Copper Recovery and Reduction of Environmental Loading from Mine Tailings by High-Pressure Leaching and SX-EW Process
Abstract
:1. Introduction
2. Materials and Methods
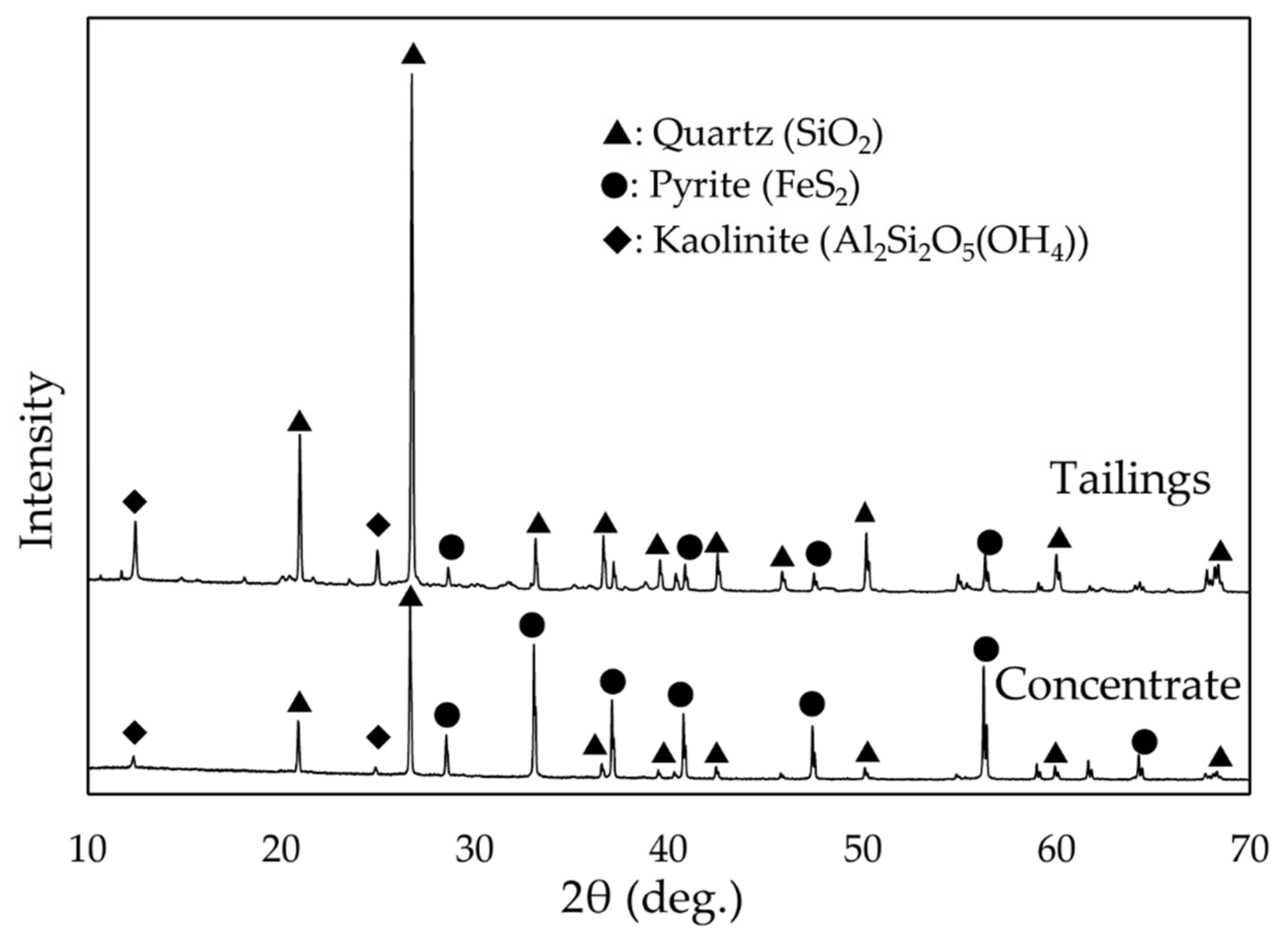
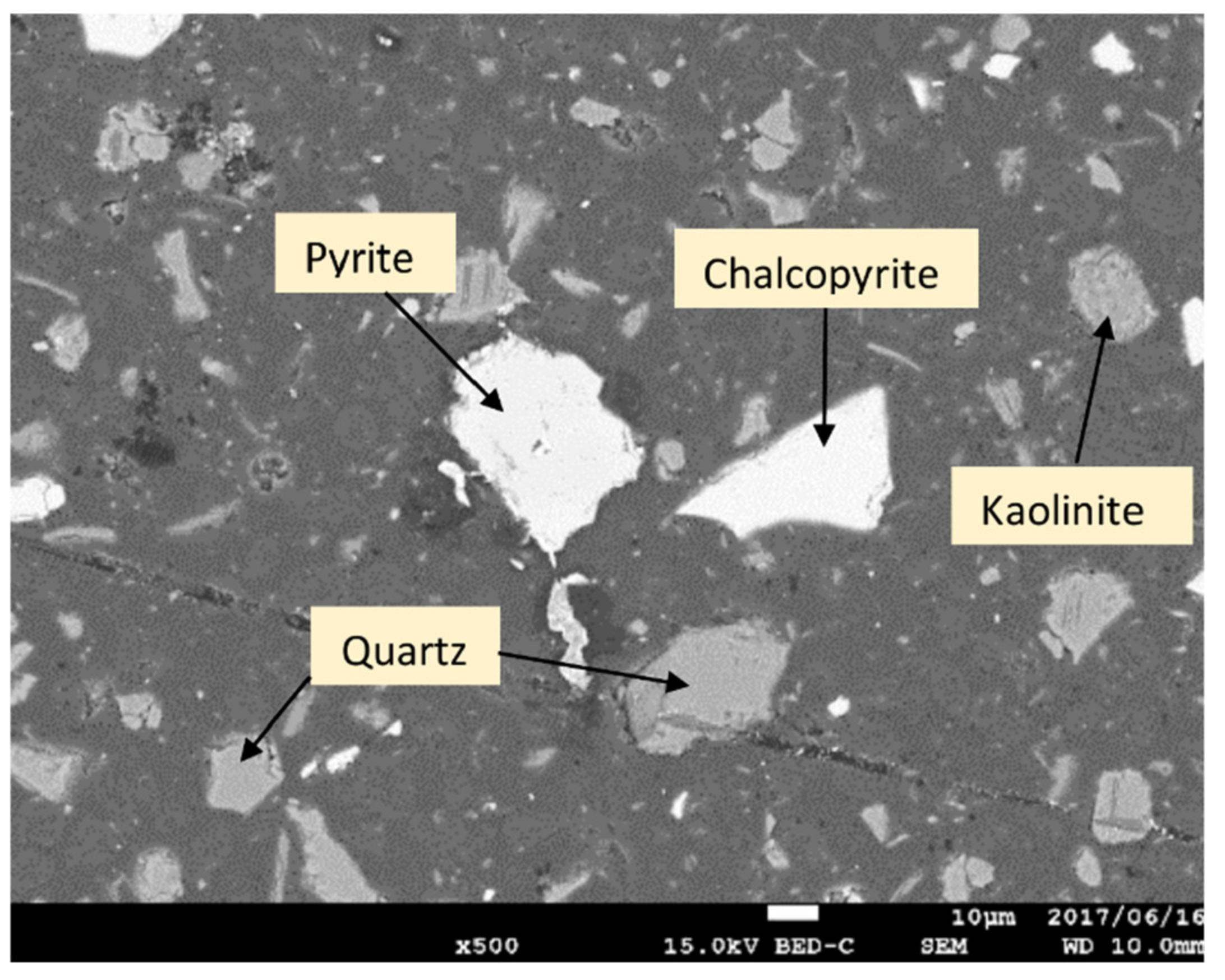
2.1. Experimental Sample
2.2. Experimental Procedure
2.2.1. High-Pressure Leaching
2.2.2. Elution Test
2.2.3. Solvent Extraction Test
2.2.4. Electrowinning Test
3. Results and Discussion
3.1. High-Pressure Leaching
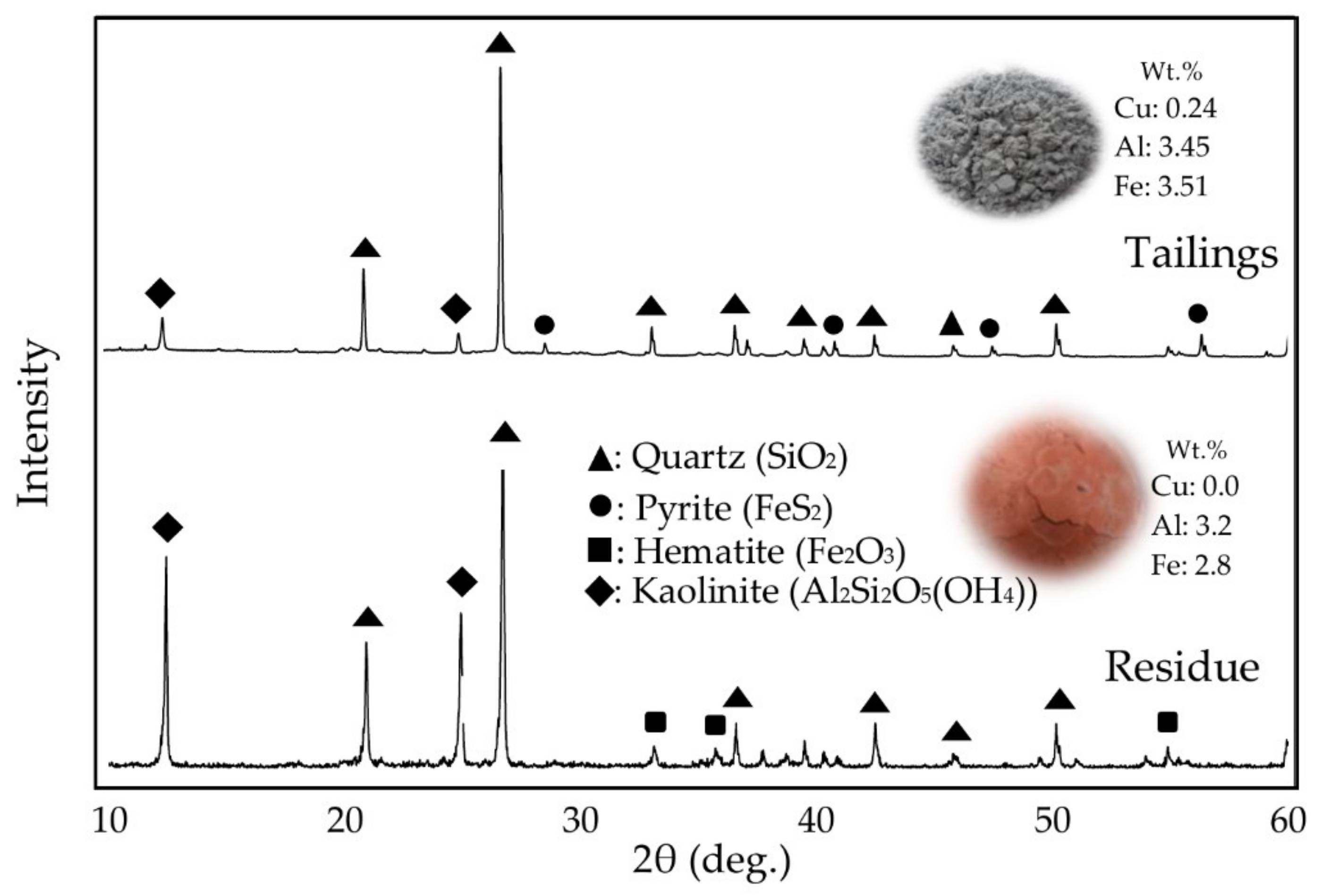
3.1.1. Leaching of Mine Tailing
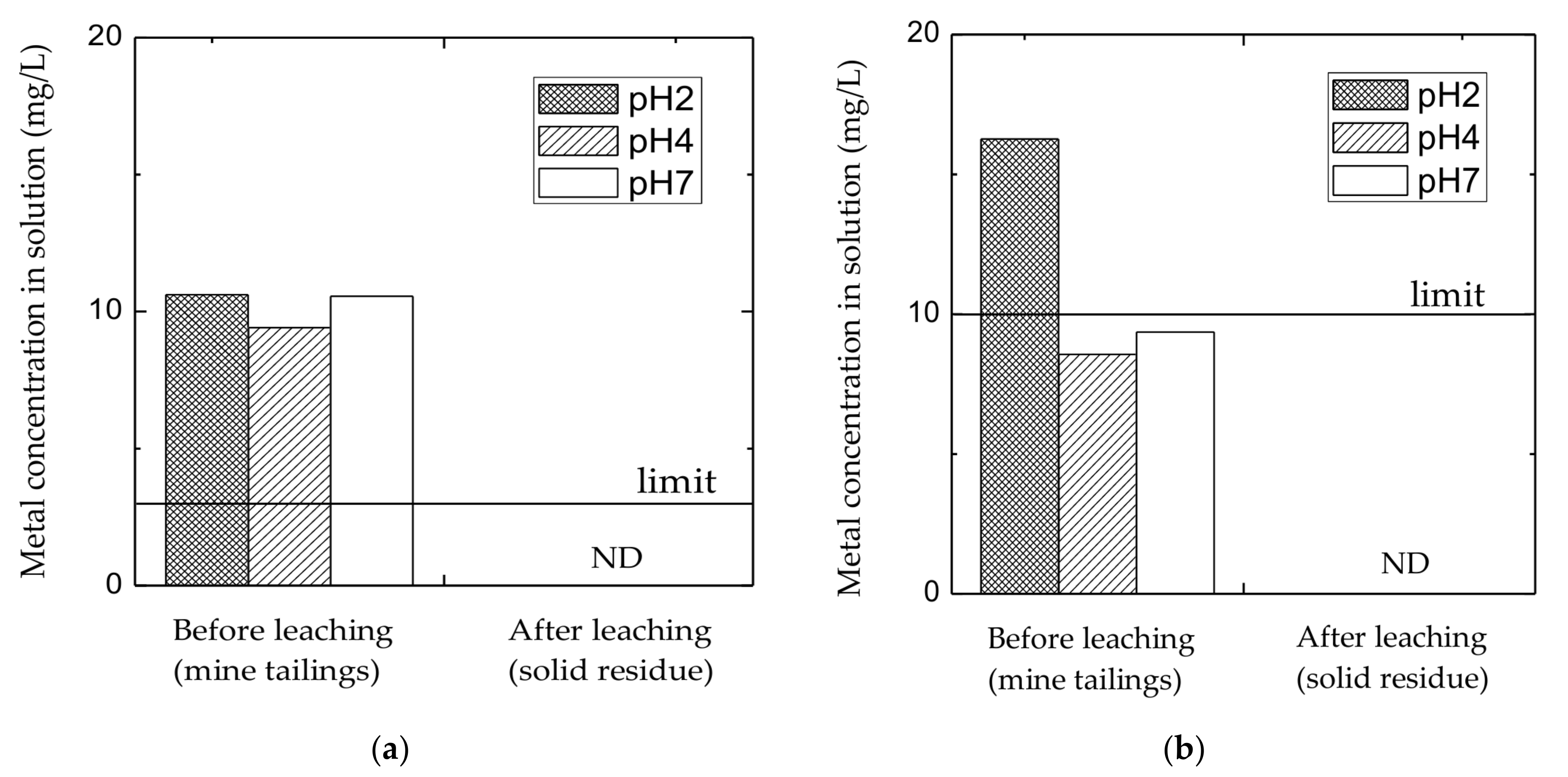
3.1.2. Elution Test of Leaching Residue Obtained from High-Pressure Leaching
3.1.3. Leaching of Concentrate from Mine Tailings
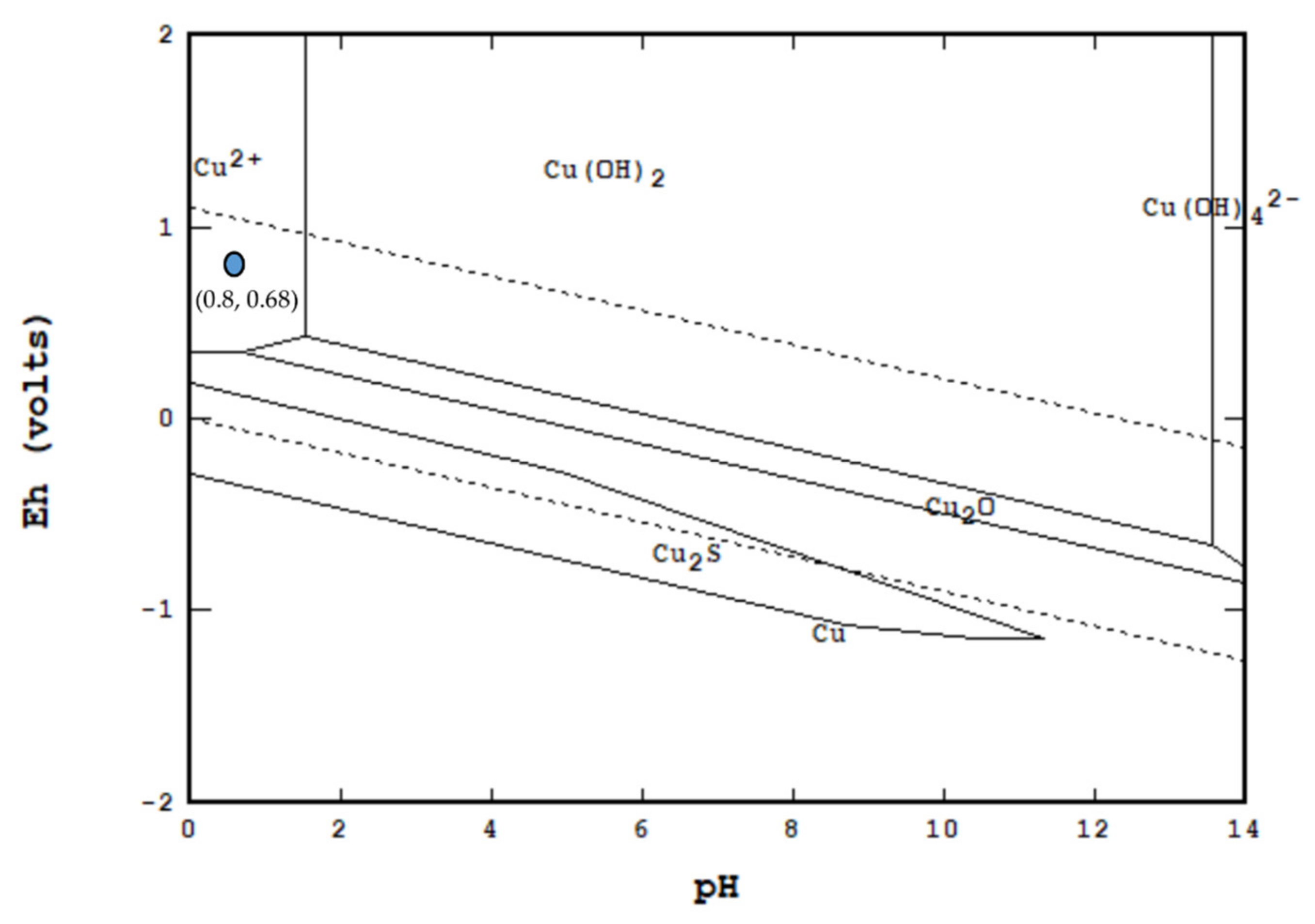
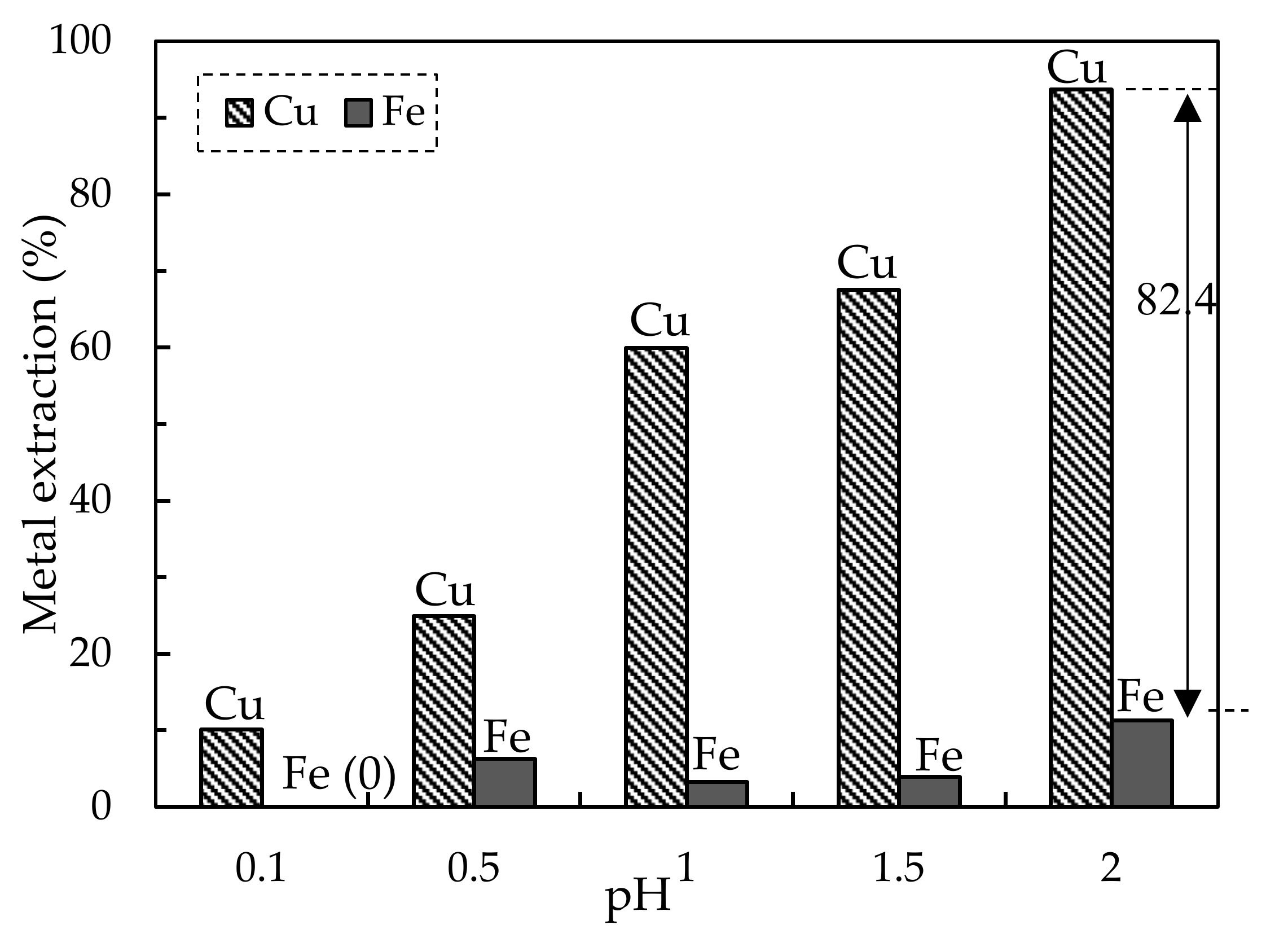
3.2. Solvent Extraction of the Pregnant Leach Solution from HPOL
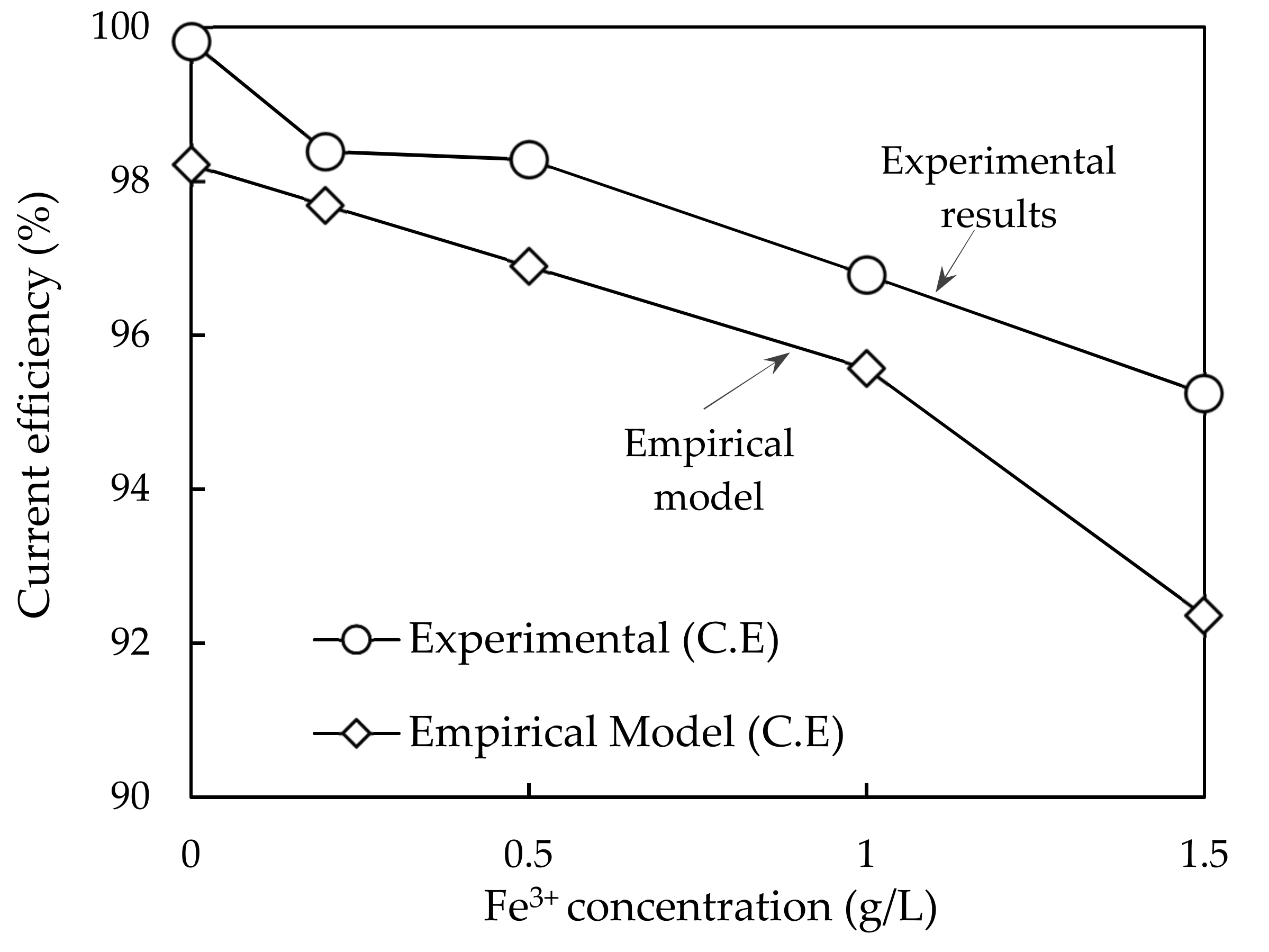
3.3. Electrowinning of the Simulated Stripped Solution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vriens, B.; Plante, B.; Seigneur, N.; Jamieson, H. Mine Waste Rock: Insights for Sustainable Hydrogeochemical Management. Minerals 2020, 10, 728. [Google Scholar] [CrossRef]
