Investigating Metal Solidification with X-ray Imaging
Abstract
:1. Introduction
2. Materials and Methods
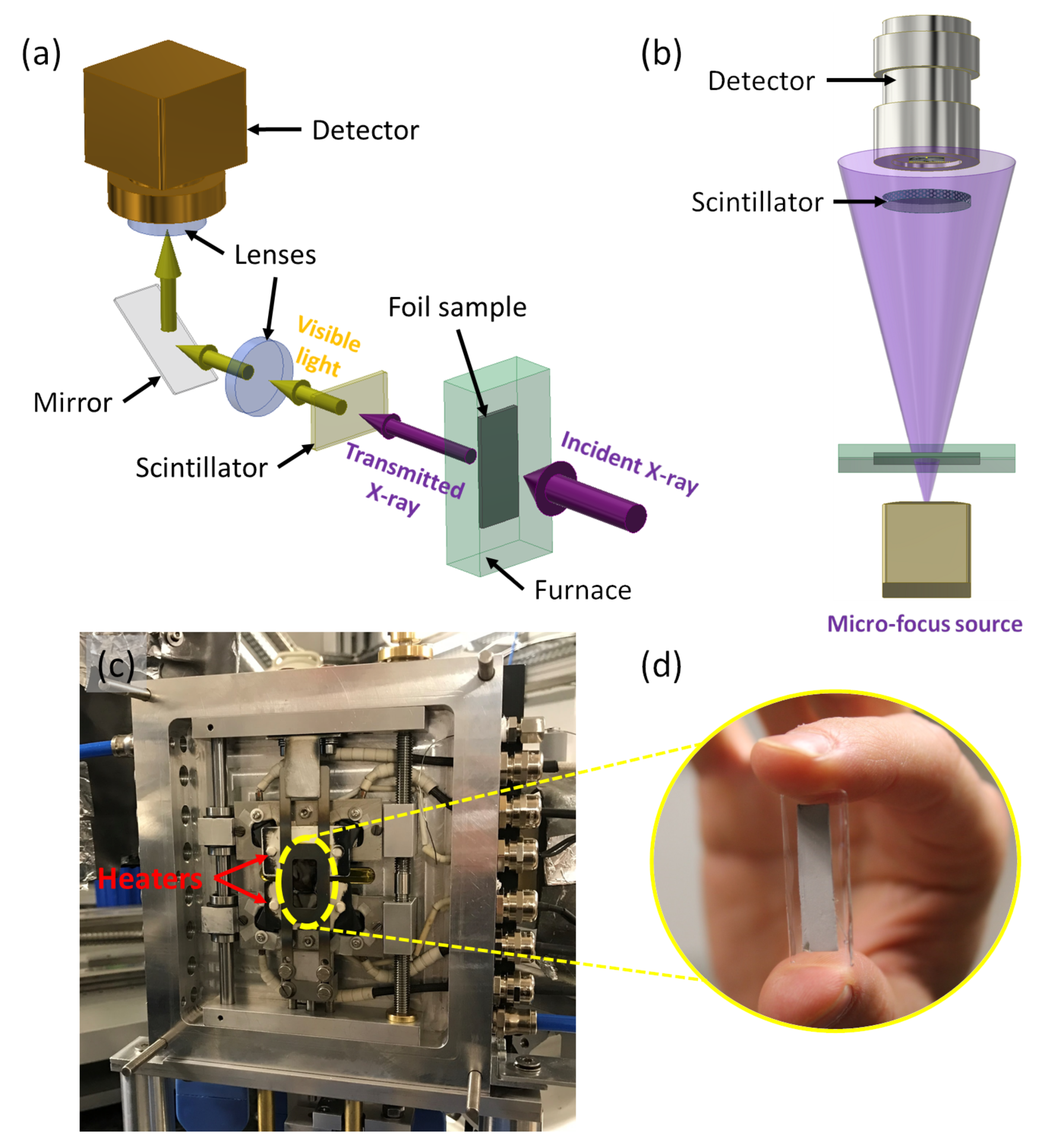
2.1. X-ray Radiography
2.2. Sample Preparation
3. Results and Discussion
3.1. Grain Refinement of -Al
3.1.1. Extrinsic Refinement: Inoculation
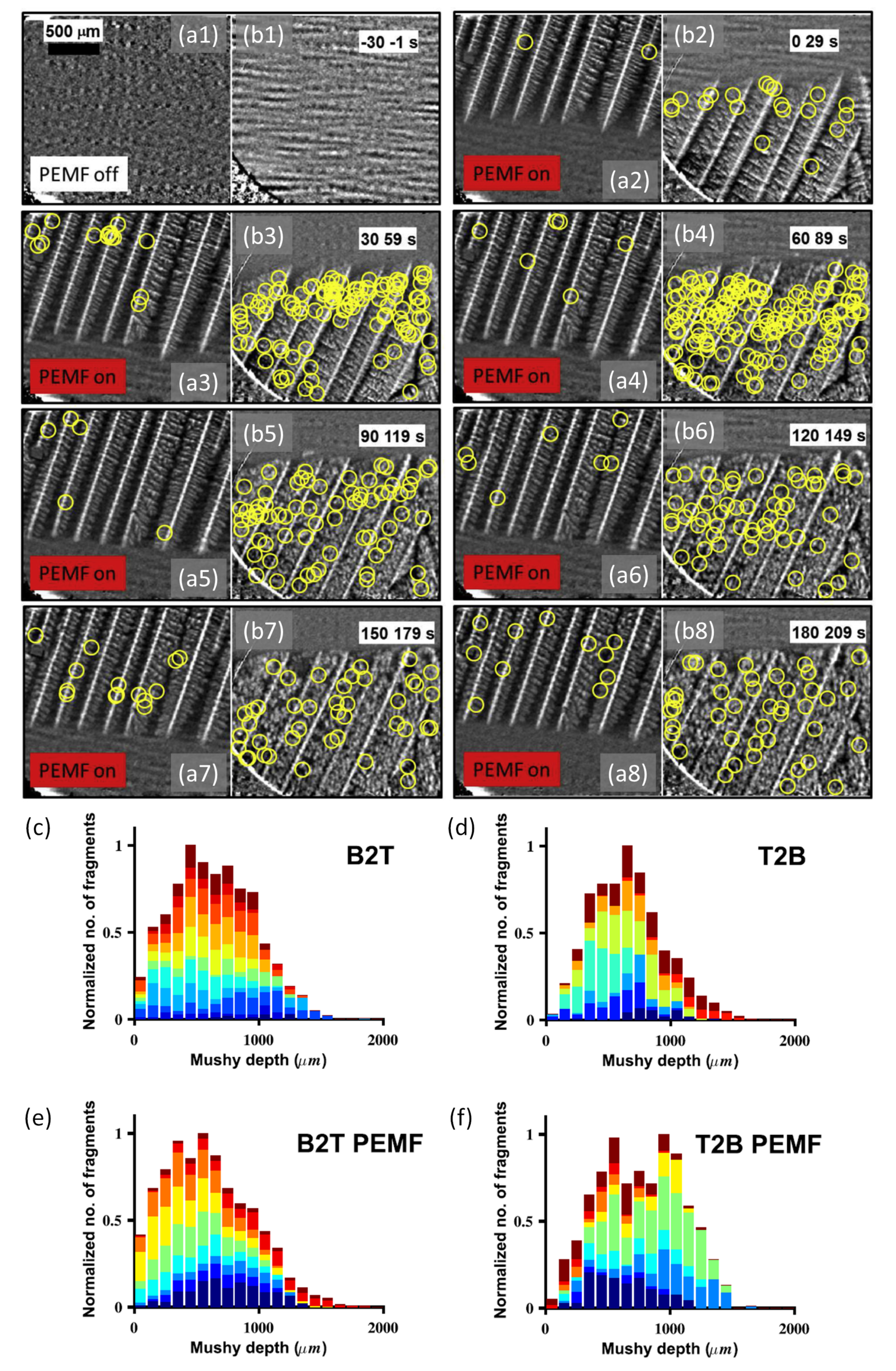
3.1.2. Intrinsic Refinement: Dendrite Fragmentation
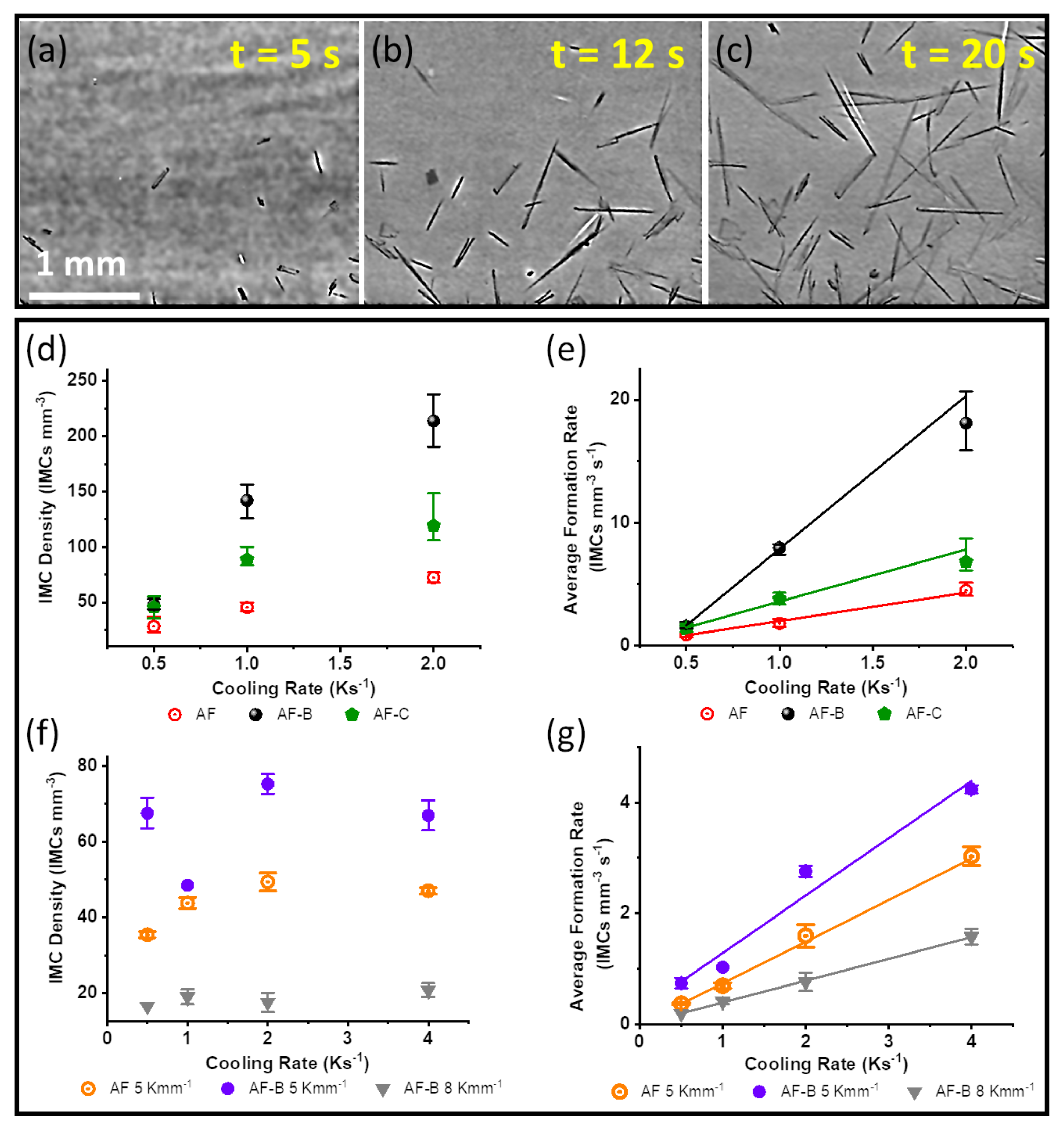
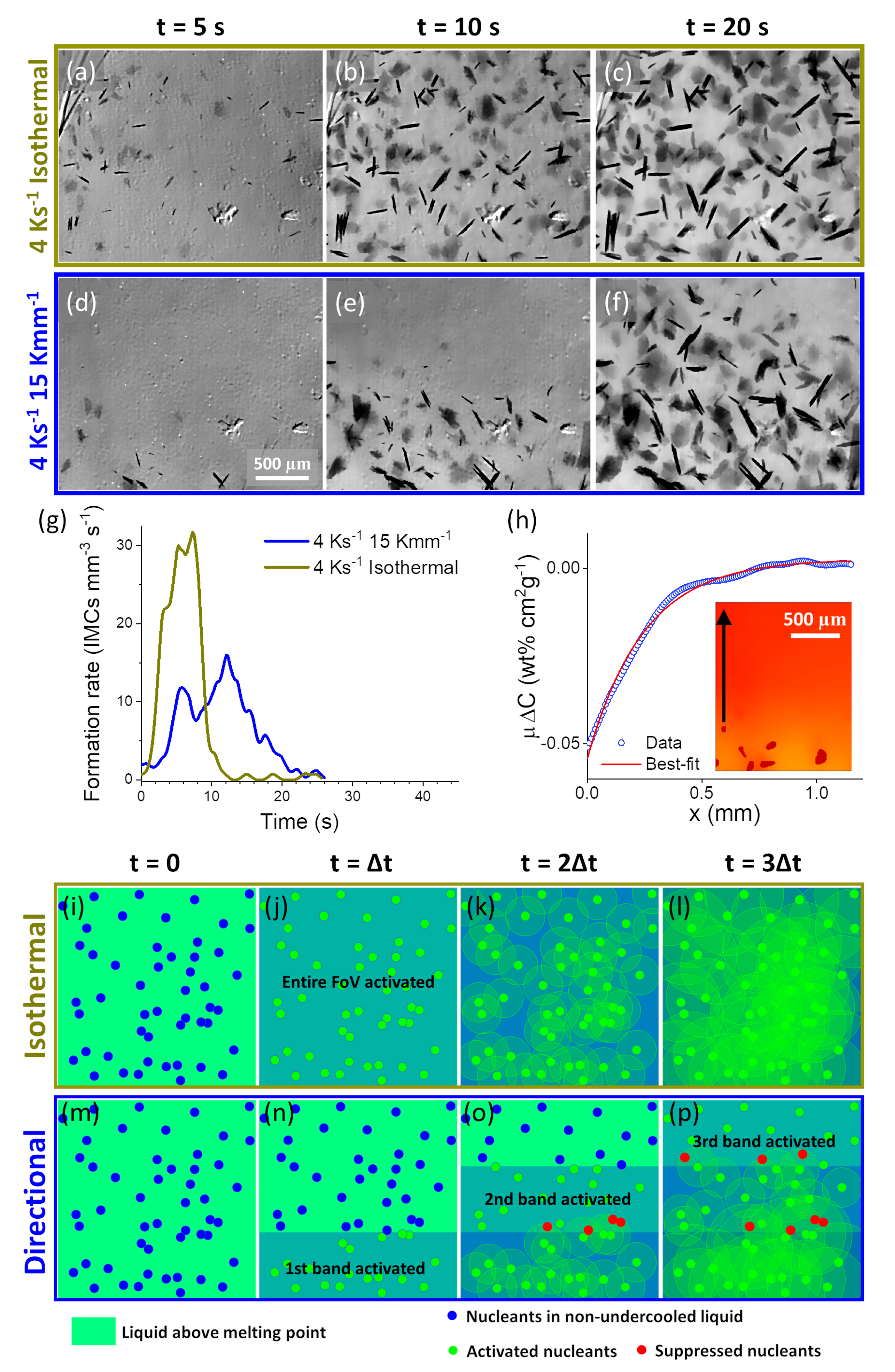
3.2. Nucleation and Growth of IMCs in Al Alloys
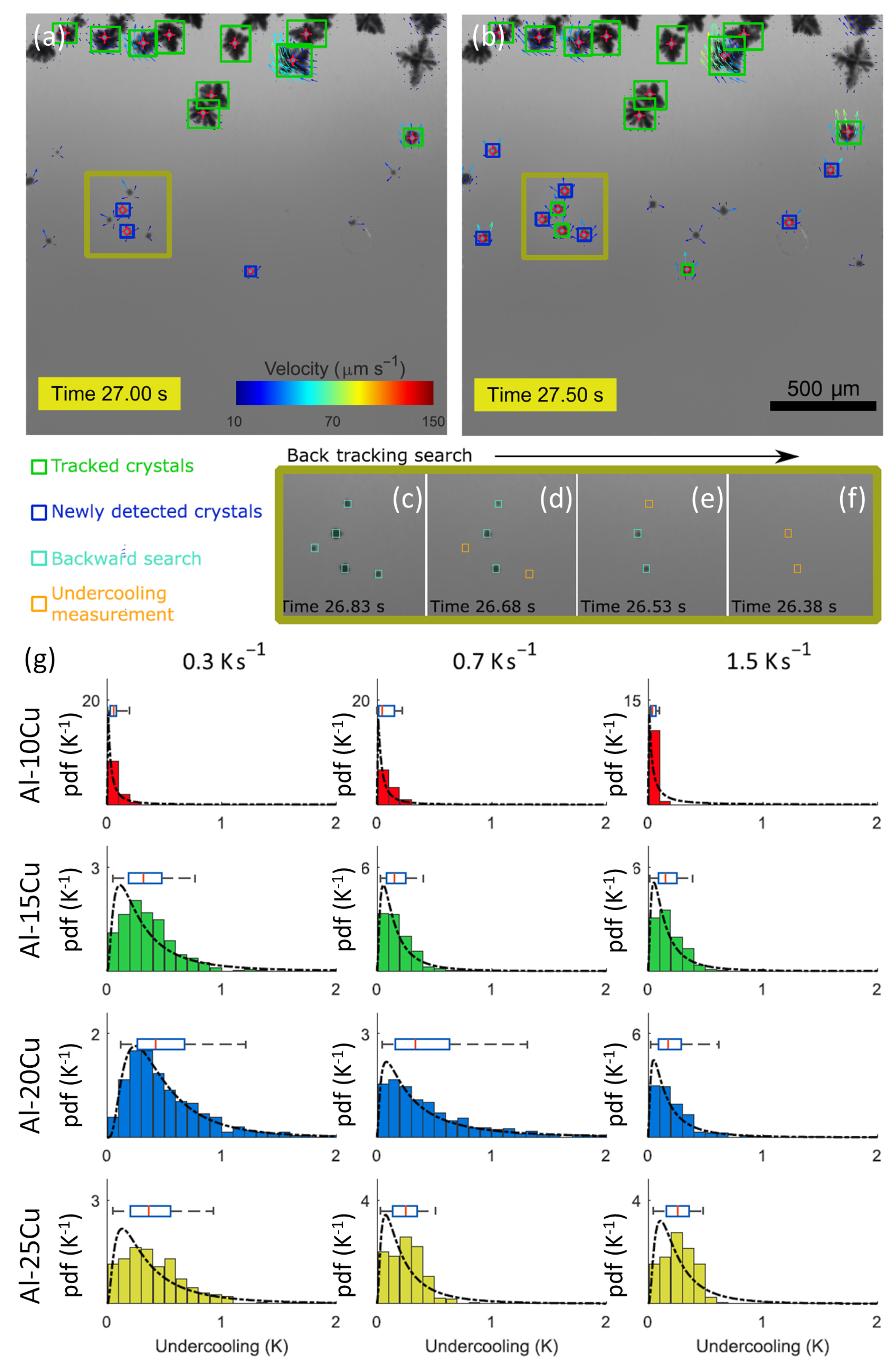
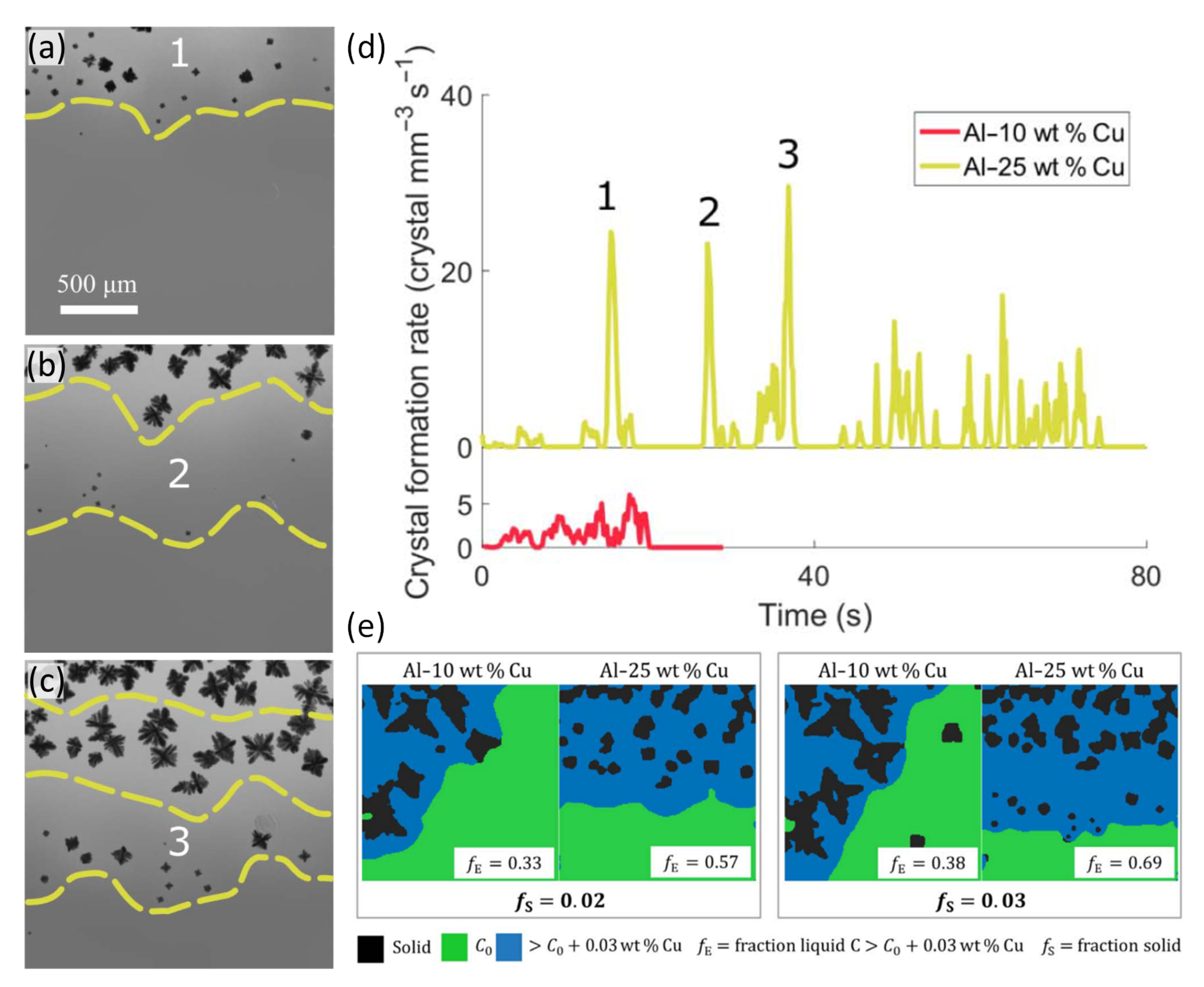
3.2.1. Nucleation of IMCs in Al Alloys
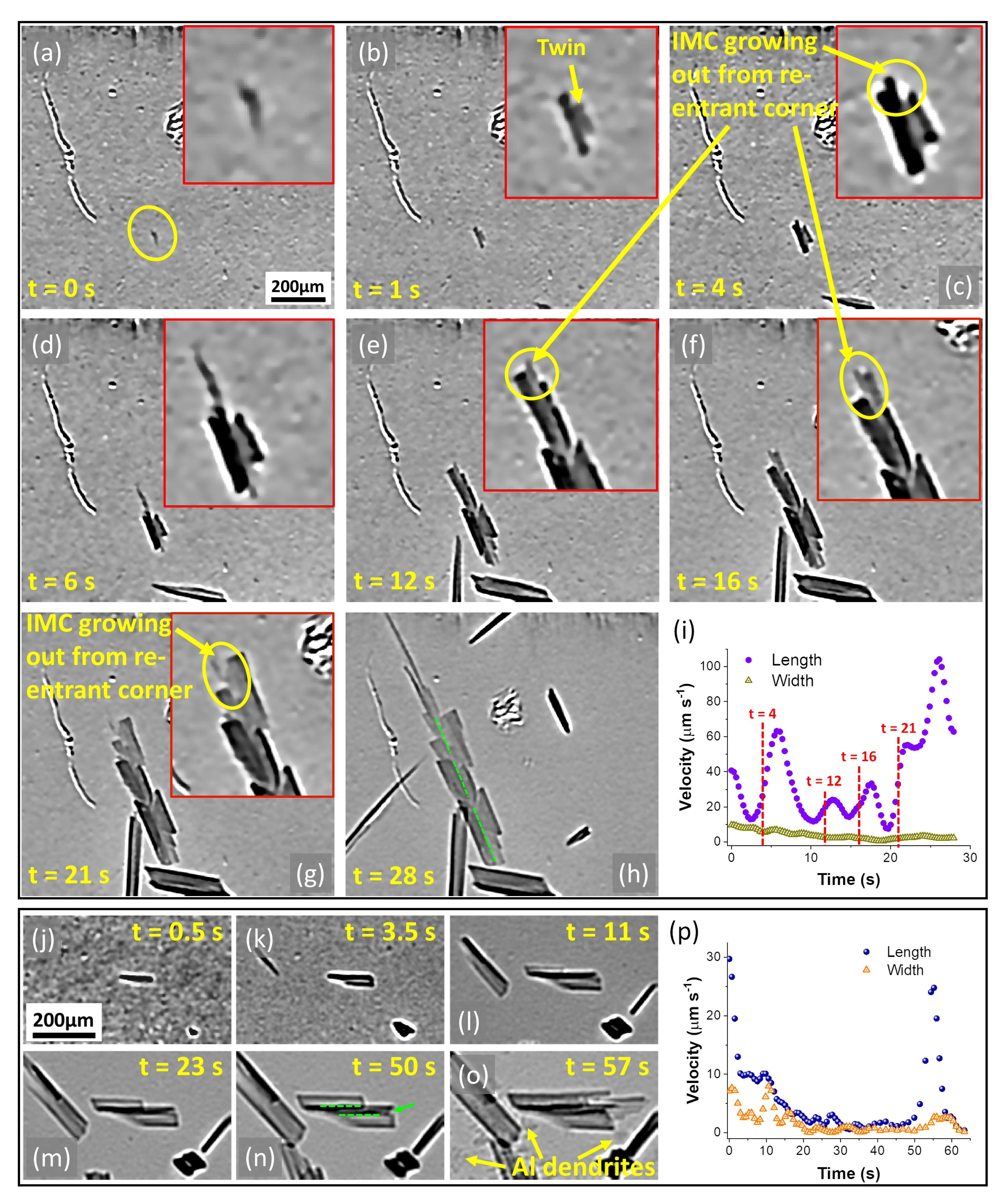
3.2.2. Faceted Growth of IMCs
3.3. Fluid Flow during Solidification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greer, A.L.; Cooper, P.S.; Meredith, M.W.; Schneider, W.; Schumacher, P.; Spittle, J.A.; Tronche, A. Grain refinement of aluminium alloys by inoculation. Adv. Eng. Mater. 2003, 5, 81–91. [Google Scholar] [CrossRef]
- Greer, A.L. Grain refinement of alloys by inoculation of melts. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2003, 361, 479–495. [Google Scholar] [CrossRef]
- Kevorkjjan, V. The recycling of standard quality wrought aluminum alloys from low-grade contaminated scrap. JOM 2010, 62, 37–42. [Google Scholar] [CrossRef]
- Kevorkijan, V. Challenges and advantages of recycling wrought aluminium alloys from lower grades of metallurgically clean scrap. Mater. Tehnol. 2013, 47, 13–23. [Google Scholar]
- Mohanty, P.S.; Gruzleski, J.E. Mechanism of grain refinement in aluminium. Acta Metall. Mater. 1995, 43, 2001–2012. [Google Scholar] [CrossRef]
- Murty, B.S.; Kori, S.A.; Chakraborty, M. Grain refinement of aluminium and its alloys by heterogeneous nucleation and alloying. Int. Mater. Rev. 2002, 47, 3–29. [Google Scholar] [CrossRef]
- Han, Y.; Shu, D.; Wang, J.; Sun, B. Microstructure and grain refining performance of Al-5Ti-1B master alloy prepared under high-intensity ultrasound. Mater. Sci. Eng. A 2006, 430, 326–331. [Google Scholar] [CrossRef]
- Li, H.T.; Wang, Y.; Fan, Z. Mechanisms of enhanced heterogeneous nucleation during solidification in binary Al–Mg alloys. Acta Mater. 2012, 60, 1528–1537. [Google Scholar] [CrossRef] [Green Version]
