Effects of Vacancy Defects on Electrical and Optical Properties of ZnO/WSe2 Heterostructure: First-Principles Study
Abstract
1. Introduction
2. Computational Methods
3. Results and Discussion
3.1. Structure and Stability
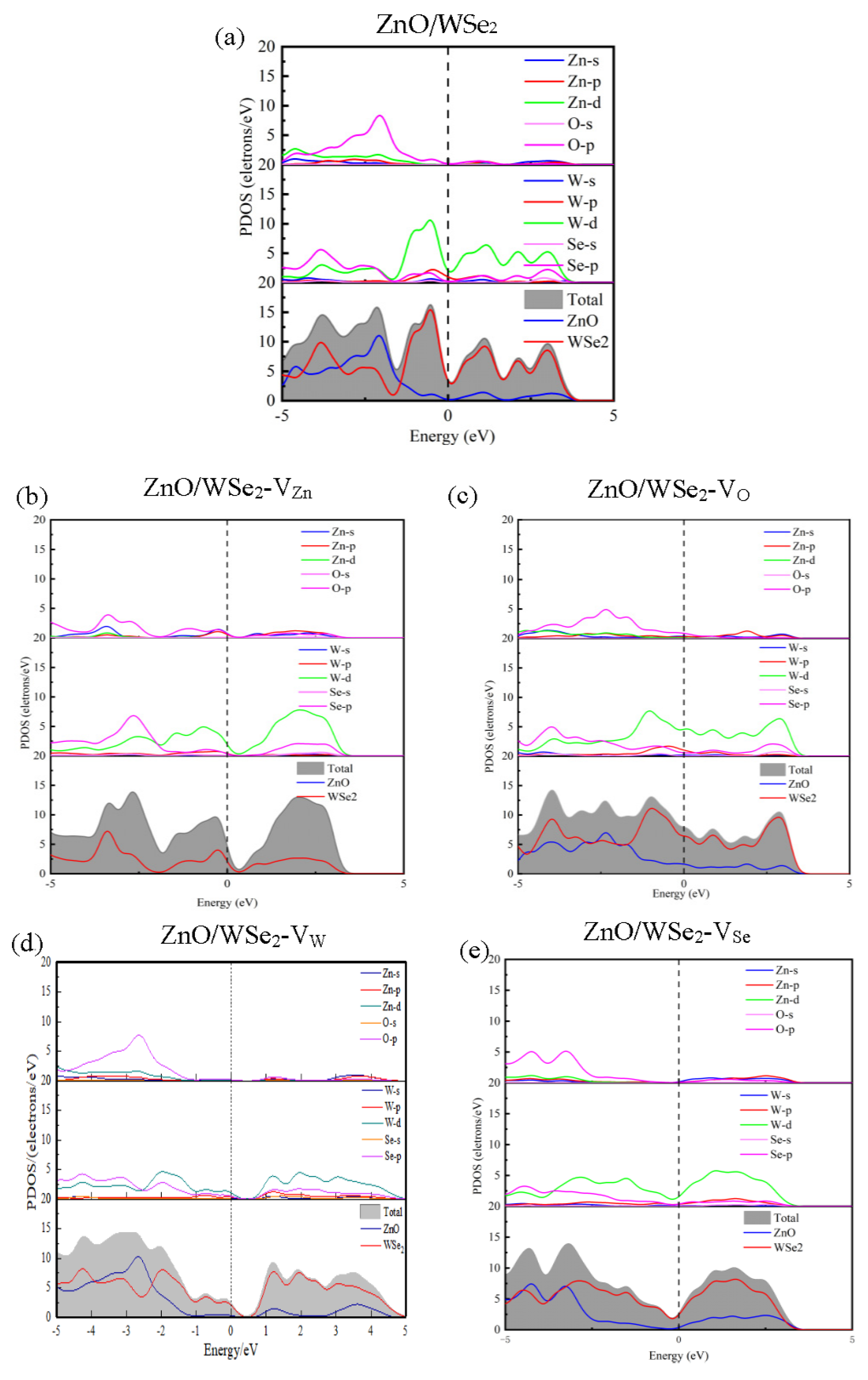
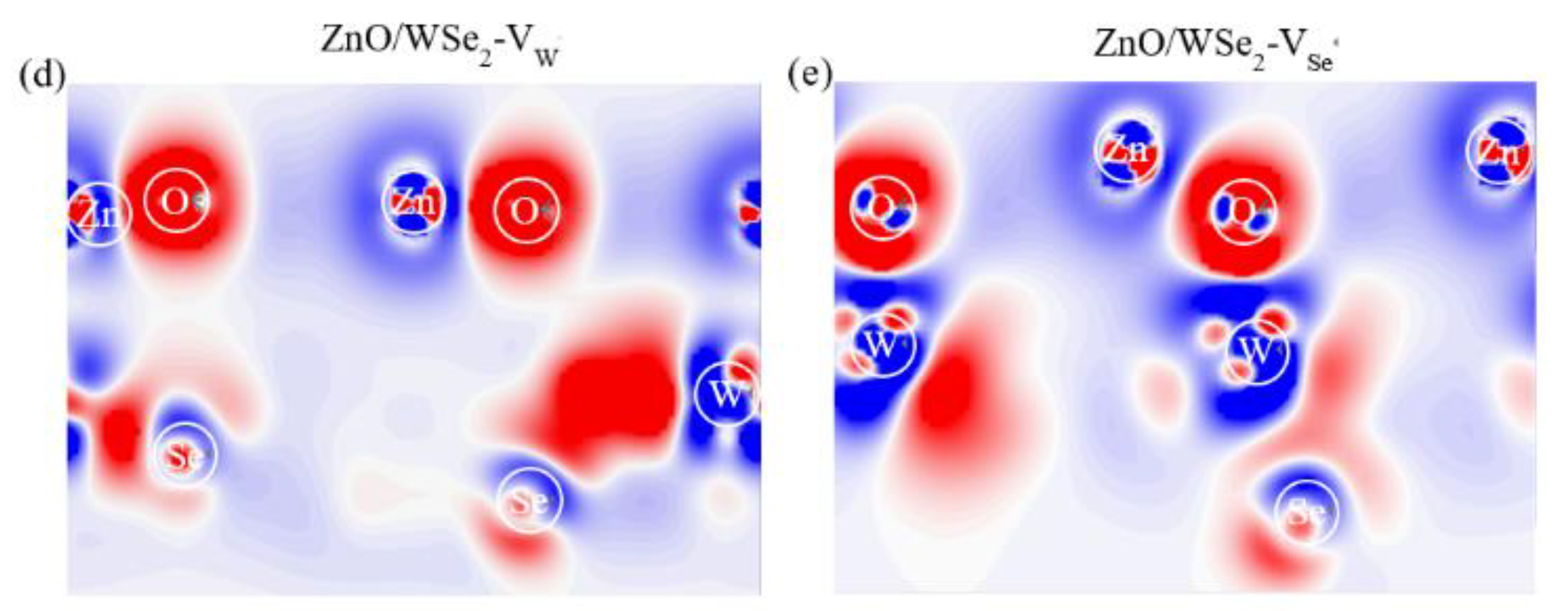
3.2. Electronic Properties
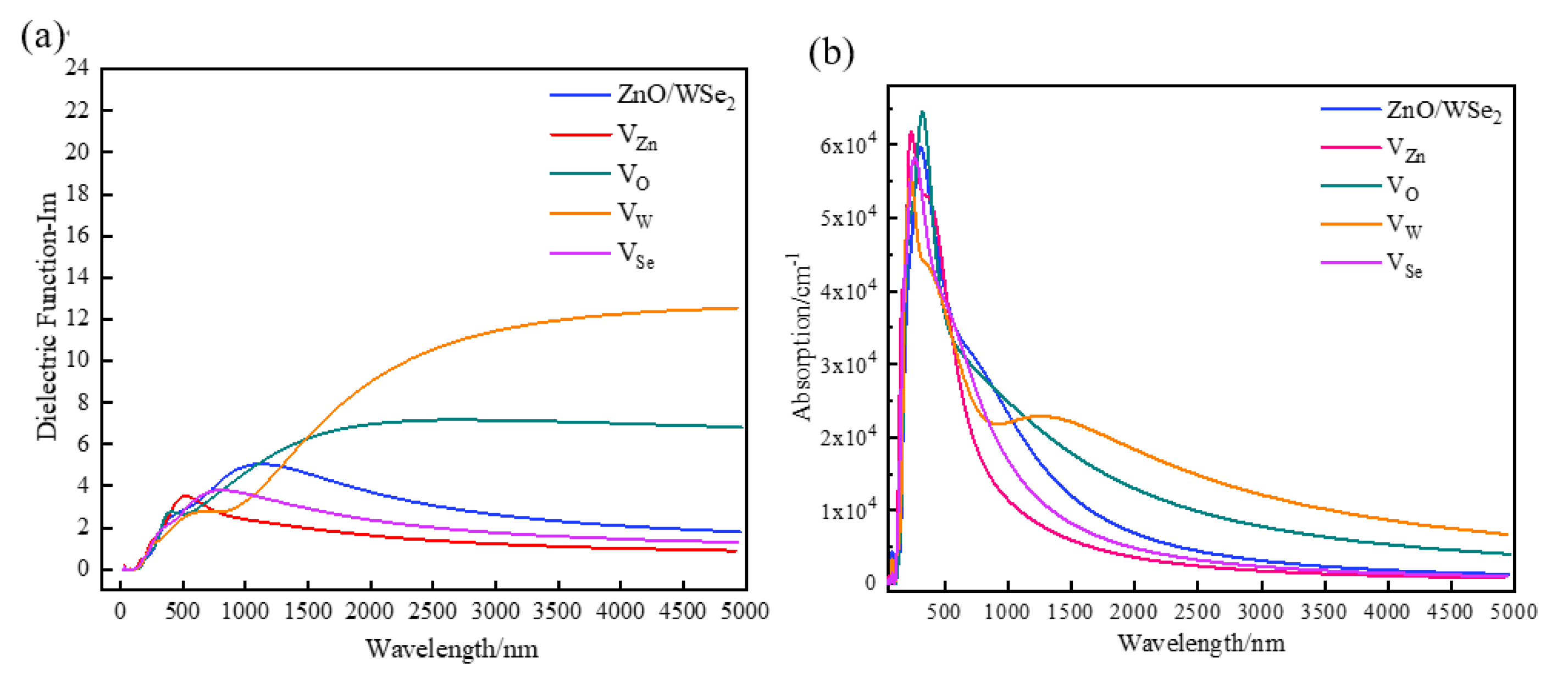
3.3. Optical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Obeid, M.M.; Bafekry, A.; Rehman, S.U.; Nguyen, C.V. A type-II GaSe/HfS2 van der Waals heterostructure as promising photocatalyst with high carrier mobility. Appl. Surf. Sci. 2020, 534, 147607. [Google Scholar] [CrossRef]
- Wang, B.-J.; Li, X.-H.; Cai, X.-L.; Yu, W.-Y.; Zhang, L.-W.; Zhao, R.-Q.; Ke, S.-H. Blue phosphorus/Mg (OH)2 van der Waals heterostructures as promising visible-light photocatalysts for water splitting. J. Phys. Chem. C 2018, 122, 7075–7080. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Vu, T.V.; Binh, N.T.; Hoat, D.; Hieu, N.V.; Nguyen, C.V.; Phuc, H.V.; Jappor, H.R.; Obeid, M. Strain-tunable electronic and optical properties of monolayer GeSe: Promising for photocatalytic water splitting applications. Chem. Phys. 2020, 529, 110543. [Google Scholar] [CrossRef]
