Recovery of Zinc from the Concentrate of Domestic Waste Processing by Vacuum Distillation
Abstract
:1. Introduction
2. Research Methods
2.1. Microstructural and Elemental Composition Analyses
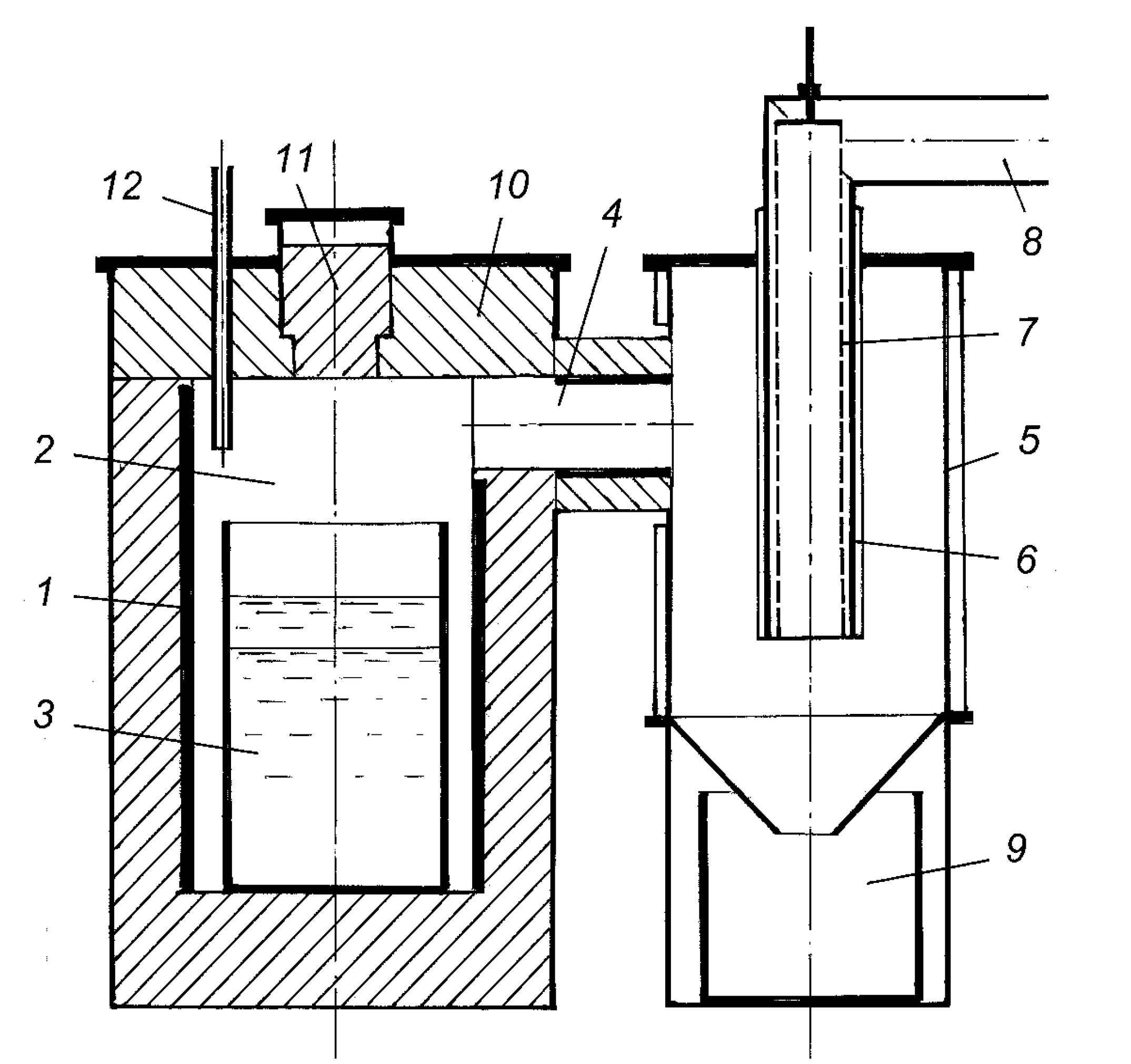
2.2. Metal Concentrate Melting
3. Object of Research
4. Choice of Concentrate Processing Technology
5. Results and Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guerrero, L.A.; Maas, G.; Hogland, W. Solid waste management challenges for cities in developing countries. Waste Manag. 2013, 33, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Hoornweg, D.; Bhada-Tata, P.; Kennedy, C. Peak Waste: When Is It Likely to Occur? J. Ind. Ecol. 2014, 19, 117–128. [Google Scholar] [CrossRef]
- Umar, T. Frameworks for reducing greenhouse gas (GHG) emissions from municipal solid waste in Oman. Manag. Environ. Qual. Int. J. 2020, 31, 945–960. [Google Scholar] [CrossRef]
- Taelman, S.E.; Tonini, D.; Wandl, A.; Dewulf, J. A Holistic Sustainability Framework for Waste Management in European Cities: Concept Development. Sustainability 2018, 10, 2184. [Google Scholar] [CrossRef] [Green Version]
- Azattyq.Org. Available online: https://rus.azattyq.org/a/kazakhstan-problemy-utilizatsii-i-pererabotki-otkhodov-v-kazakhstane/29741211.html (accessed on 13 March 2022).