- Fan, Z.; Wang, Y.; Zhang, Y.; Qin, T.; Zhou, X.R.; Thompson, G.E.; Pennycook, T.; Hashimoto, T. Grain refining mechanism in the Al/Al-Ti-B system. Acta Mater. 2015, 84, 292–304. [Google Scholar] [CrossRef]
- Lui, A.; Grant, P.S.; Stone, I.C.; O’Reilly, K.A.Q. The Role of Grain Refiner in the Nucleation of AlFeSi Intermetallic Phases During Solidification of a 6xxx Aluminum Alloy. Metall. Mater. Trans. A 2019, 50, 5242–5252. [Google Scholar] [CrossRef] [Green Version]
- Que, Z.; Mendis, C.L. Heterogeneous nucleation and phase transformation of Fe-rich intermetallic compounds in Al–Mg–Si alloys. J. Alloys Compd. 2020, 836, 155515. [Google Scholar] [CrossRef]
- Liotti, E.; Arteta, C.; Zisserman, A.; Lui, A.; Lempitsky, V.; Grant, P.S. Crystal nucleation in metallic alloys using x-ray radiography and machine learning. Sci. Adv. 2018, 4, eaar4004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Casari, D.; Mathiesen, R.H.; Li, Y. Revealing the heterogeneous nucleation behavior of equiaxed grains of inoculated Al alloys during directional solidification. Acta Mater. 2018, 149, 312–325. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Huang, H.; Fu, Y.; Zhu, G.; Shu, D.; Sun, B.; StJohn, D. An in situ investigation of the solute suppressed nucleation zone in an Al-15Cu alloy inoculated by Al-Ti-B. Scr. Mater. 2019, 167, 6–10. [Google Scholar] [CrossRef]
- Feng, S.; Liotti, E.; Lui, A.; Wilson, M.D.; Connolley, T.; Mathiesen, R.H.; Grant, P.S. In-situ X-ray radiography of primary Fe-rich intermetallic compound formation. Acta Mater. 2020, 196, 759–769. [Google Scholar] [CrossRef]
- Jia, Y.; Song, D.; Zhou, N.; Zheng, K.; Fu, Y.; Shu, D. In Situ Investigation of Si-Poisoning Effect in Al–Cu–Si Alloys Inoculated by Al–5Ti–1B. Acta Metall. Sin. (Engl. Lett.) 2021, 1, 1–11. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, Y.; Zhang, Y.; Dai, Y.; Dong, Q.; Han, Y.; Zhu, G.; Zhang, J.; Fu, Y.; Sun, B. Intermittent nucleation and periodic growth of grains under thermo-solutal convection during directional solidification of Al-Cu alloy. Acta Mater. 2021, 212, 116861. [Google Scholar] [CrossRef]