- Neto, A.H.C.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Ponce, F.A.; Bour, D.P. Nitride-based semiconductors for blue and green light-emitting devices. Nature 1997, 386, 351–359. [Google Scholar] [CrossRef]
- Green, M.A.; Bremner, S.P. Energy conversion approaches and materials for high-efficiency photovoltaics. Nat. Mater. 2017, 16, 23–34. [Google Scholar] [CrossRef]
- Polman, A.; Knight, M.; Garnett, E.C.; Ehrler, B.; Sinke, W.C. Photovoltaic materials: Present efficiencies and future challenges. Science 2016, 352, aad4424. [Google Scholar] [CrossRef]
- Green, M.A.; Hishikawa, Y.; Warta, W.; Dunlop, E.D.; Levi, D.H.; Hohl-Ebinger, J.; Ho-Baillie, A.W.H. Solar cell efficiency tables (version 50). Prog. Photovolt. Res. Appl. 2017, 25, 668–676. [Google Scholar] [CrossRef]
- Ohno, H.; Munekata, H.; Penney, T.; von Molnár, S.; Chang, L.L. Magnetotransport properties of p-type (In, Mn) As diluted magnetic III-V semiconductors. Phys. Rev. Lett. 1992, 68, 2664. [Google Scholar] [CrossRef]
- Dietl, T.; Ohno, H. Dilute ferromagnetic semiconductors: Physics and spintronic structures. Rev. Mod. Phys. 2014, 86, 187. [Google Scholar] [CrossRef]
