Thermodynamic Assessment of Ti-Al-Fe-V Quaternary System Applied to Novel Titanium Alloys Designing
Abstract
:1. Introduction
2. Literature Review
2.1. Binary Systems
2.2. Ternary Systems
3. Thermodynamic Modeling
3.1. Pure Elements
3.2. Solution Phases
3.3. Intermetallic Compounds
4. Thermodynamic Optimization
4.1. Ti-Fe-V System
4.2. Ti-Al-Fe-V System
5. Alloy Design
- (1)
- As a strengthening element of the α phase, the α single-phase region of a keeps expanding with increasing Al content. Figure 12 shows that when the Al content is 6.0 wt%, the largest α single-phase region is observed. Therefore, if you want to design α titanium alloys of the Ti-Al-Fe-V system, 6.0 wt% Al can be preferred. At this time, the ranges of Fe and V are 0–3.05 wt% and 0–10.68 wt%, respectively. The recommended alloy composition is Ti-6Al-3.5V-1Fe, and its actual Mo equivalent is −0.755, which is considered to be 0.
- (2)
- V and Fe, as β phase reinforcing elements, can enlarge the β phase region. To obtain β titanium alloy, the content of elements V and Fe should be within the appropriate range as much as possible. Figure 10 shows that when the Al content is 3.0 wt%, the largest β single-phase region is observed. Therefore, if you want to design β titanium alloys of the Ti-Al-Fe-V system, 3.0 wt% Al can be preferred. At this time, the ranges of Fe and V are greater than 6.29 wt% and 22.02 wt%, respectively. The recommended alloy composition is Ti-3Al-22.75V-6.5Fe, and its Mo equivalent is 31.
- (3)
- Although α titanium alloys and β titanium alloys can also be designed when the Al content is 4.5 wt%, the range of options did not reach the optimal when compared with the other two concentrations. Therefore, when the Al content is 4.5 wt%, as shown in Figure 11, priority should be given to design α + β titanium alloys. At this time, the ranges of Fe and V are 2.76–6.58 wt% and 9.66–23.03 wt%, respectively. The recommended alloy composition is Ti-4.5Al-10.5V-3Fe, and its Mo equivalent is 11.2.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, K. The future of metals. Science 2010, 328, 319–320. [Google Scholar] [CrossRef] [PubMed]
- Boyer, R.R. Attributes, characteristics, and applications of titanium and its alloys. JOM 2017, 3, 15–18. [Google Scholar] [CrossRef]
- Qiu, C.; Fones, A.; Hamilton, H.G.C.; Adkins, N.J.E.; Attallah, M.M. A new approach to develop palladium-modified Ti-based alloys for biomedical applications. Mater. Des. 2016, 109, 98–111. [Google Scholar] [CrossRef]
- He, P.Z.; Huang, S.M.; Guo, W.; Wang, Y.F.; He, L. The present situation of the word sponge titanium industry and the reflection on the future development of China. Prog. Titan. Ind. 2017, 34, 1–4. [Google Scholar]