- Tayebi-Khorami, M.; Edraki, M.; Corder, G.; Golev, A. Re-Thinking Mining Waste Through an Integrative Approach Led by Circular Economy Aspirations. Minerals 2019, 9, 286. [Google Scholar] [CrossRef] [Green Version]
- Schlesinger, M.E.; King, M.J.; Sole, K.C.; Davenport, W.G. Extractive Metallurgy of Copper, 5th ed.; Elsevier: London, UK, 2011; pp. 68, 356, 358 and 361. [Google Scholar]
- Muravyov, M.I.; Bulaev, A.G.; Kondrat’eva, T.F. Complex treatment of mining and metallurgical wastes for recovery of base metals. Miner. Eng. 2014, 64, 63–66. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Lei, C.; Yana, B.; Xiao, X. Metal recovery from the copper sulfide tailing with leaching and fractional precipitation technology. Hydrometallurgy 2014, 147, 178–182. [Google Scholar] [CrossRef]
- Antonijevi’c, M.M.; Dimitrijevi’c, M.D.; Stevanovi’c, Z.O.; Serbula, S.M.; Bogdanovic, G.D. Investigation of the possibility of copper recovery from the flotation tailings by acid leaching. J. Hazard. Mater. 2008, 158, 23–34. [Google Scholar] [CrossRef]