- Feng, S.; Liotti, E.; Lui, A.; Wilson, M.D.; Grant, P.S. Nucleation bursts of primary intermetallic crystals in a liquid Al alloy studied using in situ synchrotron X-ray radiography. Acta Mater. 2021, 221, 117389. [Google Scholar] [CrossRef]
- Xu, Y.; Casari, D.; Du, Q.; Mathiesen, R.H.; Arnberg, L.; Li, Y. Heterogeneous nucleation and grain growth of inoculated aluminium alloys: An integrated study by in-situ X-radiography and numerical modelling. Acta Mater. 2017, 140, 224–239. [Google Scholar] [CrossRef]
- Wang, N.; Tang, Y.; Wu, Y.; Zhang, Y.; Dai, Y.; Zhang, J.; Zhang, R.; Xu, Y.; Sun, B. Dynamic evolution of microstructure morphology in thin-sample solidification: Deep learning assisted synchrotron X-ray radiography. Mater. Charact. 2021, 181, 111451. [Google Scholar] [CrossRef]
- Liotti, E.; Lui, A.; Vincent, R.; Kumar, S.; Guo, Z.; Connolley, T.; Dolbnya, I.P.; Hart, M.; Arnberg, L.; Mathiesen, R.H.; et al. A synchrotron X-ray radiography study of dendrite fragmentation induced by a pulsed electromagnetic field in an Al–15Cu alloy. Acta Mater. 2014, 70, 228–239. [Google Scholar] [CrossRef]
- Salloum-Abou-Jaoude, G.; Nguyen-Thi, H.; Reinhart, G.; Mathiesen, R.H.; Zimmermann, G.; Voss, D. Characterization of motion of dendrite fragment by X-ray radiography on Earth and under microgravity environment. Mater. Sci. Forum 2014, 790–791, 311–316. [Google Scholar] [CrossRef]
- Liotti, E.; Lui, A.; Kumar, S.; Guo, Z.; Bi, C.; Connolley, T.; Grant, P.S. The spatial and temporal distribution of dendrite fragmentation in solidifying Al-Cu alloys under different conditions. Acta Mater. 2016, 121, 384–395. [Google Scholar] [CrossRef]
- Wang, F.; Eskin, D.; Mi, J.; Wang, C.; Koe, B.; King, A.; Reinhard, C.; Connolley, T. A synchrotron X-radiography study of the fragmentation and refinement of primary intermetallic particles in an Al-35 Cu alloy induced by ultrasonic melt processing. Acta Mater. 2017, 141, 142–153. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, B.; Song, D.; Zheng, D.; Sun, Z.; Xie, C.; Zhang, W. Effect of Compound Fields of Ultrasonic Vibration and Applied Pressure on the 3D Microstructure and Tensile Properties of Recycled Al-Cu-Mn-Fe-Si Alloys. Materials 2019, 12, 3904. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wang, C.; Koe, B.; Schlepütz, C.M.; Irvine, S.; Mi, J. Synchrotron X-ray imaging and ultrafast tomography in situ study of the fragmentation and growth dynamics of dendritic microstructures in solidification under ultrasound. Acta Mater. 2021, 209, 116796. [Google Scholar] [CrossRef]
- Nelson, T.; Cai, B.; Warnken, N.; Lee, P.; Boller, E.; Magdysyuk, O.; Green, N. Gravity effect on thermal-solutal convection during solidification revealed by four-dimensional synchrotron imaging with compositional mapping. Scr. Mater. 2020, 180, 29–33. [Google Scholar] [CrossRef]
- Reinhart, G.; Grange, D.; Abou-Khalil, L.; Mangelinck-Noël, N.; Niane, N.; Maguin, V.; Guillemot, G.; Gandin, C.A.; Nguyen-Thi, H. Impact of solute flow during directional solidification of a Ni-based alloy: In-situ and real-time X-radiography. Acta Mater. 2020, 194, 68–79. [Google Scholar] [CrossRef]
- Liotti, E.; Lui, A.; Connolley, T.; Grant, P.S. Probing interdendritic flow and hot tearing during solidification using real time X-ray imaging and droplet tracking. Acta Mater. 2021. submitted. [Google Scholar]
- Mathiesen, R.H.; Arnberg, L.; Nguyen-Thi, H.; Billia, B. In Situ X-Ray Video Microscopy as a Tool in Solidification Science. JOM 2012, 64, 76–82. [Google Scholar] [CrossRef]
- Rakete, C.; Baumbach, C.; Goldschmidt, A.; Samberg, D.; Schroer, C.G.; Breede, F.; Stenzel, C.; Zimmermann, G.; Pickmann, C.; Houltz, Y.; et al. Compact x-ray microradiograph for in situ imaging of solidification processes: Bringing in situ x-ray micro-imaging from the synchrotron to the laboratory. Rev. Sci. Instrum. 2011, 82, 105108. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.G.; Browne, D.J.; Mirihanage, W.U.; Mathiesen, R.H. Combined in situ X-ray radiographic observations and post-solidification metallographic characterisation of eutectic transformations in Al–Cu alloy systems. Acta Mater. 2013, 61, 4559–4571. [Google Scholar] [CrossRef]