- Gazder, A.A.; Vu, V.Q.; Saleh, A.A.; Markovsky, P.E.; Ivasishin, O.M.; Davies, C.H.J.; Pereloma, E.V. Recrystallisation in a cold drawn low cost beta titanium alloy during rapid resistance heating. J. Alloys Compd. 2014, 585, 245–259. [Google Scholar] [CrossRef]
- Zadra, M.; Girardini, L. High-performance, low-cost titanium metal matrix composites. Mater. Sci. Eng. A 2014, 608, 155–163. [Google Scholar] [CrossRef]
- Santos, P.F.; Niinomi, M.; Cho, K.; Nakai, M.; Liu, H.; Ohtsu, N.; Hirano, M.; Ikeda, M.; Narushima, T. Microstructures, mechanical properties and cytotoxicity of low cost beta Ti-Mn alloys for biomedical applications. Acta Biomater. 2015, 26, 366–376. [Google Scholar] [CrossRef]
- Bodunrin, M.O.; Chown, L.N.; Omotoyinbo, J.A. Development of low-cost titanium alloys: A chronicle of challenges and opportunities. Mater. Today Proc. 2021, 38, 5–7. [Google Scholar] [CrossRef]
- Robertson, I.M.; Schaffer, G.B. Review of densification of titanium based powder systems in press and sinter processing. Powder Metall. 2010, 53, 146–162. [Google Scholar] [CrossRef]
- Bryan, D. ATI 425® Alloy Formability: Theory and Application. Mater. Sci. Forum 2014, 783–786, 543–548. [Google Scholar] [CrossRef]
- Zhang, C.; Yin, H.; Zhang, R.; Jiang, X.; Liu, G.; Du, Y. Experimental and thermodynamic investigation of gradient zone formation for Ti(C,N)-based cermets sintered in nitrogen atmosphere. Ceram. Int. 2017, 43, 12089–12094. [Google Scholar] [CrossRef]
- Kattner, U.R.; Lin, J.C.; Chang, Y.A. Thermodynamic assessment and calculation of the Ti-Al system. Metall. Mater. Trans. A 1992, 23, 2081–2090. [Google Scholar] [CrossRef]
- Okamoto, H. Al-Ti (aluminum-titanium). J. Phase Equilib. 1993, 14, 764. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, S.L.; Chang, Y.A.; Kattner, U.R. A thermodynamic description of the Ti-Al system. Intermetallics 1997, 5, 471–482. [Google Scholar] [CrossRef]
- Braun, J.; Ellner, M. Phase equilibria investigations on the aluminum-rich part of the binary system Ti-Al. Metall. Mater. Trans. A 2001, 32, 1037–1047. [Google Scholar] [CrossRef]
- Ohnuma, I.; Fujita, Y.; Mitsui, H.; Ishikawa, K.; Kainuma, R.; Ishida, K. Phase equilibria in the Ti-Al binary system. Acta Mater. 2000, 48, 3113–3123. [Google Scholar] [CrossRef]
- Kostov, A.; Friedrich, B.; Zivkovic, D. Thermodynamic calculations in alloys Ti-Al, Ti-Fe, Al-Fe and Ti-Al-Fe. J. Min. Metall. Sect. B 2008, 44, 49–61. [Google Scholar] [CrossRef]
- Witusiewicz, V.T.; Bondar, A.A.; Hecht, U.; Rex, S.; Velikanova, T.Y. The Al-B-Nb-Ti system III: Thermodynamic re-evaluation of the constituent binary system Al-Ti. J. Alloys Compd. 2008, 465, 64–77. [Google Scholar] [CrossRef]
- Ohtani, H.; Hillert, M. A thermodynamic assessment of the Fe-N-Ti system. Calphad 1991, 15, 41–52. [Google Scholar] [CrossRef]
- Kumar, K.C.H.; Wollaiits, P.; Delaey, L. Thermodynamic reassessment and calculation of Fe-Ti phase diagram. Calphad 1994, 18, 223–234. [Google Scholar] [CrossRef]