- Narayana, T. Municipal solid waste management in India: From waste disposal to recovery of resources? Waste Manag. 2009, 29, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, A.; Zotov, V.; Musinova, N. Engineering solutions in the construction of production facilities for waste processing. MATEC Web Conf. 2018, 193, 02010. [Google Scholar] [CrossRef]
- Reschovsky, J.D.; Stone, S.E. Market Incentives to Encourage Household Waste Recycling: Paying for What You Throw Away. J. Policy Anal. Manag. 1994, 13, 120. [Google Scholar] [CrossRef]
- Bernd, F. The “Big Six” metallurgical topics at IME supporting circular economy—Recycling metals: Opportunities for the Norwegian industry. In Proceedings of the Workshop Conference “Norwegian-German Business Cooperation”, Aachen, Germany, 7 May 2019. [Google Scholar] [CrossRef]
- Muchova, L.; Rem, P.C. Metal content and recovery of MSWI bottom ash in Amsterdam. WIT Trans. Ecol. Environ. 2006, 92, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Šyc, M.; Simon, F.G.; Hykš, J.; Braga, R.; Biganzoli, L.; Costa, G.; Funari, V.; Grosso, M. Metal recovery from incineration bottom ash: State-of-the-art and recent developments. J. Hazard. Mater. 2020, 393, 122433. [Google Scholar] [CrossRef]
- Kilian, G.; Bernd, F. VeMRec—Metallurgische herausforderungen beim recycling von NE-Metallkonzentraten aus abfallverbrennungs-rostasche. In Proceedings of the Conference “Mineralische Nebenprodukte und Abfälle—Aschen, Schlacken, Stäube und Baurestmassen”, Berlin, Germany, 4–5 May 2015. [Google Scholar] [CrossRef]
- Malyshev, V.P.; Turdukozhaeva, A.M.; Ospanov, E.A.; Sarkenov, B. Evaporation and Boiling of Simple Substances; Scientific World: Moscow, Russia, 2010; p. 304. [Google Scholar]
- Dai, Y.-N.; Yang, B. Vacuum Metallurgy of Non-Ferrous Metals; Metallurgical Industry Press: Beijing, China, 2000; Volume 3, pp. 516–543. [Google Scholar]
- Timucin, M. Thermodynamic properties of liquid copper-lead alloys. Met. Mater. Trans. A 1980, 11, 503–510. [Google Scholar] [CrossRef]
- Trebukhov, S.A.; Volodin, V.N.; Ulanova, O.V.; Nitsenko, A.V.; Burabaeva, N.M. Thermodynamics of formation and evaporation of solutions of the tin-zinc system. Russ. J. Inorg. Chem. 2021, 66, 1722–1729. [Google Scholar] [CrossRef]
- Trebukhov, S.A.; Volodin, V.N.; Ulanova, O.V.; Nitsenko, A.V.; Burabaeva, N.M. Thermodynamics of formation and evaporation of lead-tin alloys. Kompleks. Ispol’zovanie Miner. Syr’a 2021, 316, 82–90. [Google Scholar] [CrossRef]
- Lyakishev, N.P. (Ed.) Diagrams of the State of Double Metal Systems: Guide; Mechanical Engineering: Moscow, Russian, 1997; Volume 2, p. 1024. [Google Scholar]
- Lyakishev, N.P. (Ed.) Diagrams of the State of Double Metal Systems: Guide; Book 1; Mechanical Engineering: Moscow, Russian, 2001; Volume 3, p. 872. [Google Scholar]
- Lyakishev, N.P. (Ed.) Diagrams of the State of Double Metal Systems: Guide; Book 2; Mechanical Engineering: Moscow, Russian, 2000; Volume 3, p. 448. [Google Scholar]
- Volodin, V.N.; Tuleushev, Y.Z.; Kenzhaliyev, B.K.; Trebukhov, S.A. Thermal degradation of hard alloys of the niobium-cadmium system at low pressure. Kompleks. Ispol’zovanie Miner. Syr’a 2020, 312, 41–47. [Google Scholar] [CrossRef]
