Influence of Morphology of Intermetallic Particles on the Microstructure and Properties Evolution in Severely Deformed Al-Fe Alloys
Abstract
1. Introduction
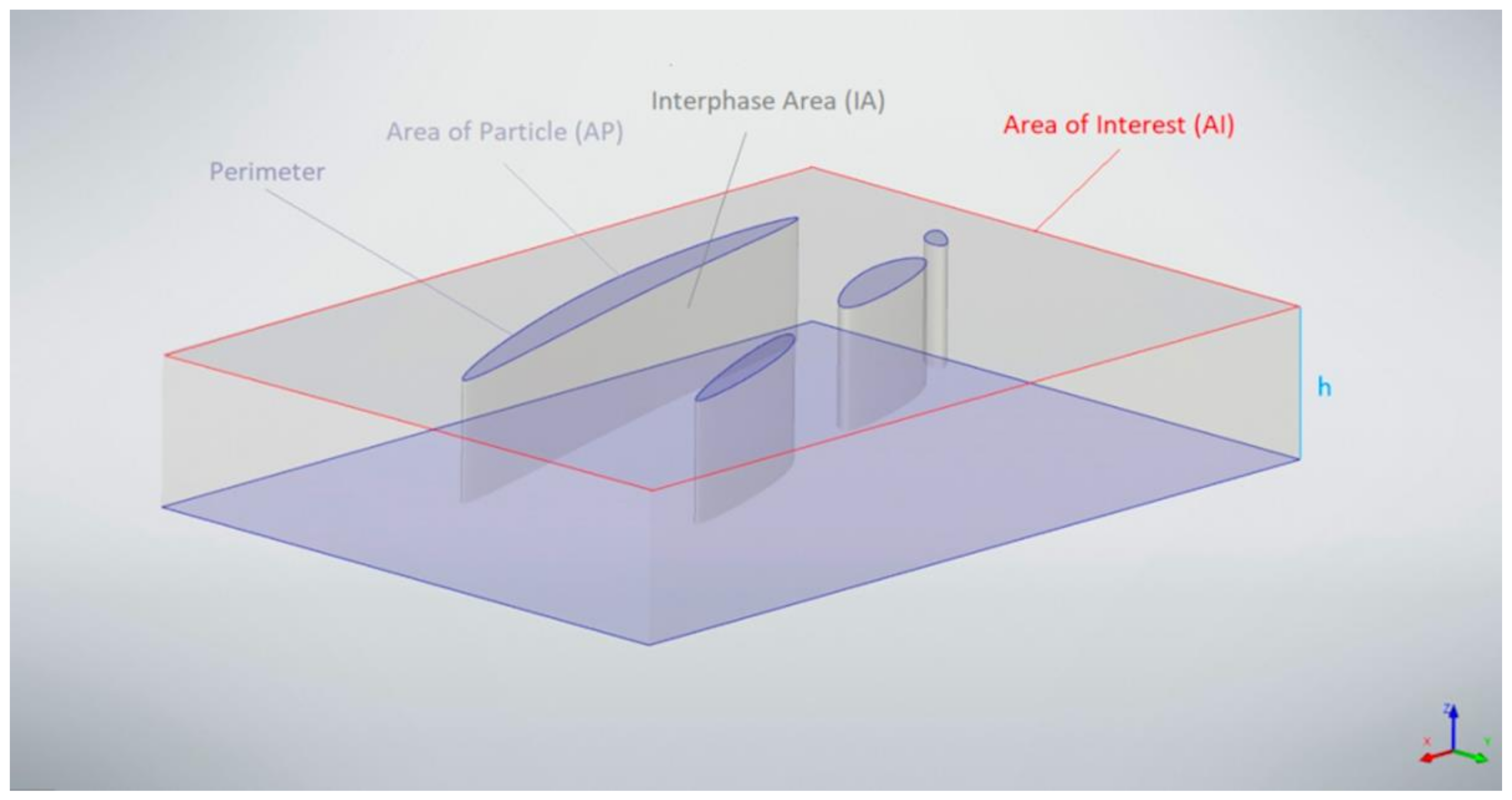
2. Materials and Methods
3. Results
3.1. Microstructure Evolution of the Alloys during HPT
3.1.1. SEM Analyses