- Nguyen-Thi, H.; Reinhart, G.; Salloum Abou Jaoude, G.; Mathiesen, R.H.; Zimmermann, G.; Houltz, Y.; Voss, D.; Verga, A.; Browne, D.J.; Murphy, A.G. XRMON-GF: A novel facility for solidification of metallic alloys with in situ and time-resolved X-ray radiographic characterization in microgravity conditions. J. Cryst. Growth 2013, 374, 23–30. [Google Scholar] [CrossRef]
- Greer, A.L.; Bunn, A.M.; Tronche, A.; Evans, P.V.; Bristow, D.J. Modelling of inoculation of metallic melts: Application to grain refinement of aluminium by Al-Ti-B. Acta Mater. 2000, 48, 2823–2835. [Google Scholar] [CrossRef]
- Fisher, J.C.; Hollomon, J.H.; Turnbull, D. Nucleation. J. Appl. Phys. 1948, 19, 775–784. [Google Scholar] [CrossRef]
- Turnbull, D.; Fisher, J.C. Rate of nucleation in condensed systems. J. Chem. Phys. 1949, 17, 71–73. [Google Scholar] [CrossRef]
- Turnbull, D. Kinetics of heterogeneous nucleation. J. Chem. Phys. 1950, 18, 198–203. [Google Scholar] [CrossRef]
- Hollomon, J.H.; Turnbull, D. Nucleation. Prog. Met. Phys. 1953, 4, 333–388. [Google Scholar] [CrossRef]
- Turnbull, D. Theory of catalysis of nucleation by surface patches. Acta Metall. 1953, 1, 8–14. [Google Scholar] [CrossRef]
- Maxwell, I.; Hellawell, A. A simple model for grain refinement during solidification. Acta Metall. 1975, 23, 229–237. [Google Scholar] [CrossRef]
- Cantor, B.; Doherty, R.D. Heterogeneous nucleation in solidifying alloys. Acta Metall. 1979, 27, 33–46. [Google Scholar] [CrossRef]
- Shu, D.; Sun, B.; Mi, J.; Grant, P.S. A quantitative study of solute diffusion field effects on heterogeneous nucleation and the grain size of alloys. Acta Mater. 2011, 59, 2135–2144. [Google Scholar] [CrossRef]
- Stjohn, D.H.; Qian, M.; Easton, M.A.; Cao, P. The Interdependence Theory: The relationship between grain formation and nucleant selection. Acta Mater. 2011, 59, 4907–4921. [Google Scholar] [CrossRef]
- Buffet, A.; Thi, H.N.; Bogno, A.; Schenk, T.; Mangelinck-Noel, N.; Reinhart, G.; Bergeon, N.; Billia, B.; Baruchel, J. Measurement of solute profiles by means of synchrotron X-ray radiography during directional solidification of Al-4 wt% Cu alloys. Mater. Sci. Forum 2010, 649, 331–336. [Google Scholar] [CrossRef]
