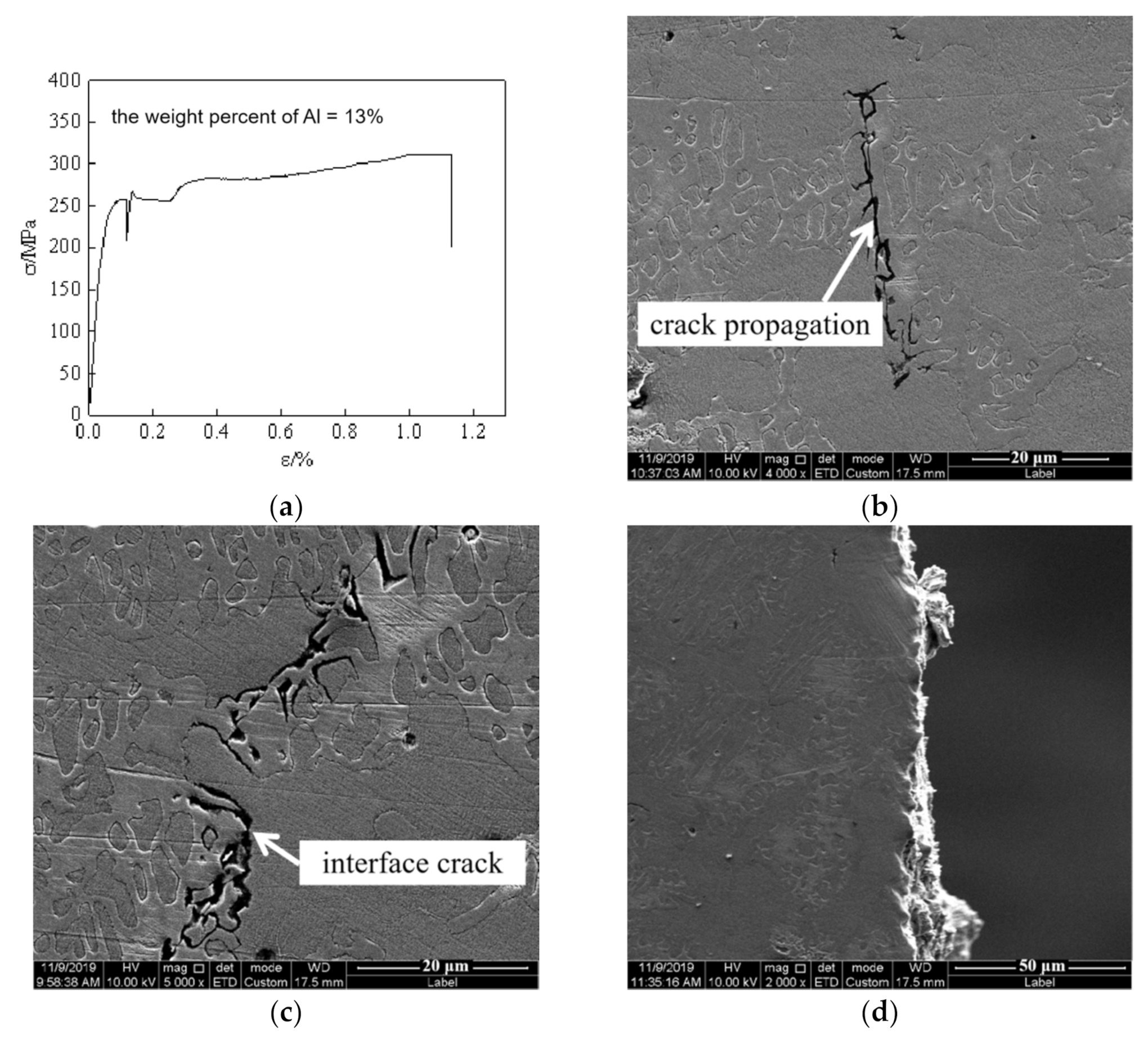
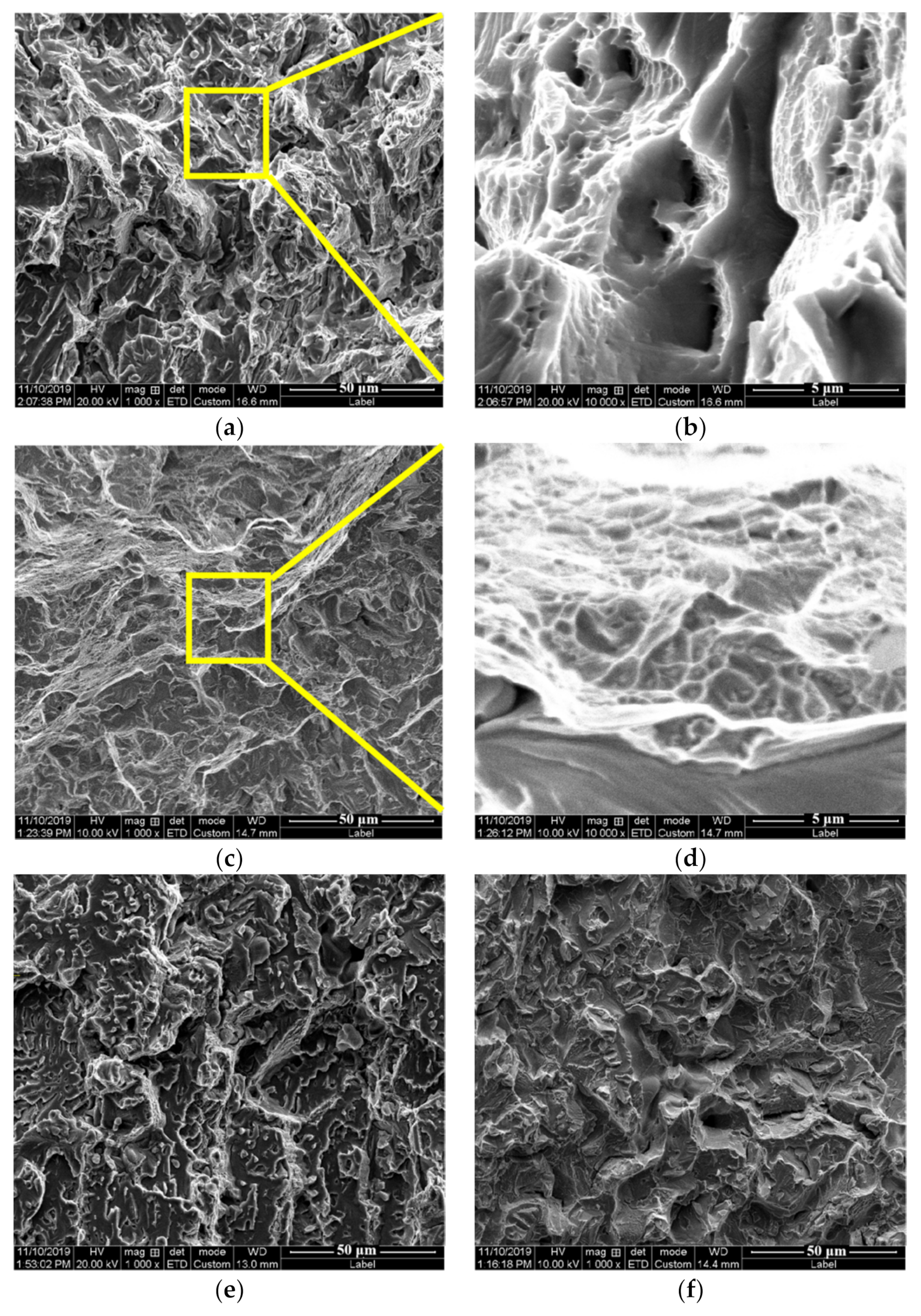
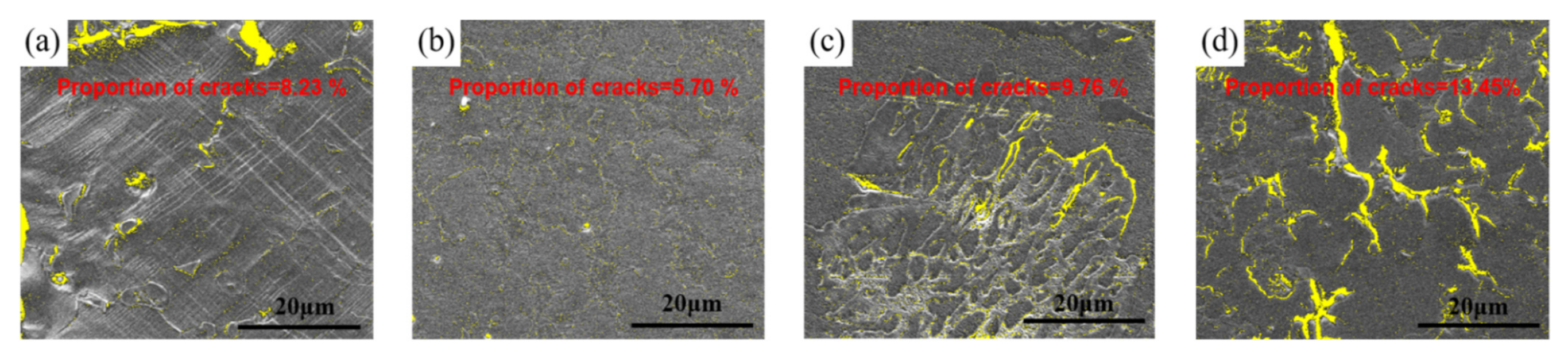
3.2. Mechanical Properties
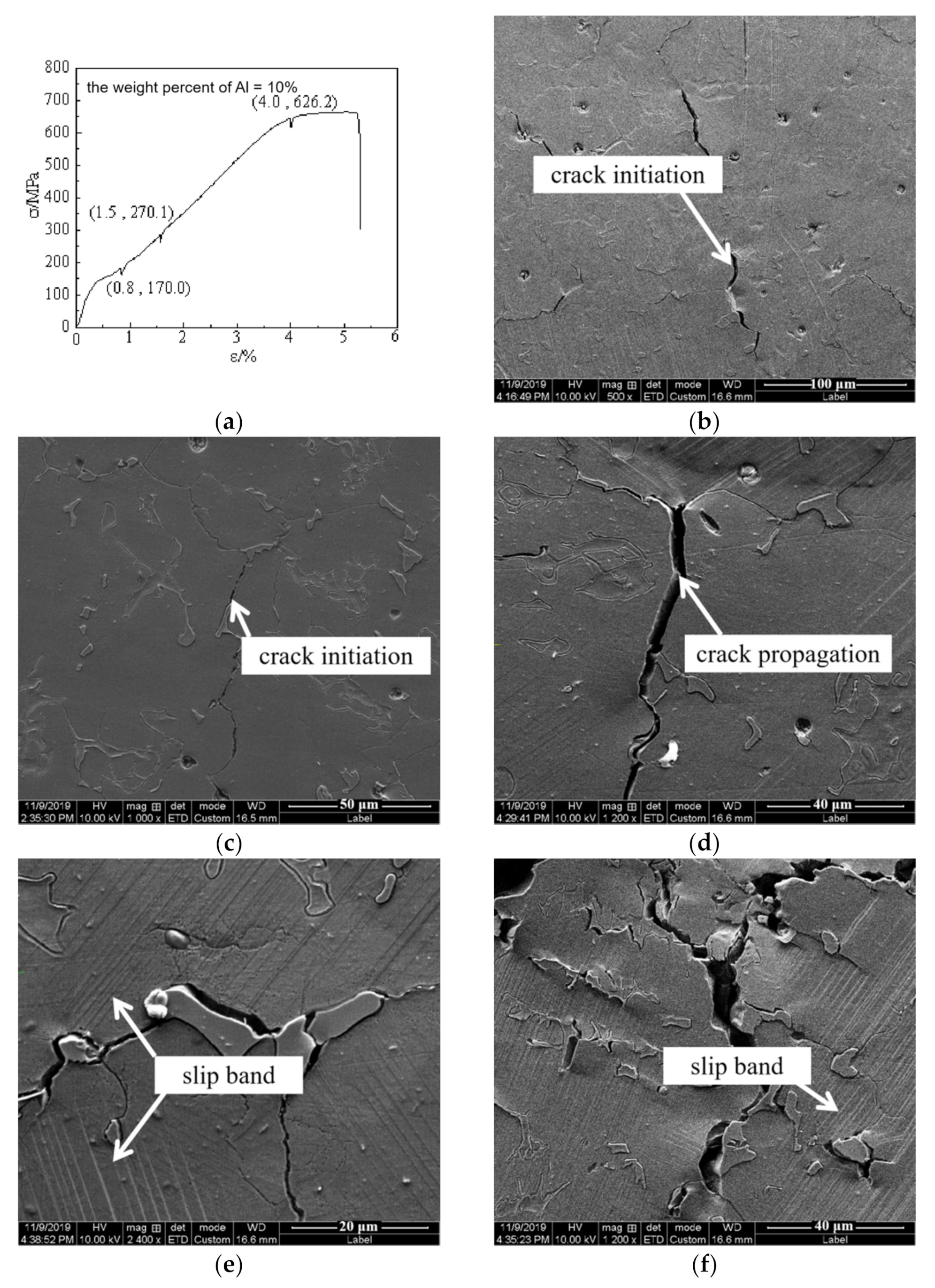
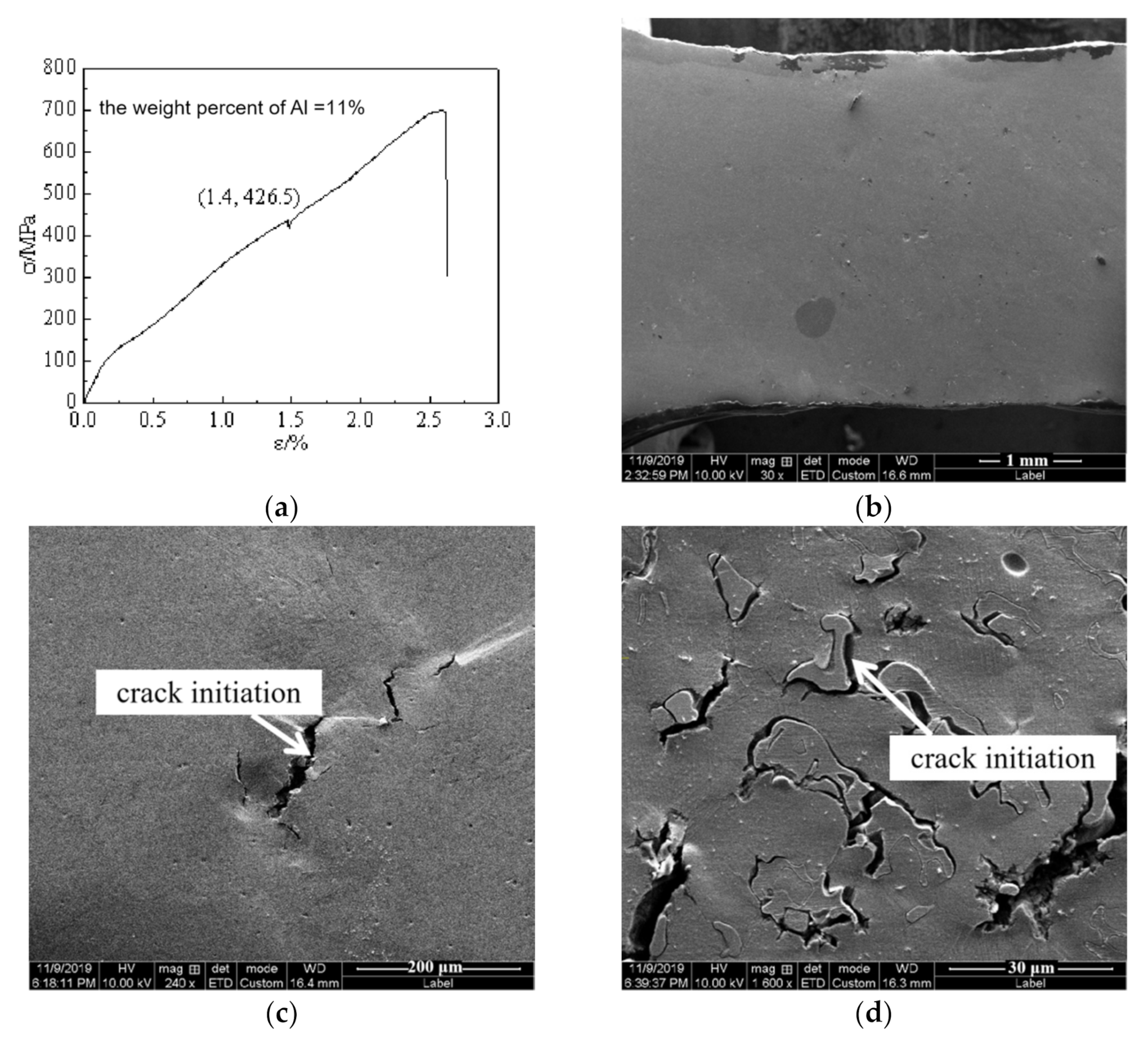
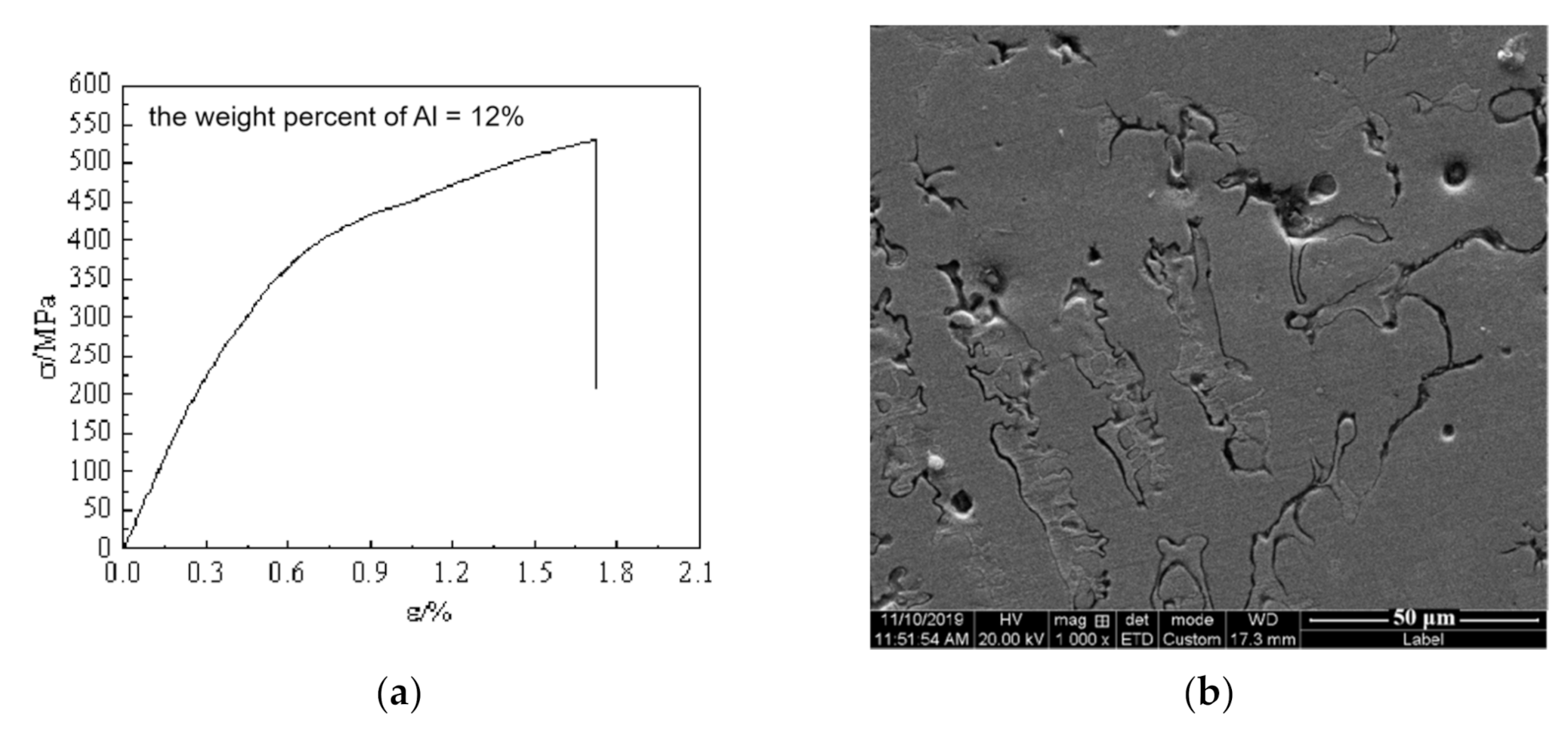
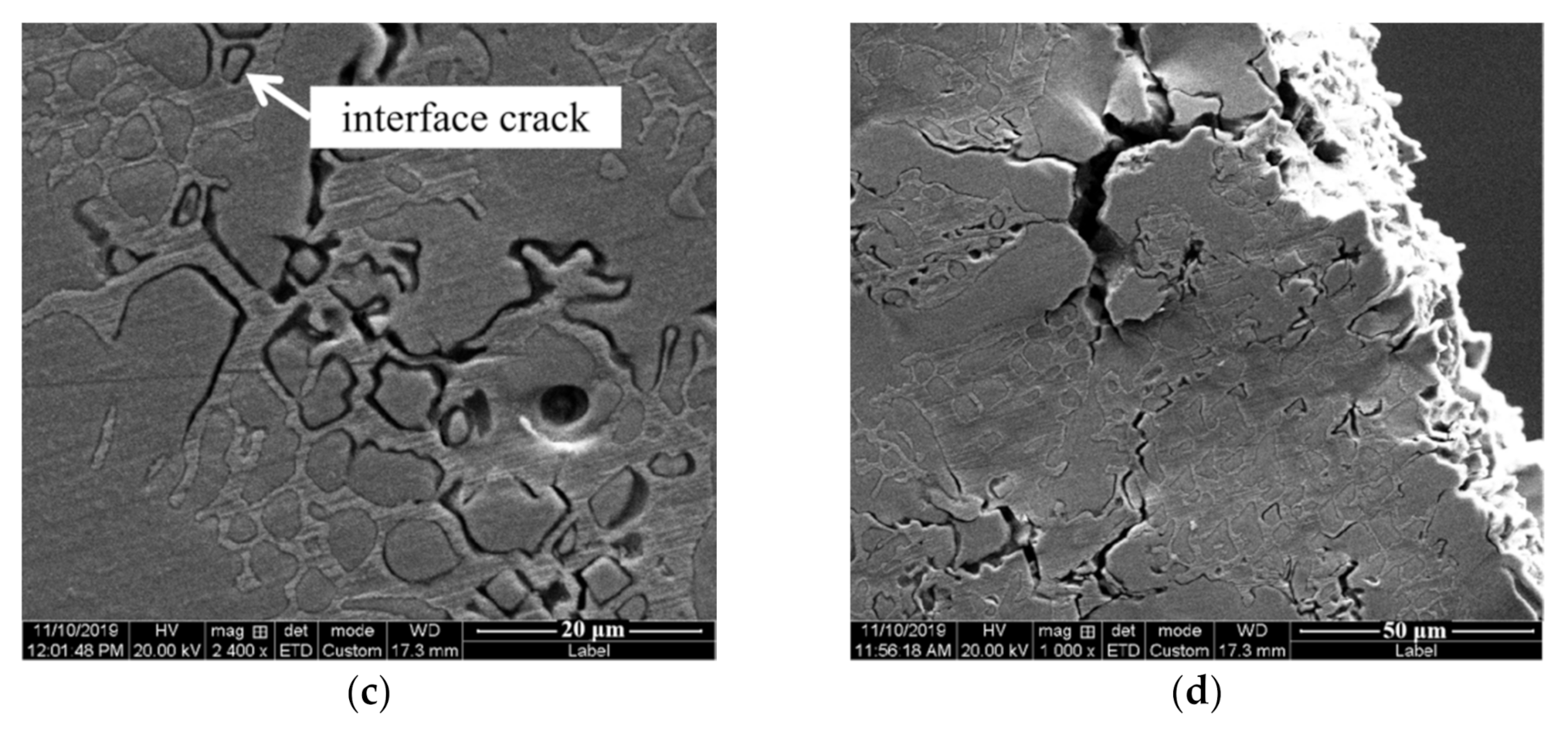
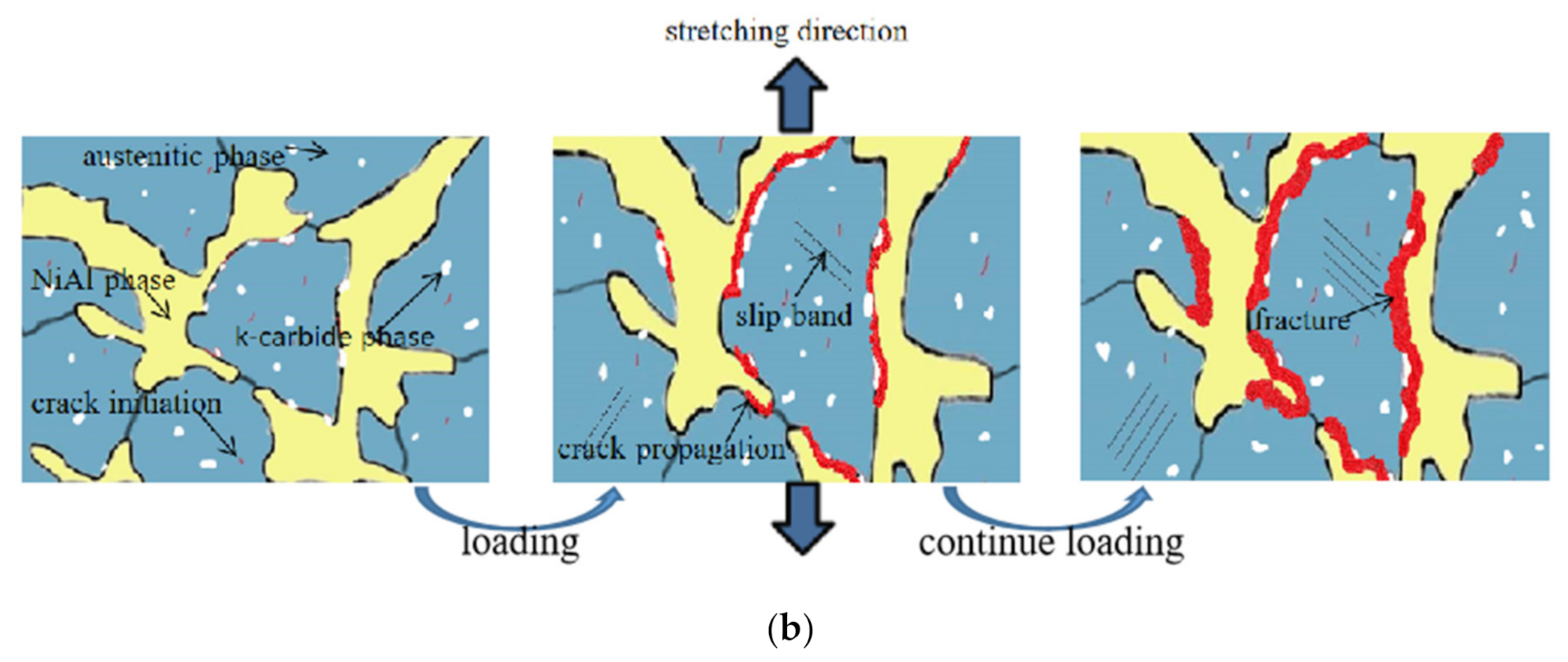
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, J.; Omori, T.; Kainuma, R. Abnormal grain growth in Fe–Mn–Al–Ni shape memory alloy with higher Al content. Scr. Mater. 2020, 187, 355–359. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Y.; Zhao, B.; Guo, Z.; Tian, X.; Yang, W.; Hou, H. Multi-component phase-field simulation of microstructural evolution and elemental distribution in Fe–Cu–Mn–Ni–Al alloy. Calphad 2020, 69, 101759. [Google Scholar] [CrossRef]
- Chang, S.C.; Hsiau, Y.H.; Jahn, M.T. Tensile and fatigue properties of Fe-Mn-Al-C alloys. J. Mat. Sci. 1989, 24, 1117–1120. [Google Scholar] [CrossRef]
- Sutou, Y.; Kamiya, N.; Umino, R.; Ohnuma, I.; Ishida, K. High-strength Fe–20Mn–Al–C-based Alloys with Low Density. ISIJ Int. 2010, 50, 893–899. [Google Scholar] [CrossRef]