3.1.2. XRD Analyses
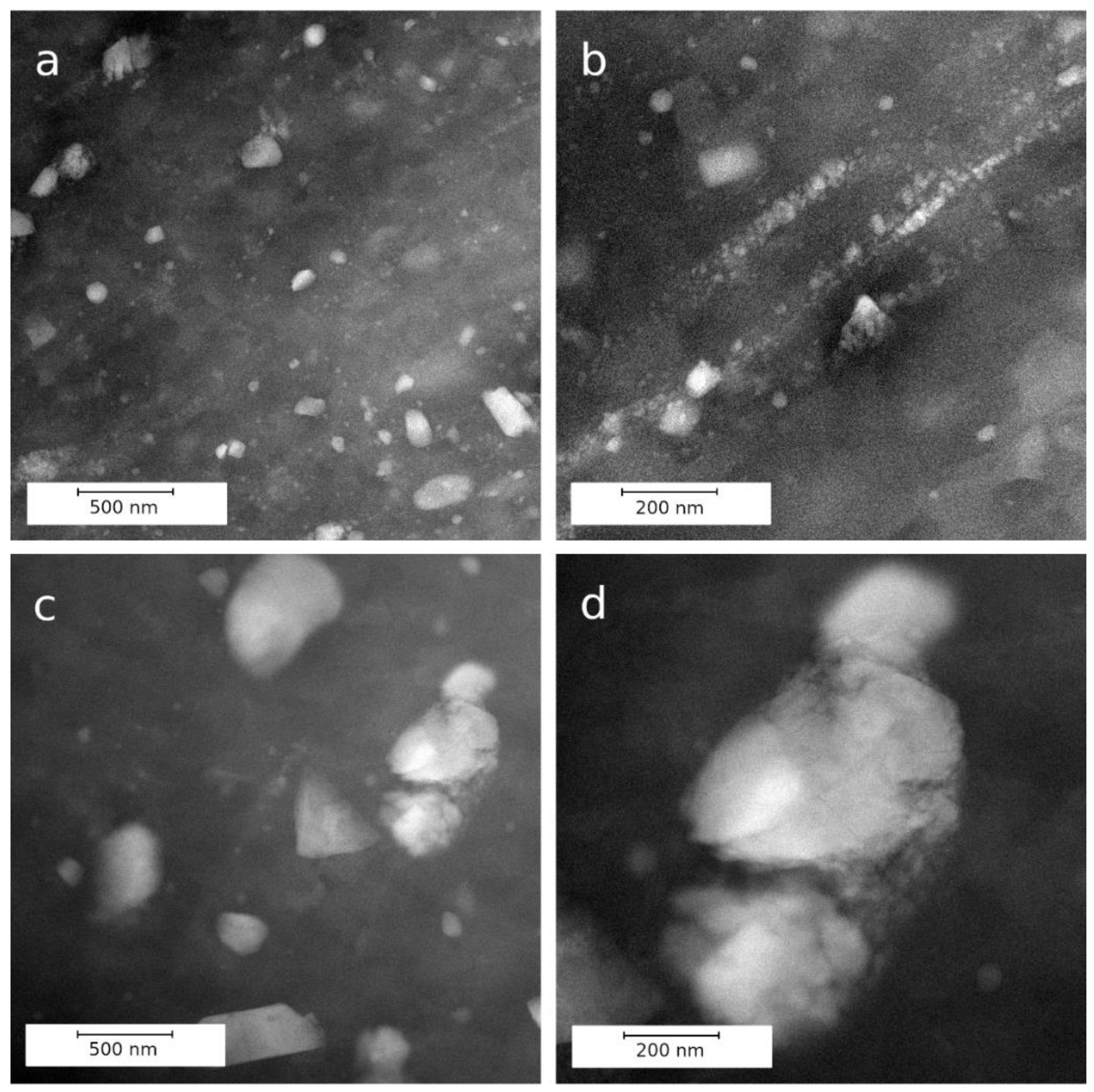
3.1.3. TEM and STEM Analyses
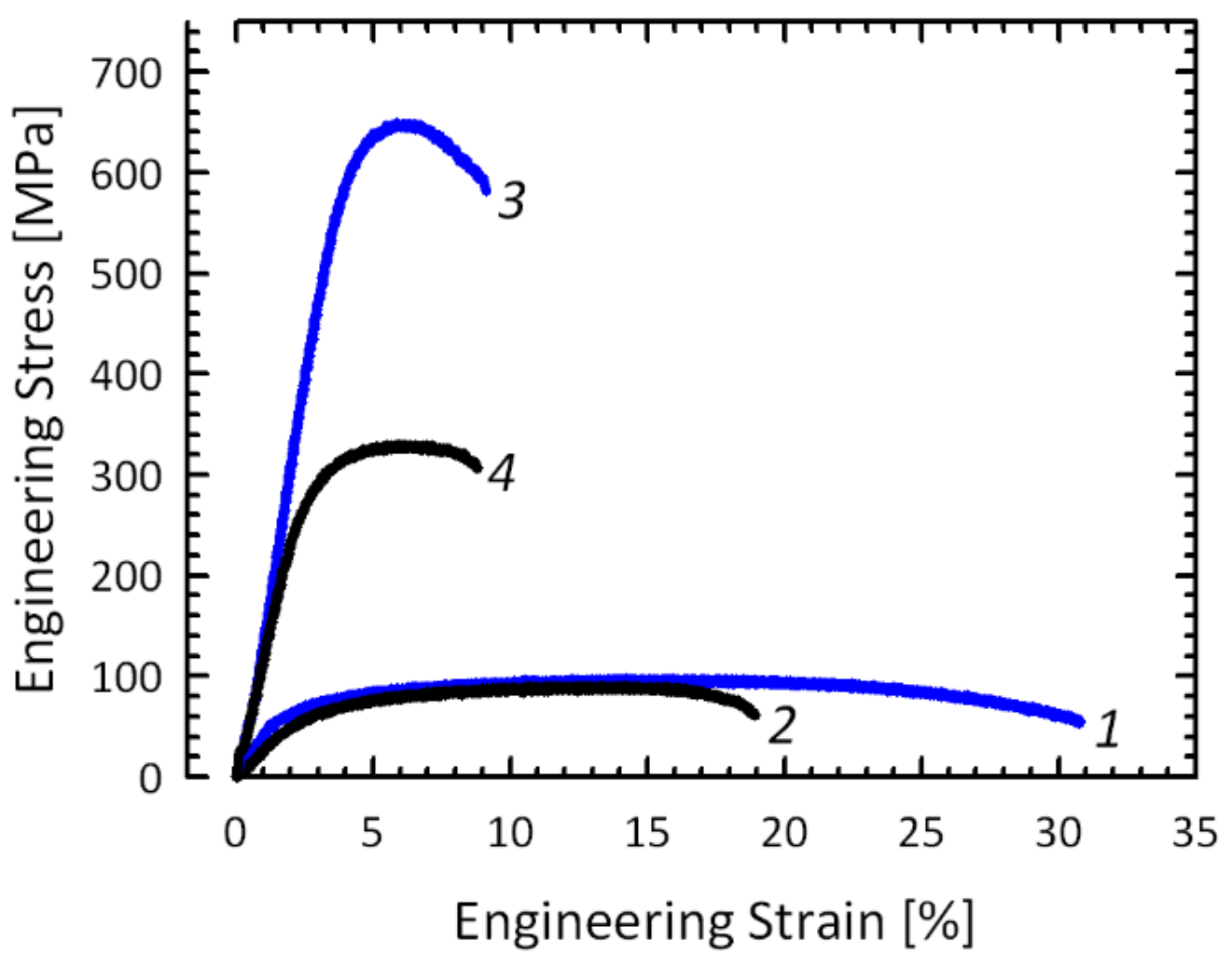
3.2. Physical and Mechanical Properties
4. Discussion
4.1. Particle Morphology
4.2. Formation of the Solid Solution
5. Conclusions
- The initial microstructure of the Al-2Fe and the Al-4Fe alloys is composed of the mixture of Al and eutectic Al6Fe and Al13Fe4 phases. In the Al-2Fe alloy the intermetallic particles are shaped as plates/needles with the length up to 150 nm, while Al-4Fe alloy contains, among the fine plate-like particles, coarser particles with the size up to tens of microns.