- Dumitrescu, L.F.S.; Hillert, M.; Saunders, N. Comparison of Fe-Ti assessments. J. Phase Equilib. 1998, 19, 441–448. [Google Scholar] [CrossRef]
- Jonsson, S. Assessment of the Fe-Ti system. Metall. Mater. Trans. B 1998, 29, 361–370. [Google Scholar] [CrossRef]
- Keyzer, J.D.; Cacciamani, G.; Dupin, N.; Wollants, P. Thermodynamic modeling and optimization of the Fe-Ni-Ti system. Calphad 2009, 33, 109–123. [Google Scholar] [CrossRef]
- Bo, H.; Wang, J.; Duarte, L.; Leinenbach, C.; Liu, L.B.; Liu, H.S.; Jin, Z.P. Thermodynamic re-assessment of Fe-Ti binary system. Trans. Nonferr. Met. Soc. China 2012, 22, 2204–2211. [Google Scholar] [CrossRef]
- Zeng, K.; Schmid-Fetzer, R. Thermodynamic assessment and applications of Ti-V-N system. Mater. Sci. Technol. 1998, 14, 1083–1091. [Google Scholar] [CrossRef]
- Lindwall, G.; Wang, P.; Kattner, U.R.; Campbell, C.E. The effect of oxygen on phase equilibria in the Ti-V system: Impacts on the AM processing of Ti alloys. JOM 2018, 70, 1692–1705. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, G. Thermodynamic and kinetic modeling of the Cr-Ti-V system. J. Phase Equilib. 2002, 23, 310–328. [Google Scholar] [CrossRef]
- Jacobs, M.H.G.; Schmid-Fetzer, R. Phase behavior and thermodynamic properties in the system Fe-Al. Calphad 2009, 33, 170–178. [Google Scholar] [CrossRef]
- Phan, A.T.; Paek, M.K.; Kang, Y.B. Phase equilibria and thermodynamics of the Fe-Al-C system: Critical evaluation, experiment and thermodynamic optimization. Acta Mater. 2014, 79, 1–15. [Google Scholar] [CrossRef]
- Sundman, B.; Ohnuma, I.; Dupin, N.; Kattner, U.R.; Fries, S.G. An assessment of the entire Al-Fe system including D03 ordering. Acta Mater. 2009, 57, 2896–2908. [Google Scholar] [CrossRef]
- Kroupa, A.; Mazalova, M.; Richter, K.W. The reassessment of the Al-V system and new assessment of the Al-Si-V system. Calphad 2017, 59, 47–60. [Google Scholar] [CrossRef]
- Gong, W.P.; Du, Y.; Huang, B.Y.; Schmid-Fetzer, R.; Zhang, C.F.; Xu, H.H. Thermodynamic reassessment of the Al-V system. Z. Met. 2004, 95, 978–986. [Google Scholar] [CrossRef]
- Lee, B.J.; Lee, D.N. A thermodynamic study on the V-C and Fe-V systems. Calphad 1991, 15, 283–291. [Google Scholar]
- Andersson, J.O. A thermodynamic evaluation of the iron-vanadium system. Calphad 1983, 7, 305–315. [Google Scholar] [CrossRef]
- Kumar, K.C.H.; Raghavan, V. A thermodynamic reassessment of the Fe-V system. Calphad 1991, 15, 307–314. [Google Scholar] [CrossRef]
- Zeng, L.J.; Xu, G.L.; Liu, L.B.; Bai, W.M.; Zhang, L.G. Experimental investigation of phase equilibria in the Ti-Fe-Zr system. Calphad 2018, 61, 20–32. [Google Scholar] [CrossRef]
- Hu, B.; Yao, B.; Wang, J.; Zhao, J.R.; Min, F.F.; Du, Y. Thermodynamic assessment of the Al-Mo-V ternary system. J. Min. Metall. Sect. B 2017, 53, 95–106. [Google Scholar] [CrossRef]
