Mössbauer Spectroscopy for Additive Manufacturing by Selective Laser Melting
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
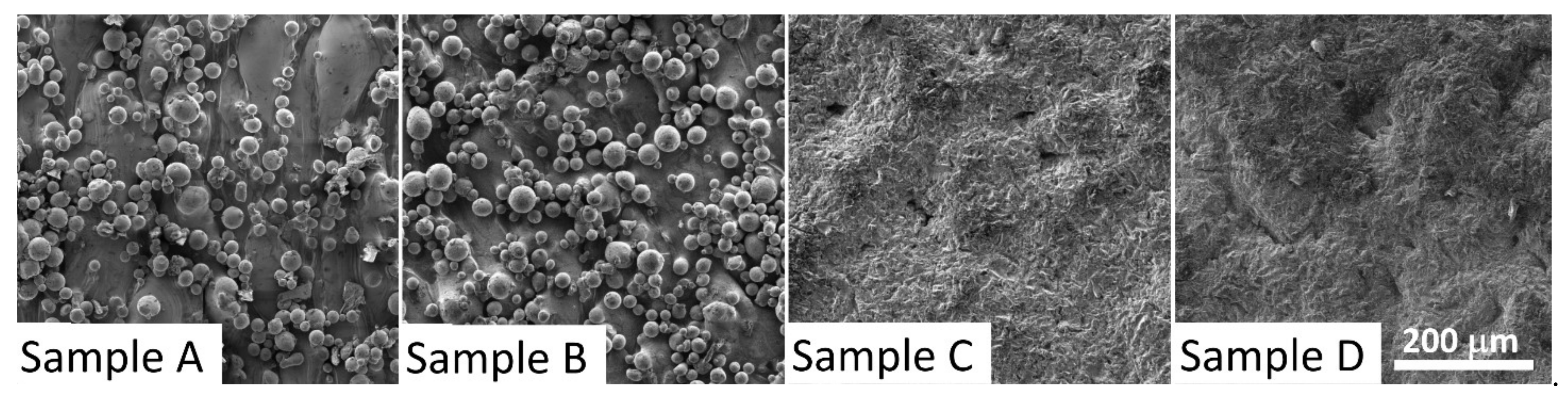
3.1. Scanning Electron Microscopy
3.2. Energy-Dispersive X-ray Spectroscopy
3.3. Mössbauer Spectroscopy
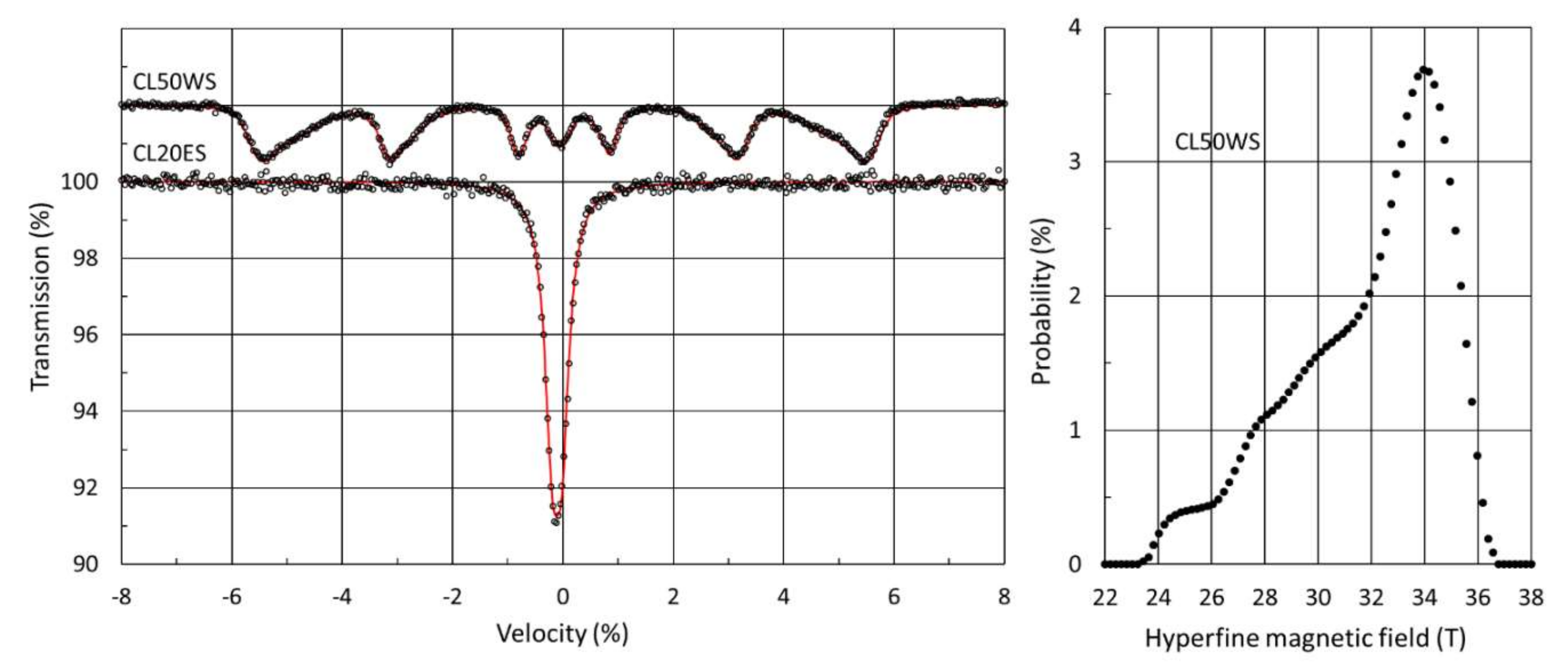
3.3.1. Mössbauer Spectroscopy of Initial Powders
3.3.2. Mössbauer Investigation of Surface Samples Manufactured from CL20ES Powder
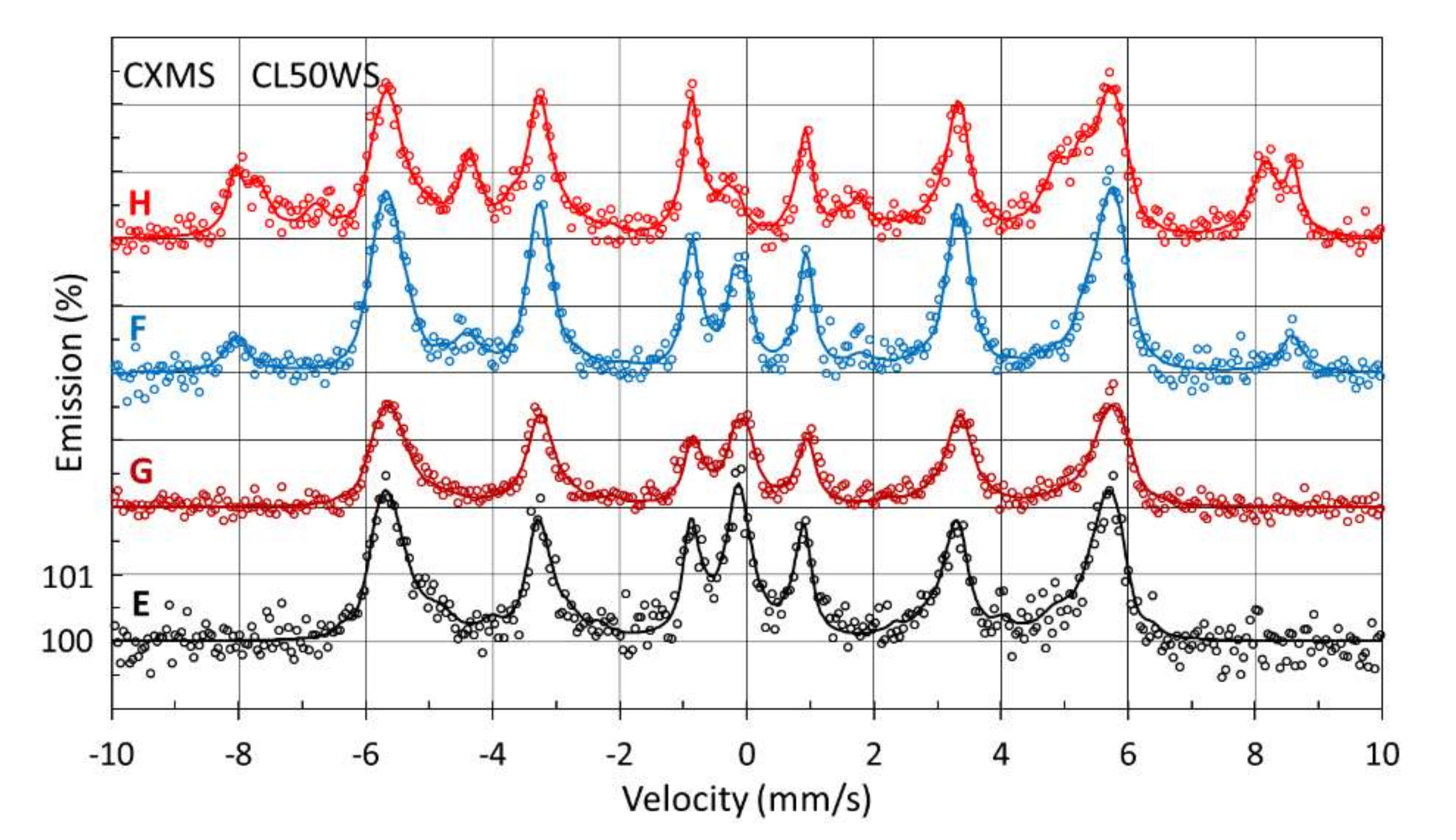
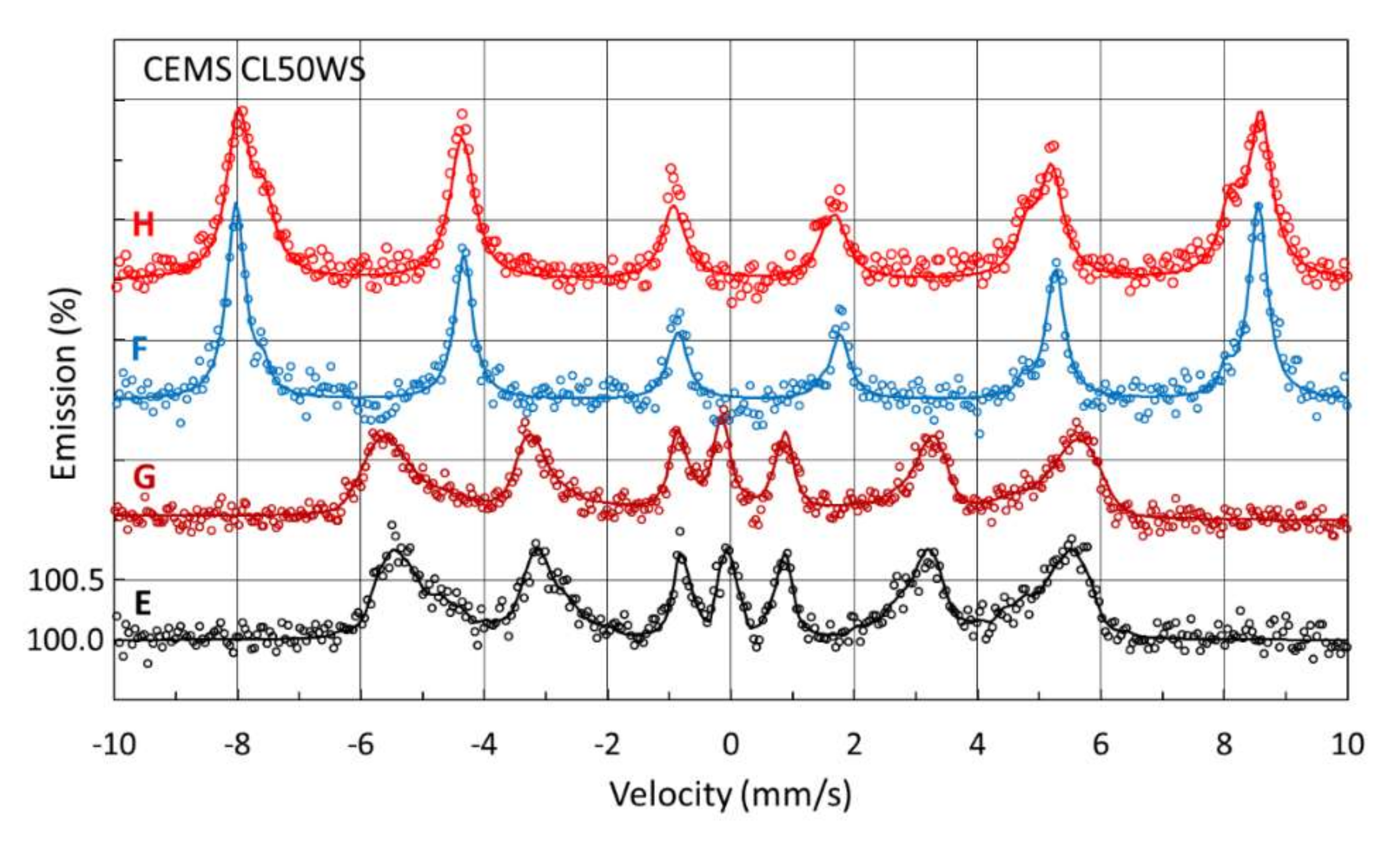
3.3.3. Mössbauer Investigation of Surface Samples Manufactured from CL50WS Powder
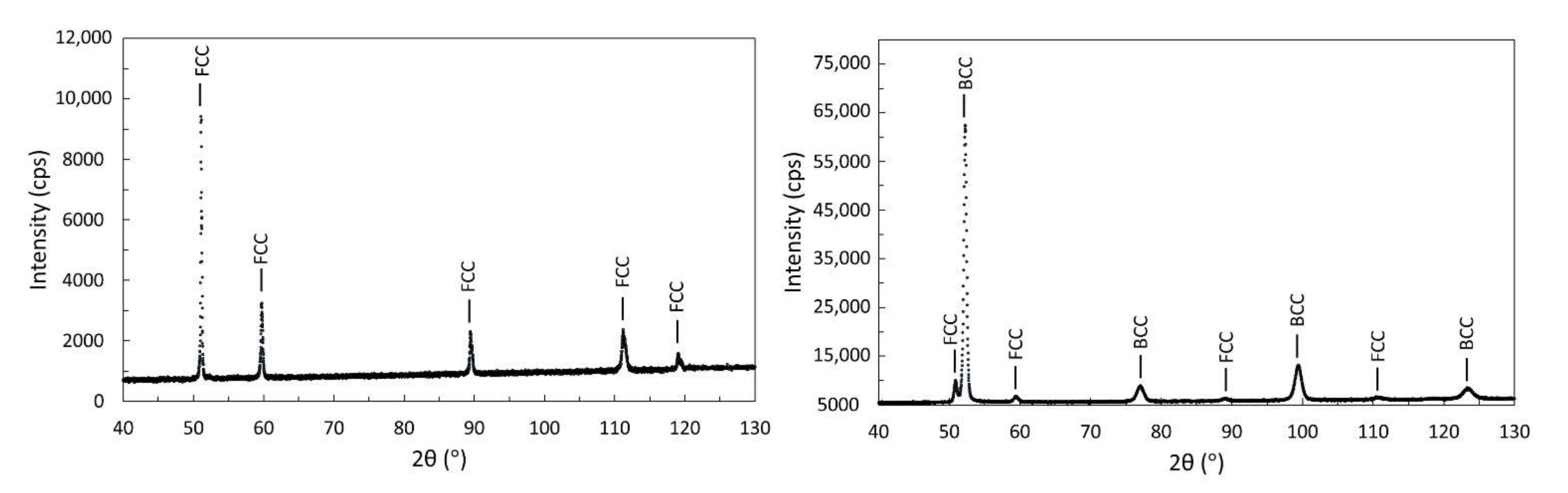
3.4. X-ray Diffraction
3.4.1. X-ray Diffraction of Initial Powders
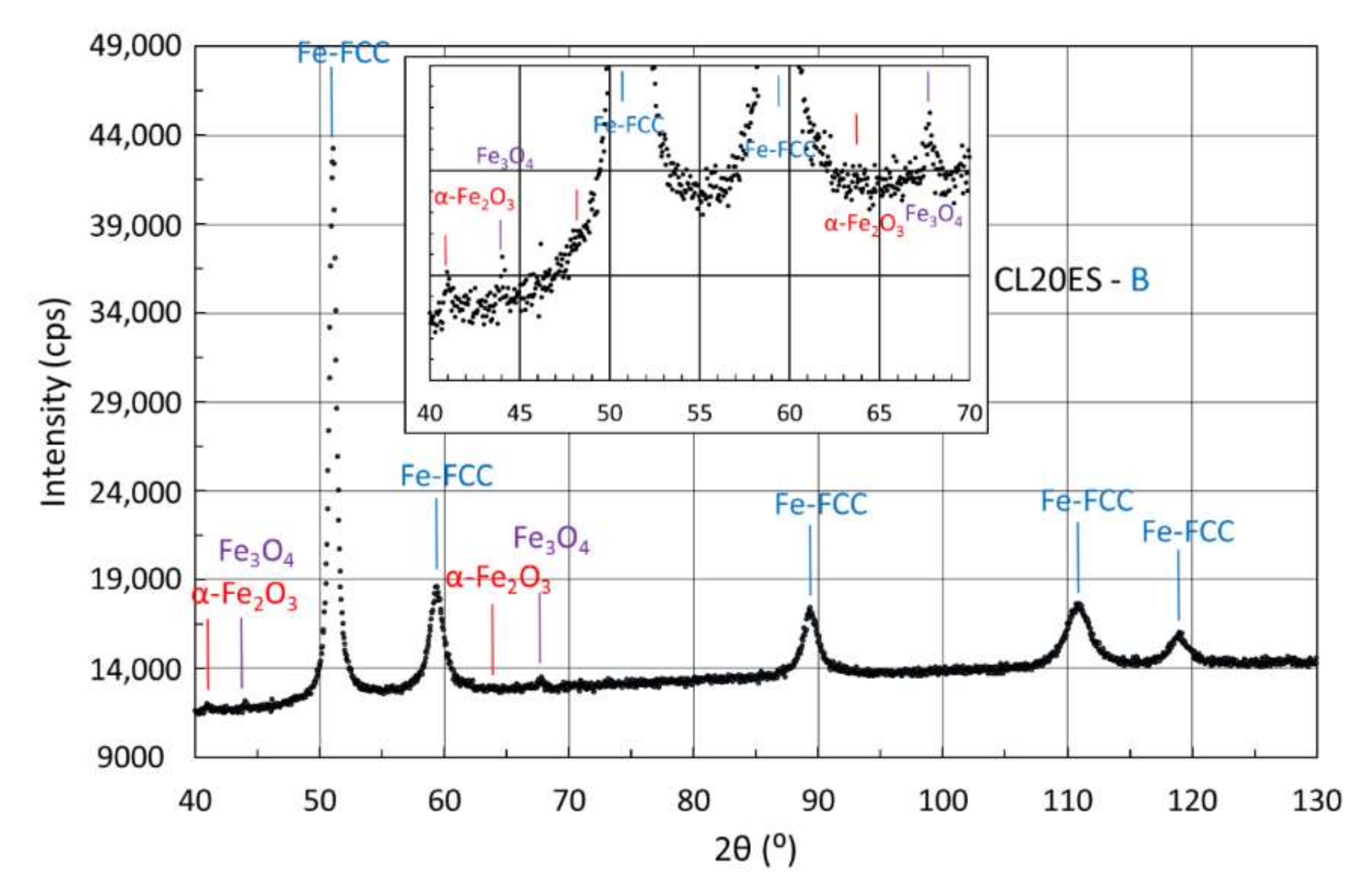
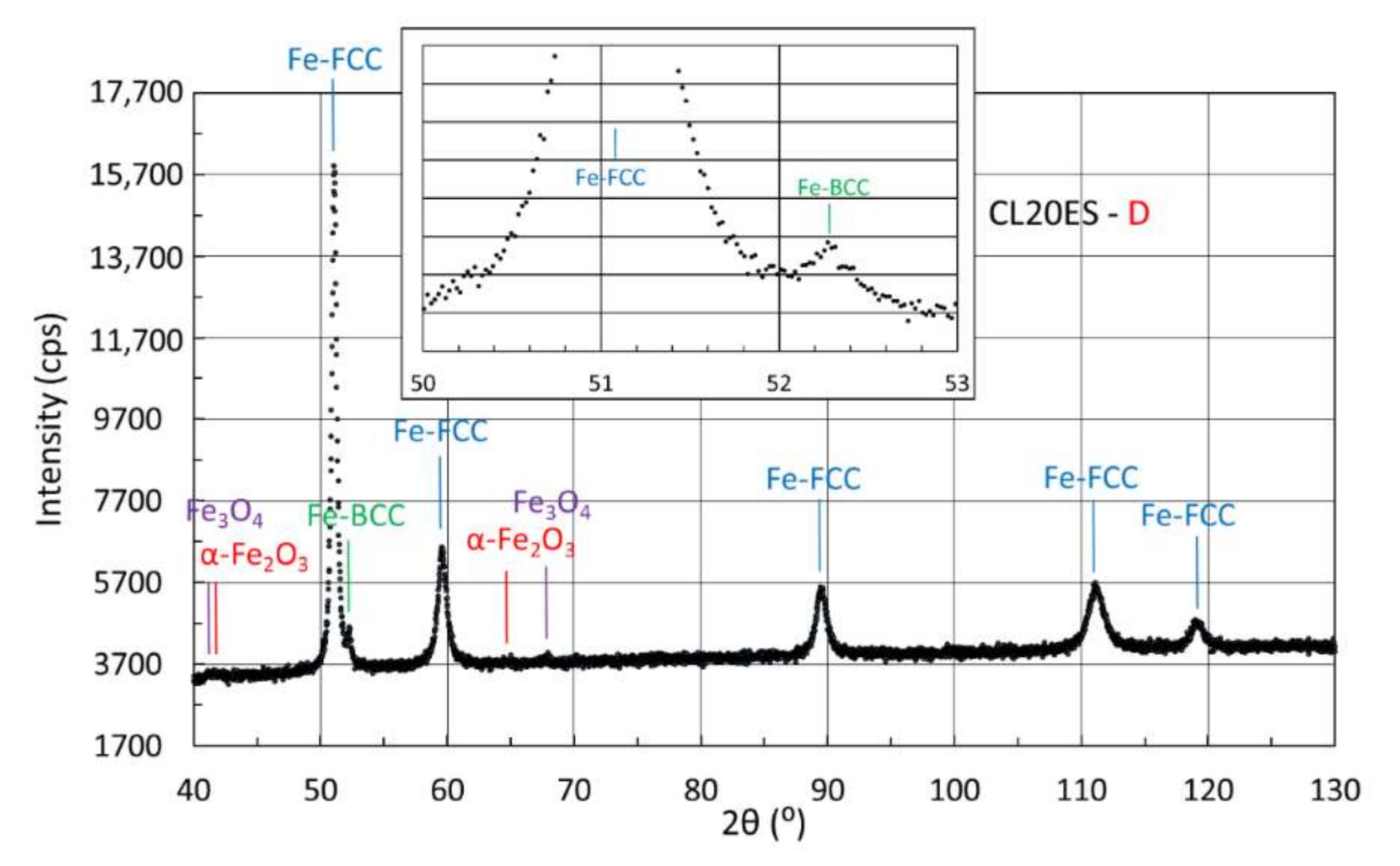
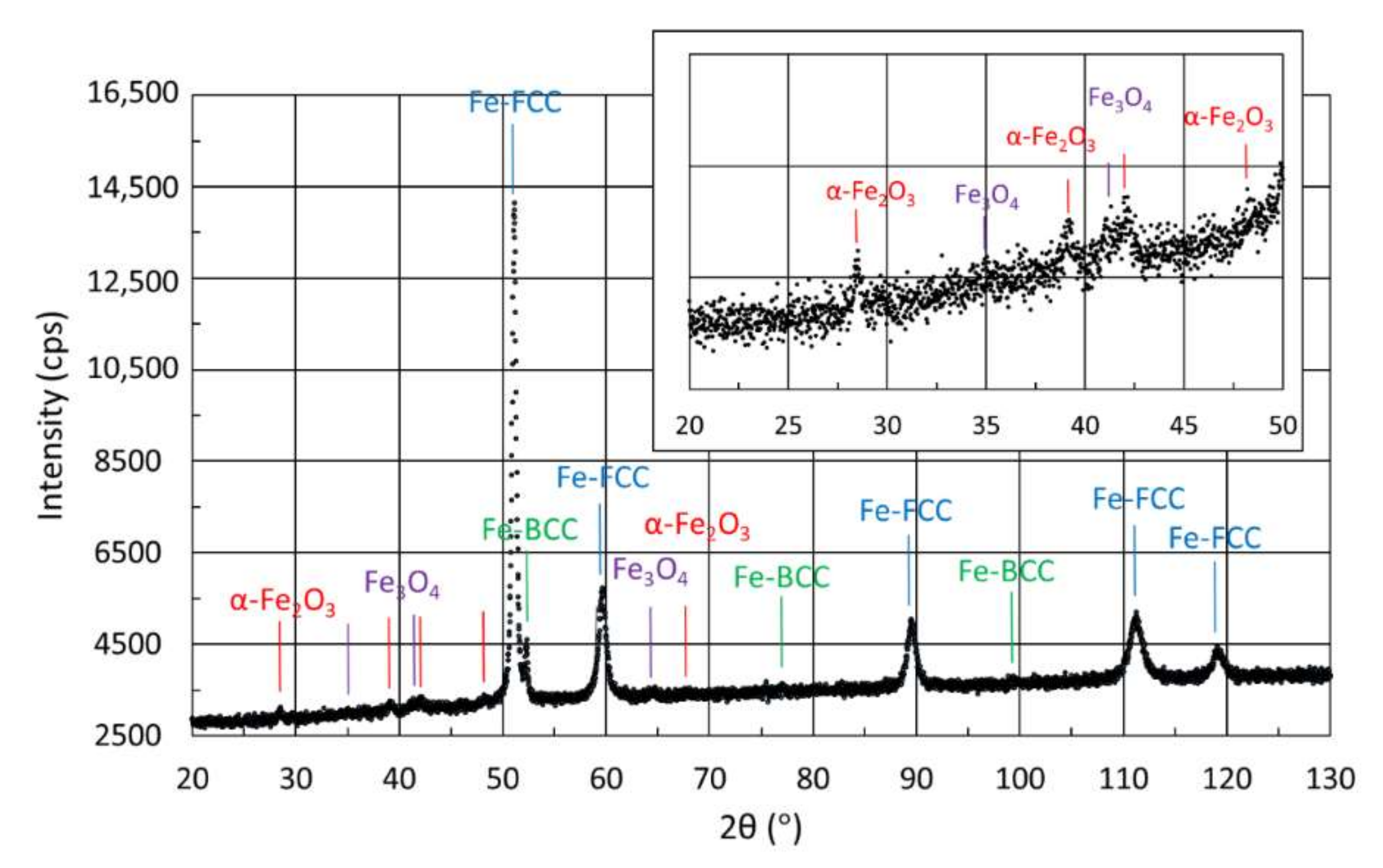
3.4.2. X-ray Diffraction of Samples Manufactured from CL20ES Powder
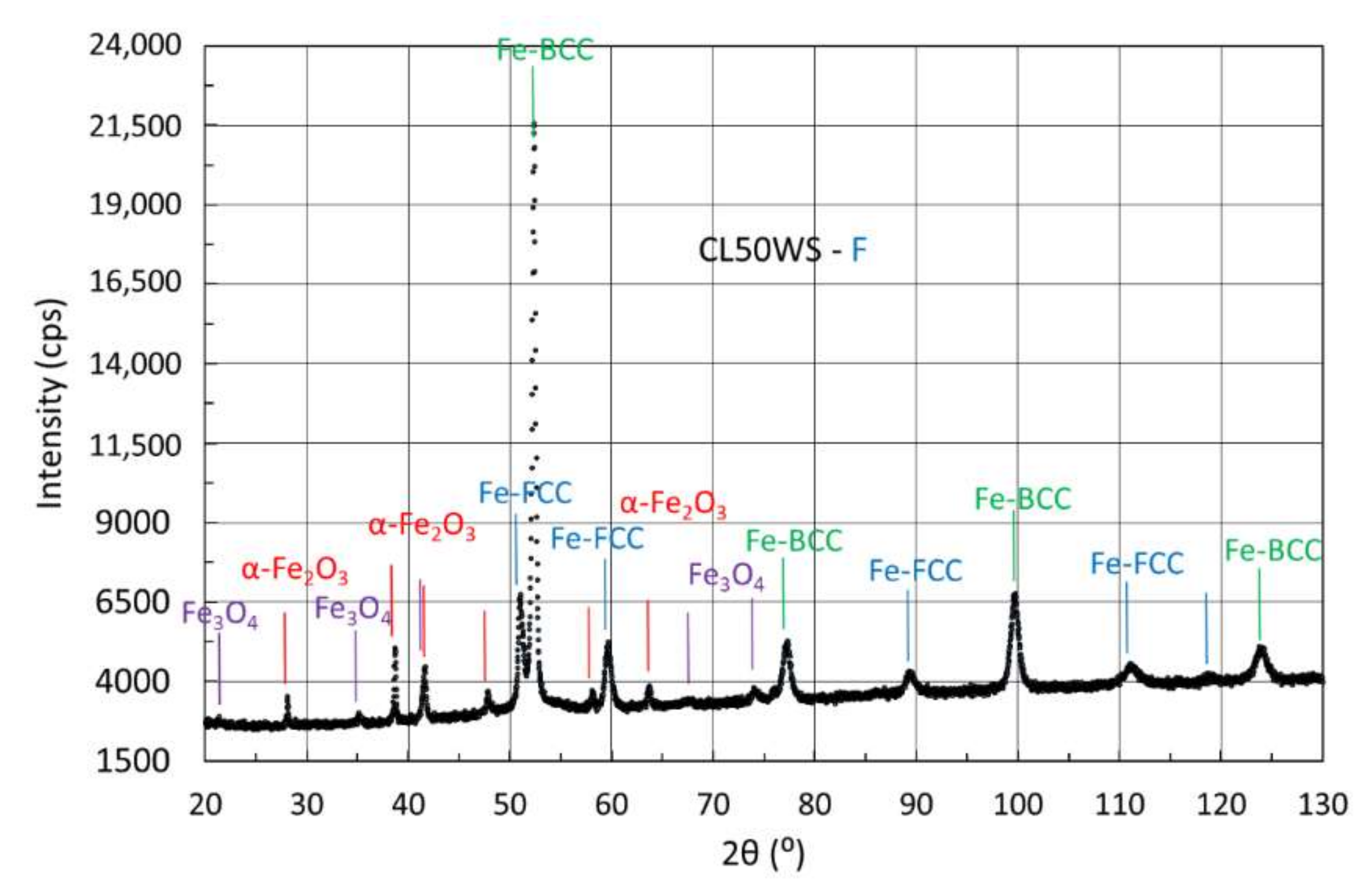
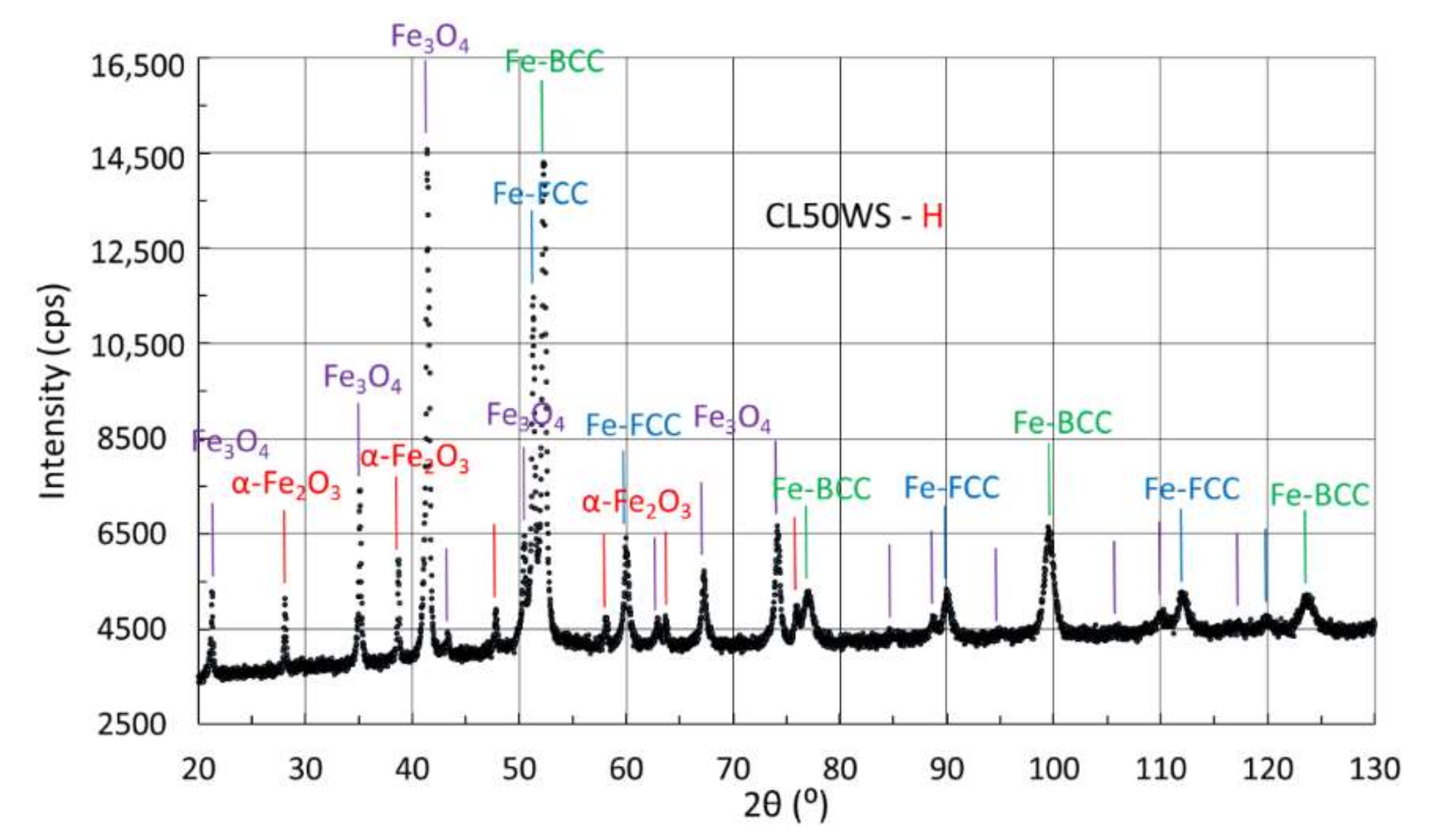
3.4.3. X-ray Diffraction of Samples Manufactured from CL50WS Powder
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alkahari, M.R.; Furumoto, T.; Ueda, T.; Hosokava, A.; Tanaka, R.; Aziz, M.S.A. Thermal conductivity of metal powder and con-solidated material fabricated via selective laser melting. Key Eng. Mater. 2012, 523–524, 244–249. [Google Scholar] [CrossRef] [Green Version]
- Kruth, J.P.; Froyen, L.; Van Vaerenbergh, J.; Mercelis, P.; Rombouts, M.; Lauwers, B. Selective laser melting of iron-based powder. J. Mater. Process. Technol. 2004, 149, 616–622. [Google Scholar] [CrossRef]
