Effects of Zr Content on the Microstructure of FeCrAl ODS Steels
Abstract
:1. Introduction
2. Experimental Details
2.1. Preparation of FeCrAl ODS Steel Samples
2.2. Microstructure Analysis Method
3. Results and Discussion
3.1. Effects of Zr Content on Grain Size and Grain Orientation
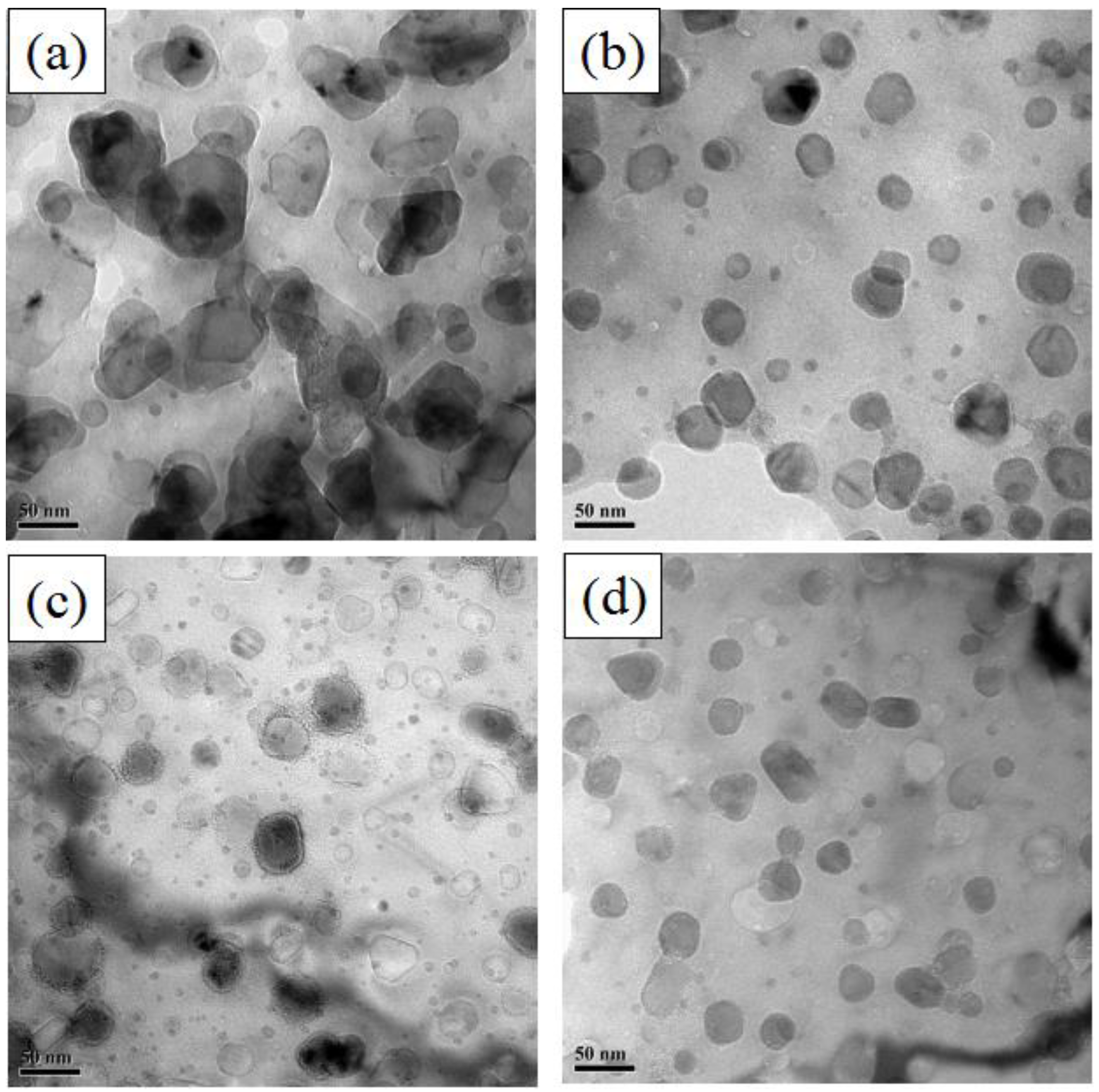
3.2. Effects of Zr Content on Dispersed Oxide Particles
3.3. Influence Mechanism of Zr Content on the Size Distribution of Nano-Precipitates
4. Conclusions
- (1)
- Zr content had a negligible effect on grain orientation distribution of FeCrAl ODS steels, and the grains of FeCrAl ODS steels with different Zr contents were all equiaxed ones with randomly distributed orientations.