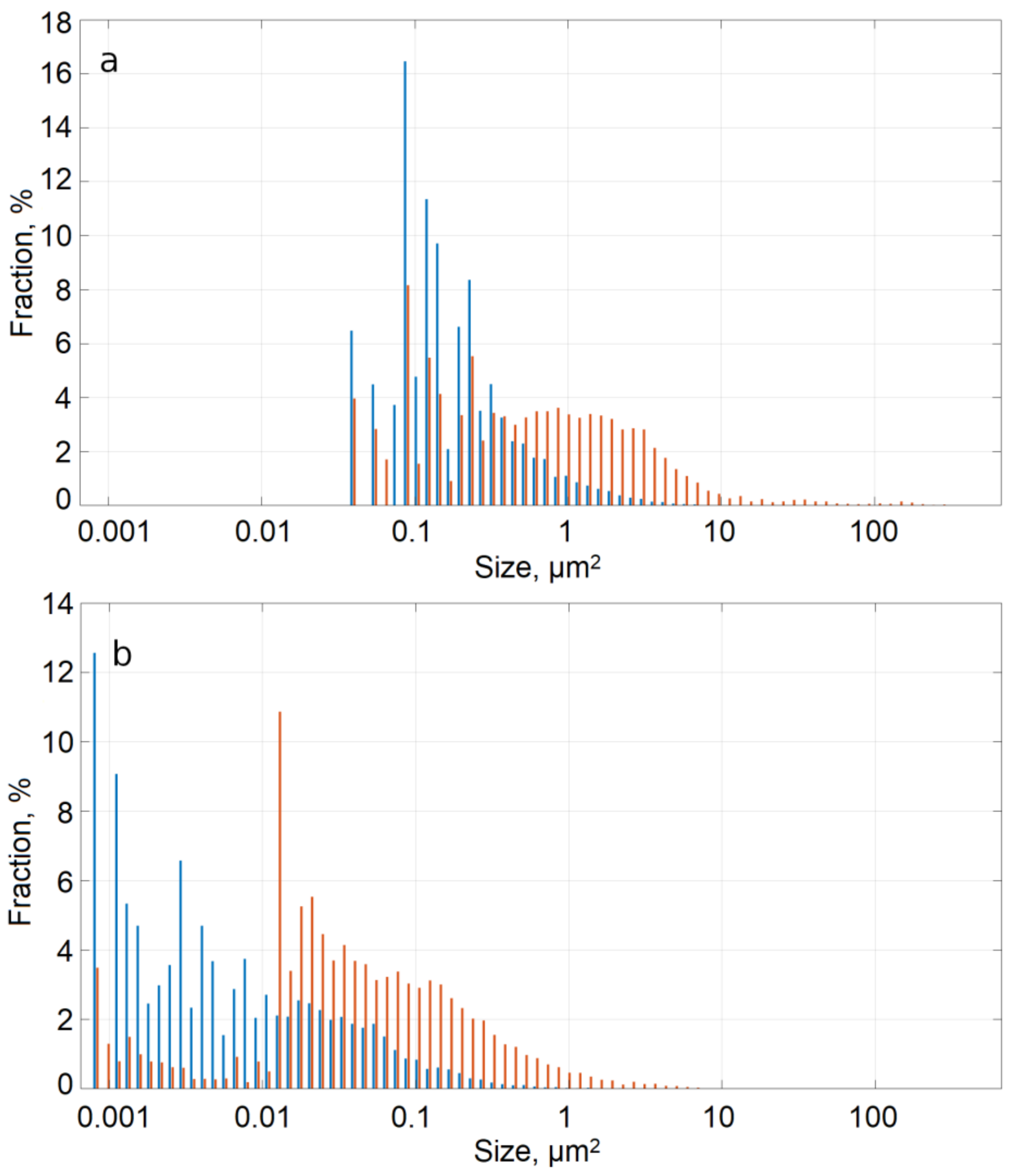
- As a result of HPT, the intermetallic particles become fragmented and redistributed in the bulk of the Al-2Fe and Al-4Fe alloys. Intermetallic particles in the Al-2Fe alloy are in the comparable size range and are uniformly distributed within the Al matrix, while in the Al-4Fe alloy the size of the particles after HPT varies from tens of nanometers to few microns, and their distribution is bimodal.