- Yadroitsev, I.; Smurov, I. Surface Morphology in selective laser melting of metal powders. Phys. Procedia 2011, 12, 264–270. [Google Scholar] [CrossRef] [Green Version]
- Murr, L.E.; Gaytan, S.M.; Ramirez, D.A.; Martinez, E.; Hernandez, J.; Amato, K.N.; Shindo, P.W.; Medina, F.R.; Wicker, R.B. Metal fabrication by additive manufacturing using laser and electron beam melting technologies. J. Mater. Sci. Technol. 2012, 28, 1–14. [Google Scholar] [CrossRef]
- Gunasekaran, J.; Sevvel, P.; Solomon, I.J. Metallic materials fabrication by selective laser melting: A review. Mater. Today Proc. 2021, 37, 252–256. [Google Scholar] [CrossRef]
- Laskin, A.; Faidel, D.; Laskin, V. Optics to control thermal effects in selective laser melting. In Laser 3D Manufacturing V; SPIE: San Francisco, CA, USA, 2018; Volume 10523. [Google Scholar] [CrossRef]
- Thombansen, U.; Gatej, A.; Pereira, M. Process observation in fiber laser-based selective laser melting. Opt. Eng. 2015, 54, 011008. [Google Scholar] [CrossRef] [Green Version]
- West, C.; Wang, X. Modeling of selective laser sintering/selective laser melting. Proceeding SPIE 2017, 10095, 6. [Google Scholar] [CrossRef]
- Principi, G. The Mössbauer Effect: A Romantic Scientific Page. Metals 2020, 10, 992. [Google Scholar] [CrossRef]
- Mashlan, M.; Linderhof, F.; Davidova, M.; Kubickova, H.; Zemtsova, E. Changes of phase composition of maraging steel 1.2709 during selective laser melting. Hyperfine Interact. 2019, 241, 11. [Google Scholar] [CrossRef]