- (2)
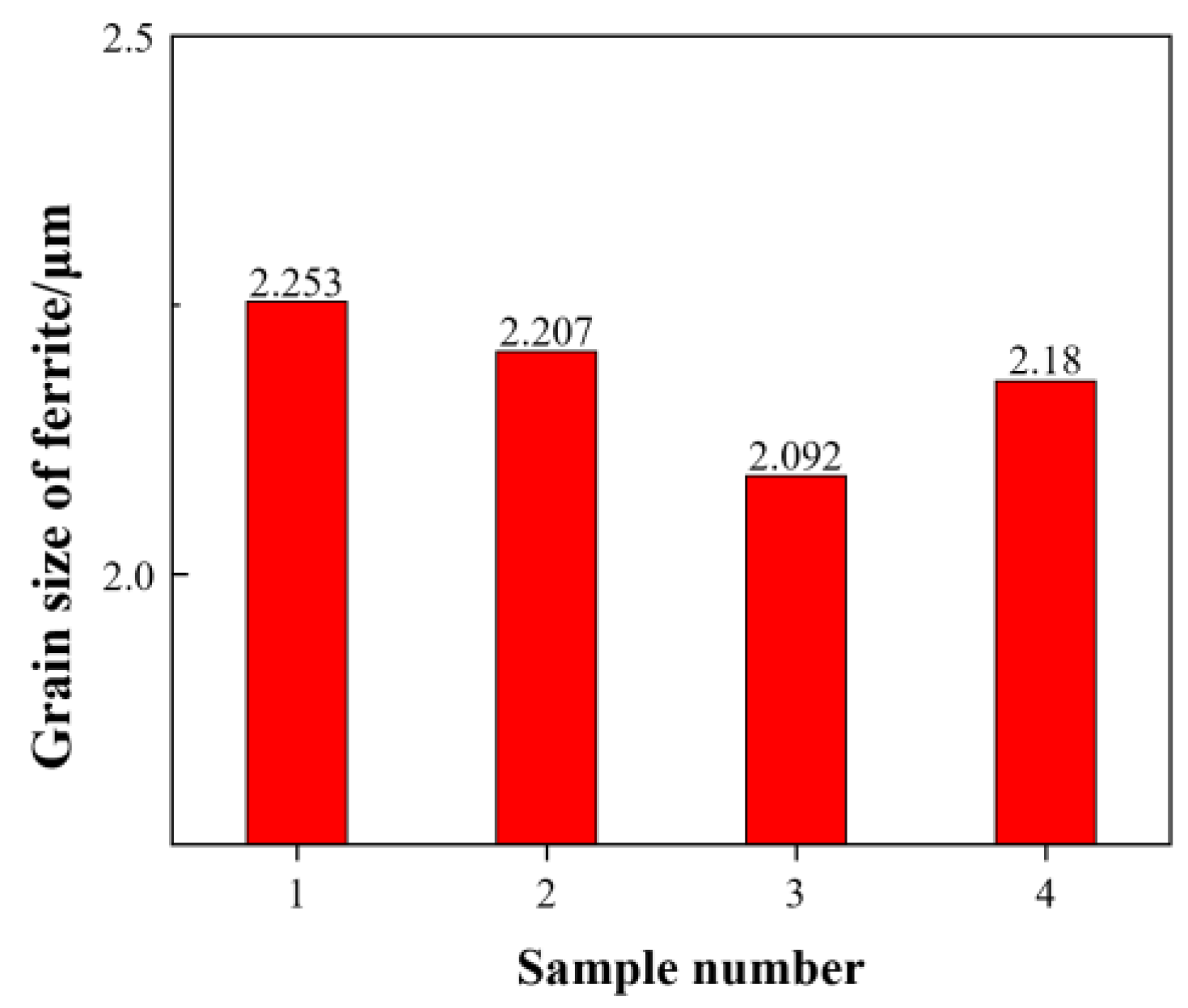
- Zr could refine grains of FeCrAl ODS steels, and its refinement mechanism was the “pinning” effect of nano-sized second phases on grain boundaries during grain growth. As the Zr content increased, the size of nano-sized precipitates increased, which could weaken the pinning ability. Hence, the grain size of FeCrAl ODS steels decreased first and then increased. The minimum average grain size was 2.092 μm when the Zr content was 0.6 wt.%.