- Volodin, V.N.; Trebukhov, S.A.; Kenzhaliyev, B.K.; Nitsenko, A.V.; Burabaeva, N.M. Melt-vapor phase diagram of the Te-S system. Russ. J. Phys. Chem. A 2018, 92, 407–410. [Google Scholar] [CrossRef]
- Kenzhaliev, B.K.; Trebukhov, S.A.; Volodin, V.N.; Trebukhov, A.A.; Tuleutai, F.K. Extraction of selenium from industrial products of metallurgical production. Kompleks. Ispol’zovanie Miner. Syr’a 2018, 307, 56–64. [Google Scholar] [CrossRef]
- Trebukhov, S.A.; Volodin, V.N.; Ulanova, O.V.; Burabaeva, N.M.; Tuleutay, F.K. Processing of polymetallic concentrate from waste recycling plants by distillation in vacuum. J. Phys. Conf. Ser. 2021, 2059, 012024. [Google Scholar] [CrossRef]
- Smirnov, M.P. Lead Refining and Processing of Intermediates; Metallurgy: Moscow, Russian, 1977; p. 280. [Google Scholar]
- Jia, G.-B.; Yang, B.; Liu, D.-C. Deeply removing lead from Pb-Sn alloy with vacuum distillation. Trans. Nonferrous Met. Soc. China 2013, 23, 1822–1831. [Google Scholar] [CrossRef]
- GOST 5017-2006; Interstate standard: Wrought tin bronzes. Grades. Introduced 1 January 2018. Standartinform: Moscow, Russian, 2008. Available online: https://docs.cntd.ru/document/1200048962 (accessed on 13 March 2022).
- GOST R 54310-2011; National Standard of the Russian Federation. Blister Copper. Specifications. Introduced 1 September 2011. Standartinform: Moscow, Russian, 2011. Available online: https://docs.cntd.ru/document/1200084147 (accessed on 13 March 2022).






| Elements | Content of Elements, wt. % | |||
|---|---|---|---|---|
| Zinc Fraction | Brass Fraction | Copper Fraction | Estimated in Concentrate | |
| Zn | 93.75 | 33.66 | 0.94 | 34.63 |
| Cu | 0.82 | 63.44 | 95.80 | 61.98 |
| Pb | — | 1.75 | 0.32 | 1.12 |
| Al | 4.58 | 0.13 | 0.53 | 0.92 |
| Sn | — | 0.40 | 0.79 | 0.44 |
| Si | 0.20 | 7.7 × 10−2 | 0.59 | 0.22 |
| Ni | 3 × 10−2 | 0.30 | 0.14 | 0.22 |
| Fe | 4 × 10−2 | 0.14 | 0.10 | 0.11 |
| P | 2 × 10−3 | 0.13 | 2.6 × 10−2 | 8.4 × 10−2 |
| Bi | — | — | 0.30 | 7.6 × 10−2 |
| Cl | 0.11 | 3.7 × 10−2 | 9.1 × 10−2 | 6.2 × 10−2 |
| S | 4.2 × 10−2 | 2.9 × 10−2 | 0.12 | 5.4 × 10−2 |
| Mg | 0.11 | 3.2 × 10−3 | 6.5 × 10−2 | 5.2 × 10−2 |
| W | 0.31 | — | — | 4.7 × 10−2 |
| Ca | 3.1 × 10−2 | — | 0.13 | 3.8 × 10−2 |
| As | — | — | 5.9 × 10−2 | 1.5 × 10−2 |
| Nb | — | — | 6 × 10−3 | 2 × 10−3 |
| Au | are not found | |||
| Ag | are not found | |||
| No. | Elements, wt. % | |||||||
|---|---|---|---|---|---|---|---|---|
| O | Al | Zn | Cu | C | Pb | Fe | S | |
| Zinc fraction | ||||||||
| Point 1 | 13.85 | 18.40 | 67.75 | – | – | – | – | – |
| Point 2 | 6.38 | 17.01 | 76.13 | 0.48 | – | – | – | – |
| Point 3 | 12.96 | 14.85 | 71.70 | 0.49 | – | – | – | – |
| Point 4 | – | – | 91.15 | 0.83 | 8.01 | – | – | – |
| Point 5 | – | 1.31 | 78.08 | 0.68 | 19.93 | – | – | – |
| Point 6 | – | 21.44 | 75.17 | 0.39 | – | – | – | – |
| Point 7 | – | 1.13 | 97.98 | 0.89 | – | – | – | – |
| Point 8 | – | 15.56 | 83.91 | 0.55 | – | – | – | – |
| Brass fraction | ||||||||
| Point 9 | 5.00 | – | 3.50 | 4.79 | 11.32 | 75.38 | – | – |
