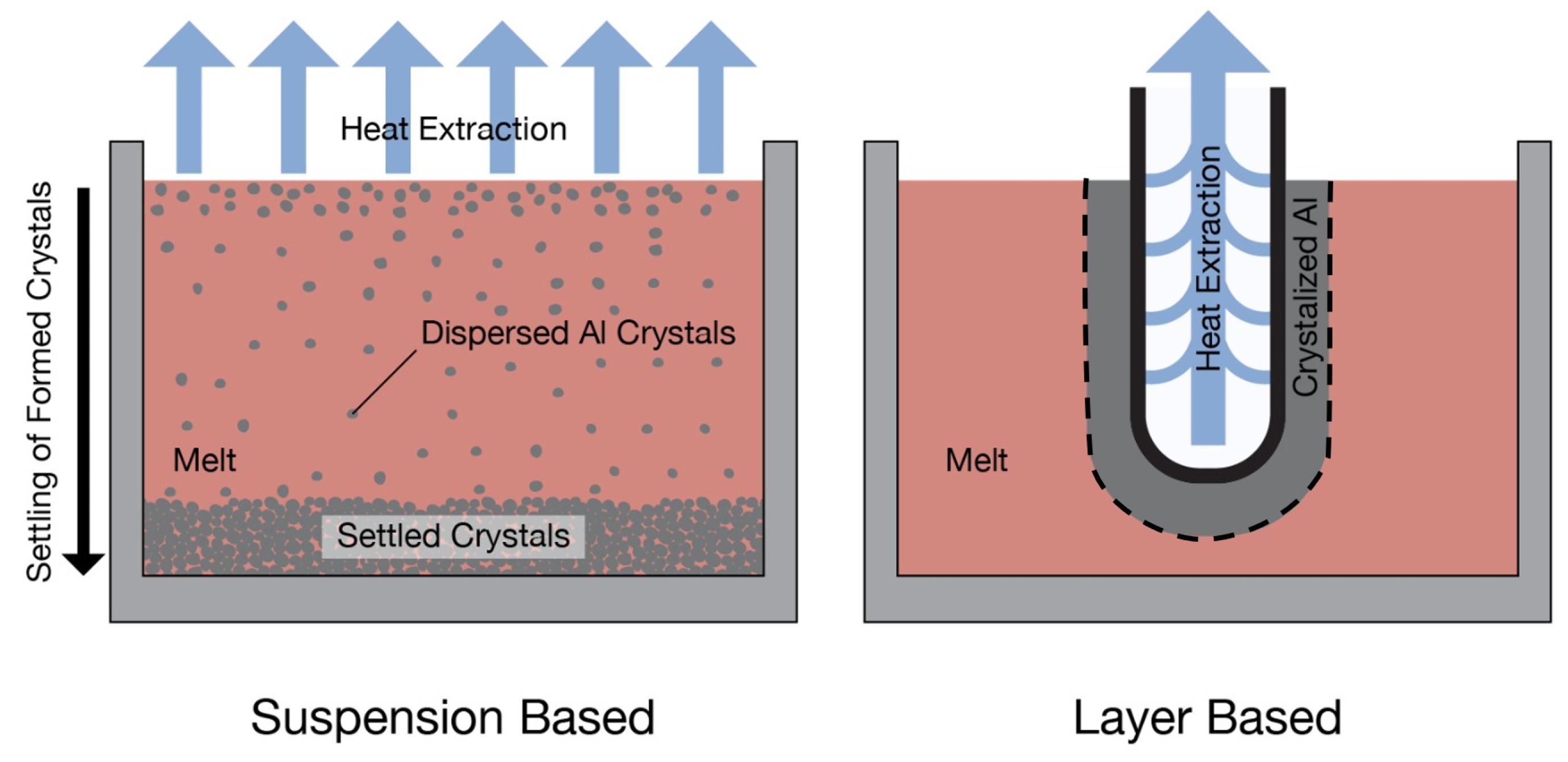
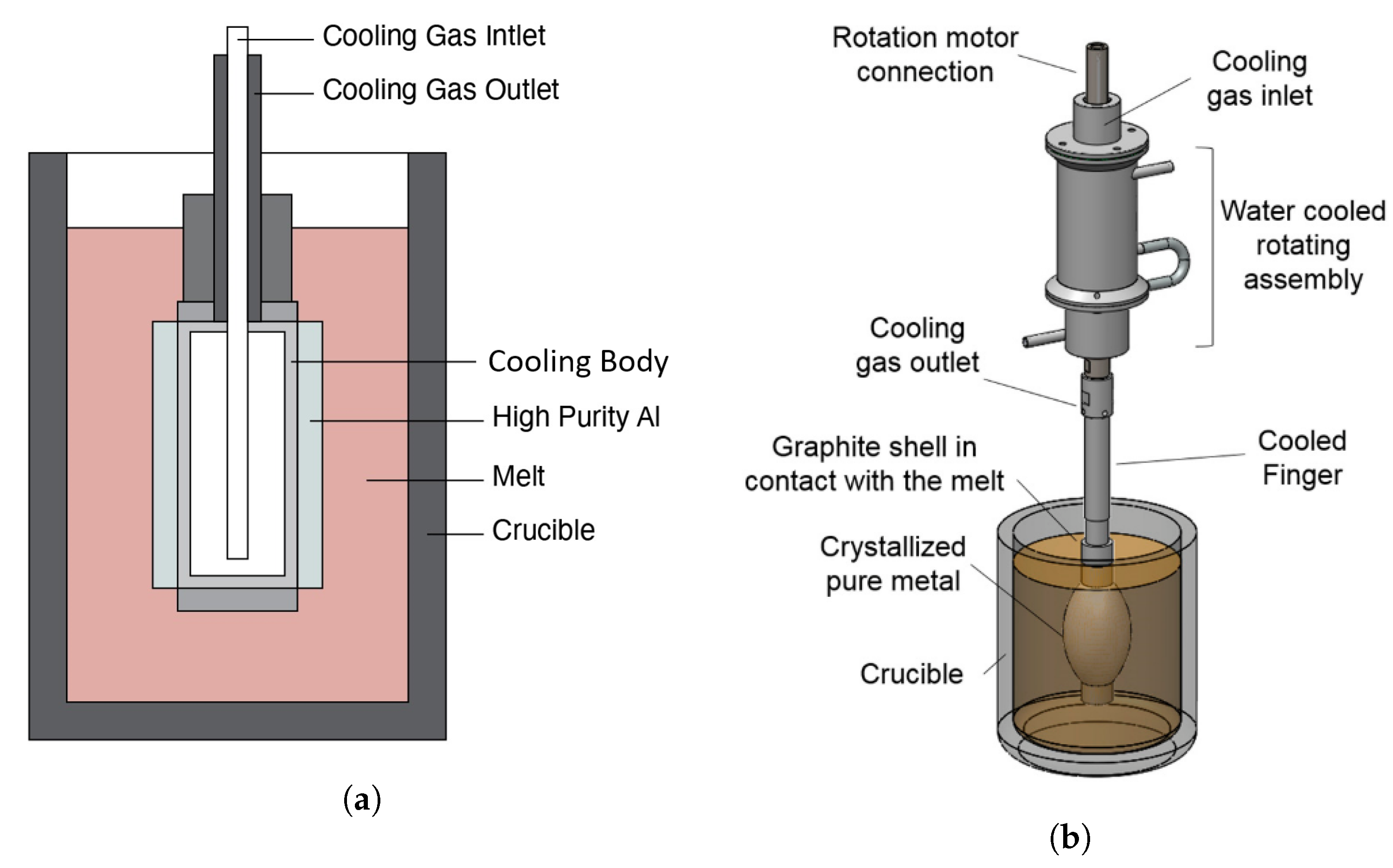
Author Contributions
Conceptualization, D.C.C., N.X. and S.F.; writing—original draft preparation, D.C.C. and N.X.; writing—review and editing, D.C.C., N.X., S.F., B.F.; supervision, S.F.; principal investigator, B.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the CNPQ-Brazilian National Council for Scientific and Technological Development for the financial support of the Brazilian scholarship holder D. Curtolo, and the CSC—Chinese Scholarship Council for the financial support of the Chinese scholarship holder N. Xiong.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Leroy, M. Alpha Rays Emitting Impurities in Ultra Pure Aluminum Evolution Through the Successive Refining Steps. J. Phys. IV 1995, 5, C7-99–C7-110. [Google Scholar] [CrossRef][Green Version]
- Zhao, H.; Lu, H. The Development of 85kA Three-Layer Electrolysis Cell for Refining of Aluminum. In Proceedings of the TMS Aluminum Committee at the TMS 2008 Annual Meeting & Exhibition, New Orleans, LA, USA, 9–13 March 2008; pp. 533–540. [Google Scholar]
- Lindsay, S.J. Very high-purity aluminum: An historical perspective. JOM 2014, 66, 217–222. [Google Scholar] [CrossRef]
- Wang, Z. The properties of high pure aluminum (Part A). Light Met. 2004, 8, 3–6. (In Chinese) [Google Scholar] [CrossRef]
- Head Electronic Co Limited. Guideline for Wire Bonding. Available online: http://www.headpcb.com/html/2018/news&blog_0612/157.html (accessed on 28 June 2021).