- (3)
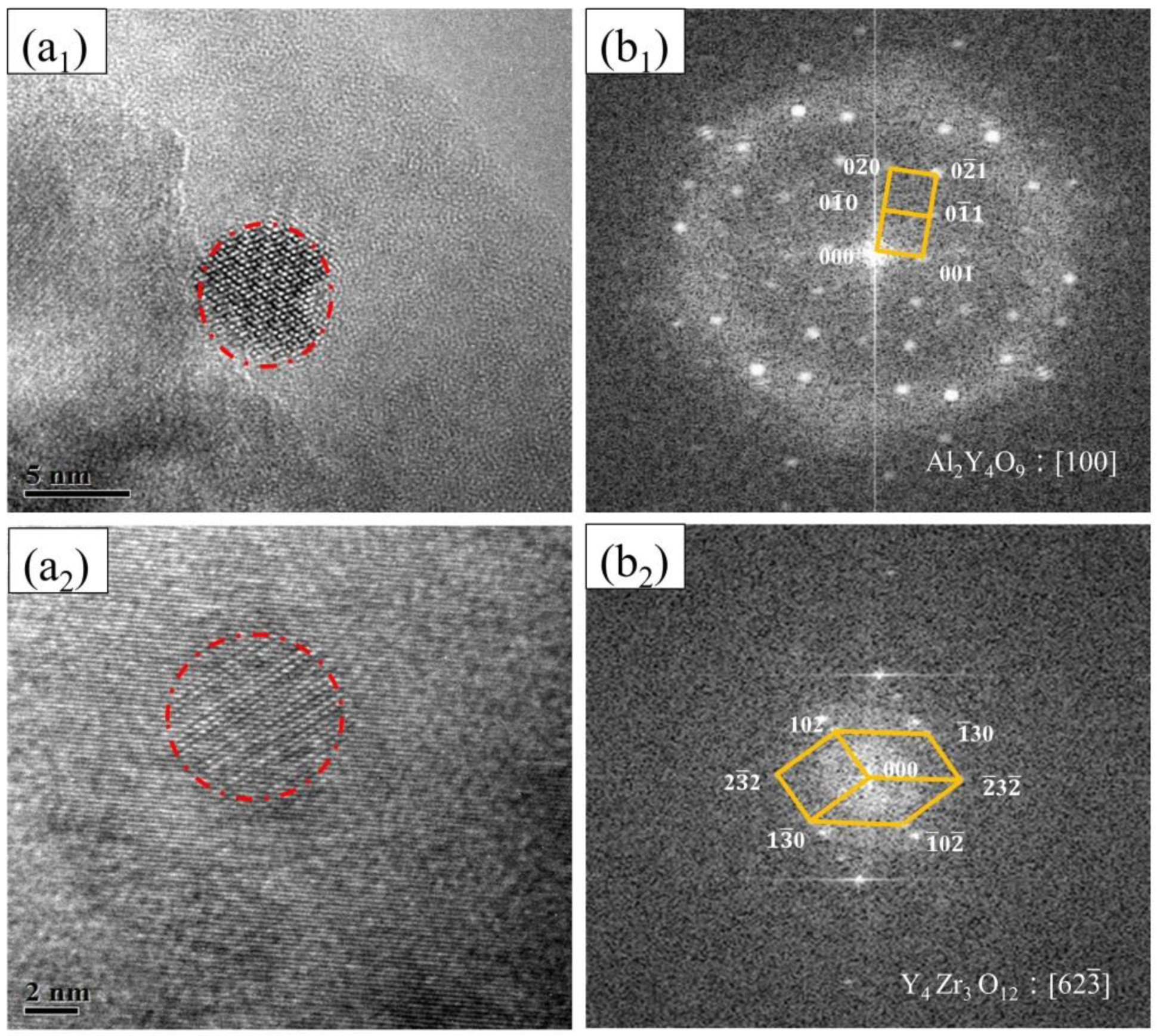
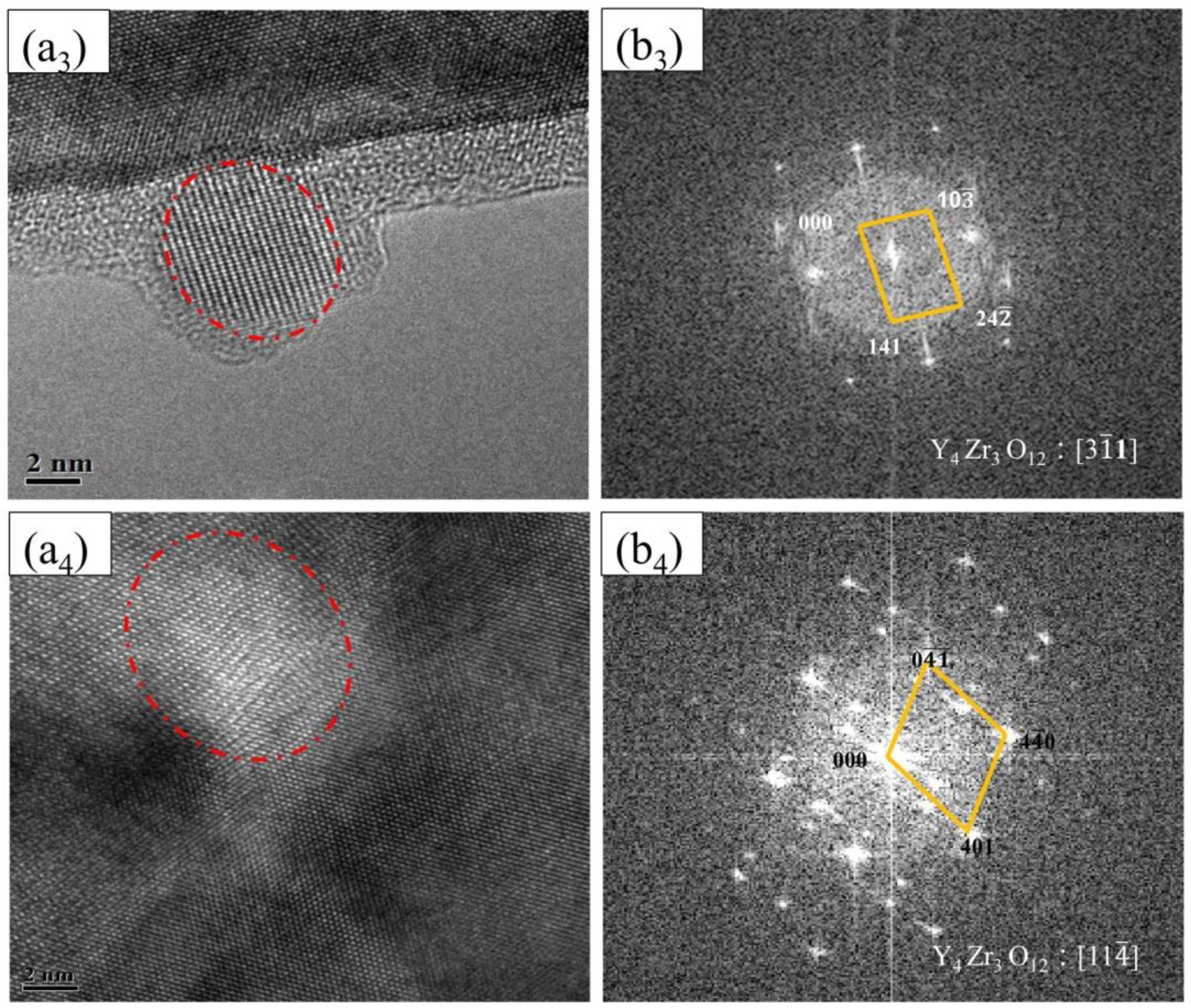
- The dispersed nanoparticles in the matrix of FeCrAl ODS steel without Zr addition were mainly the Al2Y4O9 phase of a monoclinic crystal system, and those in the matrix of FeCrAl ODS steels with Zr addition were mainly the Y4Zr3O12 phase of a hexagonal crystal system. The dispersed nanoparticles in FeCrAl ODS steels had a large average diameter per unit area.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, J.; Qiu, S.; Du, P.; Sun, Y.; Wang, H. Research Progress in the FeCrAl Alloys for Accident Tolerant Fuel Cladding. Mater. Rep. 2017, 31, 47–51. [Google Scholar]
- Ott, L.J.; Robb, K.R.; Wang, D. Preliminary assessment of accident-tolerant fuels on LWR performance during normal operation and under DB and BDB accident conditions. J. Nucl. Mater. 2014, 448, 520–533. [Google Scholar] [CrossRef]
- Field, K.G.; Gussev, M.N.; Yamamoto, Y.; Snead, L.L. Deformation behavior of laser welds in high temperature oxidation resistant Fe–Cr–Al alloys for fuel cladding applications. J. Nucl. Mater. 2014, 454, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Duriagina, Z.A.; Ryzhak, D.D.; Kulyk, V.V.; Tepla, T.L.; Lemishka, I.A.; Bohun, L.I. Microstructure and electrochemical properties of the vanadium alloys after low-temperature nitrogen plasma treatment. Arch. Mater. Sci. Eng. 2020, 1, 5–12. [Google Scholar] [CrossRef]
- Duriagina, Z.A.; Romanyshyn, M.R.; Kulyk, V.V.; Kovbasiuk, T.M.; Trostianchyn, A.M.; Lemishka, I.A. The character of the structure formation of model alloys of the Fe-Cr-(Zr, Zr-B) system synthesized by powder metallurgy. J. Achiev. Mater. Manuf. Eng. 2020, 2, 49–57. [Google Scholar] [CrossRef]