- Tyczynski, P.; Siemiatkowski, Z.; Bak, P.; Warzocha, K.; Rucki, M.; Szumiata, T. Performance of Maraging Steel Sleeves Produced by SLM with Subsequent Age Hardening. Materials 2020, 13, 3408. [Google Scholar] [CrossRef]
- Gainov, R.R.; Faidel, D.; Behr, W.; Natour, G.; Pauly, F.; Willms, H.; Vagizov, F.G. Investigation of LPBF A800H steel parts using Computed Tomography and Mössbauer spectroscopy. Addit. Manuf. 2020, 32, 101035. [Google Scholar] [CrossRef]
- Linderhof, F.; Mashlan, M.; Doláková, H.; Ingr, T.; Ivanova, T. Surface Micromorphology and Structure of Stainless and Maraging Steel Obtained via Selective Laser Melting: A Mössbauer Spectroscopy Study. Metals 2021, 11, 1028. [Google Scholar] [CrossRef]
- Garbucicchio, M.; Palombarini, G. Surface structures produced in 1C-1.5Cr and 0.38C-Ni-Cr-Mo steels by high-power CO2 laser processing. J. Mater. Sci. 1986, 21, 75–82. [Google Scholar] [CrossRef]
- Afanasieva, L.E.; Ratkevich, G.V.; Ivanova, A.I.; Novoselova, M.V.; Zorenko, D.A. On the Surface Micromorphology and Structure of Stainless Steel Obtained via Selective Laser Melting. J. Surf. Investig. X-Ray Synchrotron Neutron Tech. 2018, 12, 1082–1087. [Google Scholar] [CrossRef]
- Kale, A.B.; Kim, B.K.; Kim, D.I.; Castle, E.G.; Reece, M.; Choi, S.H. An investigation of the corrosion behavior of 316L stainless steel fabricated by SLM and SPS techniques. Mater. Charact. 2020, 163, 110204. [Google Scholar] [CrossRef]
