Recovery and Utilization of Lead in Lead–Containing Waste Residue from Electrolytic Manganese Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Devices and Materials
2.2. Experimental Process
2.3. Experimental Principle

2.4. Survey Indicators
- (1)
- Current efficiency. The percentage of the ratio of the actual amount of metal precipitated on the cathode to the theoretical amount of metal precipitated during electrolysis. The theoretical precipitation is calculated according to Faraday’s law, and so, the current efficiency is actually a measure of the deviation of the electrolysis process from Faraday law.
- (2)
- Acid recovery rate. In the process of membrane electrolysis, NO3− acts as a conductive ion between the anode solution and the cathode solution under the action of electric field force, and so, the recovery rate of HNO3 is calculated by Faraday law to measure the acid enrichment and recovery ability of this process.
- (3)
- Lead recovery rate. In this experiment, the ratio of Pb2+ concentration in cathode solution to Pb2+ concentration in initial cathode solution was used as lead recovery rate.
- (4)
- Electricity consumption. In the electrolysis test, the lower the energy consumption, the better the economy.
3. Results and Discussion
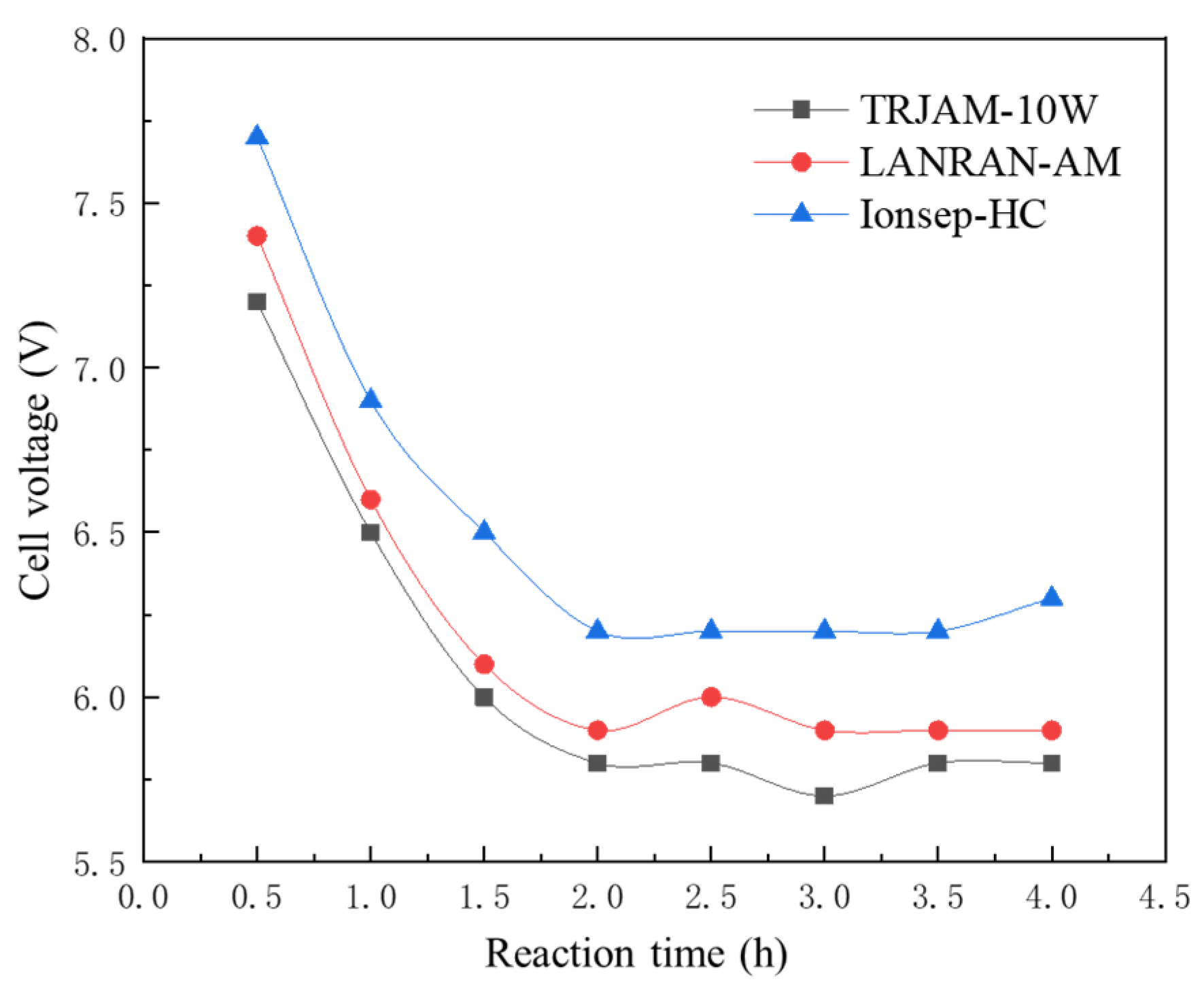
3.1. Selection of Ion Exchange Membrane
3.2. Selection of Anode Materials
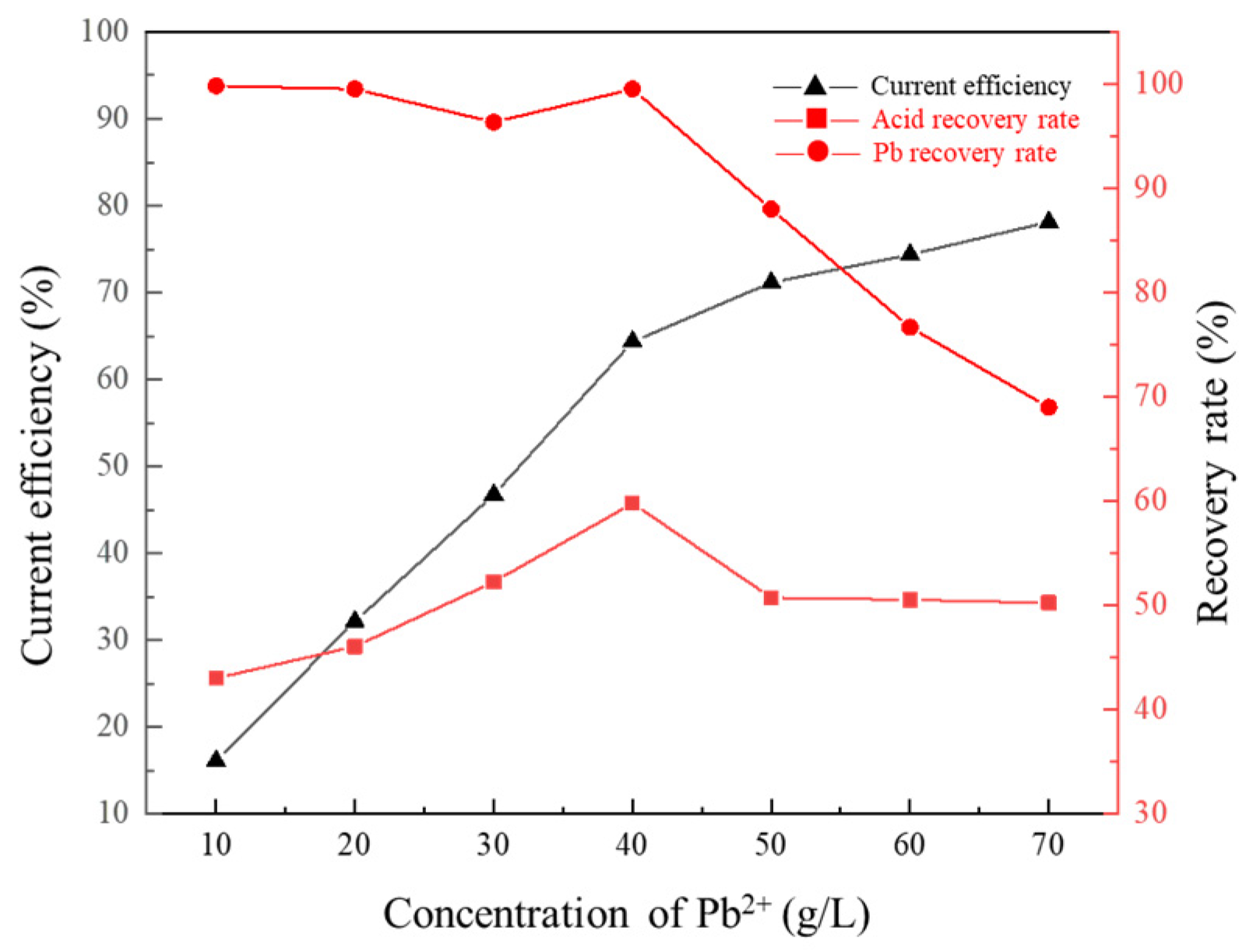
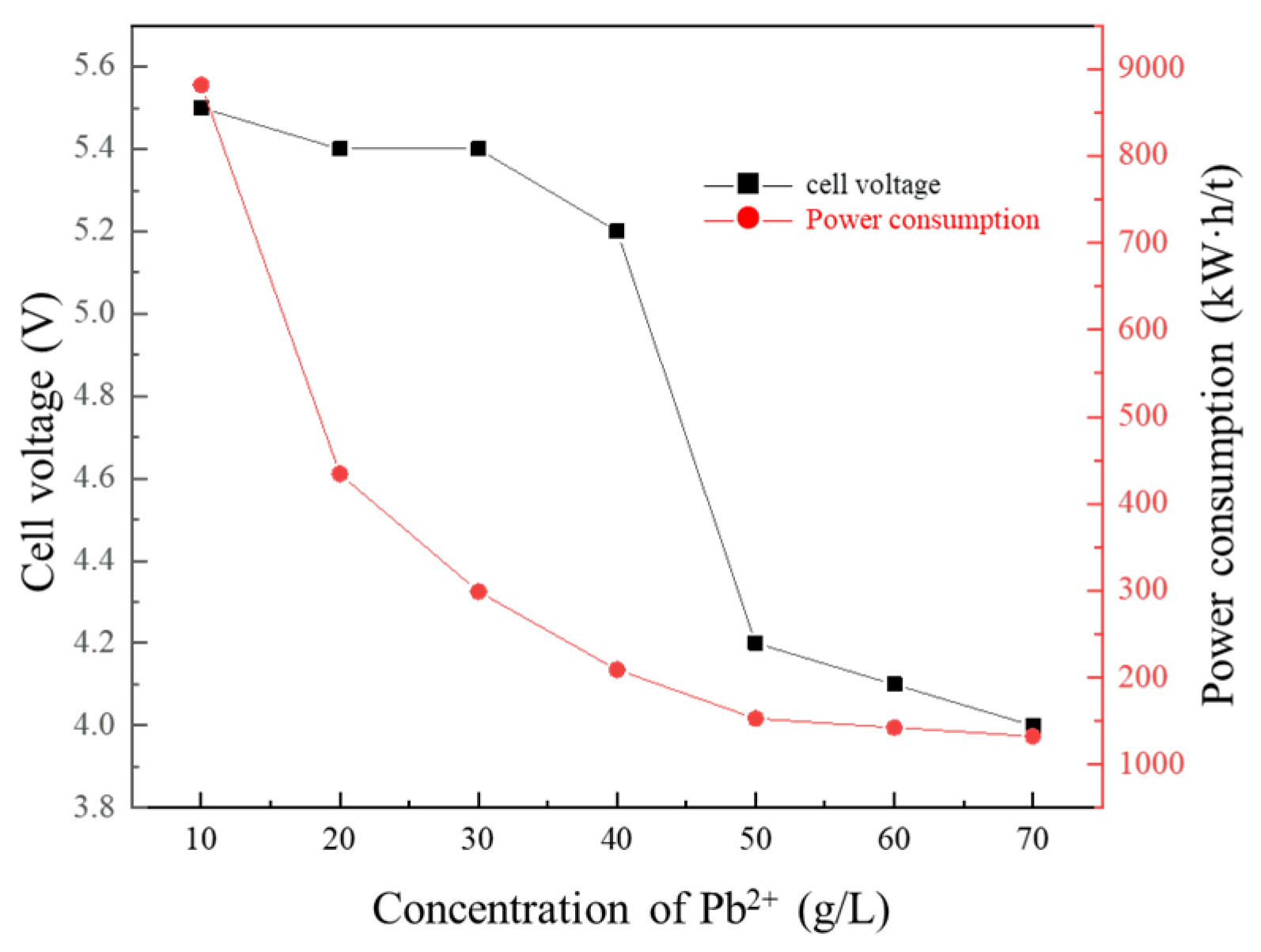
3.3. Effect of Pb2+ Concentration of Cathode Solution on Membrane Electrolysis
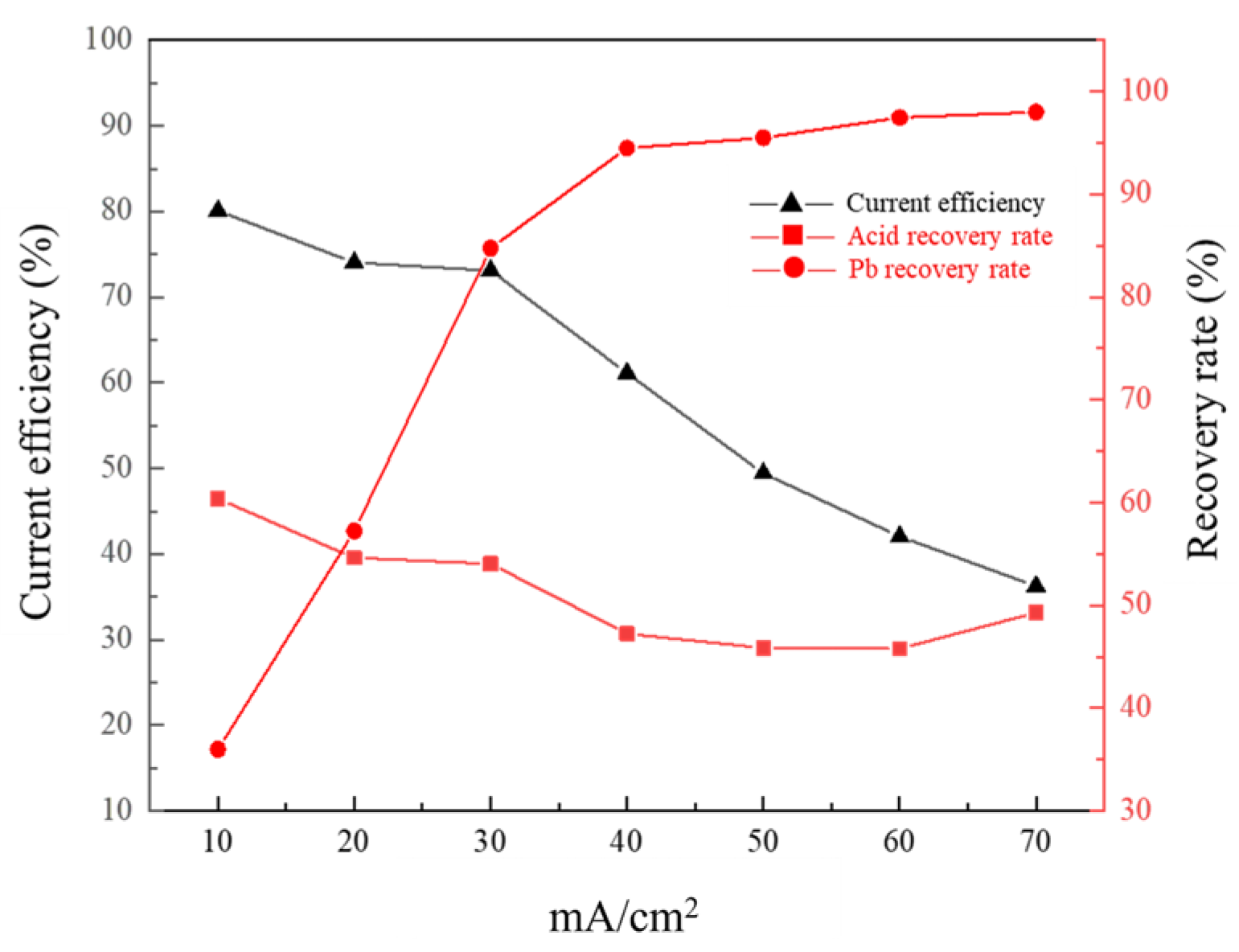
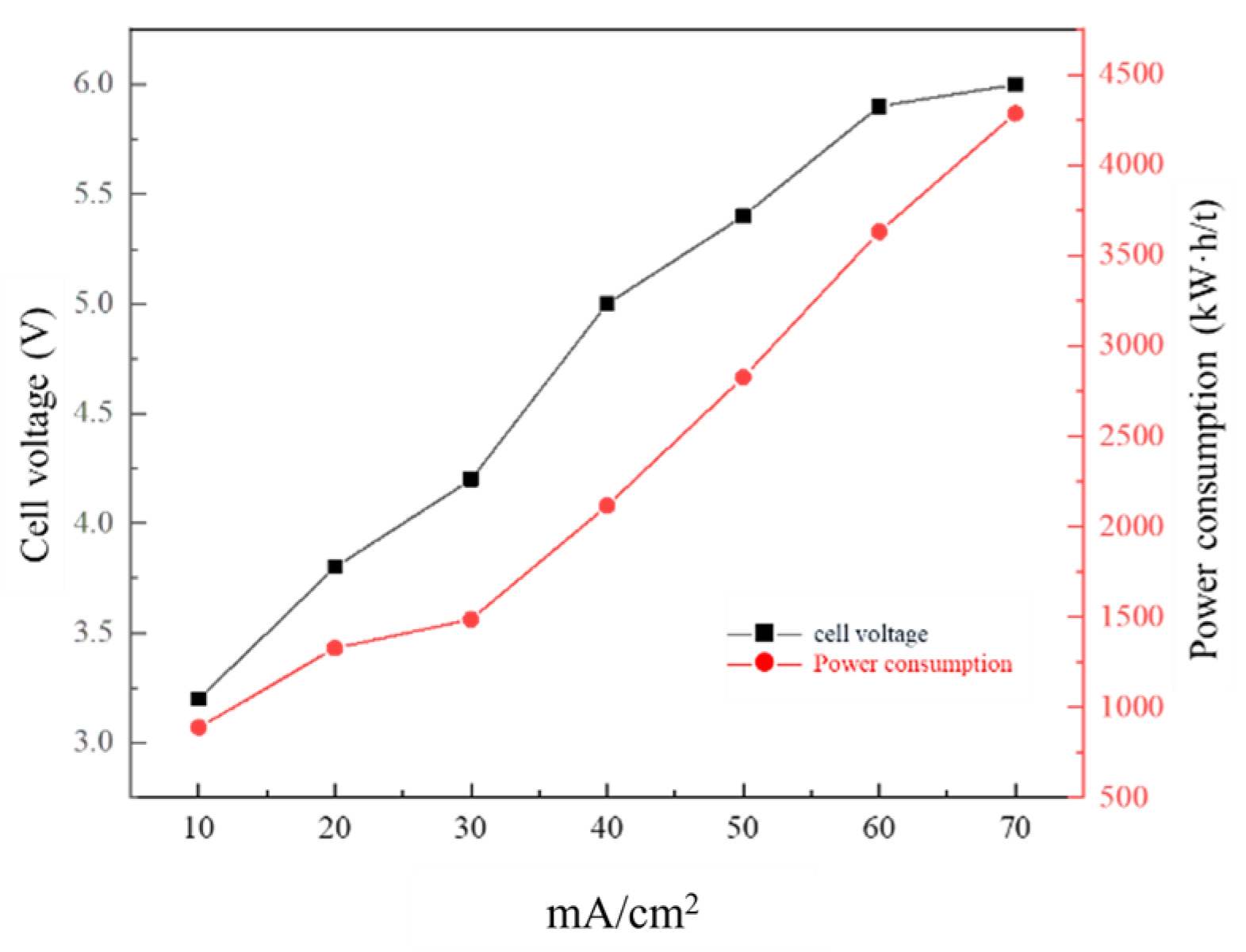
3.4. Effect of Current Density on Membrane Electrolysis
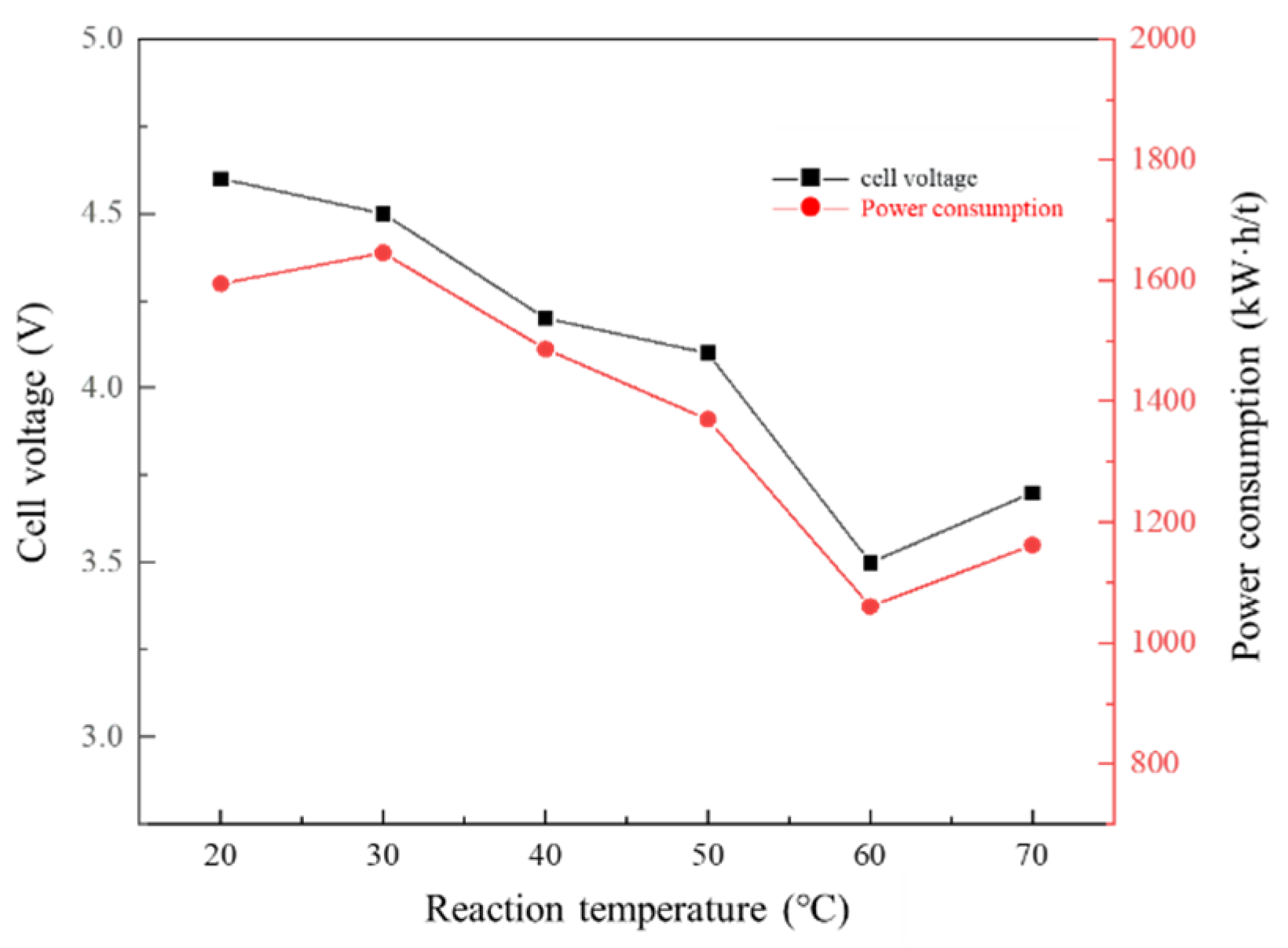
3.5. Effect of Reaction Temperature on Membrane Electrolysis
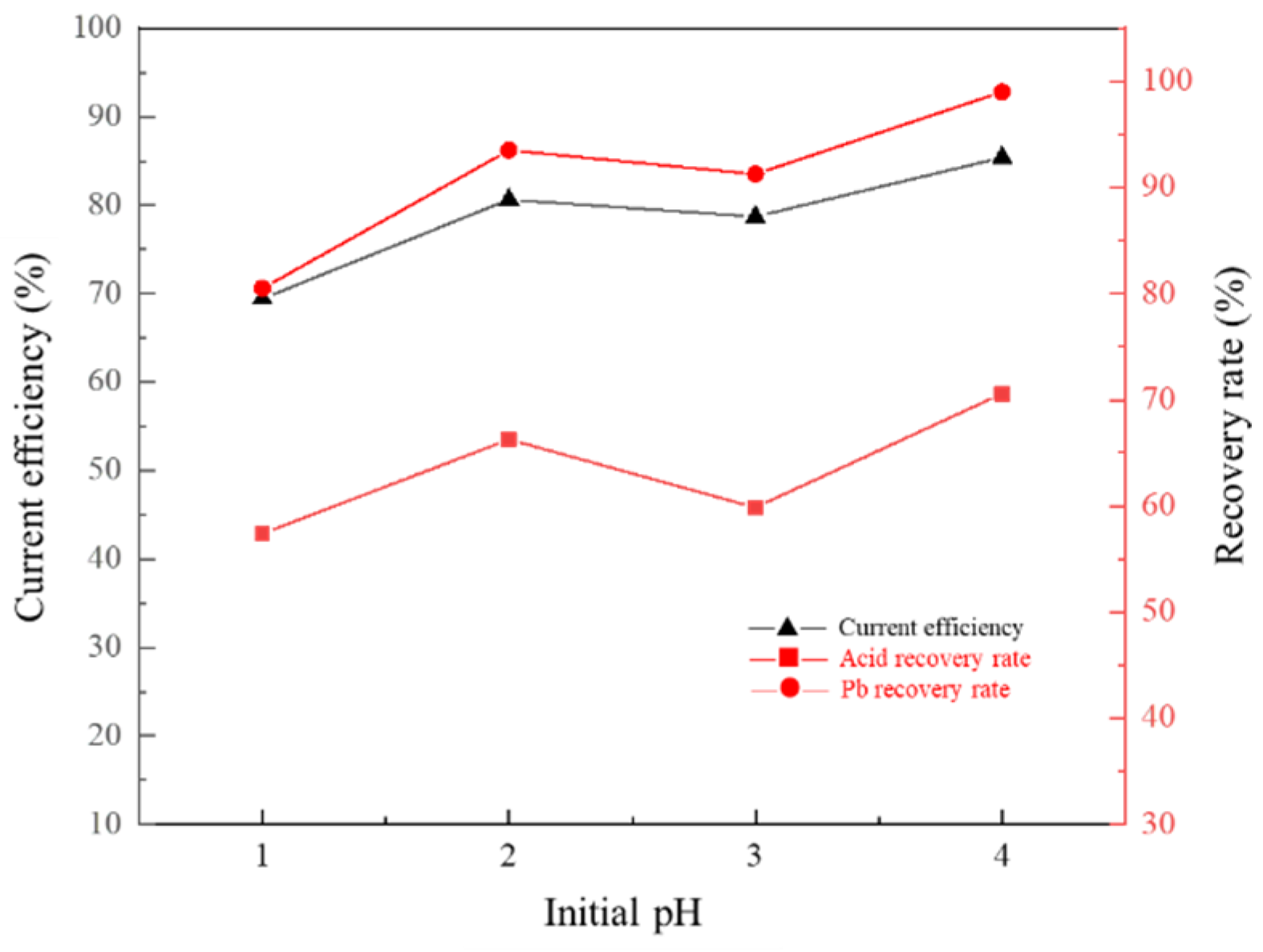
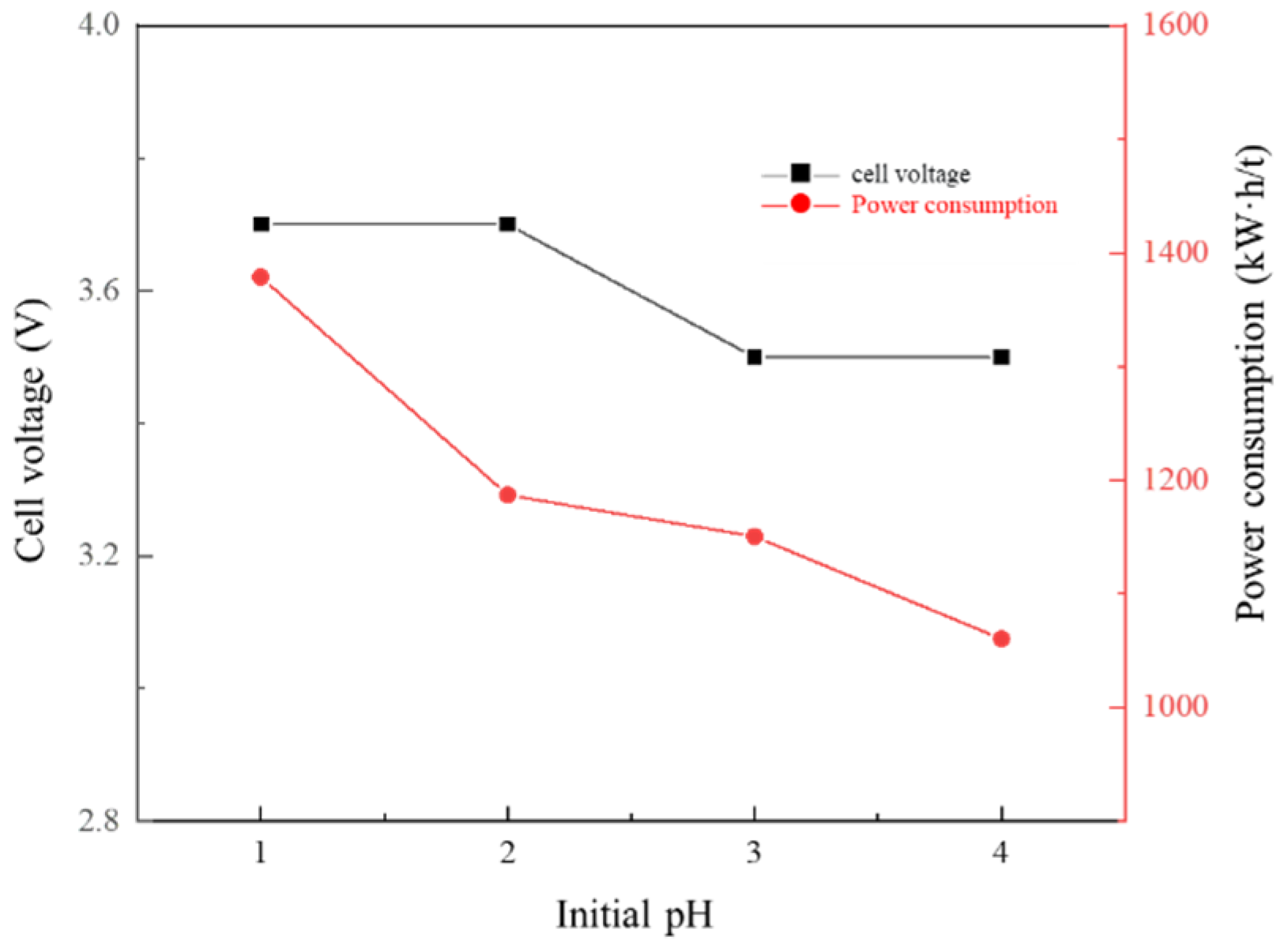
3.6. Effect of Cathode Solution pH on Membrane Electrolysis
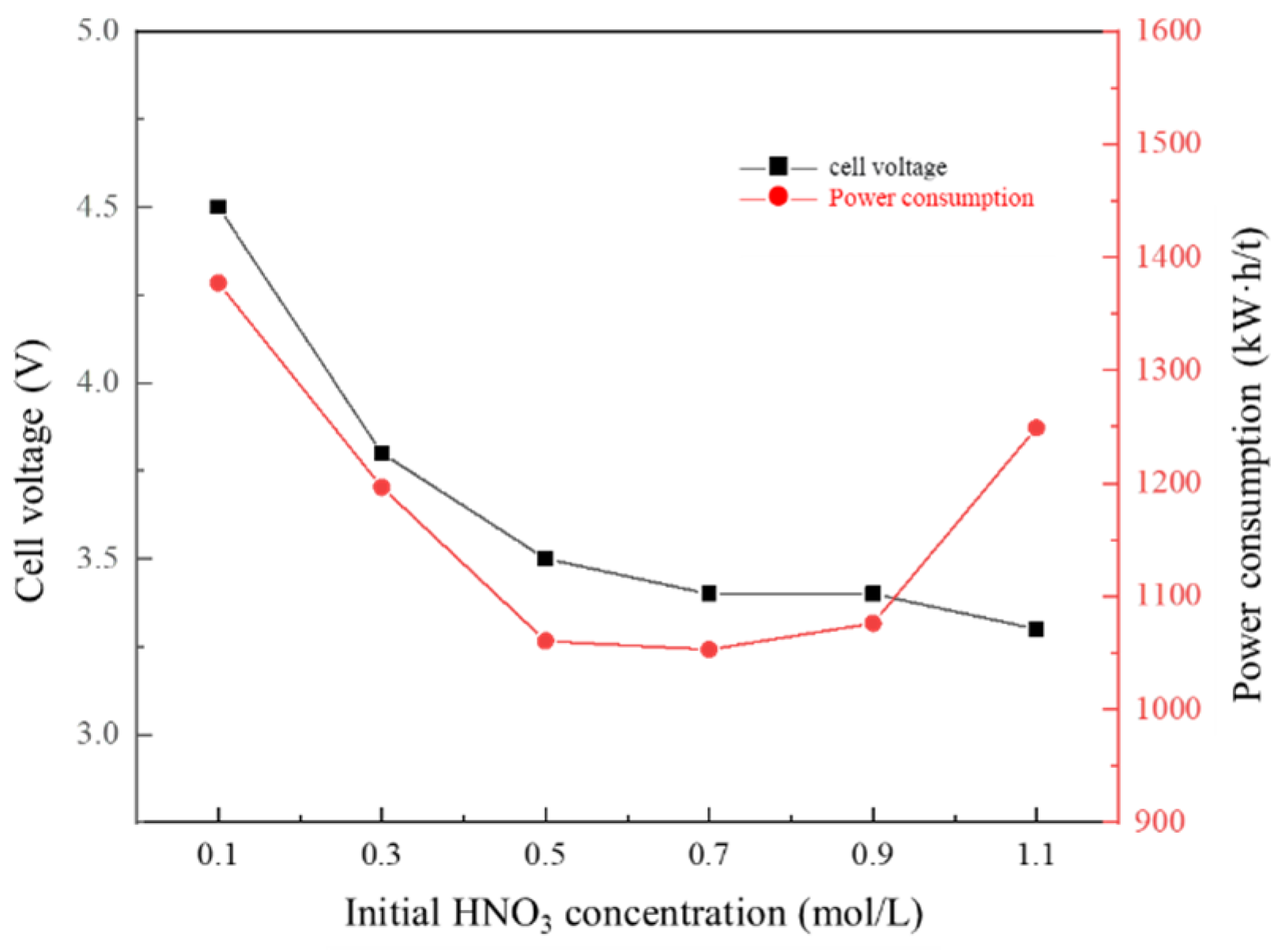
3.7. Effect of HNO3 Concentration of Anode Solution on Membrane Electrolysis
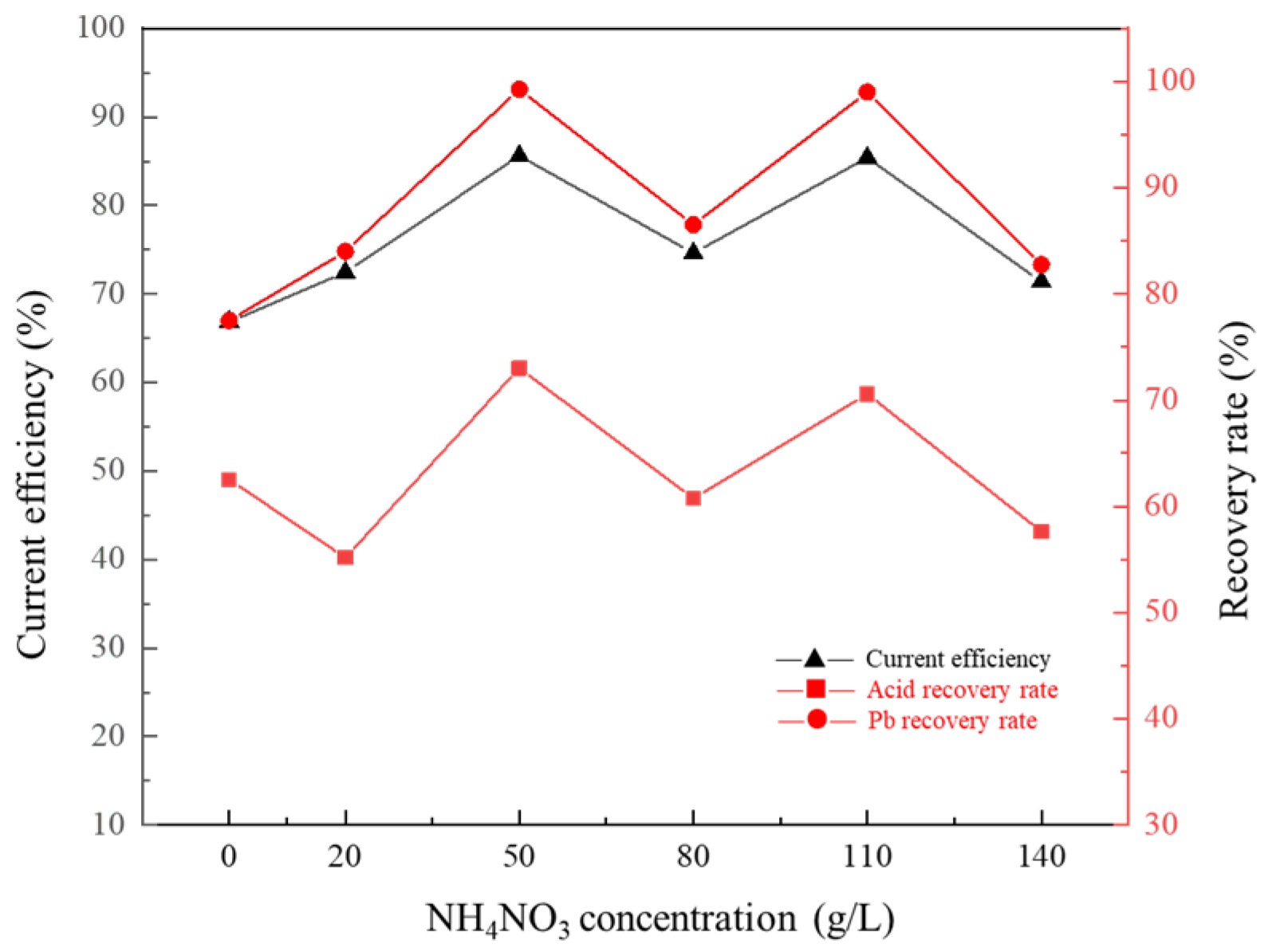