- Zhao, C.C.; Yang, S.Y.; Lu, Y.; Guo, Y.H.; Wang, C.P.; Liu, X.J. Experimental investigation and thermodynamic calculation of the phase equilibria in the Fe-Ni-V system. Calphad 2014, 46, 80–86. [Google Scholar] [CrossRef]
- Palm, M.; Inden, G.; Thomas, N. The Fe-Al-Ti system. J. Phase Equilib. 1995, 16, 209–222. [Google Scholar] [CrossRef]
- Mabuchi, H.; Nagayama, H.; Tsuda, H.; Matsui, T.; Morii, K. Formation of ternary L1_2 intermetallic compound and phase relation in the Al-Ti-Fe system. Mater. Trans. JIM 2000, 41, 733–738. [Google Scholar] [CrossRef] [Green Version]
- Kainuma, R.; Ohnuma, I.; Ishikawa, K.; Ishida, K. Stability of B2 ordered phase in the Ti-rich portion of Ti-Al-Cr and Ti-Al-Fe ternary systems. Intermetallics 2000, 8, 869–875. [Google Scholar] [CrossRef]
- Ducher, R.; Stein, F.; Viguier, B.; Palm, M.; Lacaze, J. A re-examination of the liquidus surface of the Al-Fe-Ti system. Z. Met. 2003, 94, 396–410. [Google Scholar] [CrossRef]
- Palm, M.; Lacaze, J. Assessment of the Al-Fe-Ti system. Intermetallics 2006, 14, 1291–1303. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Minamino, Y. Ternary diffusion and thermodynamic interaction in the beta solid solutions of Ti-Al-Fe alloys at 1423 K. J. Alloys Compd. 2012, 545, 168–175. [Google Scholar] [CrossRef]
- Rafiei, M.; Enayati, M.H.; Karimzadeh, F. Thermodynamic analysis of solid solution formation in the nanocrystalline Fe-Ti-Al ternary system during mechanical alloying. J. Chem. Thermodyn. 2013, 59, 243–249. [Google Scholar] [CrossRef]
- Hu, R.M. Thermodynamic Evaluation of Ti-Al-Fe-Mn Quaternary System and Its Application in the Design of Novel Titanium Alloys. Master’s Thesis, Shanghai University, Shanghai, China, 2015. [Google Scholar]
- Hayes, F.H. The Al-Ti-V (aluminum-titanium-vanadium) system. J. Phase Equilib. 1995, 16, 163–176. [Google Scholar] [CrossRef]
- Farrar, P.A.; Margolin, H. The Titanium-rich region of the Titanium-Aluminum-Vanadium system. Trans. Am. Inst. Min. Metall. Pet. Eng. 1961, 221, 1214–1221. [Google Scholar]
- Ahmed, T.; Flower, H. Partial isothermal sections of Ti-Al-V ternary diagram. Mater. Sci. Technol. 1994, 10, 272–288. [Google Scholar] [CrossRef]
- Shao, G.; Miodownik, A.P.; Tsakiropoulos, P. ω-Phase formation in V-Al and Ti-Al-V alloys. Philos. Mag. A 1995, 71, 1389–1408. [Google Scholar] [CrossRef]
- Shao, G.; Tsakiropoulos, P.; Miodownik, A.P. Ordering and decomposition of the β phase in melt-spun TiAl1−xVx alloys. Mater. Sci. Eng. A 1996, 216, 1–10. [Google Scholar] [CrossRef]
- Chang, W.S.; Muddle, B.C. Structure and stability of a new ternary hexagonal phase in Al3Ti-based Al-Ti-V alloys. Metall. Mater. Trans. A 2003, 34, 491–501. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Du, Y. 1100 ℃ isothermal section of Ti-Al-V ternary system. Mater. Sci. Eng. Powder Metall. 2006, 11, 146–148. [Google Scholar] [CrossRef]