- Al-4Fe alloy after HPT is characterized by a larger grain size than Al-2Fe alloy. Intermetallic particles morphology in Al-2Fe alloy created the possibility for the solid solution formation, thus inhibiting the grain boundary migration. In addition, in Al-4Fe alloy intermetallic particles were distributed heterogeneously, and thus created regions in material’s volume where the movement of the grain boundaries was not inhibited.
- HPT processing leads to an increase of the UTS to ~650 MPa for the Al-2Fe alloy and to the decrease of its electrical conductivity to 40.4 %IACS. The changes in the Al-4Fe alloy have a similar character but are less pronounced due to the different morphology of intermetallic particles in the as-cast state.
- We show that Al-2Fe alloy has a uniform size distribution of the intermetallic particles after HPT because of the different particle morphology in the initial state. In particularly, the uniformity of the particle size distribution is achieved due to the higher, relative to the Al-4Fe alloy, overall interphase area in the as-cast state.
- Supersaturated solid solution of the Fe in Al is formed by HPT in Al-2Fe alloy. Al-4Fe alloy, due to lower density of interphase boundaries in the as-cast state, has fewer opportunities for the Fe to diffuse into the Al matrix, so the solid solution forms it with a significantly lower Fe concentration.
- Predesigned morphology in low-solubility alloys, coupled with deformation techniques, could be effectively used as a tool for the production of high-strength materials with a unique set of properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murashkin, M.Y.; Sabirov, I.; Sauvage, X.; Valiev, R.Z. Nanostructured Al and Cu alloys with superior strength and electrical conductivity. J. Mater. Sci. 2016, 51, 33–49. [Google Scholar] [CrossRef]
- Polmear, I.J. Light Alloys-From Traditional Alloys to Nanocrystalls, 4th ed.; Monash University: Melbourne, Australia, 2006. [Google Scholar]
- Jablonski, M.; Knych, T.; Smyrak, B. Effect of iron addition to aluminium on the structure and properties of wires used for electrical purposes. In Proceedings of the 5th International Conference on Light Metals Technology, Luneburg, Germany, 19–22 July 2011; Trans Tech Publications Ltd.: Luneburg, Germany, 2011; pp. 459–462. [Google Scholar]
- Jablonski, M.; Knych, T.; Smyrak, B. New Aluminium Alloys for Electrical Wires of Fine Diameter for Automotive Industry. Arch. Metall. Mater. 2009, 54, 671–676. [Google Scholar]
- Sekia, S.; Susai, K.; Takamura, S. Development of aluminium wire for automotive harnesses. In Proceedings of the 60th Interna-tional Wire & Cable Symposium (IWCS), Charlotte Convention Center, Charlotte, NC, USA, 7–9 November 2011; pp. 445–449. [Google Scholar]
- Horikoshi, T.; Koruda, H.; Shimizu, M.; Aoyama, S. Development of Aluminium Alloy Conductor with High Electrical Con-ductivity and Controlled Tensile Strength and Elongation. Hitachi Cable Rev. 2006, 25, 31–34. [Google Scholar]
