Heterogeneous Nucleation and Grain Initiation on a Single Substrate
Abstract
:1. Introduction
2. Simulation Approaches
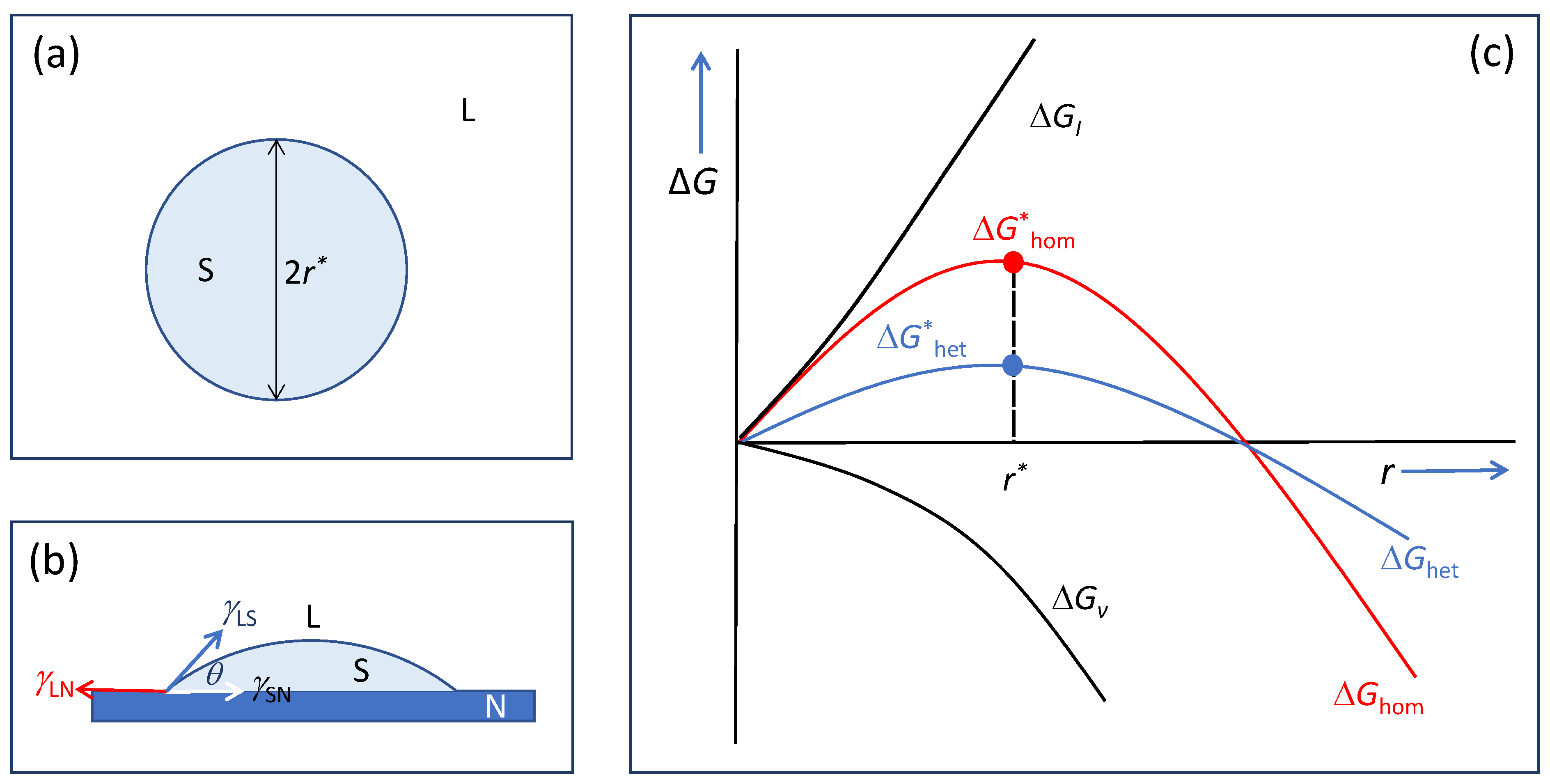
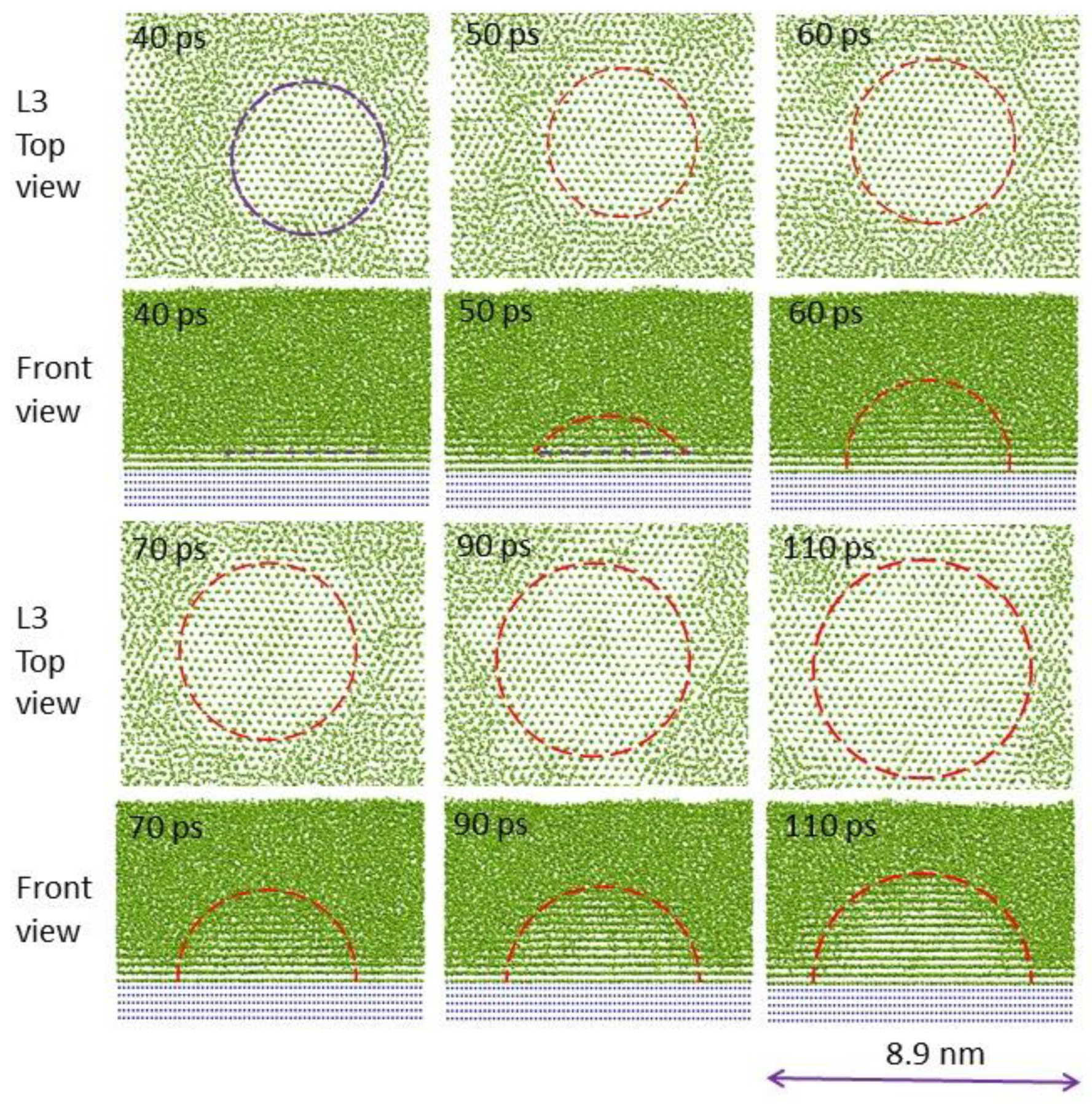
3. Heterogeneous Nucleation on a Single Substrate
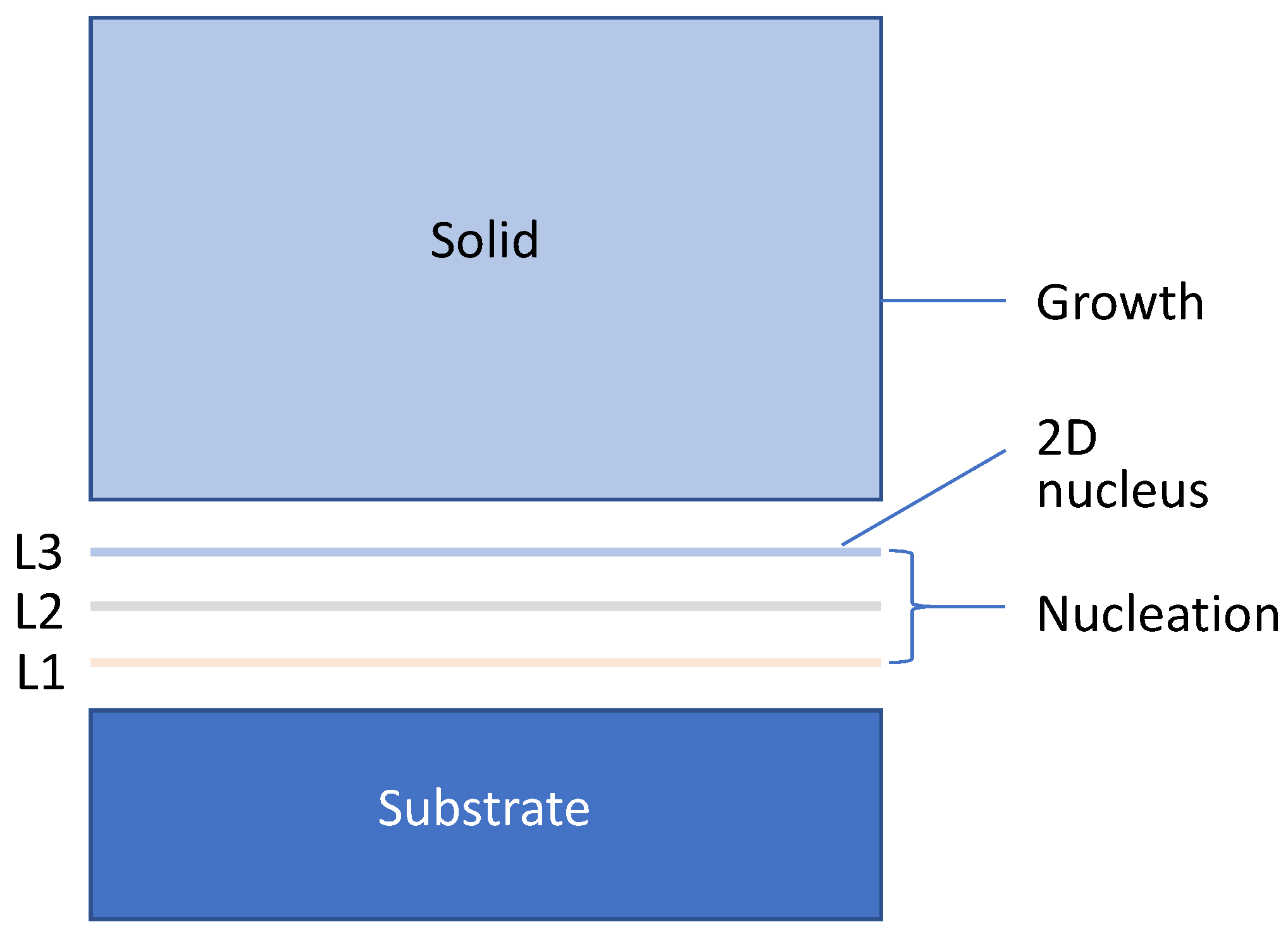
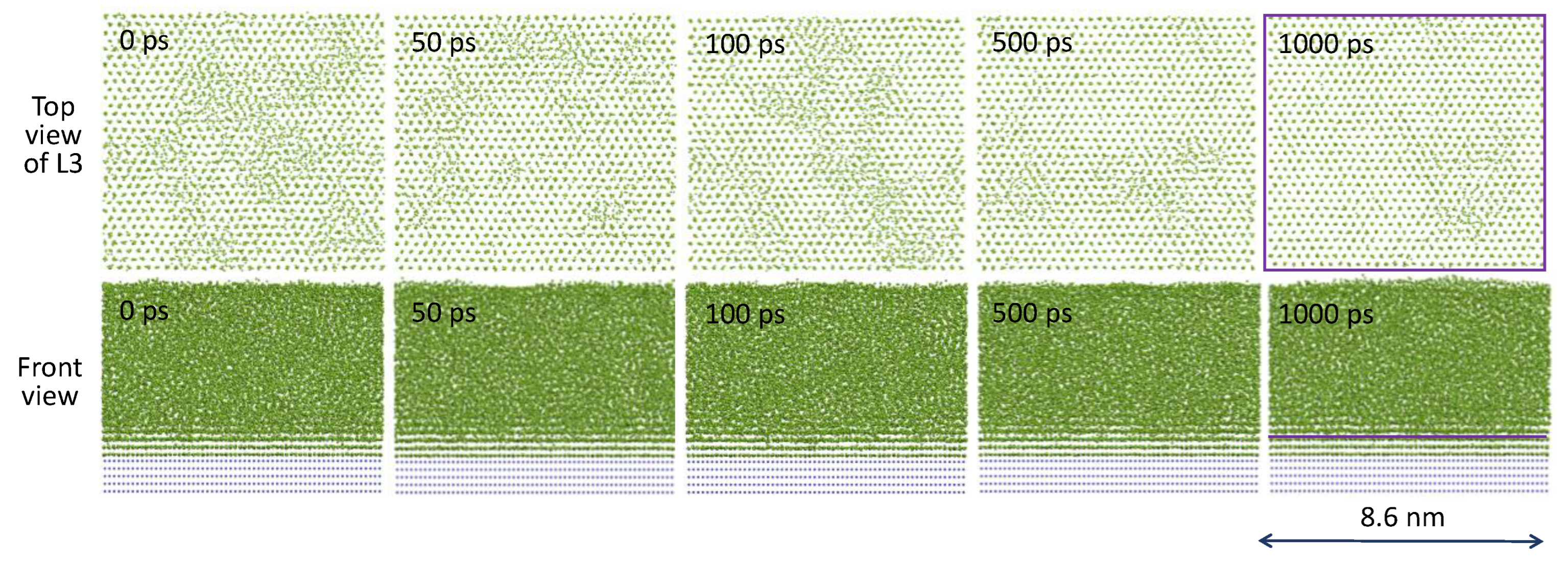
3.1. Three-Layer Nucleation Mechanism
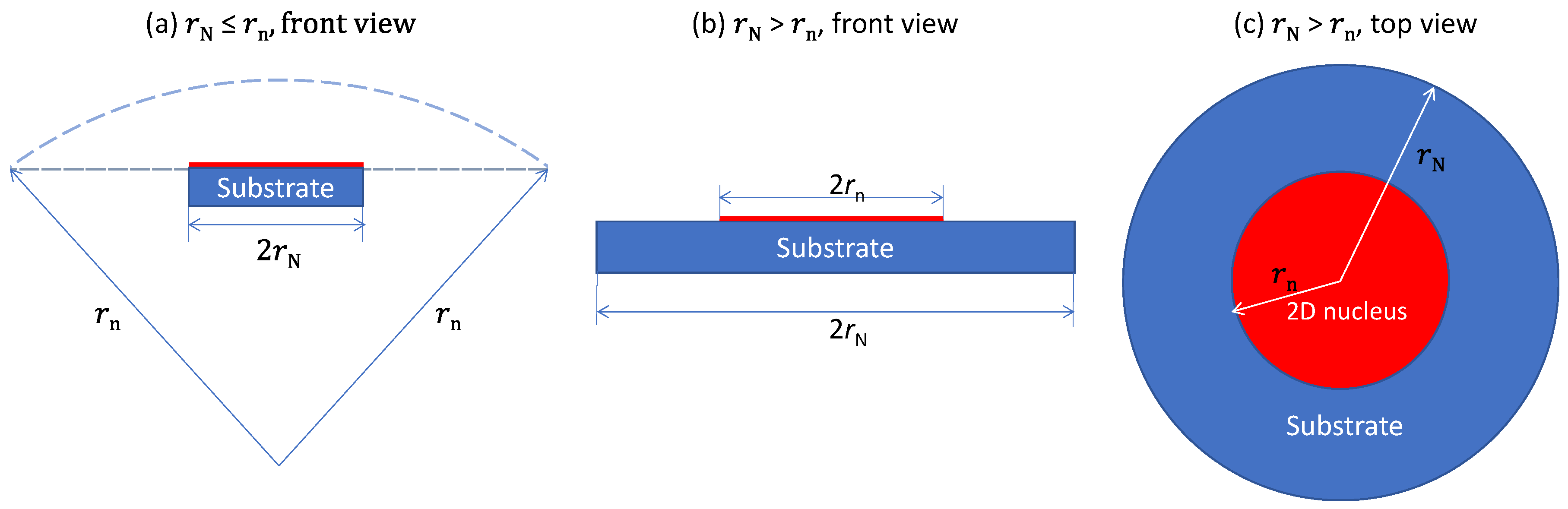
3.2. Effect of Substrate Size