- Pechousek, J.; Mashlan, M. Mössbauer spectrometer as a virtual instrument in the PXI/Compact PCI modular systém. Czech. J. Phys. 2005, 55, 853–863. [Google Scholar] [CrossRef]
- Kholmetskii, A.L.; Misevich, O.V.; Mashlan, M.; Chudakov, V.A.; Anashkevich, A.F.; Gurachevskii, V.L. Air scintillation detector for conversion electrons Mössbauer spectroscopy (CEMS). Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1997, 129, 110–116. [Google Scholar] [CrossRef]
- Klencsár, Z.; Kuzmann, E.; Vértes, A. User-friendly software for Mössbauer spectrum analysis. J. Radioanal. Nucl. Chem. Arctic. 1996, 210, 105–118. [Google Scholar] [CrossRef]
- Klencsár, Z.; Kuzmann, E.; Vértes, A. User-Friendly Program for Multifold Evaluation of Mössbauer Spectra. Hyperfine Interact. 1998, 112, 269–274. [Google Scholar] [CrossRef]
- Peckner, D.; Bernstein, I.M. Handbook of Stainless Steels; McGraw Hill: New York, NY, USA, 1977. [Google Scholar]
- Lacombe, P.; Baroux, B.; Beranger, G. Stainless Steels; Les Edition de Physique: Les Ulis, France, 1993. [Google Scholar]
- Lippold, J.C.; Kotecki, D.J. Welding Metallurgy and Weldability of Stainless Steels; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Castaing, R. Application des Sondes Electronique a Une Methode D’Analyse Ponctuelle Chimique et Cristallographique. Ph.D. Thesis, University of Paris, Paris, France, 1952. [Google Scholar]
- Cook, D.C. Strain induced martensite formation in stainless steel Metall. Mater. Trans. A 1987, 18, 201–210. [Google Scholar] [CrossRef]