- Kostov, A.; Zivkovic, D. Thermodynamic analysis of alloys Ti-Al, Ti-V., Al-V and Ti-Al-V. J. Alloys Compd. 2008, 460, 164–171. [Google Scholar] [CrossRef]
- Wang, H.; Warnken, N.; Reed, R.C. Thermodynamic and kinetic modeling of bcc phase in the Ti-Al-V ternary system. Mater. Sci. Eng. A 2010, 528, 622–630. [Google Scholar] [CrossRef]
- Lu, X.G.; Gui, N.; Qiu, A.T.; Wu, G.X.; Li, C.H. Thermodynamic Modeling of the Al-Ti-V Ternary System. Metall. Mater. Trans. A 2014, 45, 4155–4164. [Google Scholar] [CrossRef]
- Miyazaki, T.; Kozakai, T. Experimental and theoretical investigations on phase diagram of Fe-base ternary ordering alloys. J. Chim. Phys. Chim. Biol. 1997, 94, 844–848. [Google Scholar] [CrossRef]
- Schoo, K.L.; Sivaramakrishnan, C.S.; Chakrabarti, A.K. Solidification characteristics of the Al-8.3Fe-0.8V-0.9Si alloy. Metall. Mater. Trans. A 2000, 31, 1599–1610. [Google Scholar] [CrossRef]
- Zhao, P.Z.; Kozakai, T.; Miyazaki, T. Phase separations into A2 + DO3 two phases in Fe-Al-V ternary ordering alloys. J. Jpn. Inst. Met. Mater. 1989, 53, 266–272. [Google Scholar] [CrossRef] [Green Version]
- Maebashi, T.; Kozakai, T.; Doi, M. Phase equilibria in iron-rich Fe-Al-V ternary alloy system. Z. Met. 2004, 95, 1005–1010. [Google Scholar] [CrossRef]
- Wang, J.; Feng, Q.S.; Wang, S.H.; Lu, X.G.; Li, C.H. Thermodynamic modeling of Al-Fe-V ternary system. Mater. Res. Express 2019, 6, 126539. [Google Scholar] [CrossRef]
- Tsin-Khua, B.; Kornilov, I.I. Fe-V-Ti (iron-vanadium-Titanium). J. Inorg. Chem. 1960, 5, 434. (In Russian) [Google Scholar]
- Ghosh, G.; Raghavan, V. Progress in metallurgical research: Fundamental and applied aspects. In Proceedings of the International Conference, Indian Institute of Technology, Kampur, India, 11 February 1986; pp. 403–408. [Google Scholar]
- Villars, P.; Prince, A.; Okamoto, H. Handbook of Ternary Alloy Phase Diagrams; ASM International: Cleveland, OH, USA, 1995; Volume 7, pp. 10927–10934. [Google Scholar]
- Prima, S.B.; Tretyachenko, L.A. Area of homogeneity of Laves phase in the Ti-V-Fe ternary system. Sov. Powder Metall. Met. Ceram. 1987, 26, 414–415. [Google Scholar] [CrossRef]
- Massicot, B.; Joubert, J.M.; Latroche, M. Phase equilibria in the Fe-Ti-V system. Int. J. Mater. Res. 2010, 101, 1414–1423. [Google Scholar] [CrossRef]
- Guo, G.P.; Li, C.R.; Zheng, X.; Du, Z.M. Thermodynamic modeling of the Fe-Ti-V system. Calphad 2012, 38, 155–160. [Google Scholar] [CrossRef]
- Dinsdale, A.T. SGTE data for pure elements. Calphad 1991, 15, 317–425. [Google Scholar] [CrossRef]
- Redlich, O.; Kister, A.T. Algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 1948, 40, 345–348. [Google Scholar] [CrossRef]