- Guo, Q.Q.; Yang, Z.; Guo, D.; Tao, D.; Guo, Y.; Li, J.; Bai, Y. Research on the Oxidation Mechanism of Vermicular Graphite Cast Iron. Materials 2019, 12, 3130. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.D.; Hwang, S.W.; Park, K.T. Factors influencing the tensile behavior of a Fe–28Mn–9Al–0.8C steel. Mater. Sci. Eng. A 2008, 508, 234–240. [Google Scholar] [CrossRef]
- Hwang, S.W.; Ji, J.H.; Lee, E.G.; Park, K.T. Tensile deformation of a duplex Fe–20Mn–9Al–0.6C steel having the reduced specific weight. Mater. Sci. Eng. A 2011, 528, 5196–5203. [Google Scholar] [CrossRef]
- Lee, C.Y.; Jeong, J.; Han, J.; Lee, S.-J.; Lee, S.; Lee, Y.-K. Coupled strengthening in a medium manganese lightweight steel with an inhomogeneously grained structure of austenite. Acta Mater. 2015, 84, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.F.; Yang, H.; Li, J.X.; Kan, Z.W.; Huang, S.Q. Calculation model of stacking fault energy of Fe-Mn-Al-C low density steel based on thermodynamic theory. Mater. Rev. 2018, 032, 2859–2864. [Google Scholar]