| Point 10 | 6.02 | – | 3.01 | 3.74 | 13.48 | 75.75 | – | – |
| Point 11 | – | – | 36.06 | 63.94 | – | – | – | – |
| Point 12 | 1.83 | – | 36.31 | 47.79 | 13.58 | – | 0.50 | – |
| Point 13 | 4.42 | – | 3.36 | 4.32 | 11.45 | 76.48 | – | – |
| Point 14 | 4.54 | – | 5.00 | 7.27 | 11.62 | 71.57 | – | – |
| Point 15 | – | – | 36.23 | 63.77 | – | – | – | – |
| Point 16 | 15.53 | 4.39 | 21.18 | 38.57 | 20.33 | – | – | – |
| Copper fraction | ||||||||
| Point 17 | 11.69 | – | – | 82.47 | 5.84 | – | – | – |
| Point 18 | 8.42 | – | – | 84.06 | 7.52 | – | – | 0.82 |
| Point 19 | 11.97 | – | – | 65.30 | 21.91 | – | – | – |
| Point 20 | 16.15 | 1.58 | 2.62 | 50.23 | 27.78 | – | 0.90 | 0.75 |
| Point 21 | – | – | – | 100 | – | – | – | – |
| Point 22 | – | – | – | 100 | – | – | – | – |
| Products | Mass in Relation to Concentrate, % | Cu | Zn | Pb | Sn | Ni | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content, % | Distribution, % | Content, % | Distribution, % | Content, % | Distribution, % | Content, % | Distribution, % | Content, % | Distribution, % | ||
| Loaded | |||||||||||
| Concentrate | 100 | 62.30 | 100 | 33.80 | 100 | 2.15 | 100 | 0.54 | 100 | 0.22 | 100 |
| Coke breeze | 2.50 | ||||||||||
| Obtained | |||||||||||
| Copper alloy | 66.40 | 92.30 | 98.37 | 3.10 | 6.09 | 3.20 | 98.83 | 0.81 | 99.60 | 0.28 | 84.51 |
| Condensate | 31.12 | 0.64 | 0.32 | 98.70 | 90.87 | 0.04 | 0.58 | 0.006 | 0.35 | 0.002 | 0.28 |
| Losses and residue | 2.48 | 1.31 | 3.04 | 0.59 | 0.05 | 15.21 | |||||
| Copper Alloy | Copper Content, wt % | Impurities Content, wt. % | ||||||
|---|---|---|---|---|---|---|---|---|
| Zn | Pb | Sn | Ni | Bi | Sb | As | ||
| Copper alloy 1 | 90 | 4.13 | 3.17 | 0.66 | 1.22 | – | 0.001 | 0.06 |
| Copper alloy 2 | 89 | 4.87 | 4.31 | 1.55 | 0.17 | – | 0.002 | 0.03 |
| Copper alloy 3 | 93 | 3.19 | 1.56 | 1.31 | 0.31 | 0.007 | 0.003 | 0.04 |
| Copper alloy 4 | 96 | 2.21 | 0.44 | 0.22 | 0.13 | 0.037 | 0.14 | 0.06 |
| Copper alloy 5 | 96 | 2.76 | 0.24 | 0.12 | – | 0.036 | 0.13 | 0.20 |
| BrOCC4-4-2,5 alloy | 86–93 | 3.0–5.0 | 1.5–3.5 | 3.0–5.0 | 0–0.3 | 0–0.002 | 0–0.002 | – |
| BrOTCC4-4-4 alloy | 85–90 | 3.0–5.0 | 3.5–4.5 | 3.0–5.0 | 0–0.3 | 0–0.002 | 0–0.002 | – |
| MCh5 alloy | 5–97.5 | – | 0.40 | – | 1.50 | 0.040 | 0.30 | 0.30 |
| MCh6 alloy | 6–96 | – | 0.60 | – | not rationed | 0.050 | 0.35 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trebukhov, S.; Volodin, V.; Nitsenko, A.; Burabaeva, N.; Ruzakhunova, G. Recovery of Zinc from the Concentrate of Domestic Waste Processing by Vacuum Distillation. Metals 2022, 12, 703. https://doi.org/10.3390/met12050703
Trebukhov S, Volodin V, Nitsenko A, Burabaeva N, Ruzakhunova G. Recovery of Zinc from the Concentrate of Domestic Waste Processing by Vacuum Distillation. Metals. 2022; 12(5):703. https://doi.org/10.3390/met12050703
Chicago/Turabian StyleTrebukhov, Sergey, Valeriy Volodin, Alina Nitsenko, Nurila Burabaeva, and Galiya Ruzakhunova. 2022. "Recovery of Zinc from the Concentrate of Domestic Waste Processing by Vacuum Distillation" Metals 12, no. 5: 703. https://doi.org/10.3390/met12050703