- Huang, Z.; Jiang, L.; Wu, P.; Dang, Z.; Zhu, N.; Liu, Z.; Luo, H. Leaching characteristics of heavy metals in tailings and their simultaneous immobilization with triethylenetetramine functioned montmorillonite (TETA-Mt) against simulated acid rain. Environ. Pollut. 2020, 266, 115236. [Google Scholar] [CrossRef]
- Li, X.; Gao, M.; Hiroyoshi, N.; Tabelin, C.B.; Taketsugu, T.; Ito, M. Suppression of pyrite oxidation by ferric-catecholate complexes: An electrochemical study. Miner. Eng. 2019, 138, 226–237. [Google Scholar] [CrossRef]
- Alam, R.; Shang, J.Q. Effect of operating parameters on desulphurization of mine tailings by froth flotation. J. Environ. Manag. 2012, 97, 122–130. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, P.; Zhang, W.; Yuan, X.; Fan, F.; Wang, H.; Liu, J.; Wang, Z. Leaching of heavy metals from Dexing copper mine tailings pond. Trans. Nonferrous Met. Soc. China 2013, 23, 3068–3075. [Google Scholar] [CrossRef]
- Ahmadi, A.; Khezri, M.; Abdollahzadeh, A.A.; Askari, M. Bioleaching of copper, nickel and cobalt from the low grade sulfidic tailing of Golgohar Iron Mine, Iran. Hydrometallurgy 2015, 154, 1–8. [Google Scholar] [CrossRef]