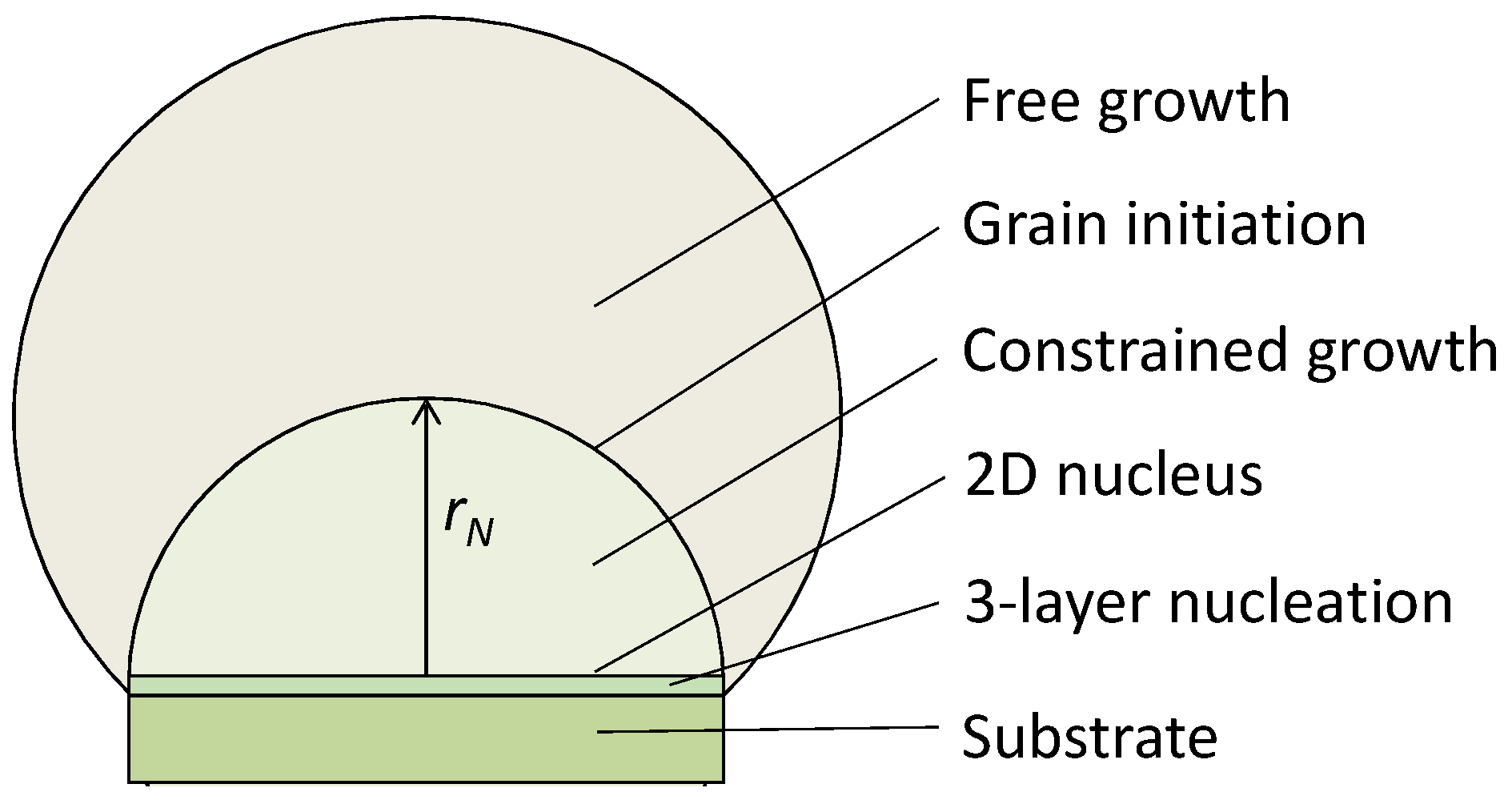
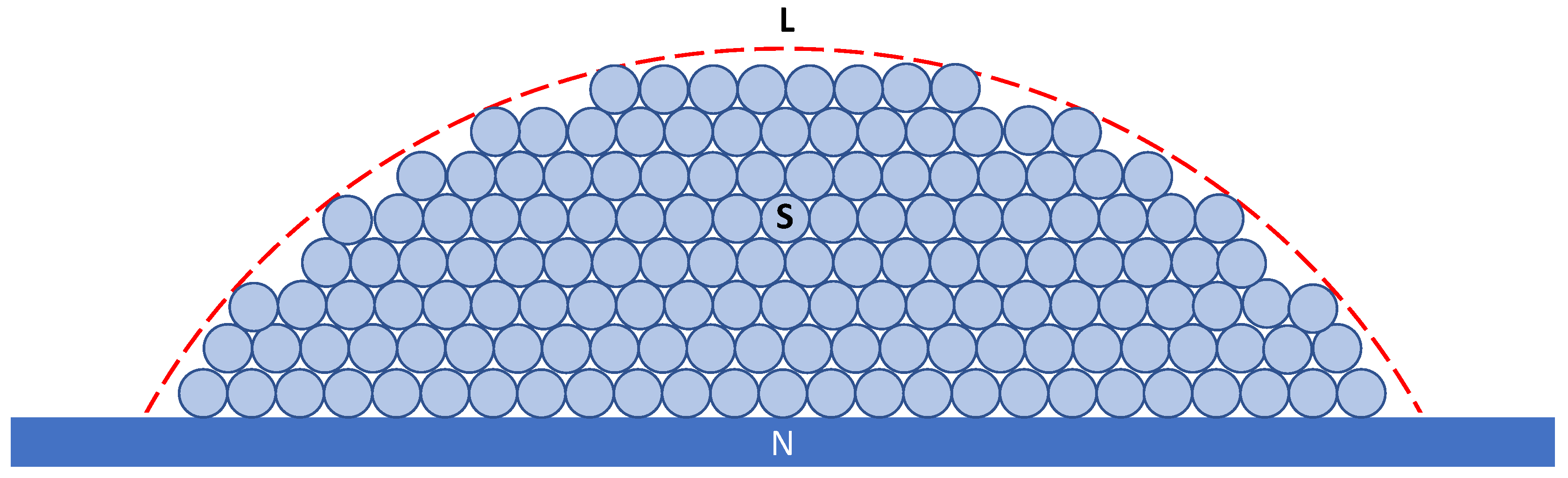
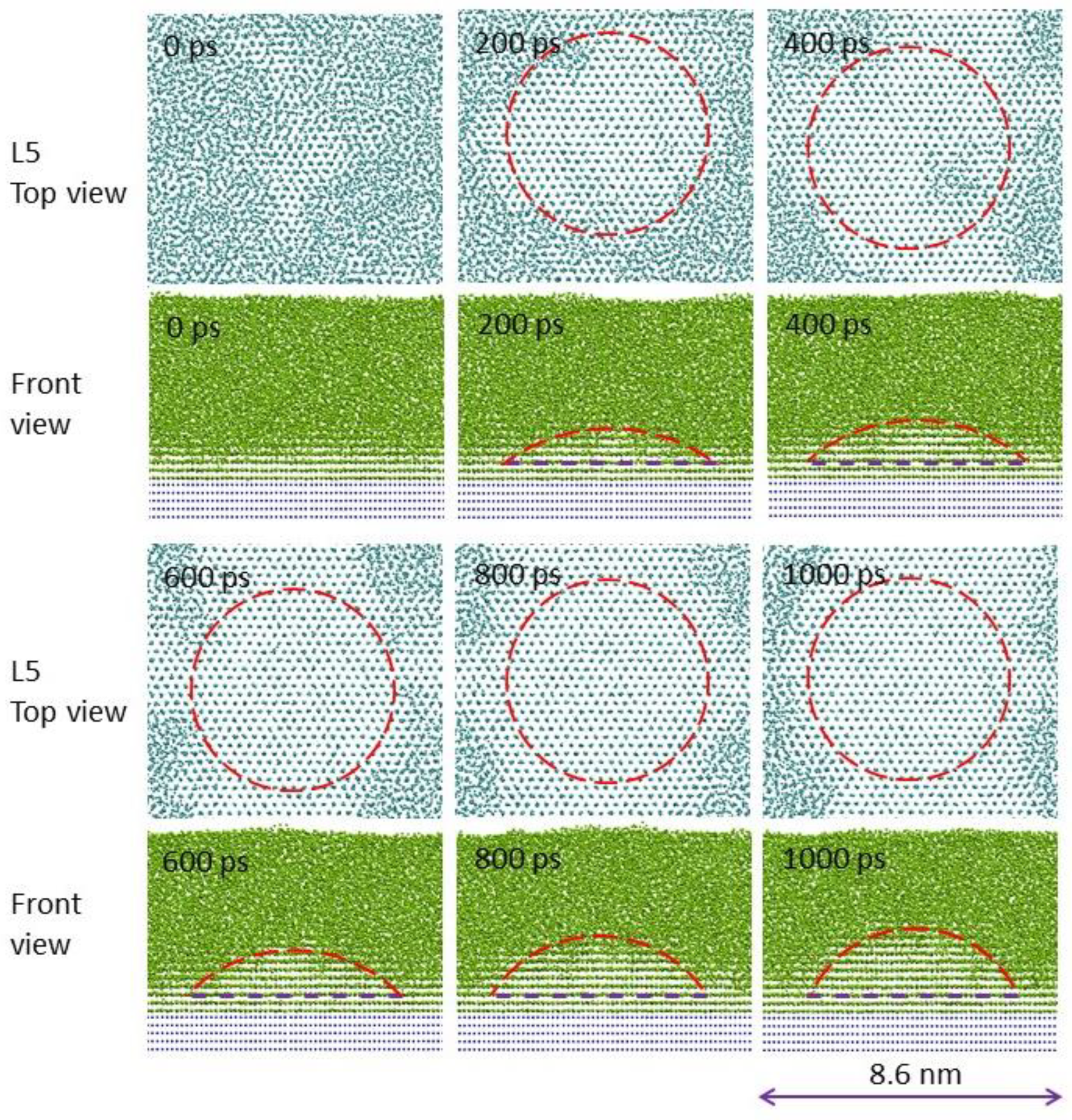
4. Grain Initiation on a Single Substrate
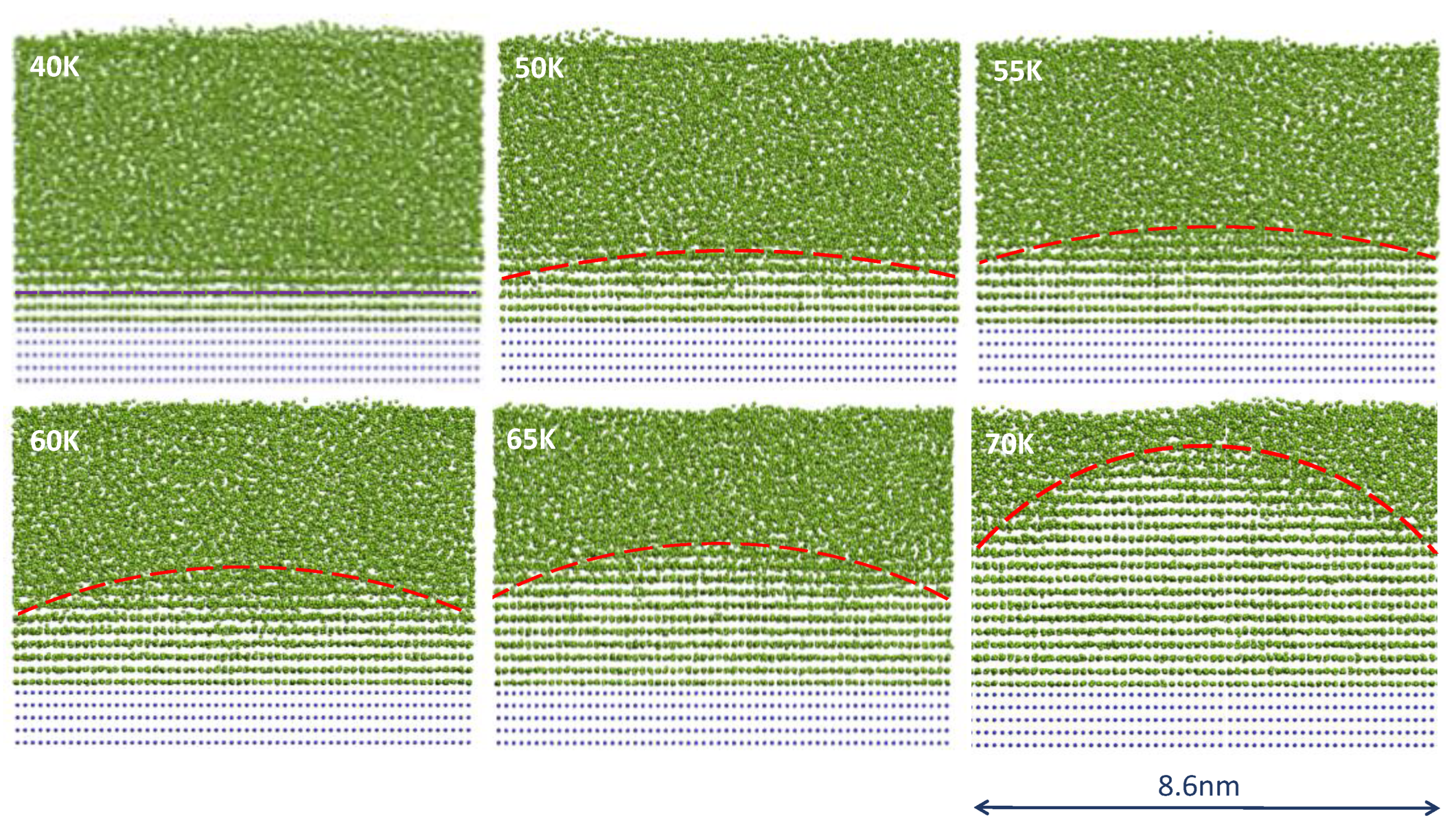
4.1. Constrained Grain Initiation
4.2. Unconstrained Grain Initiation
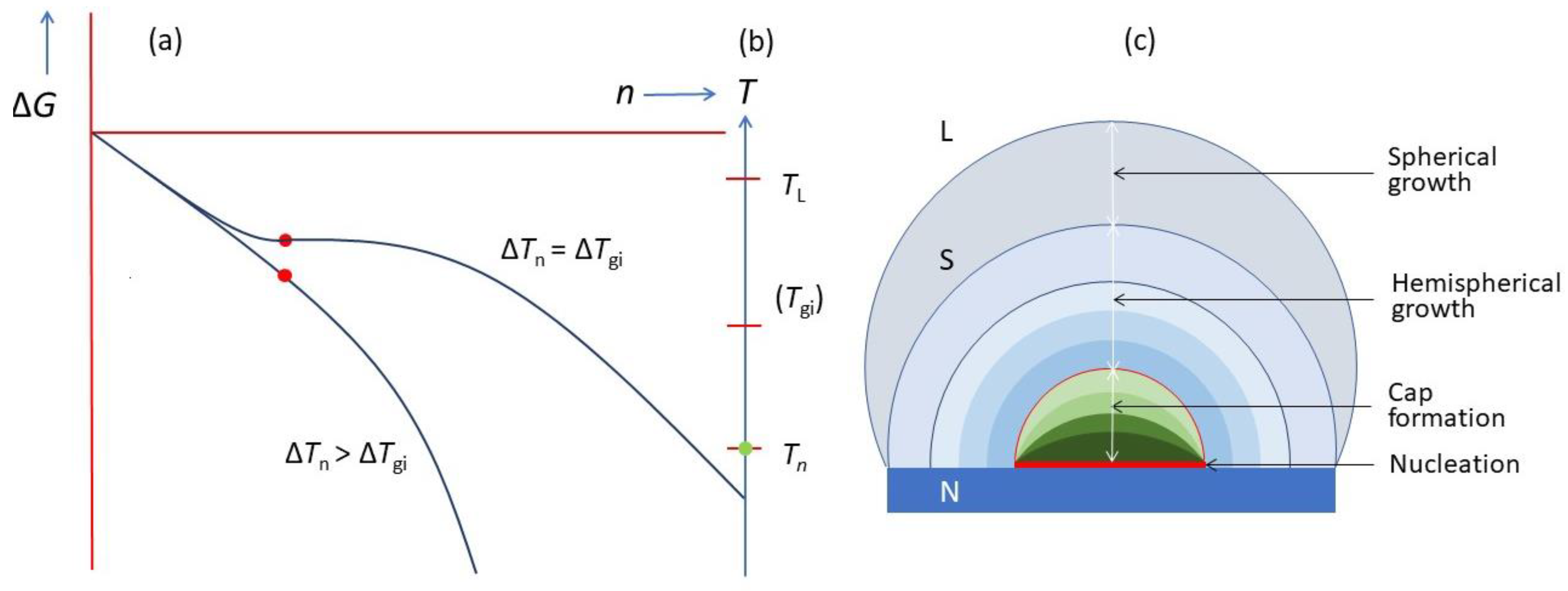
- Heterogeneous nucleation through the three-layer mechanism to generate 2D nucleus with rn being defined by the nucleation undercooling (ΔTn).