3.8. Effect of Concentration of NH4NO3 in Cathode Solution on Membrane Electrolysis
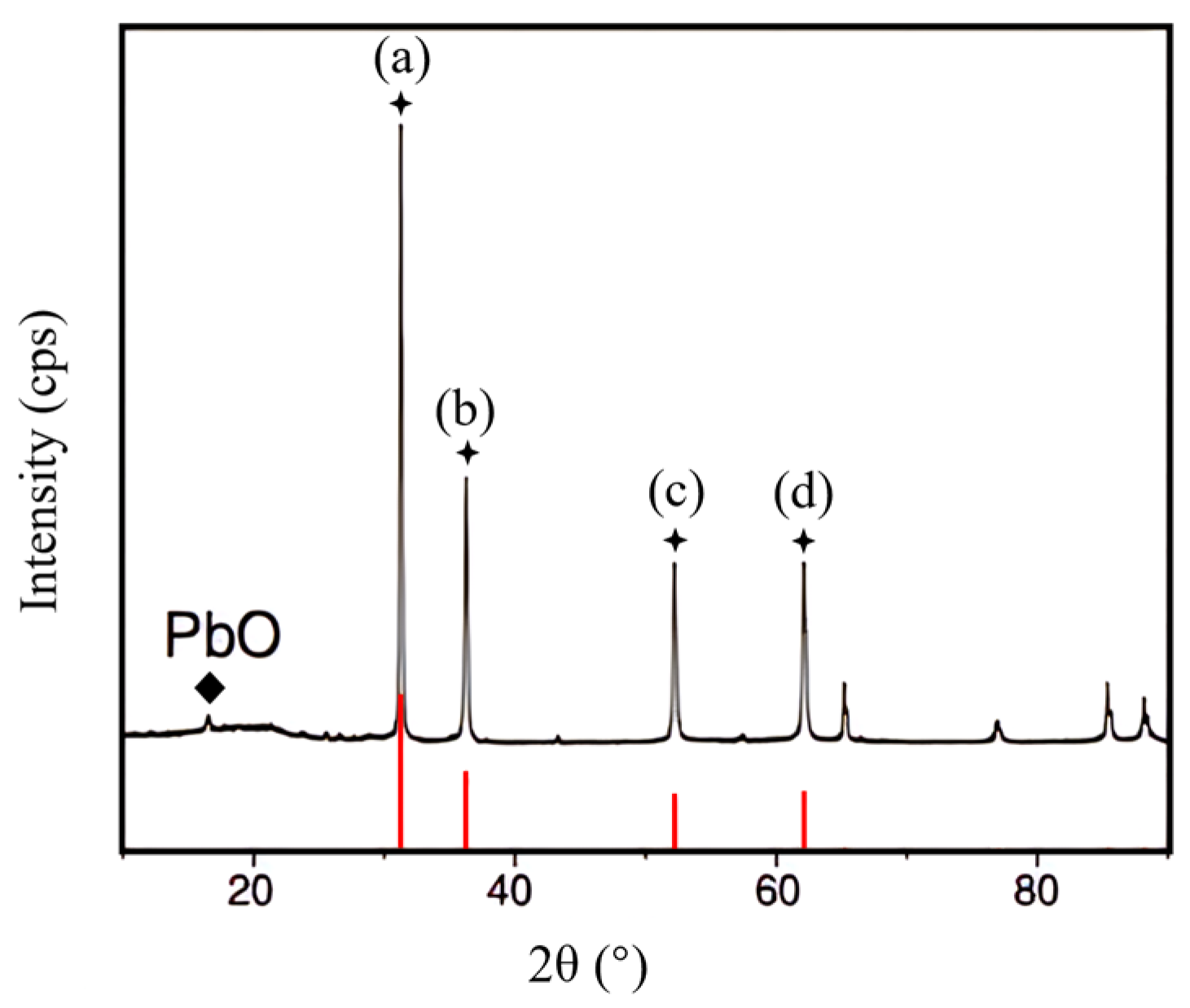
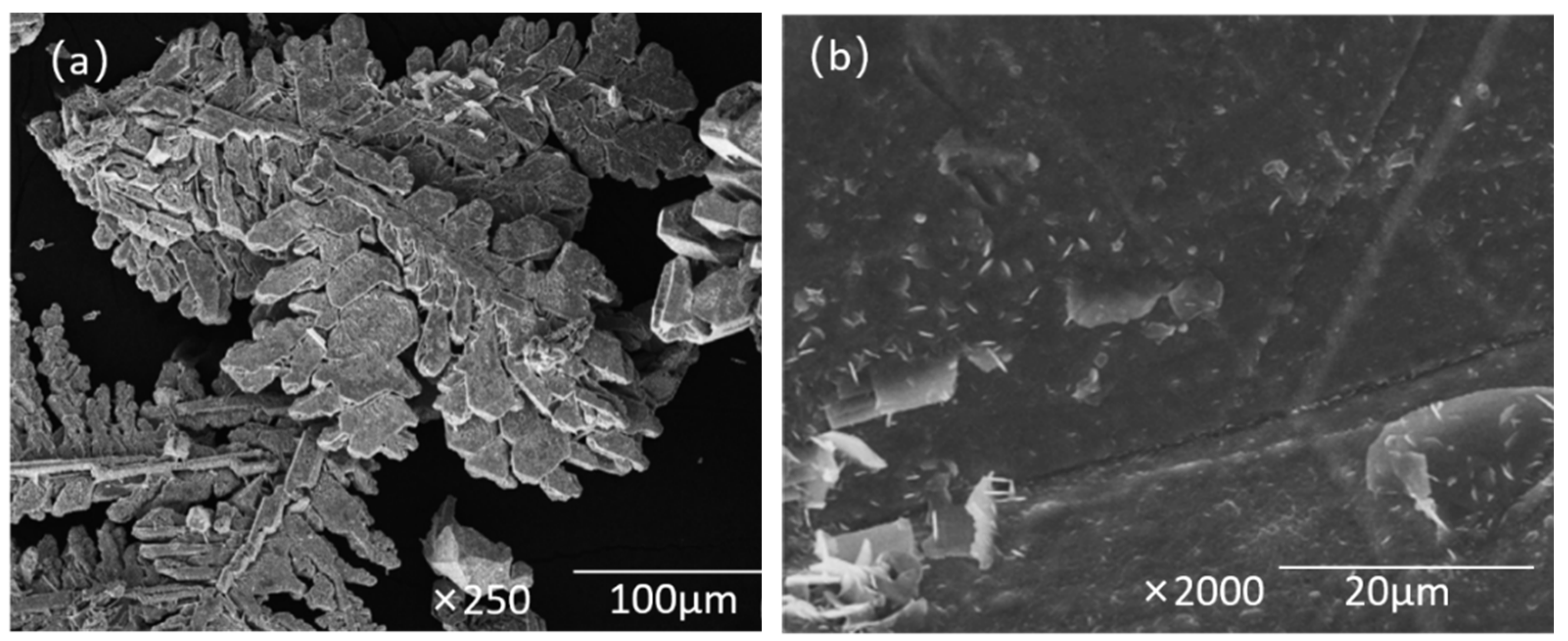
3.9. Characterization and Analysis
4. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wei, H.; Yang, Y.; Luo, D.; Wu, X.; Lu, Q.; Huang, H. A Research on Comprehensive Recycling of Electrolytic Manganese Anode Slim. China Manganese Ind. 2017, 35, 55–58. (In Chinese) [Google Scholar]
- Camarillo, R.; Llanos, J.; García-Fernández, L.; Pérez, Á.; Canizares, P. Treatment of copper (II)-loaded aqueous nitrate solutions by polymer enhanced ultrafiltration and electrodeposition. Sep. Purif. Technol. 2010, 70, 320–328. [Google Scholar] [CrossRef]
- Li, T.; Xiao, K.; Yang, B.; Peng, G.; Liu, F.; Tao, L.; Chen, S.; Wei, H.; Yu, G.; Deng, S. Recovery of Ni (II) from real electroplating wastewater using fixed-bed resin adsorption and subsequent electrodeposition. Front. Environ. Sci. Eng. 2019, 13, 91. [Google Scholar] [CrossRef]
