Determination of Phase Equilibria among δ-Fe, γ-Fe and Fe2M Phases in Fe-Cr-M (M: Hf, Ta) Ternary Systems
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. Microstructures of Heat-Treated/Quenched Samples and Phase Identification
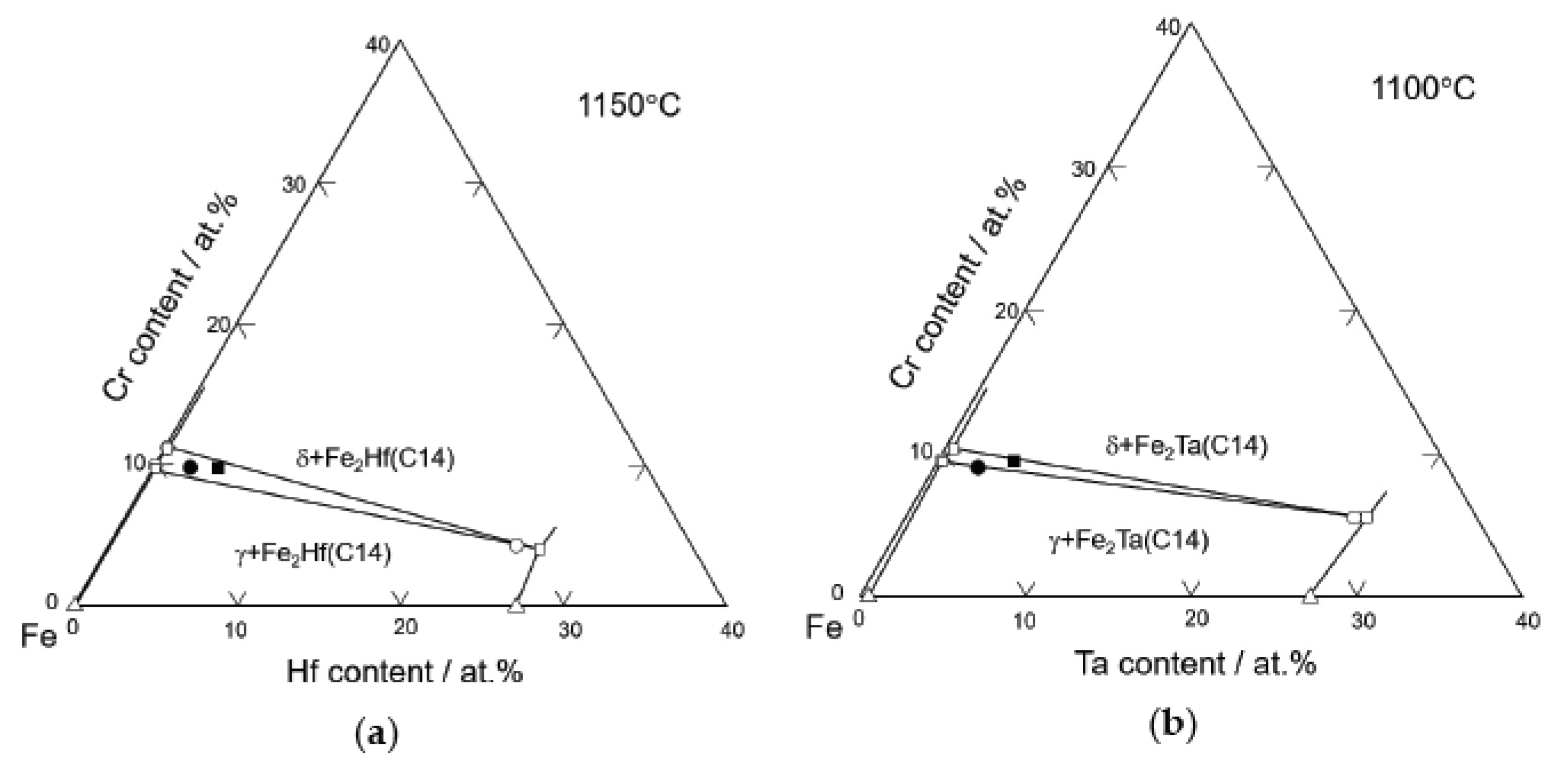
3.2. Chemical Analysis and Isothermal Sections
3.3. Calculation of Vertical Sections of Phase Diagram and Confirmation by Experiments
3.4. Formation of Fe2M Phase along the Pseudo-Eutectoid Reaction Path
4. Conclusions
- A pseudo-eutectoid trough (δ → γ + Fe2M) exists at ~1220 °C at a Hf content of 0.1% and at ~1130 °C at a Ta content of 0.6% on the vertical sections at a Cr content of 9.5% in each ternary system, respectively;
- Thermodynamic calculation with a database based on reported binary phase diagrams and the present study indicates that reducing the Cr content in the ternary alloy systems increases the temperature and the Hf/Ta contents of the pseudo-eutectoid troughs;
- The determined phase equilibria suggest that the supersaturation of Hf/Ta for the formation of γ phase is higher in the Hf doped system than in the Ta doped system at a Cr content of 9.5%, which is probably an origin of a much slower kinetics of precipitation on the eutectoid path in the latter alloy system.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Key World Energy Statistics 2020 IEA. Available online: https://www.iea.org/reports/key-world-energy-statistics-2020 (accessed on 10 January 2021).