- Barrierless spherical cap formation to create a hemisphere with a radius of rn.
- Hemispherical growth with an increasing radius to deliver a hemisphere with rLS = rN.
- Spherical growth beyond the hemisphere with rLS > rN.
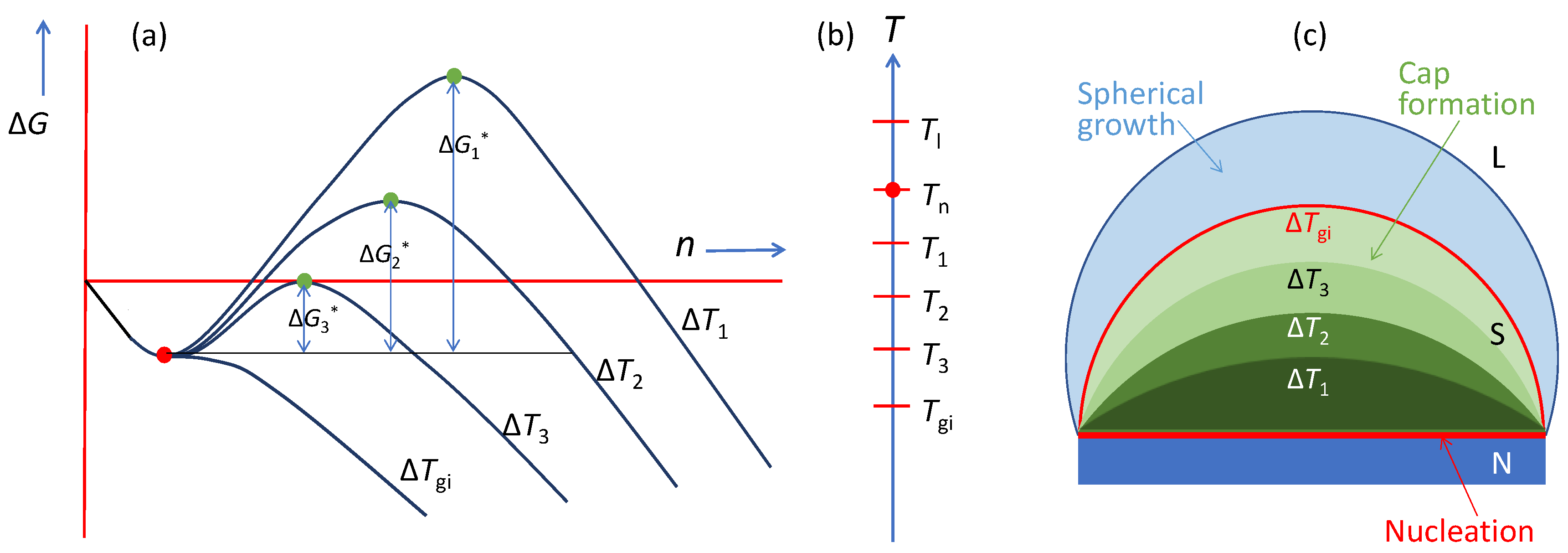
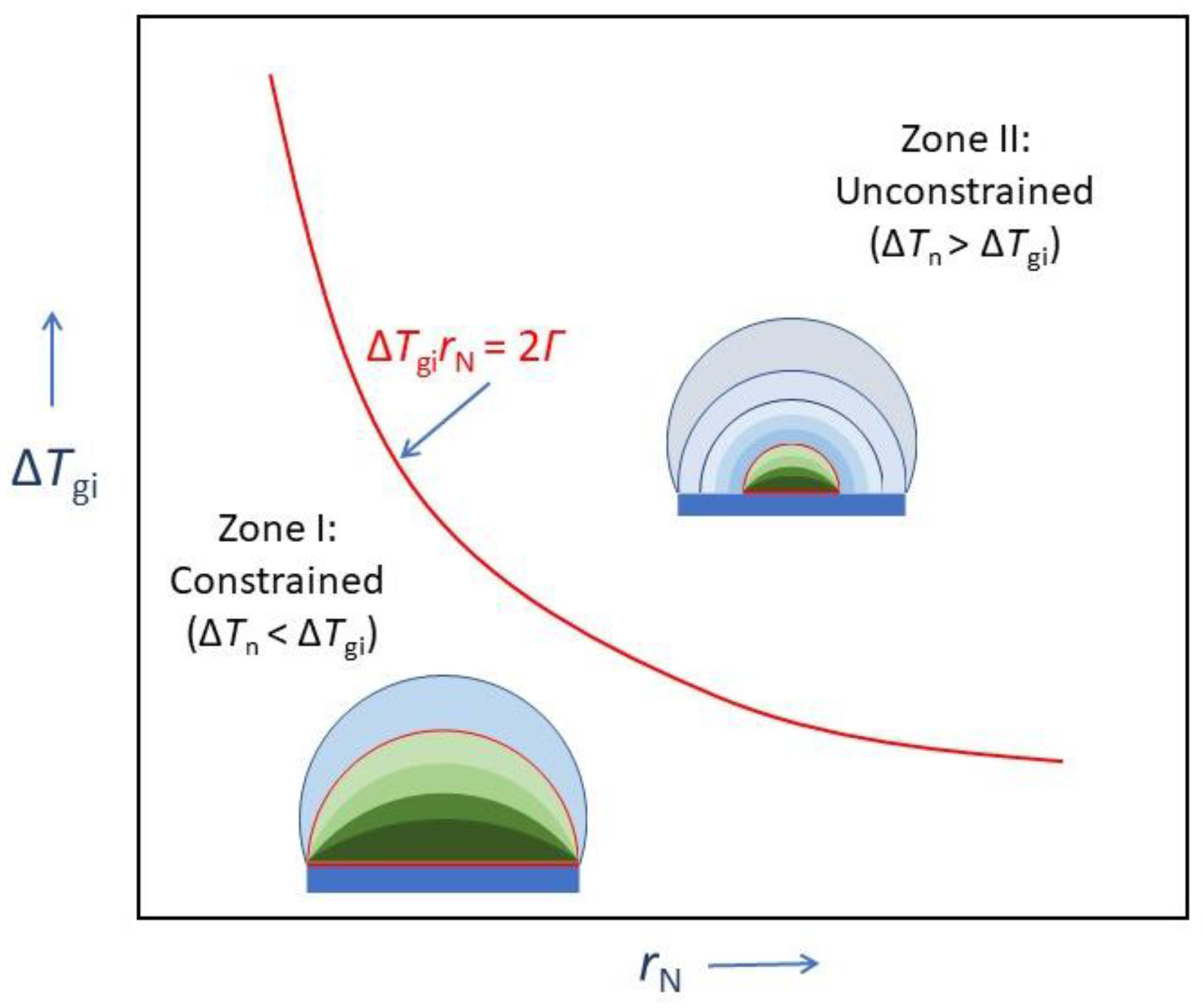
4.3. Grain Initiation Map
- Zone I: grain initiation through constrained spherical cap formation. Grain initiation in this zone is characterised by ΔTgirN < 2Γ. Thus, in