- Romero-Cano, L.A.; García-Rosero, H.; Baldenegro-Pérez, L.A.; Marín, F.C.; González-Gutiérrez, L.V. Coupled Adsorption and Electrochemical Process for Copper Recovery from Wastewater Using Grapefruit Peel. J. Environ. Eng. 2020, 146, 04020100. [Google Scholar] [CrossRef]
- Yang, Y.; Shu, J.; Zhang, L.; Su, P.; Meng, W.; Wan, Q.; Liu, Z.; Liu, R.; Chen, F.; Ming, X. Enhanced Leaching of Mn from Electrolytic Manganese Anode Slime via an Electric Field. Energy Fuels 2021, 35, 20224–20230. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Liu, B.; Huang, J.; Ye, J.; Li, Y.; Su, Z.; Wang, J. Extraction and Separation of Mn and Pb from Electrolytic Manganese Anodic Slime (EMAS) via SO2 Roasting Followed by Acid Leaching Process. JOM 2020, 72, 925–932. [Google Scholar] [CrossRef]
- Jiang, B. Study on Treatment and Recovery Technology of High Concentration Manganese Sulfate Waste Liquid with Double Membranes and Three Chambers; Lanzhou Jiaotong University: Lanzhou, China, 2019. (In Chinese) [Google Scholar]