- Figueiredo, J.; Vila, M.C.; Matos, K.; Martins, D.; Futuro, A.; Dinis, M.; Góis, J.; Leite, A.; Fiúz, A. Tailings reprocessing from Cabeço do Pião dam in Central Portugal: A kinetic approach of experimental data. J. Sustain. Min. 2018, 17, 139–144. [Google Scholar] [CrossRef]
- Lü, C.; Wang, Y.; Qian, P.; Liu, Y.; Fu, G.; Ding, J.; Ye, S.; Chen, Y. Separation of chalcopyrite and pyrite from a copper tailing by ammonium humate. Chin. J. Chem. Eng. 2018, 26, 1814–1821. [Google Scholar] [CrossRef]
- Mäkinen, J.; Salo, M.; Khoshkhoo, M.; Sundkvist, J.; Kinnunena, P. Bioleaching of cobalt from sulfide mining tailings; a mini-pilot study. Hydrometallurgy 2020, 196, 105418. [Google Scholar] [CrossRef]
- Falagán, C.; Grail, B.M.; Johnson, D.B. New approaches for extracting and recovering metals from mine tailings. Miner. Eng. 2017, 106, 71–78. [Google Scholar] [CrossRef]
- Kondrat’eva, T.F.; Pivovarova, T.A.; Bulaev, A.G.; Melamud, V.S.; Muravyov, M.I.; Usoltsev, A.V.; Vasil’ev, E.A. Percolation bioleaching of copper and zinc and gold recovery from flotation tailings of the sulfide complex ores of the Ural region, Russia. Hydrometallurgy 2012, 111, 82–86. [Google Scholar] [CrossRef]