- Nasu, S.; Gonser, U.; Preston, R.S. Defects and phases of iron in aluminium. J. Phys. Colloq. 1980, 41, 385–386. [Google Scholar] [CrossRef]
- Shabashov, V.A.; Brodova, I.G.; Mukoseev, A.G.; Sagaradze, V.V.; Litvinov, A.V. Mossbauer study of the dissolution of iron aluminides under strong cold deformation, Proceedings of the Russian Academy of Sciences. Phys. Ser. 2005, 10, 1459–1464. [Google Scholar]
- Shabashov, V.A.; Brodova, I.G.; Mukoseev, A.G.; Sagaradze, V.V.; Litvinov, A.V. Structural transformations in the Al-Fe sys-tem under intense plastic deformation. Phys. Met. Metall. 2005, 4, 66–67. [Google Scholar]
- Senkov, O.N.; Valiev, R.Z.; Pirzada, M.D.S.; Liu, J.; Froes, F.H. A new approach to hardening of Al-Fe alloys. Synthesis of Lightweight Metals: In proceedings of the 128th Annual Meeting & Exhibition of The Minerals, Metals & Materials Society. San Diego, CA, USA, 28 February–4 March 1999; pp. 129–134. [Google Scholar]
- Stolyarov, V.V.; Brodova, I.G.; Yablonskikh, T.I.; Lapovok, R. The effect of backpressure on the structure and mechanical properties of the Al-5 wt % Fe alloy produced by equal-channel angular pressing. Phys. Met. Metallogr. 2005, 100, 182–191. [Google Scholar]
- Medvedev, A.E.; Murashkin, M.Y.; Enikeev, N.A.; Valiev, R.Z.; Hodgson, P.D.; Lapovok, R. Enhancement of mechanical and electrical properties of Al-RE alloys by optimizing rare-earth concentration and thermo-mechanical treatment. J. Alloys Compd. 2018, 745, 696–704. [Google Scholar] [CrossRef]
- Medvedev, A.E.; Murashkin, M.Y.; Enikeev, N.A.; Bikmukhametov, I.; Valiev, R.Z.; Hodgson, P.D.; Lapovok, R. Effect of the eutectic Al-(Ce,La) phase morphology on microstructure, mechanical properties, electrical conductivity and heat resistance of Al-4.5(Ce,La) alloy after SPD and subsequent annealing. J. Alloys Compd. 2019, 796, 321–330. [Google Scholar] [CrossRef]
- Fadeeva, V.; Leonov, A. Amorphization and crystallization of Al-Fe alloys by mechanical alloying. Mater. Sci. Eng. A 1996, 206, 90–94. [Google Scholar] [CrossRef]
- Fadeeva, V.; Leonov, A.V.; Khodina, L. Metastable Phases in Mechanically Alloyed Al-Fe System. Mater. Sci. Forum 1995, 179–181, 397–402. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.K.; Suryanarayana, C.; Froes, F.H.S. Structural evolution in mechanically alloyed Al-Fe powders. Met. Mater. Trans. A 1995, 26, 1939–1946. [Google Scholar] [CrossRef]
- Senkov, O.N.; Froes, F.H.; Stolyarov, V.V.; Valiev, R.Z.; Liu, J. Non-equilibrium structures in aluminium-iron alloys subjected to severe plastic deformation. In Proceedings of the 1997 5th International Conference on Advanced Particulate Materials and Processes, West Palm Beach, FL, USA, 7–9 April 1997; pp. 95–102. [Google Scholar]
- Senkov, O.; Froes, F.; Stolyarov, V.; Valiev, R.; Liu, J. Microstructure of Aluminum-Iron Alloys Subjected to Severe Plastic Deformation. Scr. Mater. 1998, 38, 1511–1516. [Google Scholar] [CrossRef]
- Stolyarov, V.; Lapovok, R.; Brodova, I.; Thomson, P. Ultrafine-grained Al–5 wt.% Fe alloy processed by ECAP with backpressure. Mater. Sci. Eng. A 2003, 357, 159–167. [Google Scholar] [CrossRef]