- Shabashov, V.A.; Gavrilov, N.V.; Kozlov, K.A.; Makarov, A.V.; Titova, S.G.; Voronin, V.I. Structure of the Surface Layers of Metastable Austenitic Stainless Steel Nitriden in Electron Beam Plasma. Phys. Met. Metallogr. 2018, 119, 755–763. [Google Scholar]
- Machala, L.; Tuček, J.; Zbořil, R. Polymorphous Transformations of Nanometric Iron(III) Oxide: A Review. Chem. Mater. 2011, 23, 3255–3272. [Google Scholar] [CrossRef]
- Boniardi, M.; Casaroli, A. Stainless Steels; Gruppo Lucefin: Esine, Italy, 2014. [Google Scholar]
- Kraus, G. Steels Processing, Structure, and Performance, 2nd ed.; ASM International, Materials Park: Russell Township, OH, USA, 2015; 705p. [Google Scholar]
- Schaeffler, A. Constitution Diagram for Stainless Steel Weld Metal. Metal Prog. 1949, 56, 680. [Google Scholar]






| Steel Powder | Element Concentration (wt %) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | C | Si | Mn | P | S | Cr | Mo | Ni | Ti | Co | |
| CL20ES | Bal | ≤ 0.03 | 0–1.0 | 0–2.0 | ≤ 0.045 | ≤ 0.03 | 16.5–18.5 | 2.0–2.5 | 10.0–13.0 | - | - |
| CL50WS | Bal | ≤ 0.03 | ≤ 0.1 | ≤ 0.15 | ≤ 0.01 | ≤ 0.01 | ≤ 0.25 | 4.5–5.2 | 17.0–9.0 | 0.8–1.2 | 8.5–10.0 |
| Material | Without Sandblasting and Without Annealing | Without Sandblasting and With Annealing | With Sandblasting and Without Annealing | With Sandblasting and With Annealing |
|---|---|---|---|---|
| CL20ES | Sample A | Sample B | Sample C | Sample D |
| CL50WS | Sample E | Sample F | Sample G | Sample H |
| Sample | Accelerating Voltage 11 kV | Accelerating Voltage 20 kV | ||||||
|---|---|---|---|---|---|---|---|---|
| Fe (%) | Cr (%) | Ni (%) | Other (%) | Fe (%) | Cr (%) | Ni (%) | Other (%) | |