- Gutierrez-Urrutia, I.; Raabe, D. Influence of Al content and precipitation state on the mechanical behavior of austenitic high-Mn low-density steels. Scr. Mater. 2013, 68, 343–347. [Google Scholar] [CrossRef]
- Hou, L.L.; Hui, J.T.; Yao, Y.H.; Chen, J.; Liu, J.N. Effects of Boron Content on microstructure and mechanical properties of AlFeCoNiBx High Entropy Alloy Prepared by vacuum arc melting. Vacuum 2019, 164, 212–218. [Google Scholar] [CrossRef]
- Koutsky, J.; Kletečka, Z. Vacuum arc re-melting and its influence on mechanical properties of steels. Vacuum 1969, 19, 187–189. [Google Scholar] [CrossRef]
- Karantzalis, A.E.; Lekatou, A.; Georgatis, E.; Arni, Z.; Dracopoulos, V. Solidification observations of vacuum arc melting processed Fe–Al–TiC composites: TiC precipitation mechanisms. Mater. Charact. 2011, 62, 1196–1204. [Google Scholar] [CrossRef]
- Liu, E.Y.; Zhang, J.H.; Chen, S.; Du, S.M.; Du, H.L.; Cai, H.; Wang, L.L. High temperature negative wear behaviour of VN/Ag composites induced by expansive oxidation reaction. Ceram. Int. 2021, 47, 15901–15909. [Google Scholar] [CrossRef]