- Sarkar, J.; Saimoto, S.; Mathew, B.; Gilman, P.S. Microstructure, texture and tensile properties of aluminum-2 at.% neodymium alloy as used in flat panel displays. J. Alloy. Compd. 2009, 479, 719–725. [Google Scholar] [CrossRef]
- Onishi, T.; Iwamura, E.; Takagi, K.; Yoshikawa, K. Influence of adding transition metal elements to an aluminum target on electrical resistivity and hillock resistance in sputter-deposited aluminum alloy thin films. J. Vac. Sci. Technol. A 1996, 14, 2728–2735. [Google Scholar] [CrossRef]
- Wang, Z. Talk about the purity aluminum (II). Met. World 2004, 4, 36–37. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, J.; Sun, B.; He, B.; Mao, H.; Chen, G.; Ge, A. Principle and control of new-style purification equipment of 5N high purity aluminum. Chin. J. Mech. Eng. 2006, 42, 64–68. (in Chinese). [Google Scholar] [CrossRef]
- Sankaran, V.A.; Rees, F.L.; Avant, C.S. Electrolytic capacitor life testing and prediction. In Proceedings of the 1997 IEEE Industry Applications Conference Thirty-Second IAS Annual Meeting, New Orleans, LA, USA, 5–9 October 1997; Volume 2, pp. 1058–1065. [Google Scholar] [CrossRef]
- Chen, Y.; Santos, A.; Ho, D.; Wang, Y.; Kumeria, T.; Li, J.; Wang, C.; Losic, D. On The Generation of Interferometric Colors in High Purity and Technical Grade Aluminum: An Alternative Green Process for Metal Finishing Industry. Electrochim. Acta 2015, 174, 672–681. [Google Scholar] [CrossRef]
- Wang, Z. Production, market and application of high-purity aluminum in the world. Nonferr. Met. Process. 2004, 33, 1–6. [Google Scholar] [CrossRef]
- Miele, P.; ten Kate, H.H. The superconducting magnet system for the ATLAS detector at CERN. Fusion Eng. Des. 2001, 58–59, 195–203. [Google Scholar] [CrossRef]
- Yamagiwa, M.; Yu, Q.; Fujita, M.; Shinohara, M.; Murakami, Y. Reliability study of high-temperature-resistant mounting structure using high-purity aluminum for power devices. J. Jpn. Inst. Electron. Packag. 2009, 12, 238–247. [Google Scholar] [CrossRef][Green Version]
- Dawless, R.K.; Troup, R.L.; Meier, D.L.; Rohatgi, A. Production of extreme-purity aluminum and silicon by fractional crystallization processing. J. Cryst. Growth 1988, 89, 68–74. [Google Scholar] [CrossRef]
- Al-Medainy, H.S.; Al-Mohawes, N.A.; Chou, H.P. Determination of uranium and thorium concentrations in integrated circuit packaging materials. J. Radioanal. Nucl. Chem. Lett. 1989, 137, 341–350. [Google Scholar] [CrossRef]
- Mahata, A.; Asle Zaeem, M.; Baskes, M.I. Understanding homogeneous nucleation in solidification of aluminum by molecular dynamics simulations. Model. Simul. Mater. Sci. Eng. 2018, 26, 025007. [Google Scholar] [CrossRef]
- The London Metal Exchange. London Metal Exchange Daily Price for Primary Aluminum. Available online: https://www.lme.com/en-GB/Metals/Non-ferrous/Aluminium#tabIndex=0 (accessed on 28 June 2021).