| C | 66 ± 2 | 18 ± 1 | 12 ± 1 | 4 ± 1 | 66 ± 2 | 18 ± 1 | 12 ± 1 | 4 ± 1 |
| D | 63 ± 2 | 23 ± 1 | 10 ± 1 | 4 ± 1 | 66 ± 2 | 17 ± 1 | 13 ± 1 | 4 ± 1 |
| Sample | Accelerating Voltage 11 kV | Accelerating Voltage 20 kV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fe (%) | Ni (%) | Co (%) | Mo (%) | Ti (%) | Fe (%) | Ni (%) | Co (%) | Mo (%) | Ti (%) | |
| G | 65 ± 2 | 18 ± 1 | 9 ± 1 | 4 ± 1 | 4 ± 1 | 65 ± 2 | 17 ± 1 | 9 ± 1 | 5 ± 1 | 4 ± 1 |
| H | 80 ± 2 | 1 ± 1 | 18 ± 1 | 0.5 ± 1 | 0.5 ± 1 | 90 ± 2 | 1 ± 1 | 8 ± 1 | 0.5 ± 1 | 0.5 ± 1 |
| Powder | Phase | IS (mm/s) | QS (mm/s) | FWHM (mm/s) | B (T) | A (%) |
|---|---|---|---|---|---|---|
| CL20ES | FCC | −0.11 ± 0.01 | 0.18 ± 0.01 | 0.32 ± 0.01 | - | 100 |
| CL50WS | FCC | −0.06 ± 0.01 | 0.16 ± 0.02 | 0.34 ± 0.02 | - | 8 ± 2 |
| BCC | 0.02 ± 0.01 | - | 0.27 ± 0.01 | 31.7 * | 92 ± 2 |
| Method | Sample | Phase | IS (mm/s) | QS (mm/s) | W (mm/s) | B (T) | A (%) |
|---|---|---|---|---|---|---|---|
| CXMS | A | FCC | −0.13 ± 0.01 | 0.17 ± 0.01 | 0.28 ± 0.01 | - | 100 |
| B | FCC | −0.13 ± 0.01 | 0.18 ± 0.01 | 0.29 ± 0.01 | - | 100 | |
| C | FCC | −0.14 ± 0.01 | 0.17 ± 0.01 | 0.30 ± 0.01 | - | 100 | |
| D | FCC | −0.13 ± 0.01 | 0.17 ± 0.01 | 0.31 ± 0.01 | - | 100 | |
| DD | FCC | −0.11 ± 0.01 | 0.16 ± 0.01 | 0.28 ± 0.01 | - | 95 ± 2 | |
| BCC | −0.06 ± 0.04 | - | 0.37 ± 0.10 | 34.3 ± 0.5 | 5 ± 2 | ||
| CEMS | A | FCC | −0.12 ± 0.01 | 0.15 ± 0.01 | 0.25 ± 0.01 | - | 100 |
| B | FCC | −0.09 ± 0.01 | 0.09 ± 0.02 | 0.27 ± 0.02 | - | 39 ± 2 | |