- Bai, Y.P.; Li, J.P.; Cheng, C.; Yang, Z. Study on microstructure and oxidation behavior of Fe–xMn–14Al–8Ni–C alloy prepared by vacuum arc melting. Mater. Res. Express. 2020, 7, 096521. [Google Scholar] [CrossRef]
- Cheng, C. Study on Fabrication Processing and Properties of Fe-Mn-Al Alloy with Low Density and High Performance. Xi’an Technol. Univ. 2020, 82, 13–25. (In Chinese) [Google Scholar]
- Chausov, M.G.; Maruschak, P.O.; Hutsaylyuk, V.; Sniezek, L.; Pylypenko, A.P. Effect of complex combined loading mode on the fracture toughness of titanium alloys. Vacuum 2018, 147, 51–57. [Google Scholar] [CrossRef]
- Maruschak, P.O.; Konovalenko, I.V.; Bishchak, R.T. Effect of thermal fatigue cracks on brittle-ductile deformation and failure of cbcm roller surface layers. Metallurgist 2012, 56, 30–36. [Google Scholar] [CrossRef]
- Pouraliakbar, H.; Khalaj, G.; Jandaghi, M.R.; Khalaj, M.J. Study on Correlation of Toughness with chemical composition and tensile test results in microalloyed API pipeline steels. J. Min. Metall. Sect. B Metall. 2015, 51, 173–178. [Google Scholar] [CrossRef]