- Lutandula, M.S.; Maloba, B. Recovery of cobalt and copper through reprocessing of tailings from flotation of oxidized ores. J. Environ. Chem. Eng. 2013, 1, 1085–1090. [Google Scholar] [CrossRef]
- Urosevic, D.M.; Dimitrijevic, M.D.; Jankovic, Z.D.; Antic, D.V. Recovery of Copper from Copper Slag and Copper Slag Flotation Tailings by Oxidative Leaching. Physicochem. Probl. Miner. Process. 2015, 51, 73–82. [Google Scholar] [CrossRef]
- Muravyov, M.I.; Fomchenko, N.V. Biohydrometallurgical treatment of old flotation tailings of sulfide ores containing non-nonferrous metals and gold. Miner. Eng. 2018, 122, 267–276. [Google Scholar] [CrossRef]
- Turan, M.D.; Orhan, R.; Turan, M.; Nizamoğlu, H. Use of Ammonia Salts in Selective Copper Extraction from Tailings. Min. Metall. Explor. 2020, 37, 1349–1356. [Google Scholar] [CrossRef]
- Stopić, R.S.; Friedrich, B.G. Pressure hydrometallurgy: A new chance to non-polluting processes. Mil. Tech. Cour. 2011, 59, 29–44. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Altansukh, B.; Haga, K.; Stevanović, Z.; Jonović, R.; Avramović, L.; Urosević, D.; Takasaki, Y.; Masuda, N.; Ishiyama, D.; et al. Development of copper recovery process from flotation tailings by a combined method of high-pressure leaching‒solvent extraction. J. Hazard. Mater. 2018, 352, 192–203. [Google Scholar] [CrossRef]
- Han, B.; Altansukh, B.; Haga, K.; Stevanovi´c, Z.; Radojka, J.; Markovic, R.; Avramovic, L.; Obradovic, L.; Takasaki, Y.; Masuda, N.; et al. Copper upgrading and recovery process from mine tailing of Bor region, Serbia using flotation. Soc. Mater. Eng. Resour. Jpn. 2014, 20, 225–229. [Google Scholar] [CrossRef] [Green Version]
- McDonald, R.G.; Muir, D.M. Pressure oxidation leaching of chalcopyrite. Part, I. Comparison of high and low temperature reaction kinetics and products. Hydrometallurgy 2007, 86, 191–205. [Google Scholar] [CrossRef]
- Ministry of the Environment, Government of Japan. Uniform National Effluent Standards (Last Update: 21 October 2015). Available online: https://www.env.go.jp/en/water/wq/nes.html (accessed on 30 March 2021).