| α-Fe2O3 | 0.37 ± 0.01 | −0.18 ± 0.02 | 0.47 ± 0.02 | 51.2 ± 0.5 | 61 ± 2 | ||
| C | FCC | −0.11 ± 0.01 | 0.17 ± 0.01 | 0.27 ± 0.01 | - | 100 | |
| D | FCC | −0.08 ± 0.01 | 0.13 ± 0.01 | 0.27 ± 0.01 | - | 56 ± 2 | |
| BCC | 0.00 ± 0.02 | - | 0.56 ± 0.06 | 33.3 ± 0.5 | 32 ± 2 | ||
| doublet | 0.29 ±0.04 | 0.97 ± 0.05 | 0.39 ± 0.06 | - | 12 ± 2 | ||
| DD | FCC | −0.10 ± 0.01 | 0.06 ± 0.08 | 0.35 ± 0.02 | - | 39 ± 2 | |
| BCC | −0.03 ± 0.03 | - | 0.77 ± 0.08 | 33.8 ± 0.5 | 50 ± 2 | ||
| doublet | 0.36 ± 0.03 | 0.95 ± 0.04 | 0.29 ± 0.04 | - | 11 ± 2 |
| Method | Sample | Phase | IS (mm/s) | QS (mm/s) | W (mm/s) | B (T) | A (%) |
|---|---|---|---|---|---|---|---|
| CXMS | E | FCC | −0.07 ± 0.01 | 0.12 ± 0.01 | 0.27 ± 0.02 | - | 8 ± 2 |
| BCC | 0.05 ± 0.01 | - | 0.23 ± 0.02 | 31.5 * | 92 ± 2 | ||
| F | FCC | −0.12 ± 0.01 | 0.18 ± 0.02 | 0.27 ± 0.04 | - | 8 ± 2 | |
| BCC | 0.03 ± 0.01 | - | 0.25 ± 0.01 | 34.4 * | 80 ± 2 | ||
| α-Fe2O3 | 0.35 ± 0.01 | −0.17 ± 0.04 | 0.43 ± 0.06 | 51.6 ± 0.5 | 12 ± 2 | ||
| G | FCC | −0.11 ± 0.01 | 0.19 ± 0.03 | 0.36 ± 0.04 | - | 12 ± 2 | |
| BCC | 0.02 ± 0.01 | - | 0.29 ± 0.02 | 31.9 * | 88 ± 2 | ||
| H | FCC | −0.13 ± 0.03 | 0.16 ± 0.04 | 0.29 ± 0.03 | - | 3 ± 2 | |
| BCC | 0.03 ± 0.01 | - | 0.22 ± 0.02 | 33.9 * | 61 ± 2 | ||
| α-Fe2O3 | 0.36 ± 0.01 | −0.18 ± 0.02 | 0.28 ± 0.04 | 51.7 ± 0.5 | 12 ± 2 | ||
| T-Fe3O4 | 0.25 ± 0.02 | - | 0.44 ± 0.07 | 49.4 ± 0.5 | 15 ± 2 | ||
| O-Fe3O4 | 0.64 ± 0.03 | - | 0.48 ± 0.10 | 46.2 ± 0.5 | 9 ± 2 | ||
| CEMS | E | FCC | −0.07 ± 0.02 | 0.11 ± 0.08 | 0.24 ± 0.08 | - | 7 ± 2 |