- Bogno, A.; Nguyen-Thi, H.; Buffet, A.; Reinhart, G.; Billia, B.; Mangelinck-Noël, N.; Bergeon, N.; Baruchel, J.; Schenk, T. Analysis by synchrotron X-ray radiography of convection effects on the dynamic evolution of the solid–liquid interface and on solute distribution during the initial transient of solidification. Acta Mater. 2011, 59, 4356–4365. [Google Scholar] [CrossRef]
- Reinhart, G.; Mangelinck-Noël, N.; Nguyen-Thi, H.; Schenk, T.; Gastaldi, J.; Billia, B.; Pino, P.; Härtwig, J.; Baruchel, J. Investigation of columnar–equiaxed transition and equiaxed growth of aluminium based alloys by X-ray radiography. Mater. Sci. Eng. A 2005, 413, 384–388. [Google Scholar] [CrossRef]
- Mathiesen, R.H.; Arnberg, L.; Bleuet, P.; Somogyi, A. Crystal fragmentation and columnar-to-equiaxed transitions in Al-Cu studied by synchrotron X-ray video microscopy. Metall. Mater. Trans. A 2006, 37, 2515–2524. [Google Scholar] [CrossRef]
- Dong, Q.; Zhang, J.; Dong, J.; Xie, H.; Li, Z.; Dai, Y.; Liu, Y.; Sun, B. In situ observation of columnar-to-equiaxed transition in directional solidification using synchrotron X-radiation imaging technique. Mater. Sci. Eng. A 2011, 530, 271–276. [Google Scholar] [CrossRef]
- Ruvalcaba, D.; Mathiesen, R.H.; Eskin, D.G.; Arnberg, L.; Katgerman, L. In situ observations of dendritic fragmentation due to local solute-enrichment during directional solidification of an aluminum alloy. Acta Mater. 2007, 55, 4287–4292. [Google Scholar] [CrossRef]
- Bogno, A.; Nguyen-Thi, H.; Reinhart, G.; Billia, B.; Baruchel, J. Growth and interaction of dendritic equiaxed grains: In situ characterization by synchrotron X-ray radiography. Acta Mater. 2013, 61, 1303–1315. [Google Scholar] [CrossRef]
- Murphy, A.G.; Mirihanage, W.U.; Browne, D.J.; Mathiesen, R.H. Equiaxed dendritic solidification and grain refiner potency characterised through in situ X-radiography. Acta Mater. 2015, 95, 83–89. [Google Scholar] [CrossRef]
- Murphy, A.; Mathiesen, R.; Houltz, Y.; Li, J.; Lockowandt, C.; Henriksson, K.; Zimmermann, G.; Melville, N.; Browne, D. XRMON-SOL: Isothermal equiaxed solidification of a grain refined Al–20 wt%Cu alloy. J. Cryst. Growth 2016, 440, 38–46. [Google Scholar] [CrossRef]