- Cubero-Sesin, J.M.; Horita, Z. Strengthening of Al through addition of Fe and by processing with high-pressure torsion. J. Mater. Sci. 2012, 48, 4713–4722. [Google Scholar] [CrossRef]
- Cubero-Sesin, J.M.; Horita, Z. Age Hardening in Ultrafine-Grained Al-2PctFe Alloy Processed by High-Pressure Torsion. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2015, 46, 2614–2624. [Google Scholar] [CrossRef]
- Cubero-Sesin, J.M.; In, H.; Arita, M.; Iwaoka, H.; Horita, Z. High-pressure torsion for fabrication of high-strength and high-electrical conductivity Al micro-wires. In Special Section: Ultrafinegrained Materials, 19th ed.; Mathaudhu, S.N., Estrin, Y., Horita, Z., Lavernia, E., Liao, X.Z., Lu, L., Wei, Q., Wilde, G., Zhu, Y.T., Eds.; Kluwer Academic Publishers: Amsterdam, The Netherlands, 2014; pp. 6550–6557. [Google Scholar]
- Cubero-Sesin, J.M.; Watanabe, M.; Arita, M.; Horita, Z.J. Aging and precipitation behavior in supersaturated Al-2%Fe alloy produced by high-pressure torsion. In Proceedings of the 14th International Conference on Aluminium Alloys, ICAA 2014, Trondheim, Norway, 15–19 June 2014; Trans Tech Publications Ltd.: Trondheim, Norway, 2014; pp. 766–771. [Google Scholar]
- Duchaussoy, A.; Sauvage, X.; Edalati, K.; Horita, Z.; Renou, G.; Deschamps, A.; De Geuser, F. Structure and mechanical behavior of ultrafine-grained aluminum-iron alloy stabilized by nanoscaled intermetallic particles. Acta Mater. 2019, 167, 89–102. [Google Scholar] [CrossRef]
- Medvedev, A.E.; Murashkin, M.Y.; Enikeev, N.A.; Valiev, R.Z.; Hodgson, P.D.; Lapovok, R. Optimization of Strength-Electrical Conductivity Properties in Al-2Fe Alloy by Severe Plastic Deformation and Heat Treatment. Adv. Eng. Mater. 2017, 20, 1700867. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Aleksandrov, I.V. Bulk Nanostructural Metallic Materials; Akademkniga: Moscow, Russia, 2007. [Google Scholar]
- Vorhauer, A.; Pippan, R. On the homogeneity of deformation by high pressure torsion. Scr. Mater. 2004, 51, 921–925. [Google Scholar] [CrossRef]
- Lutterotti, L.; Matthies, R.; Wenk, H.R.; Schultz, A.; Richardson, J. Combined texture and structure analysis of deformed lime-stone from time-of-flight neutron diffraction spectra. J. Appl. Phys. 1997, 81, 594–600. [Google Scholar] [CrossRef]
- Williamson, G.K.; Smallman, R.E., III. Dislocation densities in some annealed and cold-worked metals from measurements on the X-ray debye-scherrer spectrum. Philos. Mag. 1956, 1, 34–46. [Google Scholar] [CrossRef]
- ASTM E1004-09. Standard Test Method for Determing Electrical Conductivity Using The Electromagnetic (Eddy-Current) Method; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar]
- Saller, B.D.; Hu, T.; Ma, K.; Kurmanaeva, L.; Lavernia, E.J.; Schoenung, J.M. A comparative analysis of solubility, segregation, and phase formation in atomized and cryomilled Al–Fe alloy powders. J. Mater. Sci. 2015, 50, 4683–4697. [Google Scholar] [CrossRef]