| BCC | 0.03 ± 0.01 | - | 0.22 ± 0.02 | 31.9 * | 93 ± 2 | ||
| F | α-Fe2O3 | 0.36 ± 0.01 | −0.20 ± 0.01 | 0.36 ± 0.01 | 51.6 ± 0.5 | 93 ± 2 | |
| γ-Fe2O3 | 0.22 ± 0.02 | - | 0.24 ± 0.07 | 48.5 ± 0.5 | 7 ± 2 | ||
| G | FCC | −0.14 ± 0.02 | 0.11 ± 0.04 | 0.29 ± 0.03 | - | 8 ± 2 | |
| BCC | 0.01 ± 0.01 | - | 0.23 ± 0.02 | 31.4 * | 92 ± 2 | ||
| H | α-Fe2O3 | 0.35 ± 0.01 | −0.10 ± 0.01 | 0.45 ± 0.02 | 51.5 ± 0.5 | 73 ± 2 | |
| γ-Fe2O3 | 0.27 ± 0.01 | - | 0.40 ± 0.03 | 48.7 ± 0.5 | 27 ± 2 |
| Powder | Sample | FCC (%) | BCC (%) | Oxides (%) |
|---|---|---|---|---|
| CL20ES | A | 100 | - | - |
| B | 97 | - | 3 | |
| C | 100 | - | - | |
| D | 93 | 3 | 4 | |
| DD | 74 | 7 | 19 | |
| CL50WS | E | 5 | 95 | - |
| F | 24 | 61 | 15 | |
| G | 22 | 78 | - | |
| H | 20 | 45 | 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, T.; Mashlan, M.; Ingr, T.; Doláková, H.; Sarychev, D.; Sedláčková, A. Mössbauer Spectroscopy for Additive Manufacturing by Selective Laser Melting. Metals 2022, 12, 551. https://doi.org/10.3390/met12040551
Ivanova T, Mashlan M, Ingr T, Doláková H, Sarychev D, Sedláčková A. Mössbauer Spectroscopy for Additive Manufacturing by Selective Laser Melting. Metals. 2022; 12(4):551. https://doi.org/10.3390/met12040551
Chicago/Turabian StyleIvanova, Tatiana, Miroslav Mashlan, Tomáš Ingr, Hana Doláková, Dmitry Sarychev, and Anna Sedláčková. 2022. "Mössbauer Spectroscopy for Additive Manufacturing by Selective Laser Melting" Metals 12, no. 4: 551. https://doi.org/10.3390/met12040551