- Available online: https://computherm.com/ (accessed on 16 November 2021).
- Wang, S.S.; Wang, K.; Chen, G.Y.; Li, Z.; Qin, Z.W.; Lu, X.G.; Li, C.H. Thermodynamic modeling of Ti-Fe-Cr ternary system. Calphad 2017, 56, 160–168. [Google Scholar] [CrossRef]
- Muggianu, Y.M.; Bambino, M.; Bros, J.P. Enthalpy of formation of liquid Bi-Sn-Ga alloys at 723 K, choice of an analytical expression of integral and partial excess quantities of mixing. J. Chim. Phys. 1975, 72, 83–88. [Google Scholar] [CrossRef]
- Kolachev, B.; Il’in, A.A.; Ryndenkov, D. Structural diagrams of titanium alloys in the “molybdenum equivalent-aluminum equivalent” coordinates. Russ. Metall. 1997, 1, 118–128. [Google Scholar]
- Wang, H.B.; Wang, S.S.; Gao, P.Y.; Jiang, T.; Lu, X.G.; Li, C.H. Microstructure and mechanical properties of a novel near-α titanium alloy Ti6.0Al4.5Cr1.5Mn. Mater. Sci. Eng. A 2016, 672, 170–174. [Google Scholar] [CrossRef]
- Li, C.L.; Lee, D.; Mi, X.J.; Ye, W.J.; Hui, S.X.; Lee, Y.T. Effect of Al addition on ω precipitation and age hardening of Ti-Al-Mo-Fe alloys. Metall. Mater. Trans. A 2016, 47, 2454–2461. [Google Scholar] [CrossRef]
- Feng, Q.Y.; Tong, X.W.; Wang, J.; Wang, D.C.; Gao, Q. Status quo and development tendency on the research of low-cost titanium alloy. Mater. Rev. 2017, 31, 128–134. [Google Scholar]
- Zhao, Y.Q.; Liu, J.L.; Zhou, L. Segregation law of Cu, Fe and Cr in typical titanium alloy. Rare Met. Mater. Eng. 2005, 34, 531–538. [Google Scholar]
- Shang, G.Q.; Wang, X.N.; Fei, Y.; Li, J.; Zhu, Z.S.; Zhu, L.W. Experimental study on heat yreatment processing of a new low cost titanium alloy used in aviation field. Mater. Sci. Forum 2013, 747–748, 919–925. [Google Scholar] [CrossRef]
- Reynolds, A.P.; Hood, E.; Tang, W. Texture in friction stir welds of Timetal 21S. Scr. Mater. 2005, 52, 491–494. [Google Scholar] [CrossRef]
- Srinivasu, G.; Natraj, Y.; Bhattacharjee, A.; Nandy, T.K.; Nageswara Rao, G.V.S. Tensile and fracture toughness of high strength β Titanium alloy, Ti-10V-2Fe-3Al, as a function of rolling and solution treatment temperatures. Mater. Des. 2013, 47, 323–330. [Google Scholar] [CrossRef]
- Akahori, T.; Niinomi, M.; Nakai, M.; Tsutsumi, H.; Aki, S.; Itsumi, Y.; Murakami, S.; Oyama, H. Relationship between microstructures and mechanical properties in Ti-4.5Al-2Mo-1.6V-0.5Fe-0.3Si-0.03C for next-generation aircraft applications. Mater. Trans. 2013, 54, 783–790. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.G.; Cai, H.J.; Hui, S.X. Microstructure and mechanical properties of a novel Ti-Al-Cr-Fe titanium alloy after solution treatment. J. Alloys Compd. 2015, 640, 253–259. [Google Scholar] [CrossRef]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys: Fundamentals and Applications; WILEY-VCH Verlag GmbH & Co.: Weinheim, Germany, 2003. [Google Scholar]