- Chaira, S.R.D. Development of nano-structured duplex and ferritic stainless steels by pulverisette planetary milling followed by pressureless sintering. Mater. Charact. 2015, 99, 220–229. [Google Scholar]
- Chaira, S.R.D. Phase transformation and microstructure study of nano-structured austenitic and ferritic stainless steel powders prepared by planetary milling. Powder Technol. 2014, 259, 125–136. [Google Scholar]
- Chaira, S.R.D. Synthesis of nano-structure duplex and ferritic stainless steel powders by dry milling and its comparison with with wet milling. Arch. Metall. Mater. 2020, 65, 5–14. [Google Scholar]
- Keiser, J.R.; Brady, M.P.; Pint, B.A.; Terrani, K.A. High Temperature Oxidation of Candidate Advanced Iron-Based Alloy Cladding Materials in Steam-Hydrogen Environments. Trans. Am. Nucl. Soc. 2012, 106, 1126–1127. [Google Scholar]
- Dryepondt, S.N.; Hoelzer, D.T.; Pint, B.A.; Unocic, K.A. Development of ODS FeCrAl alloys for accident-tolerant fuel cladding. J. Nucl. Mater. 2015, 501, 59–71. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Pint, B.A.; Terrani, K.A.; Field, K.; Yang, Y.; Snead, L. Development and property evaluation of nuclear grade wrought FeCrAl fuel cladding for light water reactors. J. Nucl. Mater. 2015, 467, 703–716. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Chen, L.; Cao, S.; Jia, H.D.; Zhou, Z.J. Research Progress on Microstructure Design and Control of ODS Steels Applied to Advanced Nuclear Energy Systems. Mater. Rep. 2019, 33, 78–89. [Google Scholar]
- Dou, P.; Kimura, A.; Okuda, T. Polymorphic and coherency transition of Y-Al complex oxide particles with extrusion temperature in an Al-alloyed high-Cr oxide dispersion strengthened ferritic steel. Acta Mater. 2011, 59, 992–1002. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, Z.; Mo, K.; Wang, P.; Miao, Y.; Li, S.; Wang, M.; Liu, X.; Gong, M.; Almer, J.; et al. The microstructure and mechanical properties of Al-containing 9Cr ODS ferritic alloy. J. Alloys Compd. 2015, 648, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Hsiung, L.L.; Fluss, M.J.; Tumey, S.J.; Choi, B.W.; Serruys, Y.; Willaime, F.; Kimura, A. Formation mechanism and the role of nanoparticles in Fe-Cr ODS steels developed for radiation tolerance. Phys. Rev. B Condens. Matter 2010, 82, 184103. [Google Scholar] [CrossRef]
- Qian, Q.; Wang, Y.; Jiang, Y.; He, C.; Hu, T. Nucleation of Y-X-O (X=Al, Ti, or Zr) NCs in nano-structured ferritic alloys: A first principles comparative study. J. Nucl. Mater. 2019, 518, 140–148. [Google Scholar] [CrossRef]
- Long, Y.Q.; Liu, P.; Liu, Y.; Pan, J.S. Phase field modeling for effects of spherical and discal second-phase particles on grain growth. Chin. J. Nonferrous Met. 2009, 19, 84–89. [Google Scholar]
- Scudino, S.; Donnadieu, P.; Surreddi, K.B.; Nikolowski, K.; Stoica, M.; Eckert, J. Microstructure and mechanical properties of Laves phase-reinforced Fe-Zr-Cr alloys. Intermetallics 2009, 17, 532–539. [Google Scholar] [CrossRef]
- Donnadieu, P.; Pohlmann, C.; Scudino, S.; Blandin, J.-J.; Surreddi, K.B.; Eckert, J. Deformation at ambient and high temperature of in situ Laves phases-ferrite composites. Sci. Technol. Adv. Mater. 2014, 15, 034801. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yang, Y. Microstructure and Mechanical Properties of Laves Phase-strengthened Fe-Cr-Zr Alloys. Metall. Mater. Trans. A 2014, 46, 1188–1195. [Google Scholar] [CrossRef]


| Sample | Fe | Cr | Al | Ti | W | Y2O3 | Zr |
|---|---|---|---|---|---|---|---|
| 1 | Bal. | 13 | 4.5 | 0.4 | 2 | 0.35 | 0 |
| 2 | Bal. | 13 | 4.5 | 0.4 | 2 | 0.35 | 0.3 |