- King, T.C.; Yang, Y.P.; Liou, Y.S.; Wu, C.J. Tunable defect mode in a semiconductor-dielectric photonic crystal containing extrinsic semiconductor defect. Solid State Commun. 2012, 152, 2189–2192. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef]
- Dolui, K.; Rungger, I.; Pemmaraju, C.D.; Sanvito, S. Possible doping strategies for MoS2 monolayers: An ab initio study. Phys. Rev. B 2013, 88, 075420. [Google Scholar] [CrossRef]
- Komsa, H.-P.; Kotakoski, J.; Kurasch, S.; Lehtinen, O.; Kaiser, U.; Krasheninnikov, A. Two-dimensional transition metal dichalcogenides under electron irradiation: Defect production and doping. Phys. Rev. Lett. 2012, 109, 035503. [Google Scholar] [CrossRef]
- Segovia-Chaves, F.; Vinck-Posada, H. Effects of hydrostatic pressure on the band structure in two-dimensional semiconductor square photonic lattice with defect. Phys. B Condens. Matter 2018, 545, 203–209. [Google Scholar] [CrossRef]
- Zak, A.; Feldman, Y.; Lyakhovitskaya, V.; Leitus, G.; Popovitz-Biro, R.; Wachtel, E.; Cohen, H.; Reich, S.; Tenne, R. Alkali metal intercalated fullerene-like MS2 (M = W.; Mo) nanoparticles and their properties. J. Am. Chem. Soc. 2002, 124, 4747–4758. [Google Scholar] [CrossRef]