- Khouraibchia, Y.; Moats, M. Evaluation of copper electrowinning parameters on current efficiency and energy consumption using surface response methodology. In Proceedings of the 217th ECS Meeting, Vancouver, BC, Canada, 25–30 April 2010. [Google Scholar]
- Owais, A. Effect of electrolyte characteristics on electrowinning of copper powder. J. Appl. Electrochem. 2009, 39, 1587–1595. [Google Scholar] [CrossRef]
- Das, S.C.; Gopala Krishna, P. Effect of Fe (III) during copper electrowinning at higher current density. Int. J. Miner. Process. 1996, 46, 91–105. [Google Scholar] [CrossRef]
- Moats, M. How to evaluate current efficiency in copper electrowinning. In Separation Technologies for Minerals, Coal, and Earth Resources; Young, C.A., Luttrell, G.H., Eds.; Society for Mining, Metallurgy, and Exploration, Inc. (SME): Englewood, CO, USA, 2012; pp. 333–339. [Google Scholar]




| Grade (mass%) | |||||
|---|---|---|---|---|---|
| Elements | Cu | Fe | Al | S | SiO2 |
| Mine tailings | 0.24 | 3.51 | 3.45 | 4.88 | 61.7 |
| Concentrate from mine tailings | 0.65 | 33.20 | 2.63 | 32.72 | 23.41 |
| Cu2+ Concentration (g/L) | Current Efficiency Losses (Loss per g/L Fe (%)) |