- LI, T.; Wang, S.; Zhou, J. Experimental Study of Ion-exchange Membrane Electrolysis Nickel Sulfate Electrowinning of Nickel Metal System. Mater. Rep. 2016, 30, 432–435+439. (In Chinese) [Google Scholar]
- Guo, P. Relationship between hydrogen evolution reaction and overpotential and application of hydrogen over potential. Coal Chem. Ind. 2009, 32, 12–15. (In Chinese) [Google Scholar]
- Jia, Z.; Ma, H.; Wu, X.; Liao, S.; Wang, B. Fundamentals of electrochemistry (V)—Electrochemical kinetic and charge-transfer process for electrochemical reaction. Energy Storage Sci. Technol. 2013, 2, 402–409. (In Chinese) [Google Scholar]
- Jin, B.; Dreisinger, D.B. A green electrorefining process for production of pure lead from methanesulfonic acid medium. Sep. Purif. Technol. 2016, 170, 199–207. [Google Scholar] [CrossRef]
- Dai, F.; Huang, H.; Chen, B.; Zhang, P.; He, Y.; Guo, Z. Recovery of high purity lead from spent lead paste via direct electrolysis and process evaluation. Sep. Purif. Technol. 2019, 224, 237–246. [Google Scholar] [CrossRef]