- Laurand Associates Inc. Price of High- and Ultrahigh-Purity Aluminum. Available online: https://highpurityaluminum.com/products/pellets-slugs-shot?variant=19365073256559 (accessed on 28 June 2021).
- CAO, P. Comparison and analysis of the preparation methods of high purity aluminum. World Nonferr. Met. 2018, 11, 10–11. [Google Scholar] [CrossRef]
- Gaustad, G.; Olivetti, E.; Kirchain, R. Improving aluminum recycling: A survey of sorting and impurity removal technologies. Resour. Conserv. Recycl. 2012, 58, 79–87. [Google Scholar] [CrossRef]
- Curtolo, D.C.; Rodriguez-Rojas, M.J.; Friedrich, S.; Friedrich, B. Alternative fractional crystallization-based methods to produce high-purity aluminum. J. Mater. Res. Technol. 2021, 12, 796–806. [Google Scholar] [CrossRef]
- Hoopes, W. Process of the purification of aluminium. arXiv 1901, arXiv:1011.1669v3. [Google Scholar]
- Wang, Z. Talk about the purity aluminium (III). Met. World 2004, 5, 33–37. [Google Scholar] [CrossRef]
- Kondo, M.; Maeda, H.; Mizuguchi, M. The production of high-purity aluminum in Japan. JOM 1990, 42, 36–37. [Google Scholar] [CrossRef]
- Kong, X.; Yang, B.; Xiong, H.; Liu, D.; Xu, B. Removal of impurities from crude lead with high impurities by vacuum distillation and its analysis. Vacuum 2014, 105, 17–20. [Google Scholar] [CrossRef]
- Tao, D.P. A new model of thermodynamics of liquid mixtures and its application to liquid alloys. Thermochim. Acta 2000, 363, 105–113. [Google Scholar] [CrossRef]
- Tao, D.P. Prediction of activities of all components in the lead-free solder systems Bi-In-Sn and Bi-In-Sn-Zn. J. Alloy. Compd. 2008, 457, 124–130. [Google Scholar] [CrossRef]
- Zhang, M.; Kamavaram, V.; Reddy, R.G. New Electrolytes for Aluminum Production: Ionic Liquids. JOM 2003, 55, 54–57. [Google Scholar] [CrossRef]
- Suneesh, P.V.; Satheesh Babu, T.G.; Ramachandran, T. Electrodeposition of aluminium and aluminium-copper alloys from a room temperature ionic liquid electrolyte containing aluminium chloride and triethylamine hydrochloride. Int. J. Miner. Metall. Mater. 2013, 20, 909–916. [Google Scholar] [CrossRef]
- Gamrath, H.R.; Company, M.C. Process for the Electrolytic Deposition of Aluminum. U.S. Patent 2,849,349, 26 August 1958. [Google Scholar]
- Zheng, Y.; Peng, C.; Zheng, Y.; Tian, D.; Zuo, Y. Low-temperature electrolysis of aluminium from 1-butyl-3-methylimidazolium chloroaluminate ionic liquids with inert anode. Int. J. Electrochem. Sci. 2016, 11, 6095–6109. [Google Scholar] [CrossRef]
- Bakkar, A.; Neubert, V. A new method for practical electrodeposition of aluminium from ionic liquids. Electrochem. Commun. 2015, 51, 113–116. [Google Scholar] [CrossRef]
- Zhao, Y.; VanderNoot, T.J. Review: Electrodeposition of aluminium from nonaqueous organic electrolytic systems and room temperature molten salts. Electrochim. Acta 1997, 42, 3–13. [Google Scholar] [CrossRef]
- Böttcher, R.; Ispas, A.; Bund, A. Corrosion Behavior of Aluminum Alloys Deposited from Ionic Liquids. ECS Meet. Abstr. 2018, MA2018-02, 634. [Google Scholar]
- Hannibal, W.; Ibe, G.; Kurre, K.; Peychal-Heiling, H.; Pfundt, H.; Reuter, W.; Scharf, G.; Winkhaus, G. Entwickling eines technischen Verfahrens zur Herstellung von Reinstaluminum für die Kryoelektrotechnik, Speziell für Kryomagnete—Forschungsbericht; Technical Report; Leichtmetall-Forschungsinstitut der Vereingte Aluminum-Werke AG: Bonn, Germany, 1974. [Google Scholar]