this zone, we have ΔTn < ΔTgi, or equivalently, rn > rN. The metastable cap formed at a particular temperature is dormant, and further growth can only be made possible by increasing the undercooling to overcome the energy barrier.
- Zone II: grain initiation through unconstrained spherical cap formation. Grain initiation in this zone is characterised by ΔTgirN > 2Γ. Thus, in this zone, we have ΔTn > ΔTgi, or equivalently rn < rN. Grain initiation in this zone becomes barrierless.
5. Modelling of Heterogeneous Nucleation and Grain Initiation
5.1. Modelling of Heterogeneous Nucleation
5.2. Understanding of Grain Initiation
- is a physical property of a substrate of when ΔTn < ΔTgi. However, when ΔTn > ΔTgi, Equation (8) is no longer applicable. In this case, the grain initiation criterion becomes ΔTnrn = 2Γ. Grain initiation becomes possible when rN > rn.
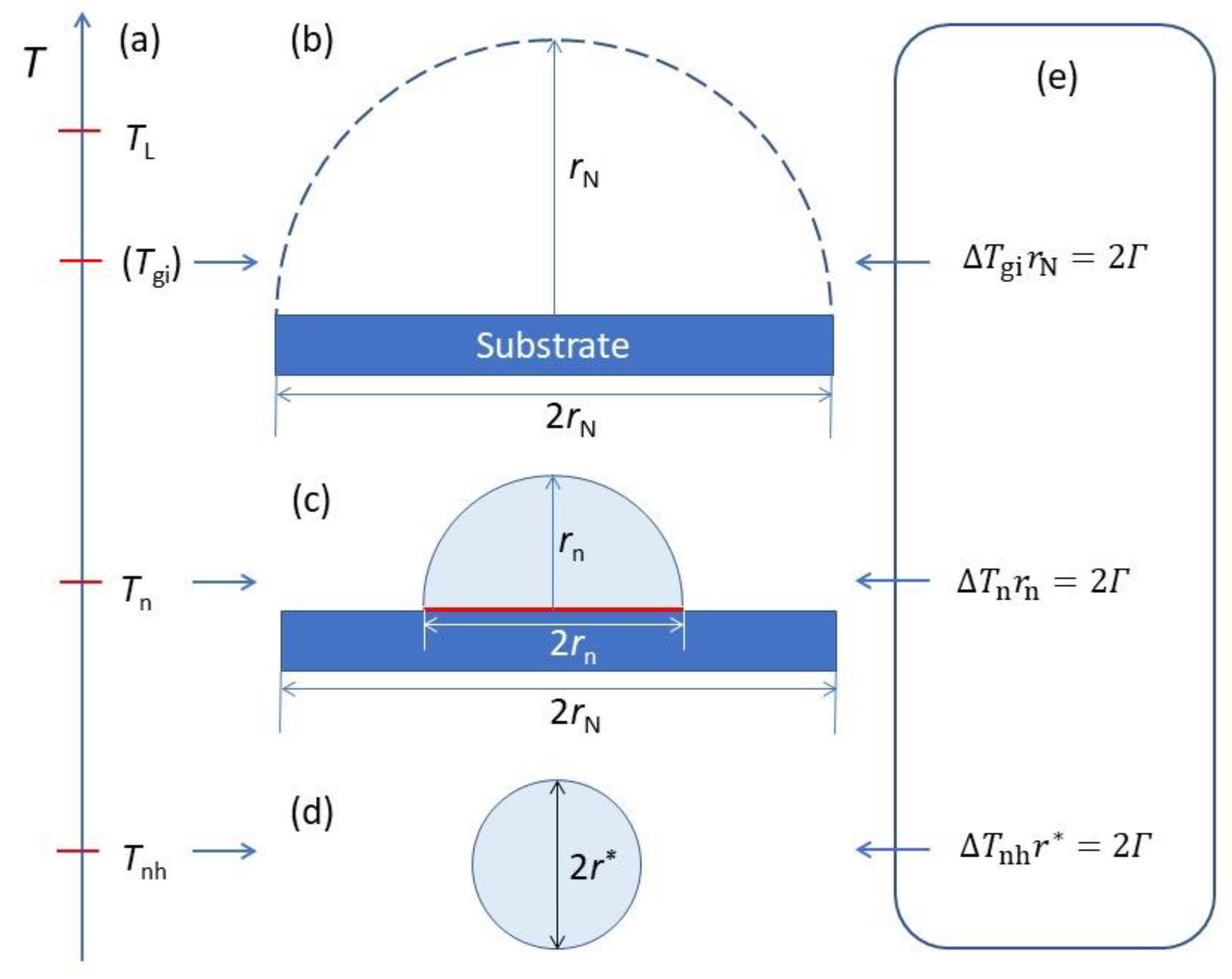
- ΔTnhr* = 2Γ vs. ΔTnrn = 2Γ vs. ΔTgirN = 2Γ: It is important to realise that ΔTnhr* = 2Γ describes the homogeneous nucleation process (3D), ΔTnrn = 2Γ describes the three-layer nucleation process (2D), while ΔTgirN = 2Γ describes the hemisphere formation (3D) on a substrate of rN, as depictured in Figure 18. The origin of the similarity between these equations is that they all describe balancing the volume free energy change with a change in interfacial energies.