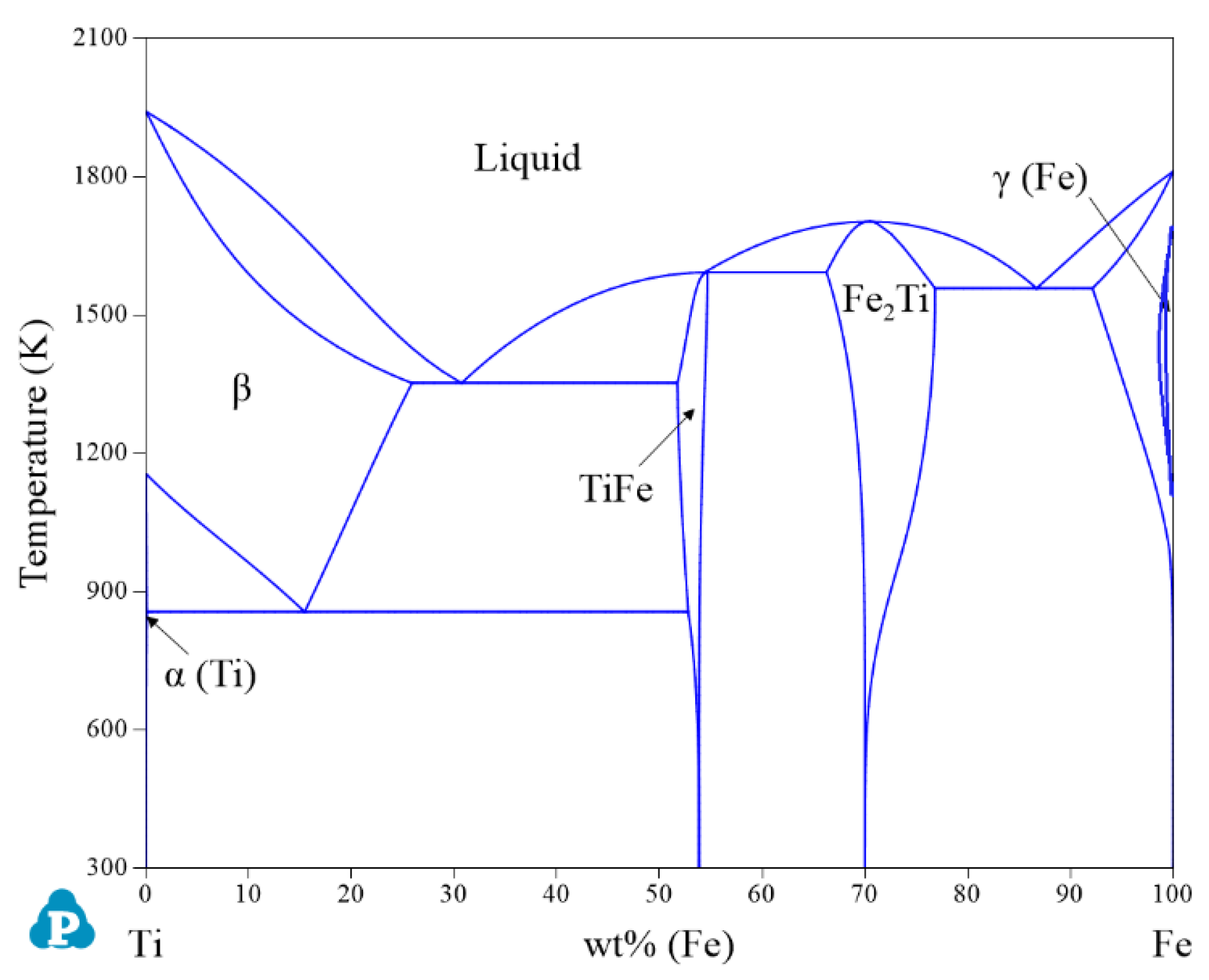
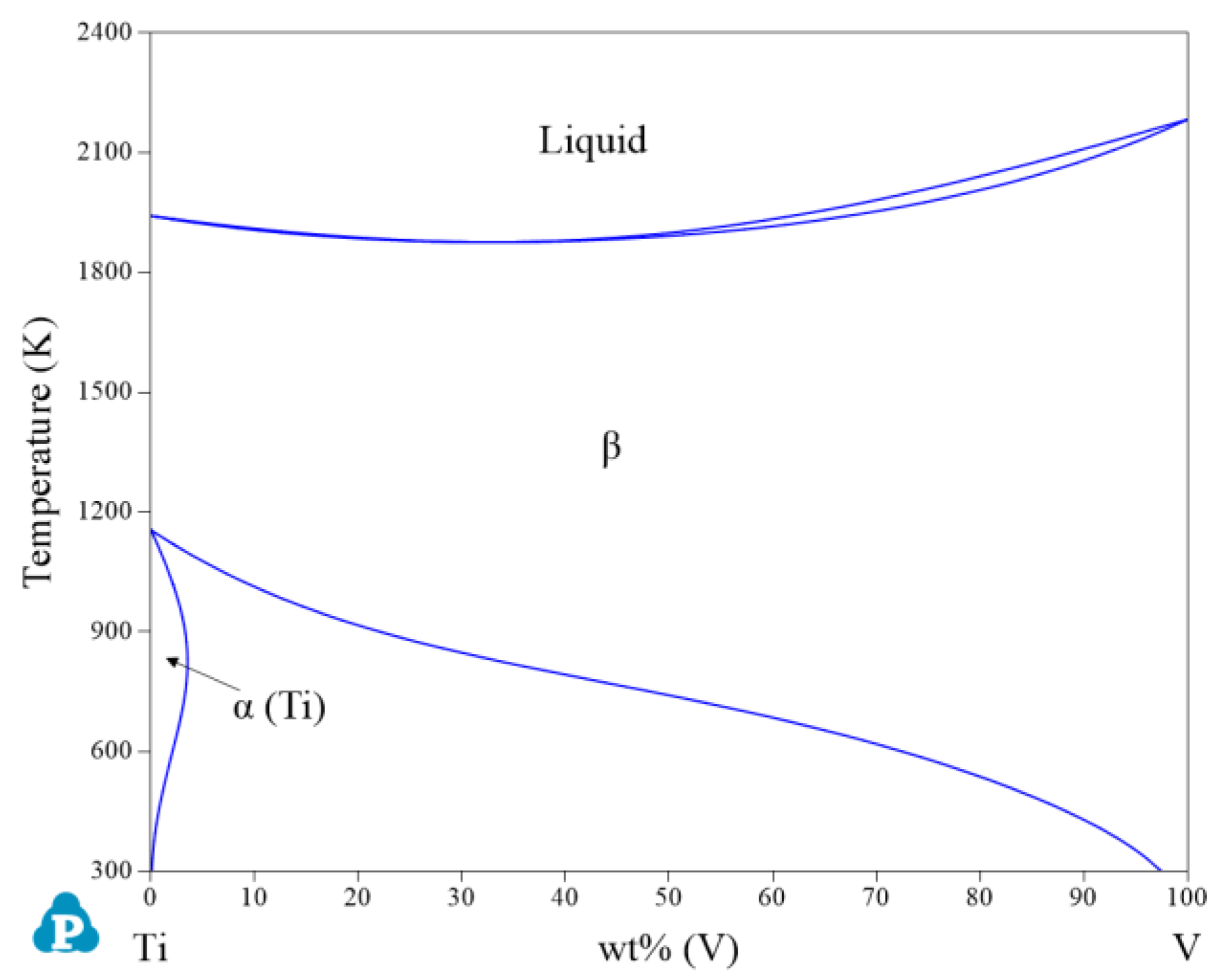
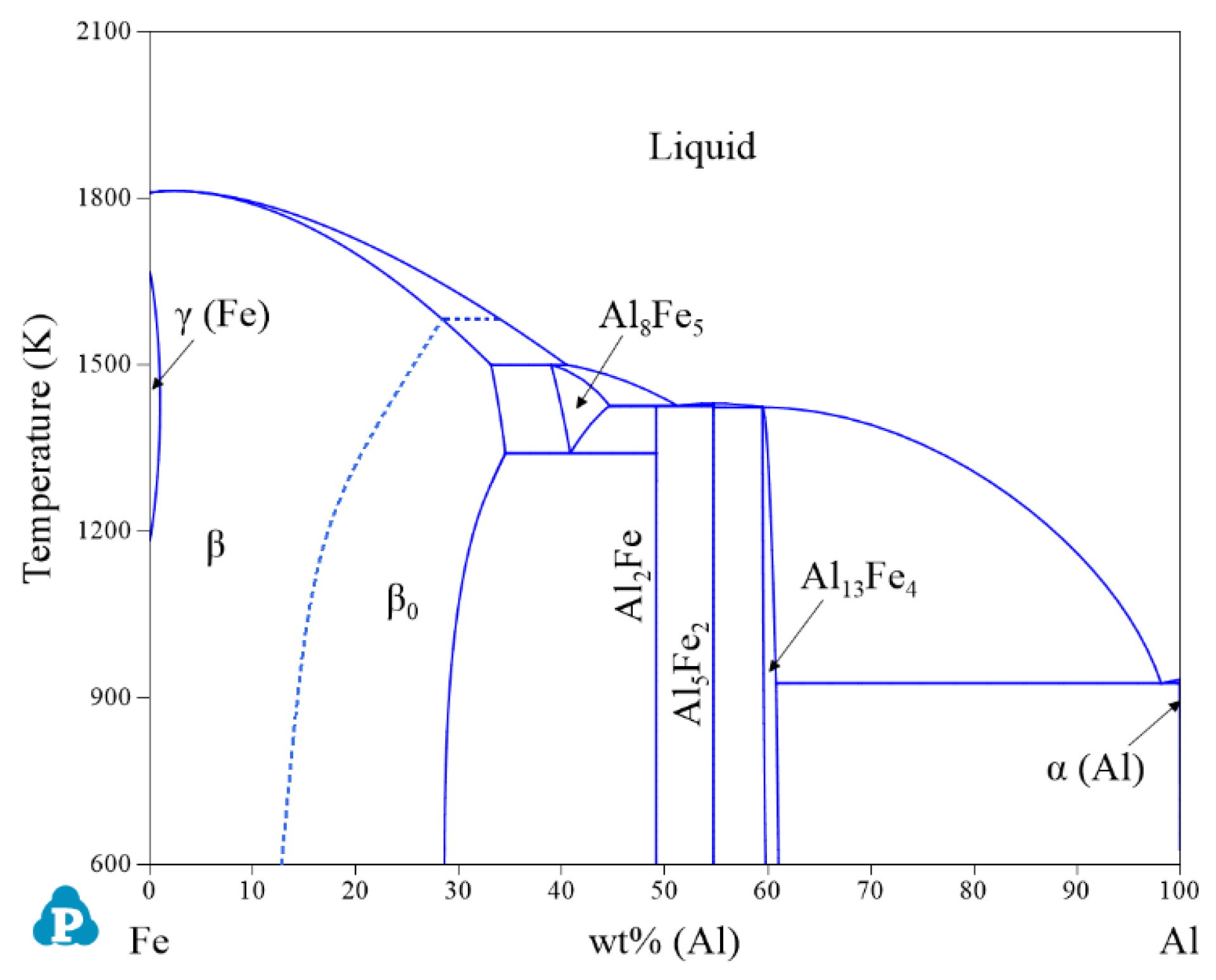

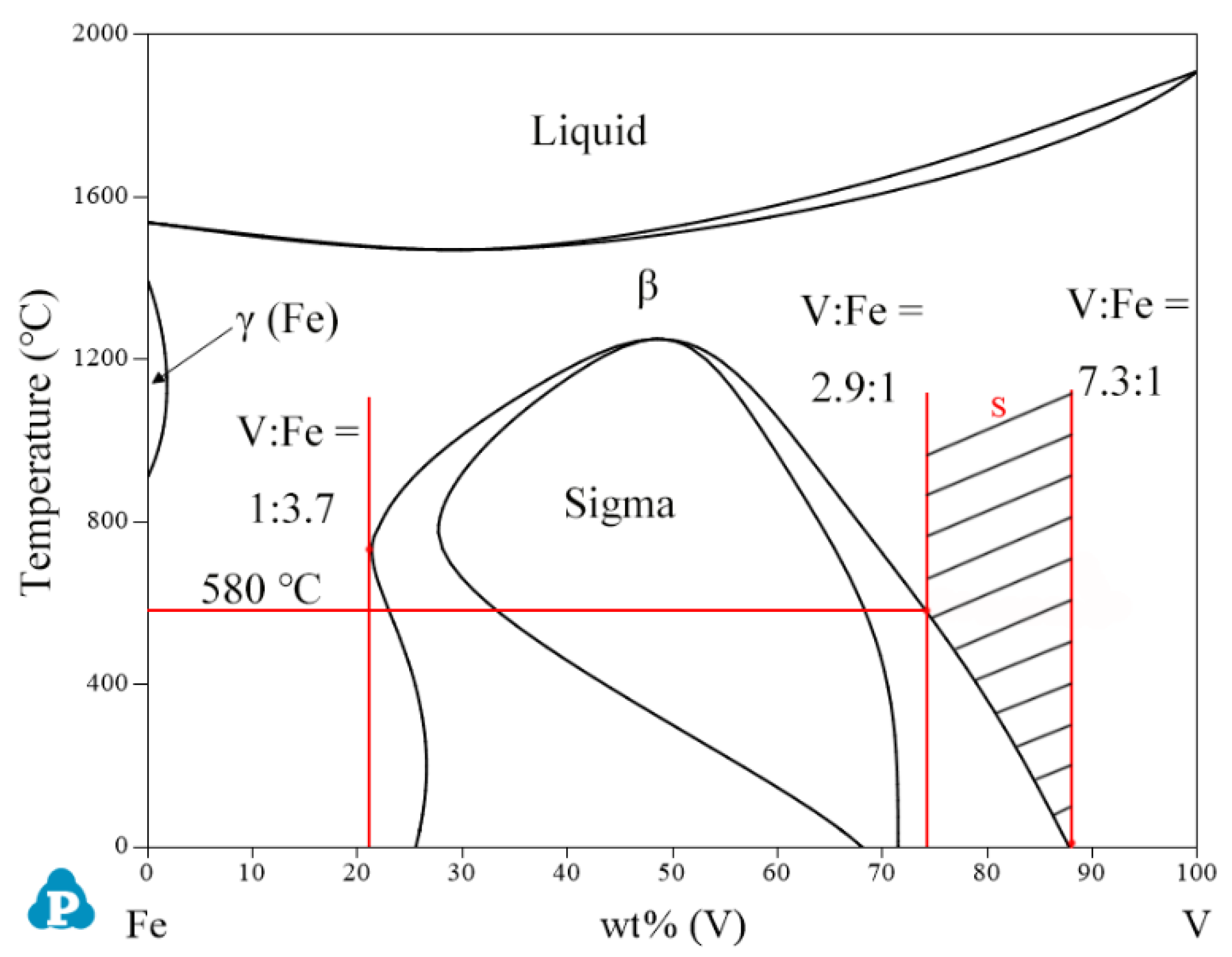

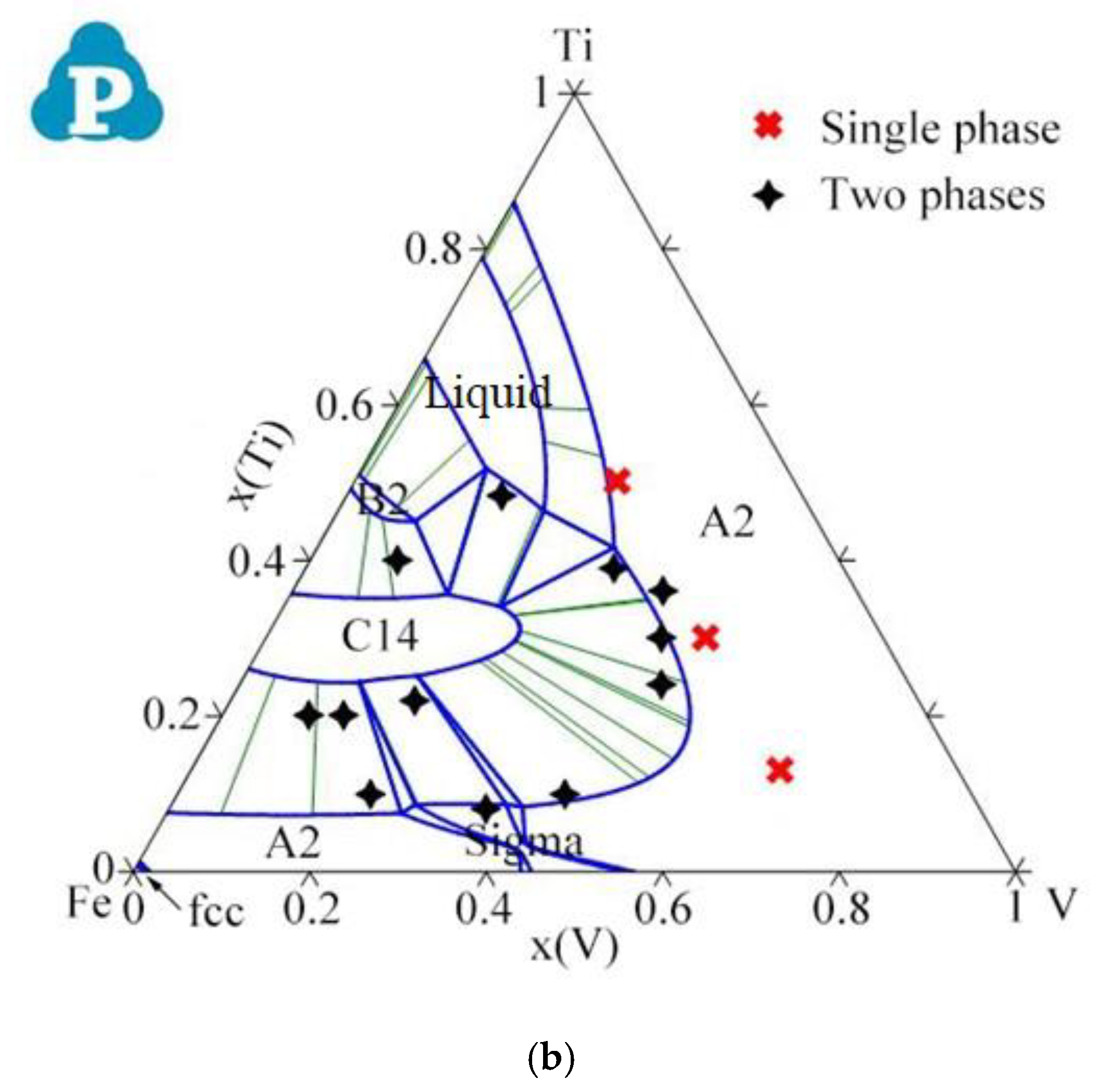

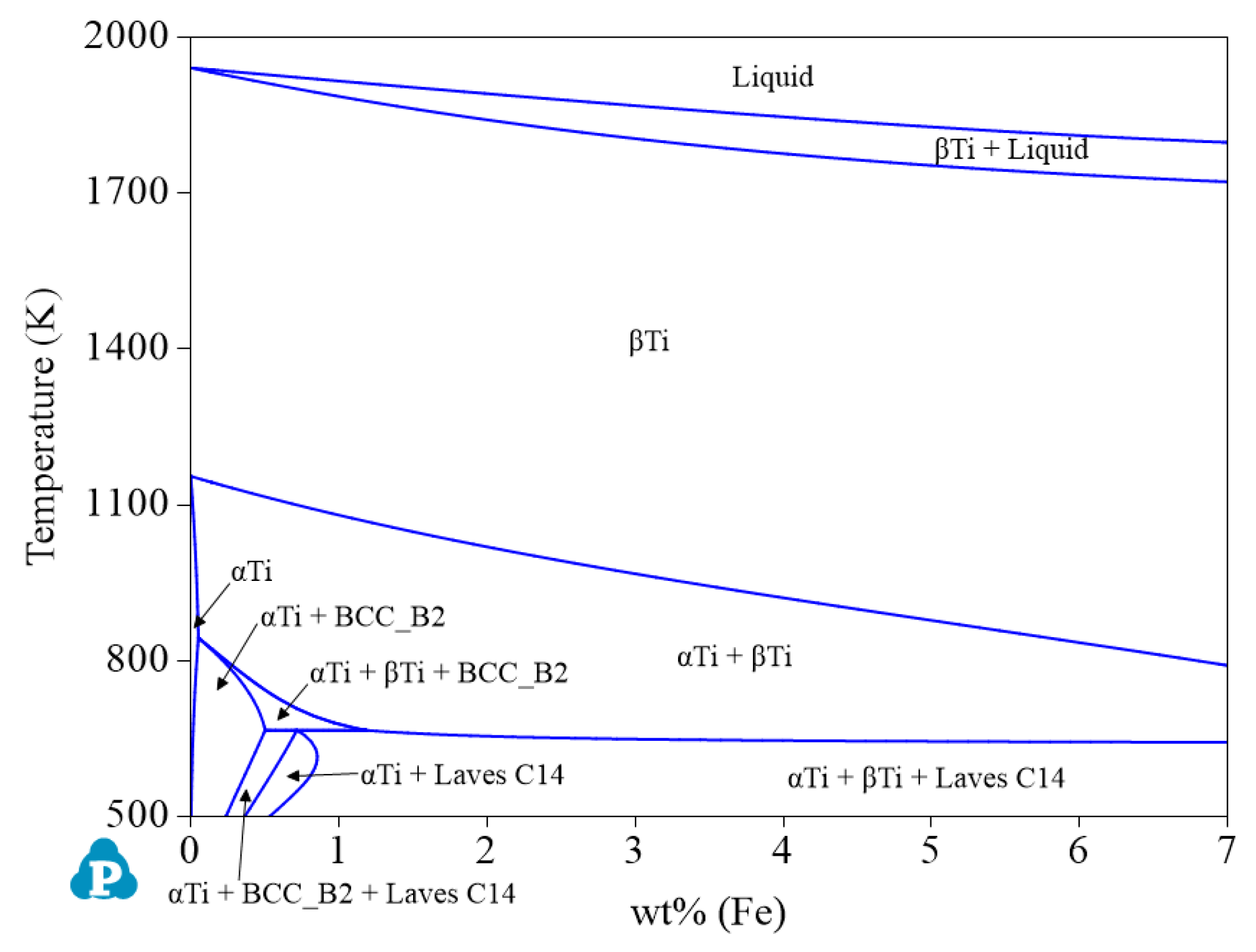
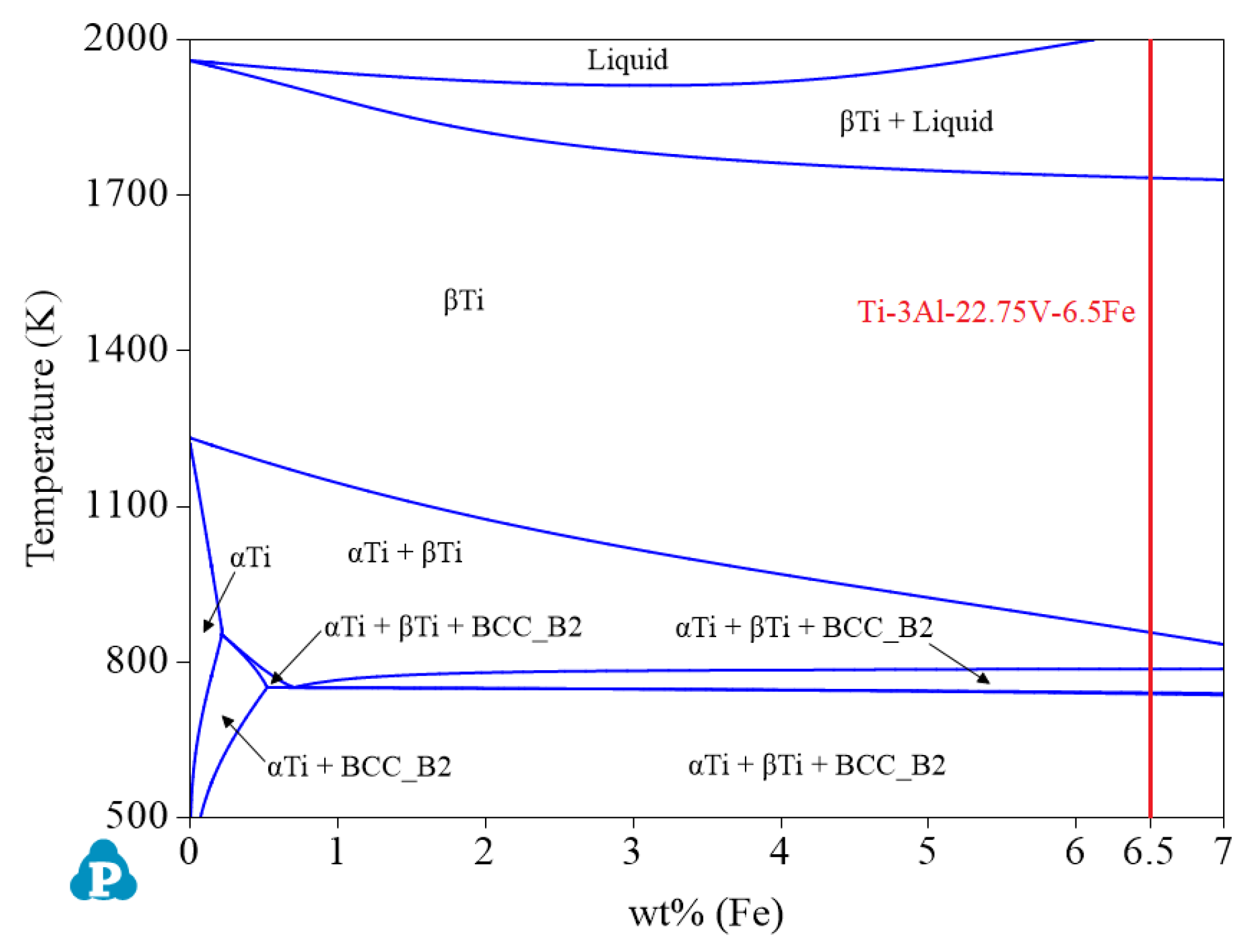
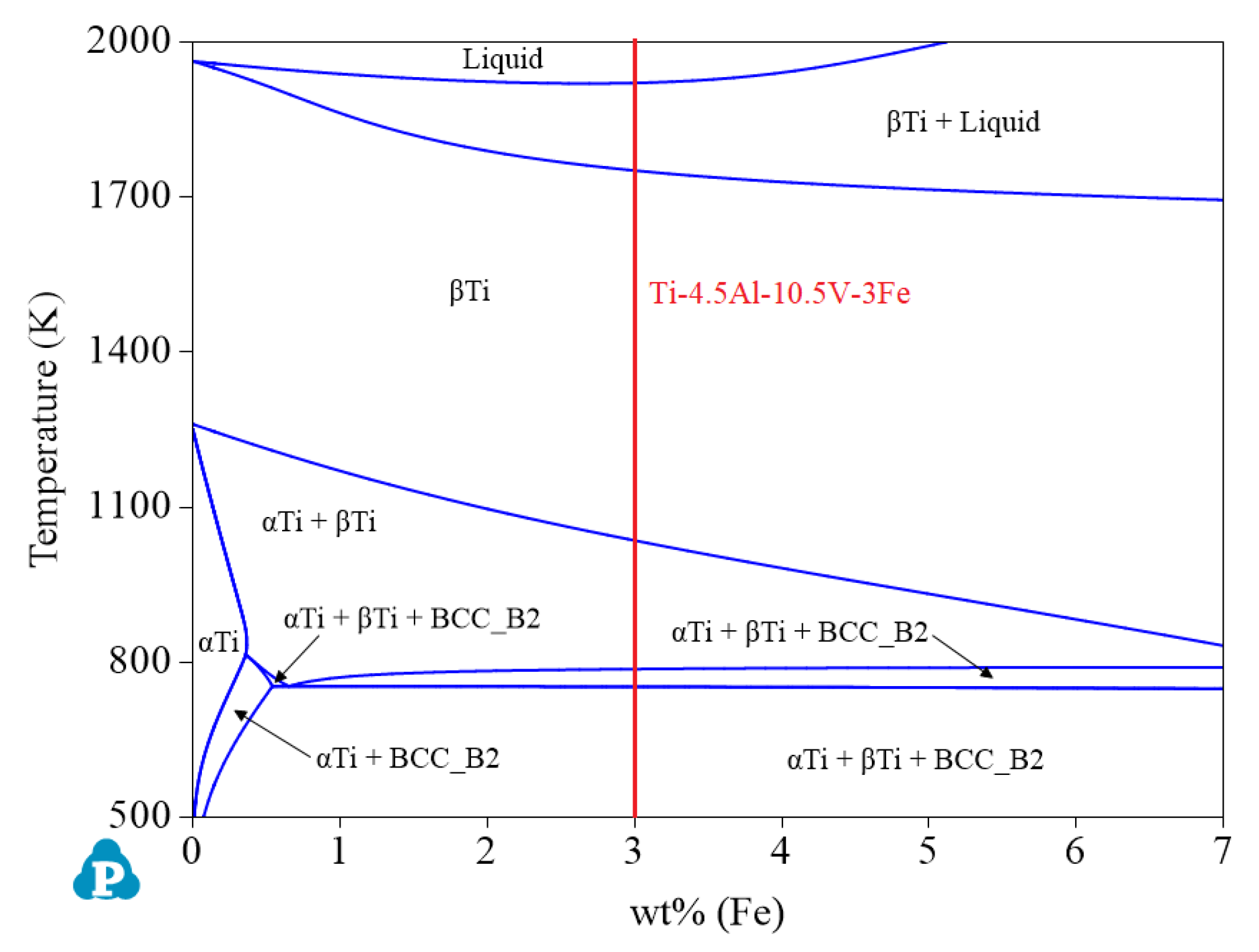

| System | Phase | Struktur-Bericht | Prototype | Person Symbol | Space Group | References |
|---|---|---|---|---|---|---|
| Ti-Al | α(Al) | FCC_A1 | Cu | cF4 | m | [18] |
| β | BCC_A2 | W | cI2 | m | [18] | |
| β0 | BCC_B2 | CsCl | cI2 | m | [18] | |
| α(Ti) | HCP_A3 | Mg | hP2 | P63/mmc | [18] | |
| Ti3Al | D019 | Ni3Sn | hP8 | P63/mmc | [18] | |
| TiAl | L10 | AuCu | tP4 | P4/mmm | [18] | |
| Ti3Al5 | - | Ti3Al5 | tP32 | P4/mbm | [18] | |
| Ti2Al5 | - | Ti2Al5 | tP28 | P4/mmm | [18] | |
| TiAl2 | - | HfGa2 | tI24 | I41/amd | [18] | |
| H_TiAl3 | D022 | TiAl3(h) | tI8 | I4/mmm | [18] | |
| L_TiAl3 | - | TiAl3(l) | tI32 | I4/mmm | [18] | |
| Ti-Fe | γ(Fe) | FCC_A1 | Cu | cF4 | m | [36] |
| β | BCC_A2 | W | cI2 | m | [36] | |
| α(Ti) | HCP_A3 | Mg | hP2 | P63/mmc | [36] | |
| TiFe | BCC_B2 | CsCl | cP2 | m | [36] | |
| Fe2Ti | C14 | MgZn2 | hP12 | P63/mmc | [36] | |
| Ti-V | β | BCC_A2 | W | cI2 | m | [27] |
| α(Ti) | HCP_A3 | Mg | hP2 | P63/mmc | [27] | |
| Fe-Al | γ(Fe) and α(Al) | FCC_A1 | Cu | cF4 | m | [30] |
| β | BCC_A2 | W | cI2 | m | [30] | |
| β0 | BCC_B2 | CsCl | cP8 | m | [30] | |
| Al8Fe5 | D82 | Cu5Zn8 | cI52 | m | [30] | |
| Al2Fe | - | Al2Fe | aP18 | P1 | [30] | |
| Al5Fe2 | - | - | oC? | Cmcm | [30] | |
| Al13Fe4 | - | - | mC102 | C2/m | [30] | |
| Al-V | α(Al) | FCC_A1 | Cu | cF4 | m | [37] |
| β | BCC_A2 | W | cI2 | m | [37] | |
| Al21V2 | - | Al21V2 | cF176 | m | [37] | |
| Al45V7 | - | Al45V7 | mC104 | C2/m | [37] | |
| Al23V4 | - | Al23V4 | hP54 | P63/mmc | [37] | |
| Al3V | - | Al3Ti | tI8 | I4/mmm | [37] | |
| Al8V5 | - | Cu5Zn8 | cI52 | 3m | [37] | |
| Fe-V | γ(Fe) | FCC_A1 | Cu | cF4 | m | [38] |
| β | BCC_A2 | W | cI2 | m | [38] | |
| sigma | D8b | σCrFe | tP30 | - | [38] |
| Phase/Model | Thermodynamic Parameters | References |
|---|---|---|
| Liquid (Fe, Ti, V)1 | [24] | |
| [24] | ||
| [35] | ||
| [35] | ||
| [27] | ||
| [27] | ||
| This work | ||
| FCC_A1 (Fe, Ti, V)1 | [24] | |
| [24] | ||
| [24] | ||
| [24] | ||
| [24] | ||
| [35] | ||
| BCC_A2 (Fe, Ti, V)1 | [24] | |
| [24] | ||
| [24] | ||
| [24] | ||
| [35] | ||
| [35] | ||
| [35] | ||
| [35] | ||
| [35] | ||
| [35] | ||
| [35] | ||
| [35] | ||
| [35] | ||
| [27] | ||
| [27] | ||
| This work | ||
| HCP_A3 (Fe, Ti, V)1 | [24] | |
| [27] | ||
| BCC_B2 (Fe, Ti, V)0.5(Fe, Ti, V)0.5 | [24] | |
| [24] | ||
| [24] | ||
| [24] | ||
| [24] | ||
| [24] | ||
| This work | ||
| This work | ||
| This work | ||
| This work | ||
| This work | ||