- Khalaj, G.; Khalaj, M.J. Modeling the correlation between yield strength, chemical composition and ultimate tensile strength of X70 pipeline steels by means of gene expression programming. Int. J. Mater. Res. 2013, 104, 697–702. [Google Scholar] [CrossRef]
- Pei, Y.B.; Huang, T.; Chen, F.X.; Zhan, M.; Gao, J.Q.; Song, Z.; Bai, L.G. In-situ observation of crack evolution in Ti/Al laminated composite. Compos. Interfaces 2020, 27, 435–448. [Google Scholar] [CrossRef]
- Qin, L.; Wang, J.; Wu, Q.; Guo, X.Z.; Tao, J. In-situ observation of crack initiation and propagation in Ti/Al composite laminates during tensile test. J. Alloy. Compd. 2017, 712, 69–75. [Google Scholar] [CrossRef]
- Chen, Z.W.; Hao, X.L.; Wang, Y.; Zhao, K. In-Situ Observation of Tensile Fracture in A357 Casting Alloys. J. Mater. Sci. Technol. 2014, 30, 139–145. [Google Scholar] [CrossRef]
- GB/T 228-2002. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Metallic Materials-Tensile Testing at Ambient Temperature (China Standard GB/T 228-2002). 2002. Available online: https://www.chinesestandard.net/PDF.aspx/GBT228-2002 (accessed on 19 April 2021).