- Grain initiation is about free growing isothermally a solid particle which is not directly connected to physical properties of the substrate, while heterogeneous nucleation is dictated by the physical properties of the substrate.
6. Summary
- A substrate wetted completely by the liquid can always induce some ordered atoms in the liquid adjacent to the liquid/substrate interface and hence can act as a nucleation site regardless of the nucleation undercooling. Under such conditions, we have γLN ≥ γSN + γLS, suggesting that Young’s equation (Equation (4)) is inapplicable to any cases for heterogeneous nucleation. Therefore, describing heterogeneous nucleation as a spherical cap formation process may not be a useful approach, since it masks some critical phenomena, such as prenucleation, formation of 2D nucleus, and constrained/unconstrained spherical cap formation.
- As a theoretical model, homogeneous nucleation theory that describes a stochastic process for the creation of a nucleus is conceptually simple and mathematically rigorous. However, it is challengeable to extend homogeneous nucleation theory to heterogeneous nucleation, which is a deterministic process. At least classical heterogeneous nucleation theory has not been helpful to generate much useful new insight except the reduction in nucleation barrier by the substrate.
- The basic atomistic mechanism for both heterogeneous nucleation and crystal growth is structural templating, which requires that any solid atom needs to be supported by the solid atoms in the layer underneath it. This fact has made us realise that curvature formation is a consequence of structural templating.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kelton, K.F.; Greer, A.L. Nucleation in Condensed Mater: Applications in Materials and Biology; Elsevier: Oxford, UK, 2010. [Google Scholar]
- Kashchiev, D. Nucleation: Theory with Applications; Butterworth-Heinemann: Oxford, UK, 2000. [Google Scholar]
- Fan, Z.; Men, H.; Wang, Y.; Que, Z.P. A new atomistic mechanism for heterogeneous nucleation in the systems with negative lattice misfit: Creating a 2D template for crystal growth. Metals 2021, 11, 478. [Google Scholar] [CrossRef]
- Gibbs, J.W. On the equilibrium of heterogeneous substances. Am. J. Sci. 1879, 16, 441–458. [Google Scholar] [CrossRef]
- Volmer, M.; Weber, A.Z. Nucleus formation in supersaturated systems. Z. Für Phys. Chem. 1926, 119, 277–301. [Google Scholar]
- Becker, R.; Döring, W. Kinetic treatment of nucleation in supersaturated vapors. Ann. Phys. 1935, 24, 719–752. [Google Scholar] [CrossRef]
- Zeldovich, J.B. On the theory of new phase formation. Cavitation. Acta Physicochim. USSR 1943, 18, 1–22. [Google Scholar]
- Cantor, B. Heterogeneous nucleation and adsorption. Philos. Trans. R. Soc. Lond. 2003, 361, 409–417. [Google Scholar] [CrossRef]
- Kim, W.T.; Zhang, D.L.; Cantor, B. Nucleation of solidification in liquid droplets. Metall. Trans. A 1991, 22A, 2487–2501. [Google Scholar] [CrossRef]
- Kim, W.T.; Cantor, B. Solidification of tin droplets embedded in an aluminium matrix. J. Mater. Sci. 1991, 26, 2868–2878. [Google Scholar] [CrossRef]
- Kim, W.T.; Cantor, B. Solidification behaviour of Pb droplets embedded in a Cu matrix. Acta Metall. 1992, 40, 3339–3347. [Google Scholar] [CrossRef]
- Kim, W.T.; Cantor, B. Heterogeneous nucleation of Al2Cu in Al-Cu eutectic liquid droplets embedded in an Al matrix. Acta Metall. Mater. 1994, 42, 3045–3053. [Google Scholar] [CrossRef]
- Stranski, I.; Kaischew, R. Über den mechanismus des gleichgewichtes kleiner kriställchen I. Z. Phys. Chem. B 1934, 26, 100–113. [Google Scholar] [CrossRef]
- Richards, W.T. The persistence and development of crystal nuclei above the melting temperature. J. Am. Chem. Soc. 1932, 54, 479–495. [Google Scholar] [CrossRef]
- Coudurier, L.; Eustathopoulos, N.; Desré, P.; Passerone, A. Rugosite atomique et adsorption chimique aux interfaces solide-liquide des systems metalliques binaires. Acta Metall. 1978, 26, 465–475. [Google Scholar] [CrossRef]
- Cantor, B. Embedded droplet measurements and an adsorption model of the heterogeneous nucleation of solidification. Mater. Sci. Eng. A 1994, 178, 225–231. [Google Scholar] [CrossRef]
- Kim, W.T.; Cantor, B. An adsorption model of the heterogeneous nucleation of solidification. Acta Metall. Mater. 1994, 42, 3115–3127. [Google Scholar] [CrossRef]
- Jones, G.P. Solidification Technology in the Foundry and Cast House; The Metals Society: London, UK, 1983; pp. 112–114. [Google Scholar]
- Jones, G.P. Solidification Processing 1987; Beech, J., Jones, H., Eds.; The Institute of Metals: London, UK, 1988; pp. 496–499. [Google Scholar]
- Fan, Z.; Wang, Y.; Zhang, Y.; Qin, T.; Zhou, X.R.; Thompson, G.E.; Pennycook, T.; Hashimoto, T. Grain refining mechanism in the Al/Al-Ti-B system. Acta Mater. 2015, 84, 292–304. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, C.M.; Zhou, L.; Hashimoto, T.; Zhou, X.; Ramasse, Q.M.; Fan, Z. Mechanism for Zr poisoning of Al-Ti-B based grain refiners. Acta Mater. 2019, 164, 428–439. [Google Scholar] [CrossRef]
- Wang, Y.; Que, Z.P.; Hashimoto, T.; Zhou, X.R.; Fan, Z. Mechanism for Si poisoning of Al-Ti-B grain refiners in Al alloys. Metall. Mater. Trans. A 2020, 51, 5743–5757. [Google Scholar] [CrossRef]
- Wang, S.H.; Wang, F.; Wang, Y.; Ramasse, Q.M.; Fan, Z. Segregation of Ca at the Mg/MgO interface and its effect on grain refinement of Mg alloys. IOP Conf. Ser. Mater. Sci. Eng. 2019, 529, 012048. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, S.H.; Niu, Z.C. Modification of γ-Al2O3/Al interface through La interfacial segregation: A strategy to harness native γAl2O3 for grain refinement. Acta Mater 2002. submitted. [Google Scholar]