| 3 | Bal. | 13 | 4.5 | 0.4 | 2 | 0.35 | 0.6 |
| 4 | Bal. | 13 | 4.5 | 0.4 | 2 | 0.35 | 1.2 |
| Sample | Zr Content (wt.%) | Mean Area (nm2) | Mean Diameter (nm) | Number Density (1023 Particle·m−3) |
|---|---|---|---|---|
| 1# | 0 | 53.55 | 7.83 | 0.656 |
| 2# | 0.3 | 45.00 | 7.15 | 1.870 |
| 3# | 0.6 | 30.20 | 5.58 | 3.330 |
| 4# | 1.2 | 46.00 | 7.21 | 1.040 |
| Sample | #1 | #2 | #3 | #4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal plane | (0) | () | () | (102) | (30) | () | () | () | (141) | () | (0) | (401) |
| Theoretical interplanar crystal spacing (nm) | 0.5253 | 0.1301 | 1.0534 | 0.4009 | 0.3188 | 0.2612 | 0.2858 | 0.1504 | 0.1804 | 0.2054 | 0.2108 | 0.2054 |
| Actual interplanar crystal spacing (nm) | 0.5227 | 0.4606 | 1.0720 | 0.3813 | 0.3097 | 0.2549 | 0.2915 | 0.1549 | 0.1821 | 0.2022 | 0.2029 | 0.2025 |
| Sample | #1 | #2 | #3 | #4 | ||||
|---|---|---|---|---|---|---|---|---|
| Crystal plane | (0)/(0) | (1)(001) | (102)/(30) | (30)/() | ()/(24) | (24)/(141) | ()/(40) | (40)/(401) |
| Theoretical value (°) | 26.51 | 63.49 | 84.85 | 40.45 | 56.47 | 31.74 | 60.85 | 60.85 |
| Measured value (°) | 27.81 | 65.15 | 84.12 | 41.34 | 57.87 | 31.91 | 60.05 | 60.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, D.; Sun, Y.; Zhang, R.; Qiu, S.; Yu, L. Effects of Zr Content on the Microstructure of FeCrAl ODS Steels. Metals 2022, 12, 1114. https://doi.org/10.3390/met12071114
Long D, Sun Y, Zhang R, Qiu S, Yu L. Effects of Zr Content on the Microstructure of FeCrAl ODS Steels. Metals. 2022; 12(7):1114. https://doi.org/10.3390/met12071114
Chicago/Turabian StyleLong, Dijun, Yongduo Sun, Ruiqian Zhang, Shaoyu Qiu, and Liming Yu. 2022. "Effects of Zr Content on the Microstructure of FeCrAl ODS Steels" Metals 12, no. 7: 1114. https://doi.org/10.3390/met12071114