- Xu, J. Preparation of Nanocrystalline Nickel by Electrodeposition and its Electrochemical Properties; Kunming University of Science and Technology: Kunming, China, 2007. (In Chinese) [Google Scholar]
- Tsurtsumia, G.; Shengelia, D.; Koiava, N.; Lezhava, T.; Gogoli, D.; Beriashvili, L.; Kakhniashvili, I. Novel hydro-electrometallurgical technology for simultaneous production of manganese metal, electrolytic manganese dioxide, and manganese sulfate monohydrate. Hydrometallurgy 2019, 186, 260–268. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Y.; Pan, J. A Clean and Highly Efficient Leaching–Electrodeposition Lead Recovery Route in HClO4 Solution. Int. J. Electrochem. Sci. 2017, 12, 6966–6979. [Google Scholar] [CrossRef]
- Zhang, H. Research on Production Recovery Process and Pilot Test from the Amberplex Metal Electrodeposition Technology; Lanzhou Jiaotong University: Lanzhou, China, 2015. (In Chinese) [Google Scholar]
- Wei, Q.; Ren, X.; Du, J.; Wei, S.; Hu, S. Study of the electrodeposition conditions of metallic manganese in an electrolytic membrane reactor. Miner. Eng. 2010, 23, 578–586. [Google Scholar] [CrossRef]