- Kim, S.; Reddy, R. Recents advances in electrodeposition technology. J. Korean Inst. Surf. Eng. 2001, 34, 553–567. [Google Scholar]
- Wu, B.; Reddy, R.G.; Rogers, R.D. Aluminum Reduction via Near Room Temperature Electrolysis in Ionic Liquids BT. In Essential Readings in Light Metals: Volume 2—Aluminum Reduction Technology; Springer: Cham, Switzerland, 2016; pp. 1100–1106. [Google Scholar] [CrossRef]
- Kurz, W.; Fisher, D.J. Fundamentals of Solidification, 3rd ed.; Trans Tech Publ.: Aedermannsdorf, Switzerland, 1989; pp. 53–55. [Google Scholar]
- Weiser, K. Theoretical calculation of distribution coefficients of impurities in germanium and silicon, heats of solid solution. J. Phys. Chem. Solids 1958, 7, 118–126. [Google Scholar] [CrossRef]
- Curtolo, D.; Friedrich, S.; Bellin, D.; Nayak, G.; Friedrich, B. Definition of a First Process Window for Purification of Aluminum via “Cooled Finger” Crystallization Technique. Metals 2017, 7, 341. [Google Scholar] [CrossRef]
- Burton, J.A.; Prim, R.C.; Slichter, W.P. The distribution of solute in crystals grown from the melt. Part I. Theoretical. J. Chem. Phys. 1953, 21, 1987–1991. [Google Scholar] [CrossRef]
- Drini, B.; Katgerman, L.; Boom, R. Metal refining with fractional crystallization: State-of-the-art and future prospects. In Proceedings of the ECI Conference on Metal Separation Technologies III; Aune, R.E., Kekkonen, M., Eds.; Helsinki University of Technology, Laboratory of Metallurgy: Espoo, Finland, 2004; pp. 34–41. [Google Scholar]
- Dawless, R.K.; Graziano, R.E. Fractional Crystallization Process. U.S. Patent 4,294,612, 13 October 1994. [Google Scholar]
- Jacobs, S.C.; Burrell, L. Purification of Aluminum. U.S. Patent 3,303,019, 7 February 1967. [Google Scholar]
- Dawless, R.K.; Jacobs, S.C. Production of Extreme Purity Aluminum. U.S. Patent 4,273,627, 16 June 1981. [Google Scholar]
- Kahveci, A.I.; Unal, A. Refining of a 5XXX series aluminumalloy scrap by alcoa fractional crystallization process. In Proceedings of the Fourth International Symposium on Recycling of Metals and Engineered Materials; Stewart, D.J., Daley, J., Stephens, R., Eds.; The Minerals, Metals & Materials Society: Pittsburgh, PA, USA, 2000; pp. 979–991. [Google Scholar]
- De Vries, Paul, A.; Wouters, H.A. Method for Fractional Crystallization of a Molten Metal. Switzerland Patent 2004/005558A1, 30 December 2004.
- Yang, G.; Govani, J.; Mei, H.; Guan, Y.; Wang, G.; Huang, M.; Mei, D. Investigation of influential factors on the purification of zone-refined germanium ingot. Cryst. Res. Technol. 2014, 49, 269–275. [Google Scholar] [CrossRef]
- Spim, J.; Bernadou, M.; Garcia, A. Numerical modeling and optimization of zone refining. J. Alloy. Compd. 2000, 298, 299–305. [Google Scholar] [CrossRef]
- Ghosh, K.; Mani, V.N.; Dhar, S. Numerical study and experimental investigation of zone refining in ultra-high purification of gallium and its use in the growth of GaAs epitaxial layers. J. Cryst. Growth 2009, 311, 1521–1528. [Google Scholar] [CrossRef]
- Ho, C.D.; Yeh, H.M.; Yeh, T.L. Optimal zone lengths in multi-pass zone-refining processes. Sep. Technol. 1996, 6, 227–233. [Google Scholar] [CrossRef]
- Ho, C.D.; Yeh, H.M.; Yeh, T.L. Simulation of multipass zone-refining processes. Int. J. Model. Simul. 2003, 23, 85–93. [Google Scholar] [CrossRef]
- Yeh, H.M.; Yeh, W.H. The Improvement of Separation in Zone Refining Processes with Zone Length Varied along the Ingot. Sep. Sci. Technol. 1979, 14, 795–803. [Google Scholar] [CrossRef]