- Kabita, K.; Maibam, J.; Sharma, B.I.; Thapa, R.K.; Brojen Singh, R.K. First principle study on pressure-induced electronic structure and elastic properties of indium phosphide (InP). Indian J. Phys. 2015, 89, 1265–1271. [Google Scholar] [CrossRef]
- Mellot-Draznieks, C. Role of computer simulations in structure prediction and structure determination: From molecular compounds to hybrid frameworks. J. Mater. Chem. 2007, 17, 4348–4358. [Google Scholar] [CrossRef]
- Lejaeghere, K.; Bihlmayer, G.; Björkman, T.; Blaha, P.; Blügel, S.; Blum, V.; Caliste, D.; Castelli, I.E.; Clark, S.J.; Corso, A.D.; et al. Reproducibility in density functional theory calculations of solids. Science 2016, 351, aad3000. [Google Scholar] [CrossRef] [PubMed]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Hu, F.; Tao, L.; Ye, H.; Li, X.; Chen, X. ZnO/WSe2 vdW heterostructure for photocatalytic water splitting. J. Mater. Chem. C 2019, 7, 7104–7113. [Google Scholar] [CrossRef]
- Zheng, H.; Li, X.-B.; Chen, N.-K.; Xie, S.-Y.; Tian, W.Q.; Chen, Y.; Xia, H.; Zhang, S.B.; Sun, H.-B. Monolayer II-VI semiconductors: A first-principles prediction. Phys. Rev. B 2015, 92, 115307. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Local and Gradient-Corrected Density Functionals, Chemical Applications of Density-Functional Theory; Laird, B.B., Ross, R.B., Ziegler, T., Eds.; American Chemical Society: Washington, DC, USA, 1996; pp. 453–462. [Google Scholar]
- Zhang, Y.; Yang, W. Comment on “Generalized gradient approximation made simple”. Phys. Rev. Lett. 1998, 80, 890. [Google Scholar] [CrossRef]
- Wu, Z.; Cohen, R.E. More accurate generalized gradient approximation for solids. Phys. Rev. B 2006, 73, 235116. [Google Scholar] [CrossRef]
- Friák, M.; Šob, M.; Vitek, V. Ab initio study of the ideal tensile strength and mechanical stability of transition-metal disilicides. Phys. Rev. B 2003, 68, 184101. [Google Scholar] [CrossRef]
- Lee, H.-S.; Mizoguchi, T.; Yamamoto, T.; Kang, S.L.; Ikuharaa, Y. First-principles calculation of defect energetics in cubic-BaTiO3 and a comparison with SrTiO3. Acta Mater. 2007, 55, 6535–6540. [Google Scholar] [CrossRef]
- Ge, F.F.; Wu, W.D.; Cao, L.H.; Wang, X.M.; Wang, H.P.; Dai, Y.; Wang, H.B.; Shen, J. The structure and defect formation energy in tetragonal PbTiO3: Ab initio calculation. Ferroelectrics 2010, 401, 154–160. [Google Scholar] [CrossRef]