- Xiang, H.; Yang, Z.; Liu, S.; Li, X.; Yang, C.; Ke, Y.; Lin, Z. Natural Pyrite-assisted Mechanochemical Recovery of Insoluble Manganese from Electrolytic Manganese Residue: Kinetics and Mechanisms. ACS EST Eng. 2023. [Google Scholar] [CrossRef]










| Substan | Concentration |
|---|---|
| Pb2+ | 2456.76 mg/L |
| pH | 0.5 |
| Ca2+ | 12.3 mg/L |
| Mn2+ | 5.63 mg/L |
| Al3+ | 1.12 mg/L |
| Membrane | Type | Ion Exchange Capacity (mmol/g) | Selective Transmittance (%) | Membrane Resistance (Ω·cm2) | Bursting Strength (Mpa) | Thickness (mm) |
|---|---|---|---|---|---|---|
| Homogeneous | TRJAM–10W | 2.0 | >95 | 3 | 0.35 | 0.20~0.30 |
| Semi–homogeneous | LANRAN–AM | 2.1 | >92 | 5~6 | 0.60 | 0.38~0.42 |
| Heterogeneous | Ionsep–HC | 2.2 | >90 | 12 | 0.60 | 0.40~0.44 |
| Anode Material | Initial Cell Voltage (V) | Current Efficiency (%) | Acid Recovery Rate (%) | Power Consumption (kW·h·t−1) |
|---|---|---|---|---|
| Titanium plate | 15.55 | 34.2 | 32.2 | 3844.9 |
| Titanium–coated ruthenium iridium | 5.83 | 63.3 | 50.6 | 2040.5 |
| titanium–coated iridium–tantalum | 6.00 | 59.9 | 45.5 | 2234.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Fan, J.; Zhou, J. Recovery and Utilization of Lead in Lead–Containing Waste Residue from Electrolytic Manganese Production. Metals 2023, 13, 1643. https://doi.org/10.3390/met13101643
Song X, Fan J, Zhou J. Recovery and Utilization of Lead in Lead–Containing Waste Residue from Electrolytic Manganese Production. Metals. 2023; 13(10):1643. https://doi.org/10.3390/met13101643
Chicago/Turabian StyleSong, Xiaosan, Jishuo Fan, and Jie Zhou. 2023. "Recovery and Utilization of Lead in Lead–Containing Waste Residue from Electrolytic Manganese Production" Metals 13, no. 10: 1643. https://doi.org/10.3390/met13101643
APA StyleSong, X., Fan, J., & Zhou, J. (2023). Recovery and Utilization of Lead in Lead–Containing Waste Residue from Electrolytic Manganese Production. Metals, 13(10), 1643. https://doi.org/10.3390/met13101643