- Pfann, W.G. Zone Melting; Wiley Series on the Science and Technology of Materials; John Wiley & Sons: New York, NY, USA; Chapman & Hall: London, UK, 1958. [Google Scholar]
- Rodway, G.; Hunt, J. Optimizing zone refining. J. Cryst. Growth 1989, 97, 680–688. [Google Scholar] [CrossRef]
- Prasad, D.S.; Munirathnam, N.R.; Rao, J.V.; Prakash, T.L. Effect of multi-pass, zone length and translation rate on impurity segregation during zone refining of tellurium. Mater. Lett. 2006, 60, 1875–1879. [Google Scholar] [CrossRef]
- Kino, T.; Kamigaki, N.; Yamasaki, H.; Kawai, J.; Deguchi, Y.; Nakamichi, I. Zone refining of aluminum. Trans. Jpn. Inst. Met. 1976, 17, 645–648. [Google Scholar] [CrossRef]
- Lee, H.Y.; Oh, J.K.; Lee, D.H. Purification of tin by zone refining with development of a new model. Metall. Trans. B 1990, 21, 455–461. [Google Scholar] [CrossRef]
- Zhang, X.; Friedrich, S.; Friedrich, B. Production of High Purity Metals: A Review on Zone Refining Process. J. Cryst. Process. Technol. 2018, 8, 33–55. [Google Scholar] [CrossRef]
- Tiller, W.; Jackson, K.; Rutter, J.; Chalmers, B. The redistribution of solute atoms during the solidification of metals. Acta Metall. 1953, 1, 428–437. [Google Scholar] [CrossRef]
- Zaiour, A.; Hamdoun, B.; Charara, J.; Roumi, M.; Zahraman, K.; Hage-Ali, M. A New Theoretical Formulation of Temperature Effect on Impurities Diffusion Coefficients in Molten Tellurium. Phys. Scr. 2005, 71, 414–418. [Google Scholar] [CrossRef]
- Munirathnam, N.R.; Prasad, D.S.; Sudheer, C.H.; Rao, J.V.; Prakash, T.L. Zone refining of cadmium and related characterization. Bull. Mater. Sci. 2005, 28, 209–212. [Google Scholar] [CrossRef]
- Liu, Y.C.; Moss, R.; Dost, S. A computational thermal analysis for the zone-refining processes of Cd and Te. J. Cryst. Growth 2006, 293, 146–156. [Google Scholar] [CrossRef]
- Kobayashi, N. Power required to form a floating zone and the zone shape. J. Cryst. Growth 1978, 43, 417–424. [Google Scholar] [CrossRef]
- Dost, S.; Liu, Y.C.; Haas, J.; Roszmann, J.; Grenier, S.; Audet, N. Effect of applied electric current on impurity transport in zone refining. J. Cryst. Growth 2007, 307, 211–218. [Google Scholar] [CrossRef]
- Hashimoto, E.; Ueda, Y. Zone refining of high-purity aluminum. Mater. Trans. JIM 1994, 35, 262–265. [Google Scholar] [CrossRef]
- Mikubo, S. The Latest Refining Technologies of Segregation Process to Produce high-purity aluminum. In Proceedings of the 12th International Conference on Aluminium Alloys, Yokohama, Japan, 5–9 September 2010; pp. 224–228. [Google Scholar]
- Ferber, M.E.; Winterberger, M.G. Process for Purification of Metals. U.S. Patent 3,671,229, 20 June 1972. [Google Scholar]
- Winterberger, M.G. Precédé de Purification de Métaux Per Ségrégation. France Patent 0091386A1, 12 October 1983. [Google Scholar]
- Shingu, H.; Arai, K.; Sakaguchi, M.; Nishide, T.; Watanabe, O.; Otsuka, R.; Tsukamoto, K. Process for Producing High-Purity Aluminum. U.S. Patent 4,469,512, 4 September 1984. [Google Scholar]
- Friedrich, S.; Coladetti Curtolo, D.; Friedrich, B. Effect of Process Parameter Variation on Purity during Rotary Fractional Crystallization of Aluminum. Open J. Met. 2017, 7, 25–38. [Google Scholar] [CrossRef]
- Hong, T.; Nurgul, I. Method for Purifying High-Purity Aluminum by Directional Solidification and Smelting Furnace Therefor. U.S. Patent 2014/0202653A1, 24 July 2014. [Google Scholar]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).