- Shang, J.; Pan, L.; Wang, X.; Li, J.; Deng, H.-X.; Wei, Z. Tunable electronic and optical properties of InSe/InTe van der Waals heterostructures toward optoelectronic applications. J. Mater. Chem. C 2018, 6, 7201–7206. [Google Scholar] [CrossRef]
- Sun, S.; Meng, F.; Wang, H.; Wang, H.; Ni, Y. Novel two-dimensional semiconductor SnP3: High stability, tunable bandgaps and high carrier mobility explored using first-principles calculations. J. Mater. Chem. A 2018, 6, 11890–11897. [Google Scholar] [CrossRef]
- Yu, H.B.; Wang, W.H.; Bai, H.Y. An electronic structure perspective on glass-forming ability in metallic glasses. Appl. Phys. Lett. 2010, 96, 081902. [Google Scholar]
- Zhang, C.; Zhou, Y.; Zhang, Y.; Zhao, S.; Fang, J.; Sheng, X.; Zhang, H. Self-assembly hierarchical silica nanotubes with vertically aligned silica nanorods and embedded platinum nanoparticles. ACS Sustain. Chem. Eng. 2017, 5, 1578–1585. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical Cells. In Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group; Nature: London, UK, 2011; pp. 26–32. [Google Scholar]