| This work | ||
| Sigma (Fe)8(V)4(Fe, Ti, V)18 | [35] | |
| [35] | ||
| This work | ||
| This work | ||
| This work | ||
| This work | ||
| This work | ||
| C14 (Fe, Ti, V)2(Fe, Ti, V)1 | [24] | |
| [24] | ||
| This work | ||
| [24] | ||
| [24] | ||
| This work | ||
| This work | ||
| This work | ||
| This work | ||
| This work | ||
| This work | ||
| This work | ||
| This work | ||
| This work | ||
| This work |
| System | Source of the Ternary Description | Constituent Binary System | Source of the Constituent Binary Description | Postscript |
|---|---|---|---|---|
| Ti-Al-Fe | Hu et al. [46] | Ti-Al | Witusiewicz et al. [18] | Adopted |
| Ti-Fe | Bo et al. [24] | |||
| Al-Fe | Sundman et al. [30] | |||
| Ti-Al-V | Lu et al. [56] | Ti-Al | Witusiewicz et al. [18] | Adopted |
| Ti-V | Ghosh et al. [27] | |||
| Al-V | Gong et al. [32] | |||
| Al-Fe-V | Wang et al. [61] | Al-Fe | Sundman et al. [30] | Adopted |
| Al-V | Gong et al. [32] | |||
| Fe-V | Kumar et al. [35] | |||
| Ti-Fe-V | Present work | Ti-Fe | Bo et al. [24] | Re-assessed in the present work |
| Ti-V | Ghosh et al. [27] | |||
| Fe-V | Kumar et al. [35] |
| Country | Trade Mark | Nominal Composition (wt%) | References |
|---|---|---|---|
| USA | Timetal 62S | Ti-6Al-1.7Fe-0.1Si | [78] |
| Timetal 21S | Ti-15Mo-2.7Nb-3Al-0.2Si | [79] | |
| Ti-1023 | Ti-10V-2Fe-3Al | [80] | |
| Japan | SP-700 | Ti-4.5Al-3V-2Mo-2Fe | [78] |
| KSTi-9 | Ti-4.5Al-2Mo-1.6V-0.5Fe-0.3Si-0.03C | [81] | |
| China | Ti8LC | Ti-6Al-1Fe-1Mo | [78] |
| Ti12LC | Ti-4.5Al-1.5Fe-6.8Mo | [78] | |
| - | Ti-3Al-3.7Cr-2Fe | [82] |
| The Content of Al (wt%) | The Content of Fe and V in α Titanium Alloys | The Content of Fe and V in α + β Titanium Alloys | The Content of Fe and V in β Titanium Alloys | |||
|---|---|---|---|---|---|---|
| Fe (wt%) | V (wt%) | Fe (wt%) | V (wt%) | Fe (wt%) | V (wt%) | |
| 3.0 | <2.48 | <8.68 | 2.48–6.29 | 8.68–22.02 | >6.29 | >22.02 |
| 4.5 | <2.76 | <9.66 | 2.76–6.58 | 9.66–23.03 | >6.58 | >23.03 |
| 6.0 | <3.05 | <10.68 | 3.05–6.86 | 10.68–24.01 | >6.86 | >24.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Q.; Duan, B.; Mao, L.; Jiao, L.; Chen, G.; Lu, X.; Li, C. Thermodynamic Assessment of Ti-Al-Fe-V Quaternary System Applied to Novel Titanium Alloys Designing. Metals 2022, 12, 444. https://doi.org/10.3390/met12030444
Feng Q, Duan B, Mao L, Jiao L, Chen G, Lu X, Li C. Thermodynamic Assessment of Ti-Al-Fe-V Quaternary System Applied to Novel Titanium Alloys Designing. Metals. 2022; 12(3):444. https://doi.org/10.3390/met12030444
Chicago/Turabian StyleFeng, Qisheng, Baohua Duan, Lu Mao, Lina Jiao, Guangyao Chen, Xionggang Lu, and Chonghe Li. 2022. "Thermodynamic Assessment of Ti-Al-Fe-V Quaternary System Applied to Novel Titanium Alloys Designing" Metals 12, no. 3: 444. https://doi.org/10.3390/met12030444