- Li, P.B.; Chen, T.J.; Qin, H. Effects of mold temperature on the microstructure and tensile properties of SiCp/2024 Al-based composites fabricated via powder thixoforming. Mater. Des. 2016, 112, 34–35. [Google Scholar] [CrossRef]
- Liu, D.G.; Ding, H.; Hu, X.; Han, D.; Cai, M.H. Dynamic recrystallization and precipitation behaviors during hot deformation of a κ-carbide-bearing multiphase Fe–11Mn–10Al–0.9C lightweight steel. Mater. Sci. Eng. A 2020, 772, 138682. [Google Scholar] [CrossRef]
- Della Ventura, N.M.; Kalácska, S.; Casari, D.; Edwards, T.E.J.; Sharma, A.; Michler, J.; Logé, R.; Maeder, X. {10ī2} twinning mechanism during in situ micro-tensile loading of pure mg: Role of basal slip and twin-twin interactions. Mater. Des. 2020, 197, 109206. [Google Scholar] [CrossRef]
- Lu, X.; Dunne, F.P.E.; Xu, Y. A crystal plasticity investigation of slip system interaction, GND density and stored energy in non-proportional fatigue in Nickel-based superalloy. Int. J. Fatigue 2020, 139, 105782. [Google Scholar] [CrossRef]
- Seol, J.B.; Park, H.S.; Park, C.G. Direct In situ Observation of Tempering-induced Austenite Decomposition and Atom Probe Analyses of k-Carbide Precipitates in Lightweight Fe-Mn-Al-C Steels. Microsc. Microanal. 2016, 22, 704–705. [Google Scholar] [CrossRef][Green Version]
- Song, W.; Wang, X.G.; Li, J.G. The formation and evolution of NiAl phase in a fourth generation nickel-based single crystal superalloy. J. Alloy. Compd. 2020, 848, 156584. [Google Scholar] [CrossRef]