- Zhu, L.; Cao, X.; Gong, C.; Jiang, A.; Cheng, Y.; Xiao, J. Preparation of Cu3N/MoS2 heterojunction through magnetron sputtering and investigation of its structure and optical performance. Materials 2020, 13, 1873. [Google Scholar] [CrossRef]
- Hassan, M.A.; Kang, J.H.; Johar, M.A.; Ha, J.-S.; Ryu, S.-W. High-performance ZnS/GaN heterostructure photoanode for photoelectrochemical water splitting applications. Acta Mater. 2018, 146, 171–175. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Li, E.H. The Optical Dielectric Function: Excitonic Effects at E0 Critical Point. J. Phys. Soc. Jpn. 2001, 70, 2164–2167. [Google Scholar] [CrossRef]
- Fadaie, M.; Shahtahmassebi, N.; Roknabad, M.R. Effect of external electric field on the electronic structure and optical properties of stanene. Opt. Quantum Electron. 2016, 48, 1–12. [Google Scholar] [CrossRef]
- Sun, M.; Chou, J.-P.; Gao, J.; Cheng, Y.; Hu, A.; Tang, W.; Zhang, G. Exceptional optical absorption of buckled arsenene covering a broad spectral range by molecular doping. ACS Omega 2018, 3, 8514–8520. [Google Scholar] [CrossRef] [PubMed]
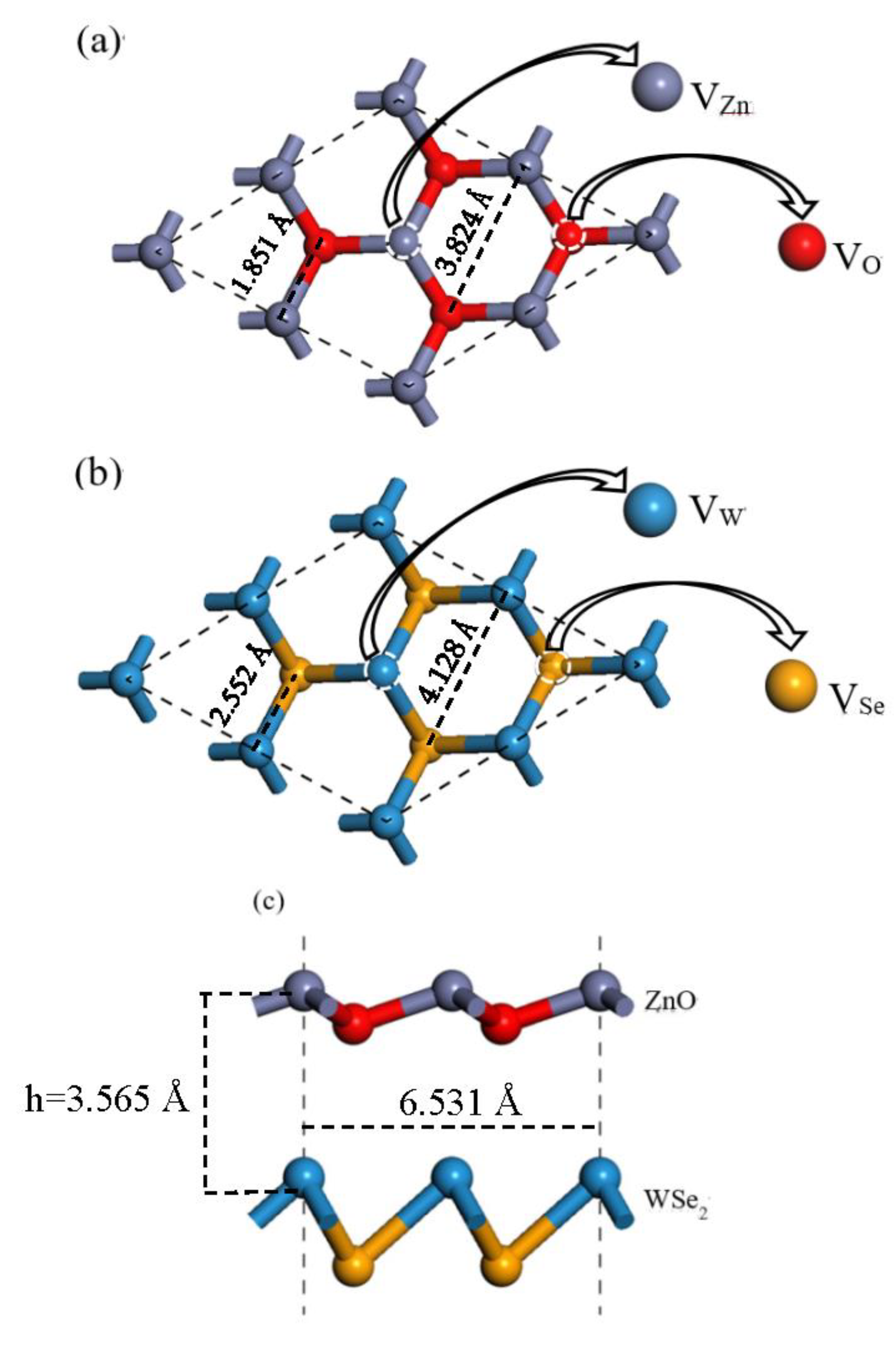
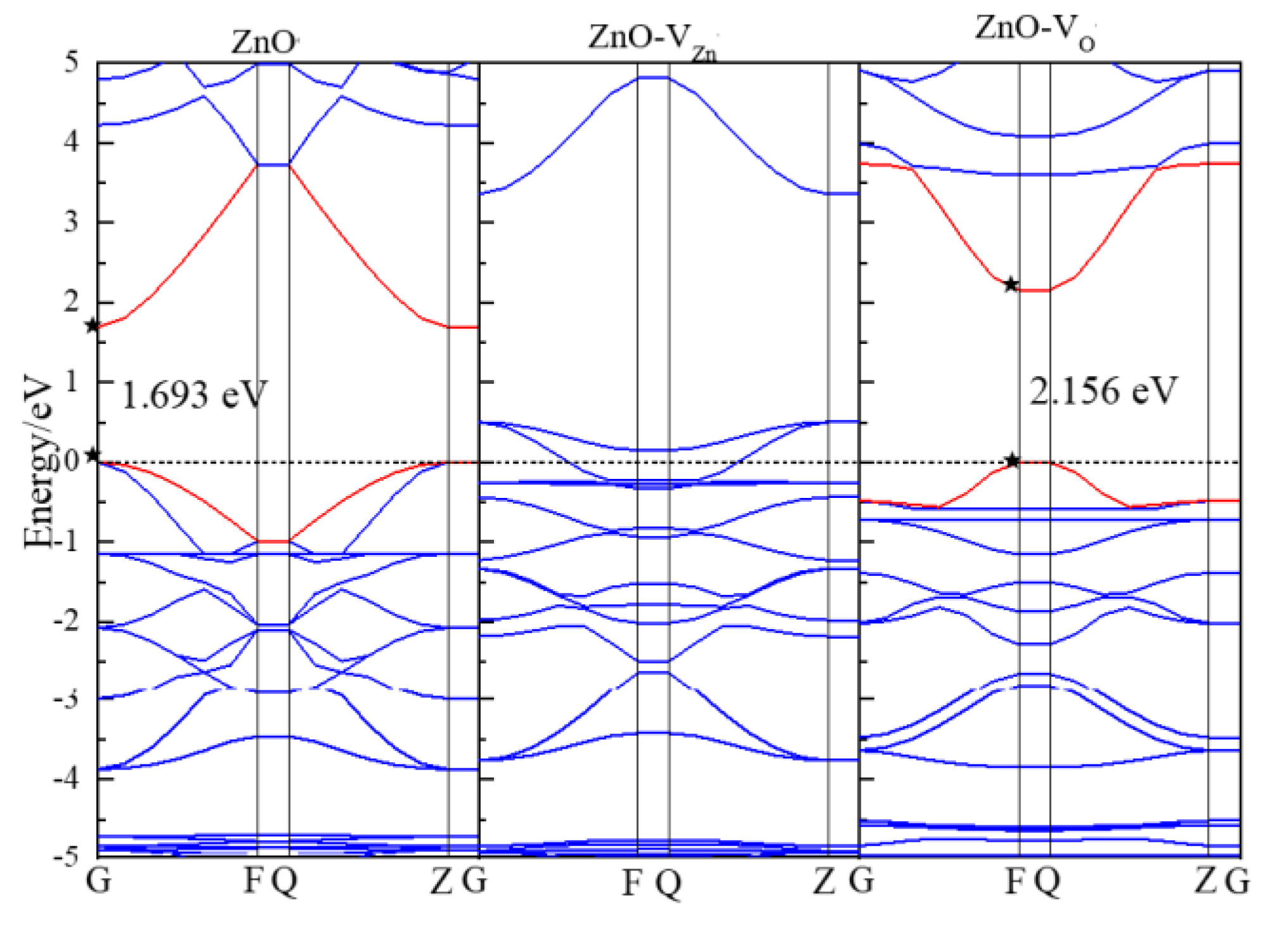
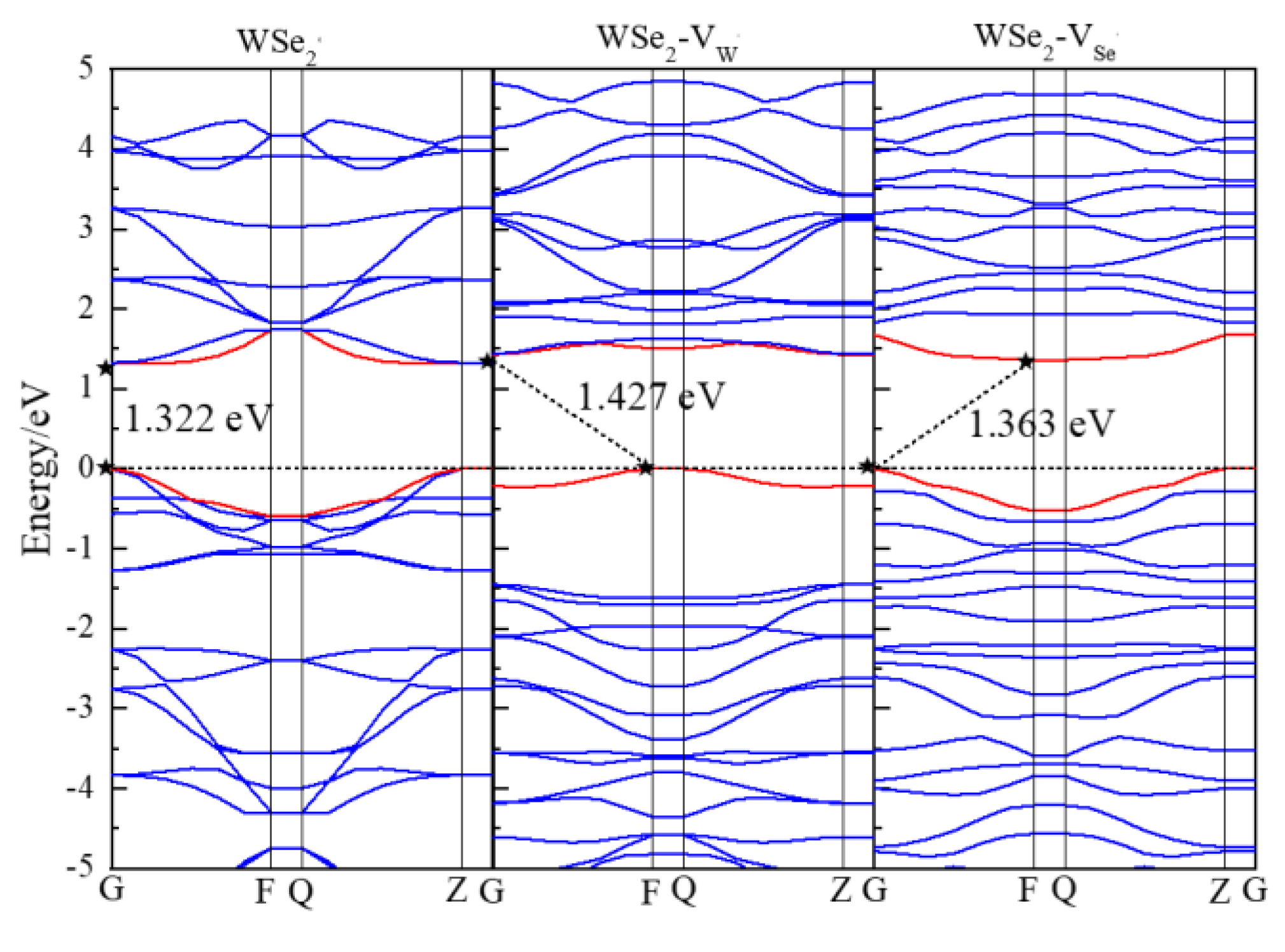
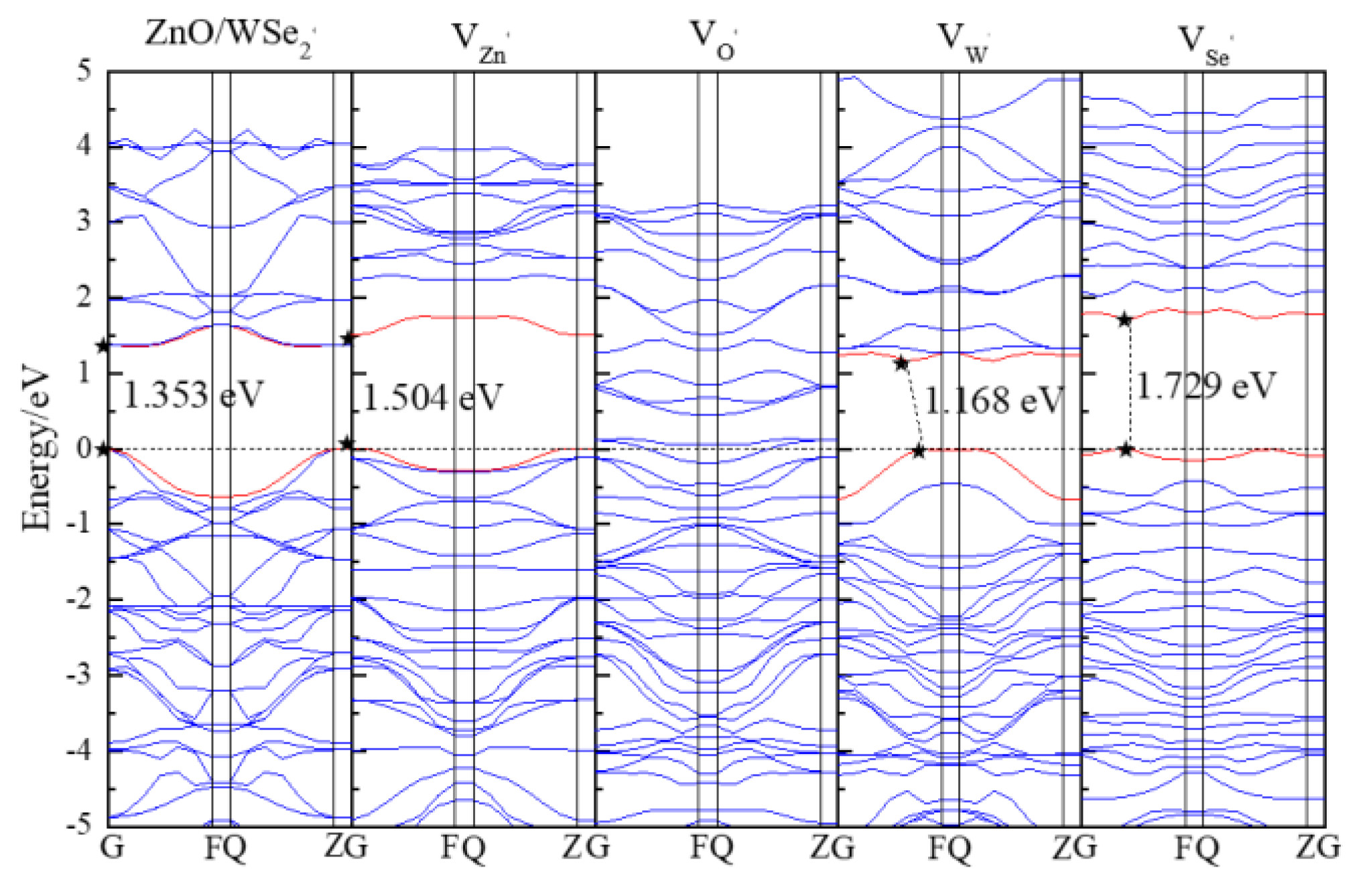