- Status of Power System Transformation 2018. Advanced Power Plant Flexibility-OECD/IEA. Available online: https://www.iea.org/reports/status-of-power-system-transformation-2018 (accessed on 23 October 2020).
- Masuyama, F. History of Power Plants and Progress in Heat Resistant Steels. ISIJ Int. 2001, 41, 612–625. [Google Scholar] [CrossRef]
- Sawada, K.; Kushima, H.; Tabuchi, M.; Kimura, K. Microstructural degradation of Gr.91 steel during creep under low stress. Mat. Sci. Eng. 2011, A528, 5511–5518. [Google Scholar] [CrossRef]
- Okamoto, H. Phase Diagrams of Binary Iron Alloys, Monograph Series on Alloy Phase Diagrams; ASM International: Novelty, OH, USA, 1993; Volume 9. [Google Scholar]
- Murata, Y.; Koyama, T.; Morinaga, M.; Miyazaki, T. Prediction of the Laves Phase Morphology in Fe–Cr–W–C Quaternary Steels with the Aid of System Free Energy Concept. ISIJ Int. 2002, 42, 1423–1429. [Google Scholar] [CrossRef]
- Abe, F. Effect of fine precipitation and subsequent coarsening of Fe2W Laves phase on the creep deformation behavior of tempered martensitic 9Cr-W steels. Metall. Mater. Trans. 2005, 36A, 321–332. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kimura, K.; Tsuzaki, K. Interphase precipitation of Fe2Hf Laves phase in a Fe-9Cr/Fe-9Cr-Hf diffusion couple. Intermetallics 2014, 46, 80–84. [Google Scholar] [CrossRef]
- Kobayashi, S.; Hibaru, T. Formation of the Fe2Hf Laves phase along the eutectoid-type reaction path of δ-Fe→γ-Fe+Fe2Hf in an Fe-9Cr based alloy. ISIJ Int. 2015, 55, 293–299. [Google Scholar] [CrossRef]
- Yuan, Z.; Kobayashi, S.; Takeyama, M. Microstructure control using the formation fo Laves phase through interphase precipitation in ferritic heat resistant steels. In Proceedings of the Joint EPRI-123HiMAT Internatioinal Conference on Advanced High-Temperature Materials, Nagasaki, Japan, 19–25 October 2019; ASM International: Nagasaki, Japan, 2019; pp. 90–95. [Google Scholar]
- Kobayashi, S.; Hara, T. Effect of different precipitation routes of Fe2Hf Laves phase on the creep rate of 9Cr-based ferritic alloys. Appl. Sci. 2021, 11, 2327. [Google Scholar] [CrossRef]
- Porter, D.A.; Eastering, K.E. Phase Transformations in Metals and Alloys, 2nd ed.; Stanley Thornes Publishers Ltd.: Gloucestershire, UK, 1992. [Google Scholar]
- Edmonds, D.V. Occurrence of fibrous vanadium carbide during transformation of an Fe-V-C steel. J. Iron Steel Inst. 1972, 210, 363–365. [Google Scholar]
- Grice, R.J.; Faulkner, R.G.; Yin, Y. Novel hafnium-containing steels for power generation. Ironmak. Steelmak. 2009, 36, 170–175. [Google Scholar] [CrossRef]
- Asakura, K.; Yamashita, Y.; Yamada, T.; Shibata, K. Effects of Ta and Nb on microstructures and mechanical properties of low activation ferritic 9Cr-2W-0.2V steel for fusion reactor. ISIJ Int. 1990, 30, 937–946. [Google Scholar] [CrossRef] [Green Version]