- Zhang, S.F.; Kim, D.U.; Jiang, W.; Tonk, M.R. A phase field model of crack propagation in anisotropic brittle materials with preferred fracture planes. Comput. Mater. Sci. 2021, 193, 110400. [Google Scholar] [CrossRef]
- Antolovich, S.D.; Armstrong, R.W.P. Lastic Strain Localization in Metals: Origins and Consequences. Progress Mater. Sci. 2014, 59, 1–160. [Google Scholar] [CrossRef]
- Höppel, H.W.; Goik, P.; Krechel, C.; Göken, M. Ex and in situ investigations on the role of persistent slip bands and grain boundaries in fatigue crack initiation. J. Mater. Res. Technol. 2017, 32, 4276–4286. [Google Scholar] [CrossRef]
- Man, J.; Petrenec, M.; Obrtlik, K. AFM and TEM Study of Cyclic Slip Localization in Fatigued Ferritic X10CrAl24 Stainless Steel. Acta Mater. 2004, 52, 5551–5561. [Google Scholar] [CrossRef]
- Risbet, M.; Feaugas, X. Some Comments about Fatigue Crack Initiation in Relation to Cyclic Slip Irreversibility. Eng. Fract. Mech. 2008, 75, 3511–3519. [Google Scholar] [CrossRef]
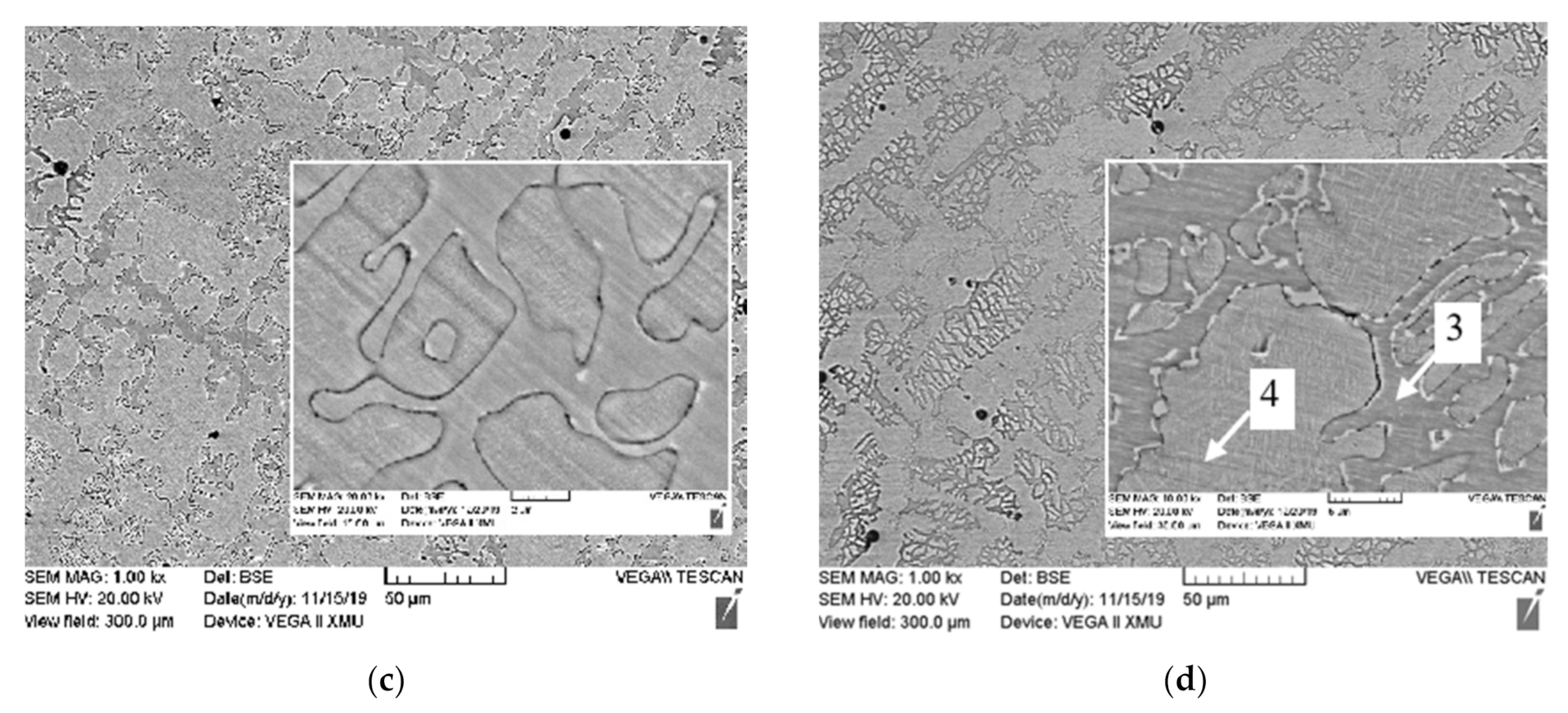
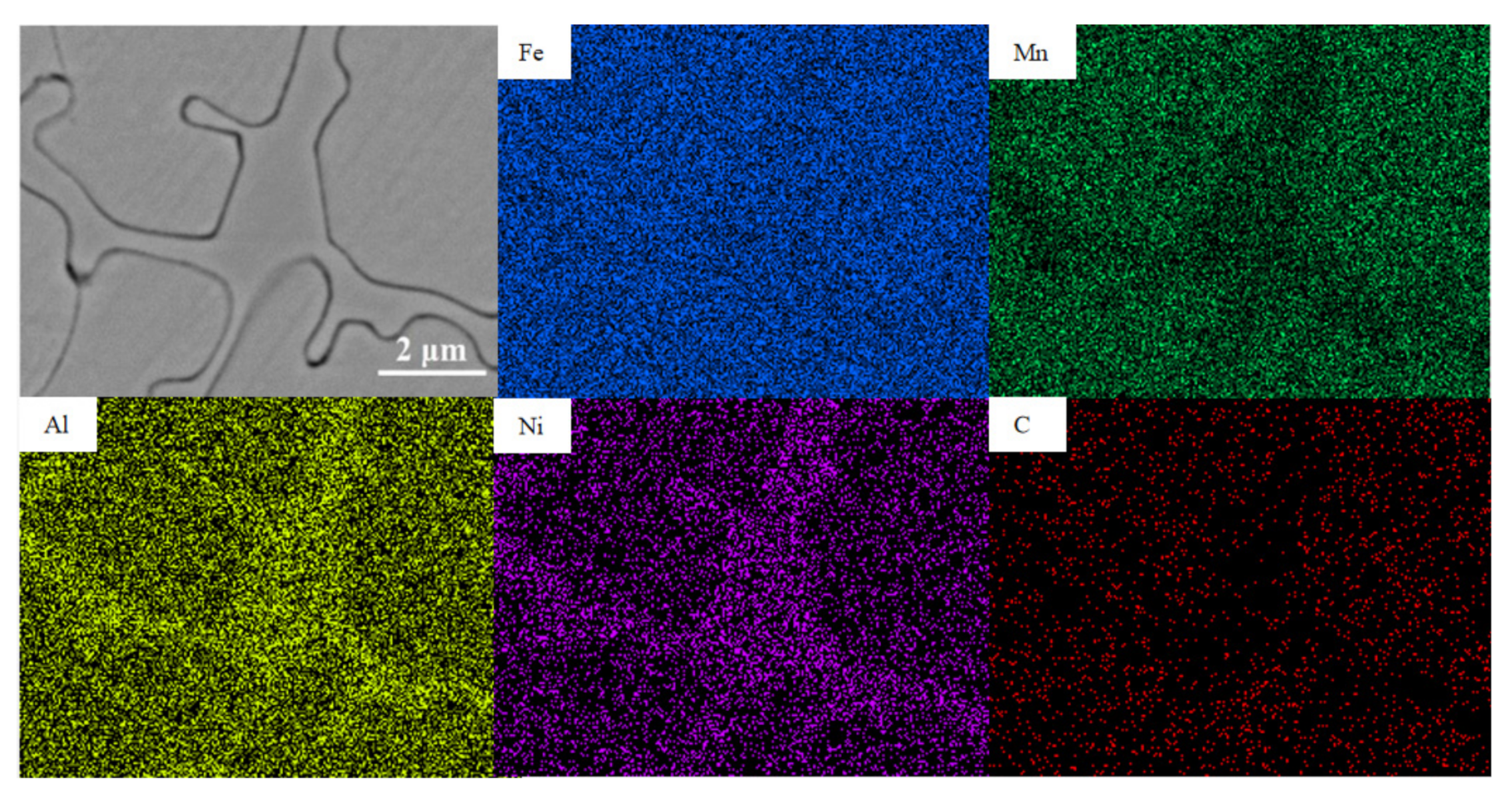
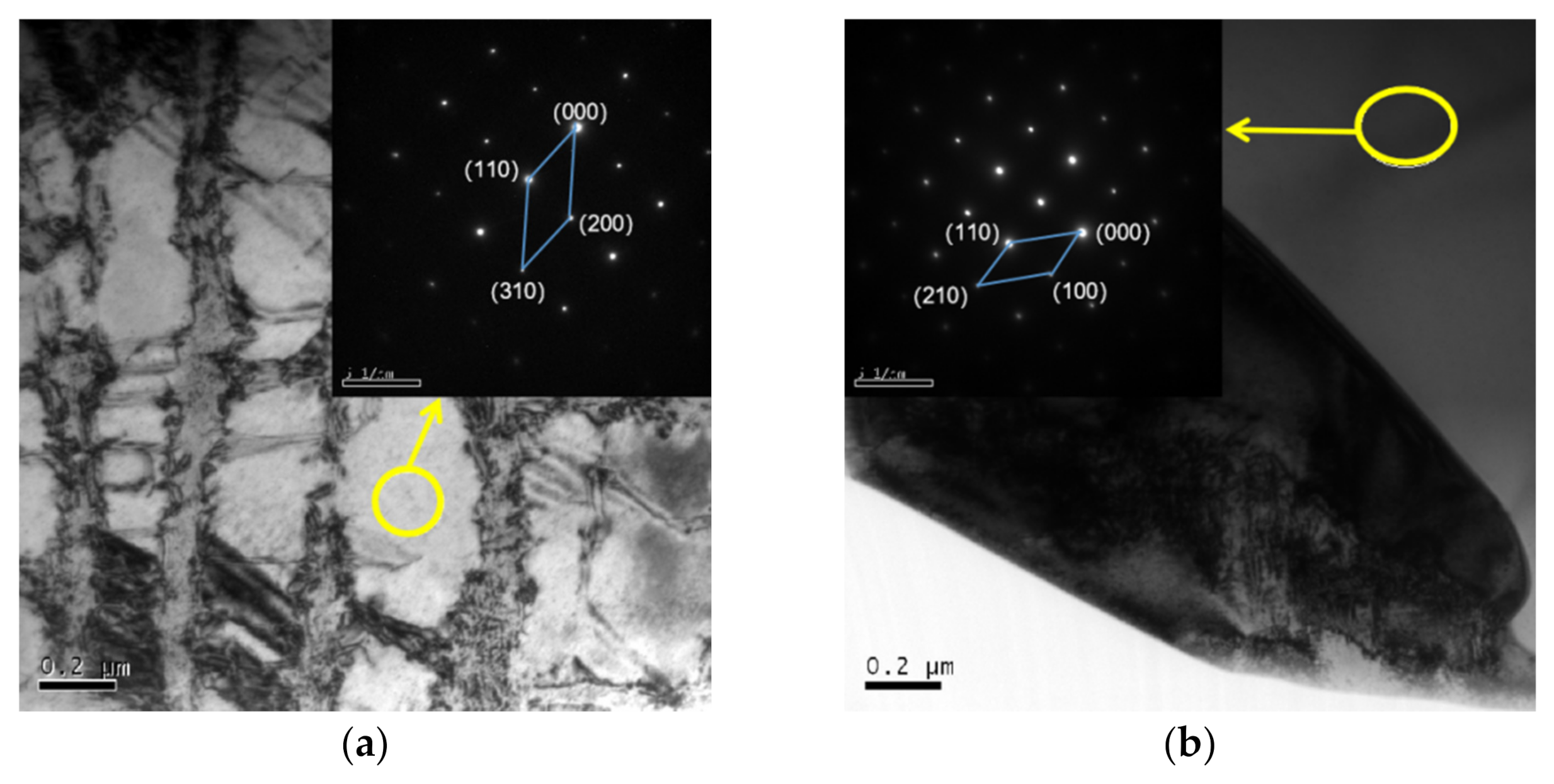
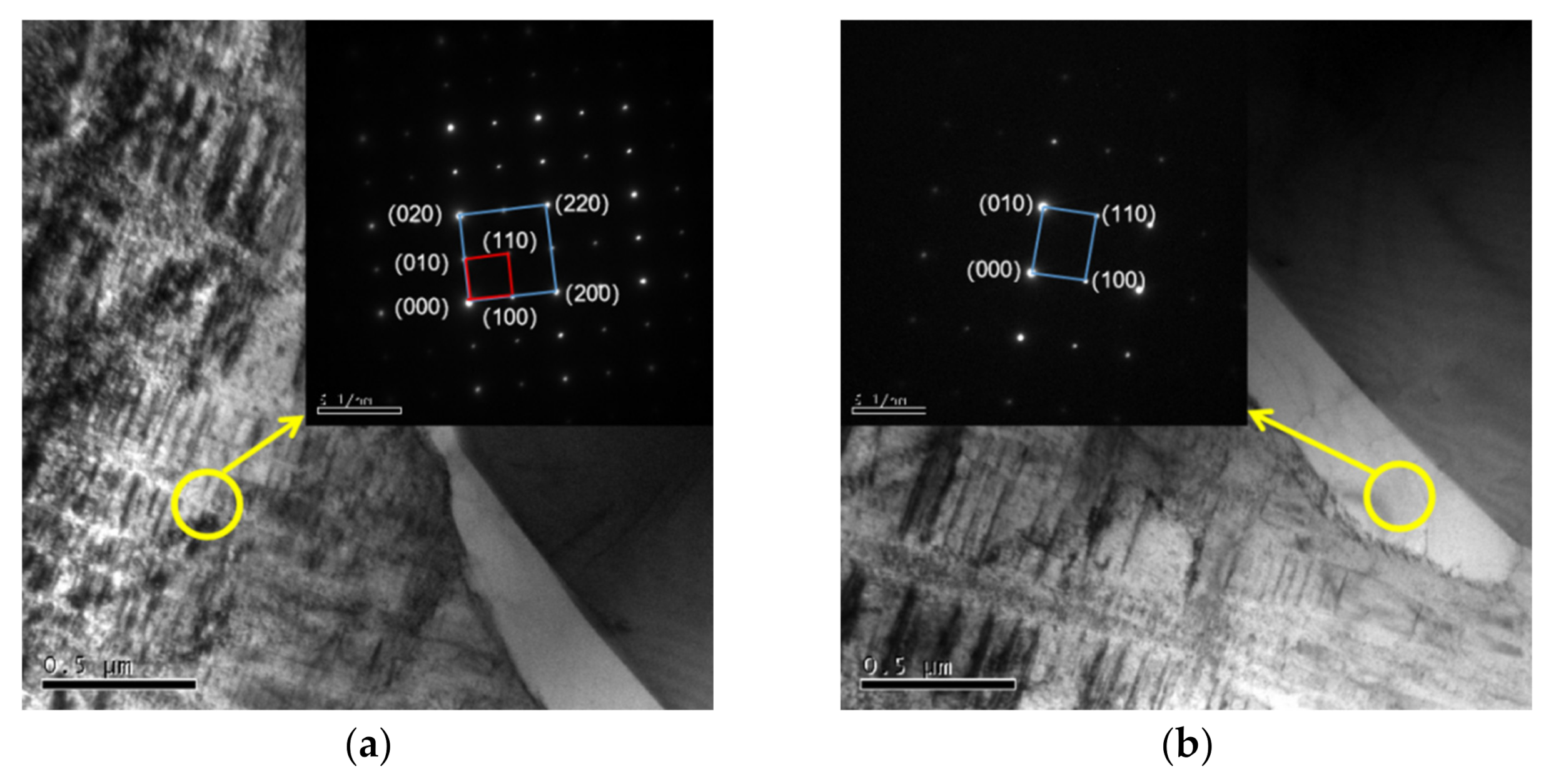


| Al Contents | Point | Element/wt.% | ||||
|---|---|---|---|---|---|---|
| Fe | Mn | Al | Ni | C | ||
| x = 11 | 1 | 39.0 | 14.1 | 16.4 | 17.6 | 12.8 |
| 2 | 50.1 | 21.5 | 9.0 | 5.7 | 13.7 | |
| x = 13 | 3 | 47.0 | 15.3 | 17.0 | 18.0 | 2.8 |