|---|---|
| 25 | 3.62 |
| 35 | 2.88 |
| 45 | 1.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godirilwe, L.L.; Haga, K.; Altansukh, B.; Takasaki, Y.; Ishiyama, D.; Trifunovic, V.; Avramovic, L.; Jonovic, R.; Stevanovic, Z.; Shibayama, A. Copper Recovery and Reduction of Environmental Loading from Mine Tailings by High-Pressure Leaching and SX-EW Process. Metals 2021, 11, 1335. https://doi.org/10.3390/met11091335
Godirilwe LL, Haga K, Altansukh B, Takasaki Y, Ishiyama D, Trifunovic V, Avramovic L, Jonovic R, Stevanovic Z, Shibayama A. Copper Recovery and Reduction of Environmental Loading from Mine Tailings by High-Pressure Leaching and SX-EW Process. Metals. 2021; 11(9):1335. https://doi.org/10.3390/met11091335
Chicago/Turabian StyleGodirilwe, Labone L., Kazutoshi Haga, Batnasan Altansukh, Yasushi Takasaki, Daizo Ishiyama, Vanja Trifunovic, Ljiljana Avramovic, Radojka Jonovic, Zoran Stevanovic, and Atsushi Shibayama. 2021. "Copper Recovery and Reduction of Environmental Loading from Mine Tailings by High-Pressure Leaching and SX-EW Process" Metals 11, no. 9: 1335. https://doi.org/10.3390/met11091335