- Harikumar, K.C.; Raghavan, V. Bcc—Fcc equilibrium in ternary iron alloys—II. J. Alloy. Phase Diagr. 1989, 5, 77–96. [Google Scholar]
- Cullity, B.D.; Stock, S.R. Elements of X-Ray Diffraction, 3rd ed.; Prentice-Hall, Inc.: Upper Saddle River, NJ, USA, 2001. [Google Scholar]






| Designation | Nominal Composition/at.% | Heat Treatment Conditions (Temp./Time) | |||
|---|---|---|---|---|---|
| Fe | Cr | Hf | Ta | ||
| 2 Hf | Bal. | 9.8 | 2.0 | - | 1150 °C/48 h |
| 4 Hf | Bal. | 9.6 | 4.0 | - | 1150 °C/20 h, 48 h |
| 2.5 Ta | Bal. | 9.2 | - | 2.5 | 1100 °C/48 h, 235 h |
| 5 Ta | Bal. | 9.6 | - | 5.0 | 1100 °C/48 h, 235 h |
| Designation | Heat Treatment | Phase Present | Lattice Parameter (Å) | |
|---|---|---|---|---|
| a | c | |||
| 4 Hf | 1150 °C/48 h | α/δ-Fe | 2.873 (5) | - |
| C14-Fe2Hf | 4.921 (0) | 8.017 (7) | ||
| 5 Ta | 1100 °C/235 h | α/δ-Fe | 2.874 (8) | - |
| C14-Fe2Ta | 4.808 (1) | 7.852 (3) | ||
| Designation | Heat Treatment | Phase Present | Chemical Composition (at.%) | ||
|---|---|---|---|---|---|
| Fe | Cr | Hf | |||
| 2 Hf | δ | Bal. | 11.1 | 0.1 | |
| 1150 °C/48 h | γ | Bal. | 9.9 | -* | |
| Fe2Hf | Bal. | 4.3 | 24.9 | ||
| 4 Hf | δ | Bal. | 11.1 | 0.1 | |
| 1150 °C/20 h | γ | Bal. | 9.8 | -* | |
| Fe2Hf | Bal. | 4.0 | 24.5 | ||
| δ | Bal. | 11.1 | 0.1 | ||
| 1150 °C/48 h | γ | Bal. | 9.8 | -* | |
| Fe2Hf | Bal. | 4.0 | 26.6 | ||
| Designation | Heat Treatment | Phase Present | Chemical Composition (at.%) | ||
|---|---|---|---|---|---|
| Fe | Cr | Ta | |||
| 2.5 Ta | 1100 °C/48 h | γ | Bal. | 9.4 | 0.3 |
| Fe2Ta | Bal. | 5.5 | 27.0 | ||
| 1100 °C/235 h | γ | Bal. | 9.3 | 0.3 | |
| Fe2Ta | Bal. | 5.6 | 26.5 | ||
| 5 Ta | 1100 °C/48 h | δ | Bal. | 10.1 | 0.6 |
| Fe2Ta | Bal. | 5.6 | 28.2 | ||
| δ | Bal. | 10.2 | 0.5 | ||
| 1100 °C/235 h | γ | Bal. | 9.3 | 0.3 | |
| Fe2Ta | Bal. | 5.6 | 27.7 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Z.; Kobayashi, S. Determination of Phase Equilibria among δ-Fe, γ-Fe and Fe2M Phases in Fe-Cr-M (M: Hf, Ta) Ternary Systems. Metals 2022, 12, 102. https://doi.org/10.3390/met12010102
Yuan Z, Kobayashi S. Determination of Phase Equilibria among δ-Fe, γ-Fe and Fe2M Phases in Fe-Cr-M (M: Hf, Ta) Ternary Systems. Metals. 2022; 12(1):102. https://doi.org/10.3390/met12010102
Chicago/Turabian StyleYuan, Zhetao, and Satoru Kobayashi. 2022. "Determination of Phase Equilibria among δ-Fe, γ-Fe and Fe2M Phases in Fe-Cr-M (M: Hf, Ta) Ternary Systems" Metals 12, no. 1: 102. https://doi.org/10.3390/met12010102
APA StyleYuan, Z., & Kobayashi, S. (2022). Determination of Phase Equilibria among δ-Fe, γ-Fe and Fe2M Phases in Fe-Cr-M (M: Hf, Ta) Ternary Systems. Metals, 12(1), 102. https://doi.org/10.3390/met12010102