| 4 | 57.5 | 26.8 | 10.1 | 1.7 | 4.0 | |
| Al Contents (wt.%) | Density (g/cm3) | Hardness (HRC) |
|---|---|---|
| x = 10 | 6.74 | 40.55 |
| x = 11 | 6.67 | 41.53 |
| x = 12 | 6.59 | 43.95 |
| x = 13 | 6.53 | 44.75 |
| Al Contents (wt.%) | Elongation (%) | Tensile Strength (MPa) |
|---|---|---|
| 10 | 4.1 | 664.4 |
| 11 | 2.6 | 702.5 |
| 12 | 1.7 | 529.7 |
| 13 | 1.1 | 310.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Li, M.; Cheng, C.; Li, J.; Guo, Y.; Yang, Z. Study on Microstructure and In Situ Tensile Deformation Behavior of Fe-25Mn-xAl-8Ni-C Alloy Prepared by Vacuum Arc Melting. Metals 2021, 11, 814. https://doi.org/10.3390/met11050814
Bai Y, Li M, Cheng C, Li J, Guo Y, Yang Z. Study on Microstructure and In Situ Tensile Deformation Behavior of Fe-25Mn-xAl-8Ni-C Alloy Prepared by Vacuum Arc Melting. Metals. 2021; 11(5):814. https://doi.org/10.3390/met11050814
Chicago/Turabian StyleBai, Yaping, Meng Li, Chao Cheng, Jianping Li, Yongchun Guo, and Zhong Yang. 2021. "Study on Microstructure and In Situ Tensile Deformation Behavior of Fe-25Mn-xAl-8Ni-C Alloy Prepared by Vacuum Arc Melting" Metals 11, no. 5: 814. https://doi.org/10.3390/met11050814
APA StyleBai, Y., Li, M., Cheng, C., Li, J., Guo, Y., & Yang, Z. (2021). Study on Microstructure and In Situ Tensile Deformation Behavior of Fe-25Mn-xAl-8Ni-C Alloy Prepared by Vacuum Arc Melting. Metals, 11(5), 814. https://doi.org/10.3390/met11050814