- Kim, D.H.; Cantor, B. Structure and decomposition behaviour of rapidly solidified Al-Fe alloys. J. Mater. Sci. 1994, 29, 2884–2892. [Google Scholar] [CrossRef]
- Nayak, S.; Wollgarten, M.; Banhart, J.; Pabi, S.; Murty, B. Nanocomposites and an extremely hard nanocrystalline intermetallic of Al–Fe alloys prepared by mechanical alloying. Mater. Sci. Eng. A 2010, 527, 2370–2378. [Google Scholar] [CrossRef]
- Sasaki, H.; Kita, K.; Nagahora, J.; Inoue, A. Nanostructures and mechanical properties of bulk Al-Fe alloys prepared by elec-tron-beam deposition. Mater. Trans. 2001, 42, 1561–1565. [Google Scholar] [CrossRef]
- Sasaki, T.; Ohkubo, T.; Hono, K. Microstructure and mechanical properties of bulk nanocrystalline Al–Fe alloy processed by mechanical alloying and spark plasma sintering. Acta Mater. 2009, 57, 3529–3538. [Google Scholar] [CrossRef]
- Shabashov, V.A.; Brodova, I.G.; Mukoseev, A.G.; Sagaradze, V.V.; Litvinov, A.V. Deformation-induced phase transformations in the Al–Fe system under intensive plastic deformation. J. Phys. Condens. Matter 2007, 19. [Google Scholar] [CrossRef]



| Alloy | State | σ0.2, MPa | σUTS, MPa | δ, % | ω, MS/m | IACS, % |
|---|---|---|---|---|---|---|
| Al-2Fe | As-cast | 55 ± 6 | 100 ± 2 | 26 ± 2 | 32.4 ± 0.2 | 55.8 ± 0.3 |
| HPT | 564 ± 12 | 649 ± 6 | 5.0 ± 0.3 | 23.4 ± 0.7 | 40.4 ± 1.7 | |
| Al-4Fe | As-cast | 70 ± 6 | 88 ± 7 | 18 ± 2 | 31.2 ± 0.2 | 54.2 ± 1.2 |
| HPT | 270 ± 19 | 340 ± 22 | 8.0 ± 0.5 | 27.8 ± 0.1 | 48.0 ± 0.6 |
| Alloy | State | Average Particle Area, µm2 | Average Particle Perimeter, µm | Perimeter-to-Area Ratio, µm−1 | RIA, µm−1 |
|---|---|---|---|---|---|
| Al-2Fe | As-cast | 0.26 ± 0.002 | 0.26 ± 0.002 | 1.00 | 0.396 |
| HPT | 0.02 ± 0.001 | 0.49 ± 0.006 | 24.50 | 1.466 | |
| Al-4Fe | As-cast | 2.55 ± 0.160 | 2.85 ± 0.180 | 1.10 | 0.234 |
| HPT | 0.25 ± 0.040 | 1.24 ± 0.020 | 4.96 | 0.460 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medvedev, A.; Murashkin, M.; Enikeev, N.; Medvedev, E.; Sauvage, X. Influence of Morphology of Intermetallic Particles on the Microstructure and Properties Evolution in Severely Deformed Al-Fe Alloys. Metals 2021, 11, 815. https://doi.org/10.3390/met11050815
Medvedev A, Murashkin M, Enikeev N, Medvedev E, Sauvage X. Influence of Morphology of Intermetallic Particles on the Microstructure and Properties Evolution in Severely Deformed Al-Fe Alloys. Metals. 2021; 11(5):815. https://doi.org/10.3390/met11050815
Chicago/Turabian StyleMedvedev, Andrey, Maxim Murashkin, Nariman Enikeev, Evgeniy Medvedev, and Xavier Sauvage. 2021. "Influence of Morphology of Intermetallic Particles on the Microstructure and Properties Evolution in Severely Deformed Al-Fe Alloys" Metals 11, no. 5: 815. https://doi.org/10.3390/met11050815
APA StyleMedvedev, A., Murashkin, M., Enikeev, N., Medvedev, E., & Sauvage, X. (2021). Influence of Morphology of Intermetallic Particles on the Microstructure and Properties Evolution in Severely Deformed Al-Fe Alloys. Metals, 11(5), 815. https://doi.org/10.3390/met11050815