- Men, H.; Fan, Z. Prenucleation induced by crystalline substrates. Metall. Mater. Trans. A 2018, 49, 2766–2777. [Google Scholar] [CrossRef]
- Fan, Z.; Men, H. A molecular dynamics study of heterogeneous nucleation in generic liquid/substrate systems with positive lattice misfit. Mater. Res. Express 2020, 7, 126501. [Google Scholar] [CrossRef]
- Men, H.; Fan, Z. Heterogeneous nucleation mechanisms in systems with large lattice misfit demonstrated by the Pb(l)/Cu(s) system. Metals 2022. submitted. [Google Scholar]
- Greer, A.L.; Bunn, A.M.; Tronche, A.; Evans, P.V.; Bristow, D.J. Modelling of inoculation of metallic melts: Application to grain refinement of aluminium by Al-Ti-B. Acta Mater. 2000, 48, 2823–2835. [Google Scholar] [CrossRef]
- Quested, T.E.; Greer, A.L. Athermal heterogeneous nucleation of solidification. Acta Mater. 2005, 53, 2683–2692. [Google Scholar] [CrossRef]
- Quested, T.E.; Greer, A.L. The effect of the size distribution of inoculant particles on as-cast grain size in aluminium alloys. Acta Mater. 2004, 52, 3859–3868. [Google Scholar] [CrossRef]
- Shu, D.; Sun, B.D.; Mi, J.; Grant, P.S. A quantitative study of solute diffusion field effects on heterogeneous nucleation and the grain size of alloys. Acta Mater. 2011, 59, 2135–2144. [Google Scholar] [CrossRef]
- Du, Q.; Li, Y.J. An extension of the Kampmann–Wagner numerical model towards as-cast grain size prediction of multicomponent aluminium alloys. Acta. Mater. 2014, 71, 380–389. [Google Scholar] [CrossRef]
- Men, H.; Jiang, B.; Fan, Z. Mechanisms of grain refinement by intensive shearing of AZ91 alloy melt. Acta Mater. 2010, 58, 6526–6534. [Google Scholar] [CrossRef]
- Men, H.; Fan, Z. Effects of solute content on grain refinement in an isothermal melt. Acta Mater. 2011, 59, 2704–2712. [Google Scholar] [CrossRef]
- Tóth, G.I.; Tegze, G.; Pusztai, T.; Gránásy, L. Heterogeneous crystal nucleation: The effect of lattice mismatch. Phys. Rev. Lett. 2012, 108, 025502. [Google Scholar] [CrossRef]
- Fujinaga, T.; Shibuta, Y. Molecular dynamics simulation of athermal heterogeneous nucleation of solidification. Comput. Mater. Sci. 2019, 164, 74–81. [Google Scholar] [CrossRef]
- Fan, Z. An epitaxial model for heterogeneous nucleation on potent substrates. Metall. Mater. Trans. A 2013, 44, 1409–1418. [Google Scholar] [CrossRef]
- Fan, Z.; Men, H. An overview of recent advances on understanding of atomistic mechanisms of heterogeneous nucleation. Metals 2022. submitted. [Google Scholar]
- Fan, Z.; Gao, F.; Jiang, B.; Que, Z.P. Impeding nucleation for more significant grain refinement. Sci. Rep. 2020, 10, 9448. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Gao, F. Grain initiation and grain refinement: An overview. Metals 2022. submitted. [Google Scholar]
- Fang, C.M.; Men, H.; Fan, Z. Effect of substrate chemistry on prenucleation. Metall. Mater. Trans. A 2018, 49, 6231–6242. [Google Scholar] [CrossRef]
- Jiang, B.; Men, H.; Fan, Z. Atomic ordering in the liquid adjacent to an atomic-level rough substrate surface. Comput. Mater. Sci. 2018, 153, 73–81. [Google Scholar] [CrossRef]
- Zope, R.R.; Mishin, Y. Interatomic potentials for atomistic simulations of the Ti-Al system. Phys. Rev. B 2003, 68, 024102. [Google Scholar] [CrossRef]
- Todorov, I.T.; Smith, W.; Trachenko, K.; Dove, M.T. Interatomic potentials for atomistic simulations of the Ti-Al system. J. Mater. Chem. 2006, 16, 1911–1918. [Google Scholar] [CrossRef]
- Jackson, K.A. The interface kinetics of crystal growth processes. Interface Sci. 2002, 10, 159–169. [Google Scholar] [CrossRef]
- Steinhardt, P.J.; Nelson, D.R.; Ronchetti, M. Bond-orientational order in liquids and glasses. Phys. Rev. B 1983, 28, 784–805. [Google Scholar] [CrossRef]
- Baumgartner, J.; Dey, A.; Bomans, P.H.H.; Coadou, C.L.; Fratzl, P.; Sommerdijk, N.A.J.M.; Faivre, D. Nucleation and growth of magnetite from solution. Nat. Mater. 2013, 12, 310–314. [Google Scholar] [CrossRef]
- Turnbull, D. Kinetics of solidification of supercooled liquid mercury droplets. J. Chem. Phys. 1952, 20, 411–424. [Google Scholar] [CrossRef]
- Turnbull, D. Theory of catalysis of nucleation by surface patches. Acta Metall. 1953, 1, 8–14. [Google Scholar] [CrossRef]
- Kurz, W.; Herlach, D.M. Solidification and Crystallization; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Pandat with Pan Al Database Version 2021. Available online: www.computherm.com (accessed on 6 December 2021).
- Boettinger, W.J.; Banerjee, D.K. Solidification. In Physical Metallurgy, 5th ed.; Laughlin, D., Hono, K., Eds.; Elsevier Science & Technology: Amsterdam, The Netherlands, 2014; p. 667. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Z.; Men, H. Heterogeneous Nucleation and Grain Initiation on a Single Substrate. Metals 2022, 12, 1454. https://doi.org/10.3390/met12091454
Fan Z, Men H. Heterogeneous Nucleation and Grain Initiation on a Single Substrate. Metals. 2022; 12(9):1454. https://doi.org/10.3390/met12091454
Chicago/Turabian StyleFan, Zhongyun, and Hua Men. 2022. "Heterogeneous Nucleation and Grain Initiation on a Single Substrate" Metals 12, no. 9: 1454. https://doi.org/10.3390/met12091454
APA StyleFan, Z., & Men, H. (2022). Heterogeneous Nucleation and Grain Initiation on a Single Substrate. Metals, 12(9), 1454. https://doi.org/10.3390/met12091454