- Quested, T.; Greer, A. The effect of the size distribution of inoculant particles on as-cast grain size in aluminium alloys. Acta Mater. 2004, 52, 3859–3868. [Google Scholar] [CrossRef]
- Bjurenstedt, A.; Casari, D.; Seifeddine, S.; Mathiesen, R.H.; Dahle, A.K. In-situ study of morphology and growth of primary α-Al(FeMnCr)Si intermetallics in an Al-Si alloy. Acta Mater. 2017, 130, 1–9. [Google Scholar] [CrossRef]
- Feng, S.; Liotti, E.; Lui, A.; Kumar, S.; Mahadevegowda, A.; O’Reilly, K.A.Q.; Grant, P.S. An in-situ method to estimate the tip temperature and phase selection of secondary Fe-rich intermetallics using synchrotron X-ray radiography. Scr. Mater. 2018, 149, 44–48. [Google Scholar] [CrossRef]
- Cai, B.; Kao, A.; Lee, P.; Boller, E.; Basevi, H.; Phillion, A.; Leonardis, A.; Pericieous, K. Growth of β intermetallic in an Al-Cu-Si alloy during directional solidification via machine learned 4D quantification. Scr. Mater. 2019, 165, 29–33. [Google Scholar] [CrossRef]
- Feng, S.; Cui, Y.; Liotti, E.; Lui, A.; Gourlay, C.M.; Grant, P.S. In-situ X-ray radiography of twinned crystal growth of primary Al13Fe4. Scr. Mater. 2020, 184, 57–62. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, W.; Koe, B.; Du, W.; Wang, M.; Wang, W.; Boller, E.; Rack, A.; Sun, Z.; Shu, D.; et al. Multiscale characterization of the nucleation and 3D structure of Al3Sc phases using electron microscopy and synchrotron X-ray tomography. Mater. Charact. 2020, 164, 110353. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, W.; Song, D.; Lin, B.; Shen, F.; Zheng, D.; Xie, C.X.; Sun, Z.; Fu, Y.; Li, R. Nucleation and growth of Fe-rich phases in Al-5Ti-1B modified Al-Fe alloys investigated using synchrotron X-ray imaging and electron microscopy. J. Mater. Sci. Technol. 2021, 80, 84–99. [Google Scholar] [CrossRef]
- Song, Z.; Magdysyuk, O.V.; Tang, L.; Sparks, T.; Cai, B. Growth dynamics of faceted Al13Fe4 intermetallic revealed by high-speed synchrotron X-ray quantification. J. Alloys Compd. 2021, 861, 158604. [Google Scholar] [CrossRef]