| Structure | ZnO | WSe2 | ZnO/WSe2 | |
|---|---|---|---|---|
| Vacancy | ||||
| VZn | 6.44 | −2.56 | ||
| VO | 8.13 | 7.90 | ||
| VW | 7.66 | 10.59 | ||
| VSe | 3.40 | 0.40 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yong, X.; Wang, A.; Deng, L.; Zhou, X.; Li, J. Effects of Vacancy Defects on Electrical and Optical Properties of ZnO/WSe2 Heterostructure: First-Principles Study. Metals 2022, 12, 1975. https://doi.org/10.3390/met12111975
Yong X, Wang A, Deng L, Zhou X, Li J. Effects of Vacancy Defects on Electrical and Optical Properties of ZnO/WSe2 Heterostructure: First-Principles Study. Metals. 2022; 12(11):1975. https://doi.org/10.3390/met12111975
Chicago/Turabian StyleYong, Xi, Ao Wang, Lichuan Deng, Xiaolong Zhou, and Jintao Li. 2022. "Effects of Vacancy Defects on Electrical and Optical Properties of ZnO/WSe2 Heterostructure: First-Principles Study" Metals 12, no. 11: 1975. https://doi.org/10.3390/met12111975
APA StyleYong, X., Wang, A., Deng, L., Zhou, X., & Li, J. (2022). Effects of Vacancy Defects on Electrical and Optical Properties of ZnO/WSe2 Heterostructure: First-Principles Study. Metals, 12(11), 1975. https://doi.org/10.3390/met12111975