- Taylor, J.A. Iron-Containing Intermetallic Phases in Al-Si Based Casting Alloys. Procedia Mater. Sci. 2012, 1, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Sha, G.; O’Reilly, K.A.Q.; Cantor, B.; Titchmarsh, J.M.; Hamerton, R. Quasi-peritectic solidification reactions in 6xxx series wrought Al alloys. Acta Mater. 2003, 51, 1883–1897. [Google Scholar] [CrossRef]
- Ji, S.; Yang, W.; Gao, F.; Watson, D.; Fan, Z. Effect of Iron in Al-Mg-Si-Mn Ductile Diecast Alloy. Light Met. 2013, 564, 130–139. [Google Scholar]
- Tiller, W.; Jackson, K.; Rutter, J.; Chalmers, B. The redistribution of solute atoms during the solidification of metals. Acta Metall. 1953, 1, 428–437. [Google Scholar] [CrossRef]
- Jackson, K.; Uhlmann, D.; Hunt, J. On the nature of crystal growth from the melt. J. Cryst. Growth 1967, 1, 1–36. [Google Scholar] [CrossRef]
- Adam, C.M.; Hogan, L.M. Crystallography of the Al-Al3 Fe eutectic. Acta Metall. 1975, 23, 345–354. [Google Scholar] [CrossRef]
- Que, Z.P.; Zhou, Y.P.; Wang, Y.; Fan, Z. Composition Templating for Heterogeneous Nucleation of Intermetailic Compounds. In Proceedings of the 6th Decennial International Conference on Solidification Processing, Old Windsor, UK, 25–28 July 2017; pp. 158–161. [Google Scholar]
- Feng, S.; Liotti, E.; Wilson, M.D.; Jowitt, L.; Grant, P.S. In situ mapping of chemical segregation using synchrotron x-ray imaging. MRS Bull. 2020, 45, 934–942. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, S.; Han, I.; Lui, A.; Vincent, R.; Ring, G.; Grant, P.S.; Liotti, E. Investigating Metal Solidification with X-ray Imaging. Metals 2022, 12, 395. https://doi.org/10.3390/met12030395
Feng S, Han I, Lui A, Vincent R, Ring G, Grant PS, Liotti E. Investigating Metal Solidification with X-ray Imaging. Metals. 2022; 12(3):395. https://doi.org/10.3390/met12030395
Chicago/Turabian StyleFeng, Shikang, Insung Han, Andrew Lui, Robin Vincent, Gideon Ring, Patrick S. Grant, and Enzo Liotti. 2022. "Investigating Metal Solidification with X-ray Imaging" Metals 12, no. 3: 395. https://doi.org/10.3390/met12030395
APA StyleFeng, S., Han, I., Lui, A., Vincent, R., Ring, G., Grant, P. S., & Liotti, E. (2022). Investigating Metal Solidification with X-ray Imaging. Metals, 12(3), 395. https://doi.org/10.3390/met12030395





