A New Approach for Increasing the Chelating Capacity of the Tartrate Ion in the Extraction of Copper from Ores
Abstract
:1. Introduction
- (i)
- To report the results obtained by introducing a new leaching methodology which consists of replacing the use of an aqueous solution of sulfuric acid by an alkaline solution rich in tartrate ions as a chelating agent for the release of copper from the oxidized mineral, to identify the presence of the complex, and to assess its stability through spectrophotometry. The selection of tartrate as a complexing agent is based on its lack of toxicity given that it is a natural component which is currently used as an ingredient in the food industry. It is commercially available at a relatively low cost and it forms complexes with transition metal cations such as Cu(II) [9] as well as lanthanide cations [10]. In fact, the interaction of tartrate with Cu(II) has been extensively discussed in the literature [11]. In addition, it is water soluble.
- (ii)
- To verify the presence of the coordination compound when metallic copper is treated with a tartrate alkaline solution in the presence of hydrogen peroxide.
- (iii)
- To assess the impact of an alkaline aqueous solution of tartrate on the dissolution of malachite (basic cupric carbonate), as well as in the oxidized ore leaching process.
2. Materials and Methods
2.1. Materials
2.2. Methodology
2.2.1. Chemical Characterization of the Processed Mineral
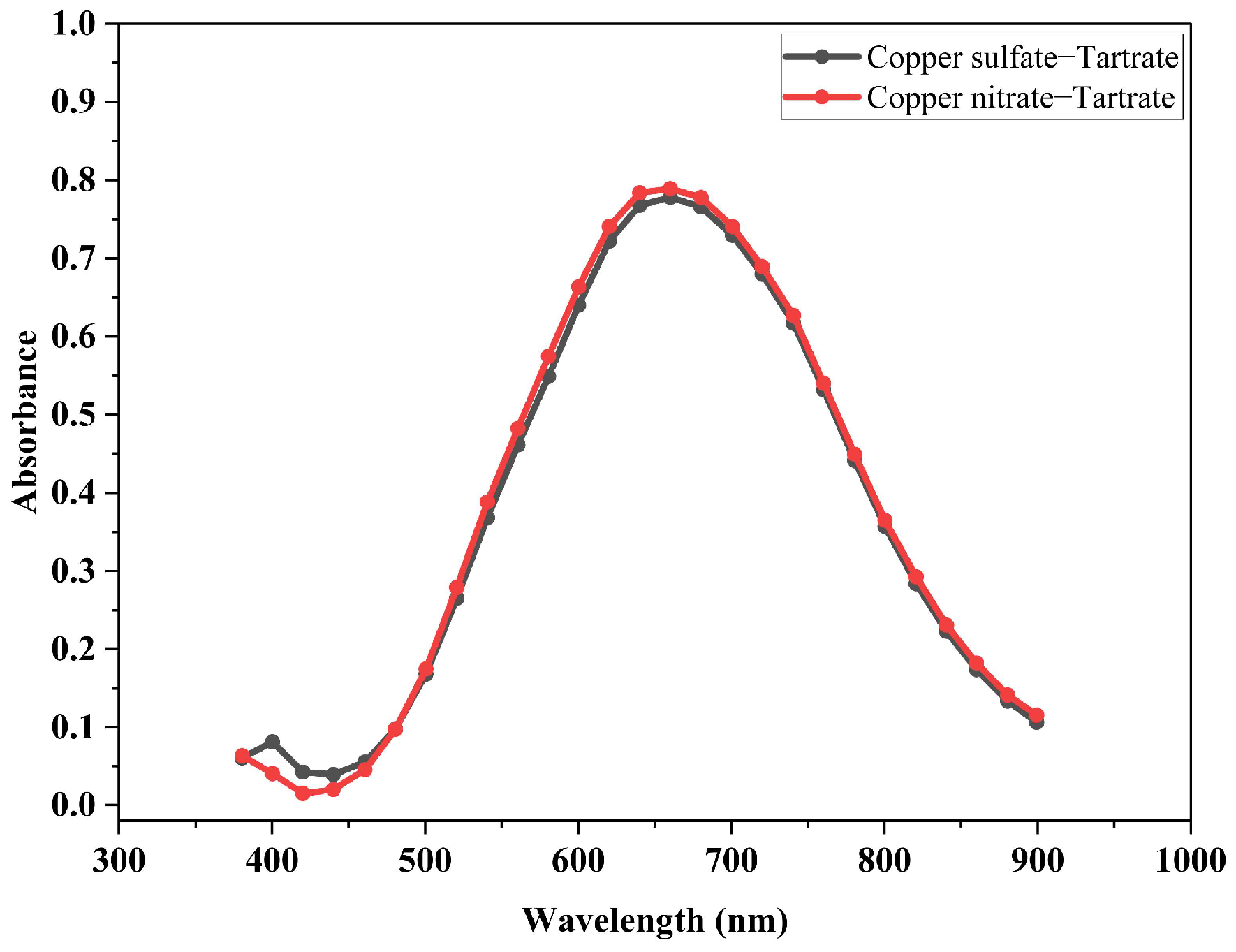
2.2.2. Identification of Complex Formation through Spectrophotometric Examination
2.2.3. Stoichiometry of the Coordination Compound
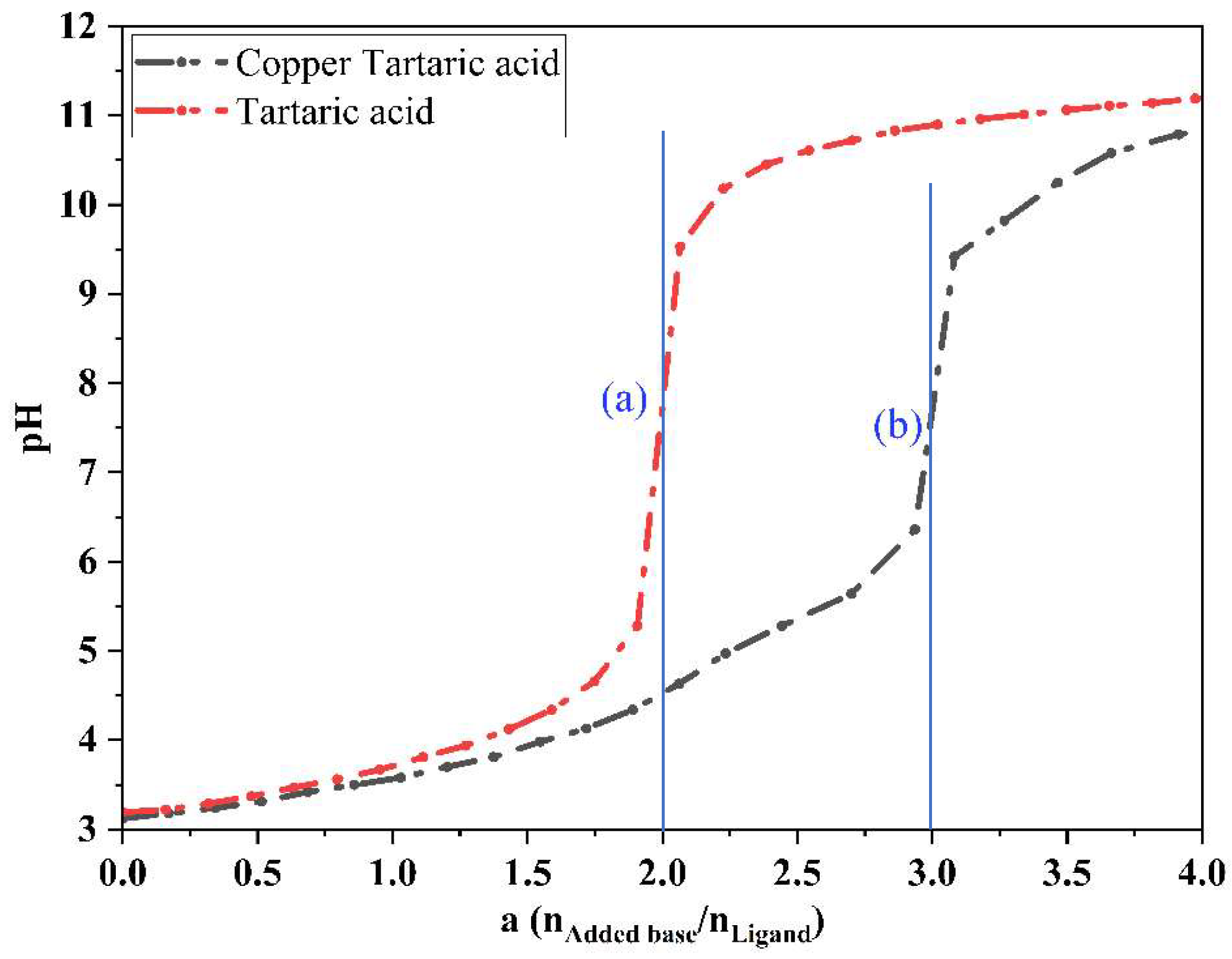
2.2.4. Potentiometric Measurements of Free Tartaric Acid in the Absence and Presence of Copper Ions at 298.15 K
2.2.5. Verification of Oxidation and Complexation of Metallic Copper in the Presence of Tartrate and Hydrogen Peroxide in an Alkaline Medium
2.2.6. Dissolution of Basic Cupric Carbonate (Malachite) by an Alkaline Aqueous Solution of Tartrate
2.2.7. Evaluation of the Removal Capacity of Tartrate for Copper
3. Results and Discussion
3.1. Chemical Characterization of the Processed Mineral
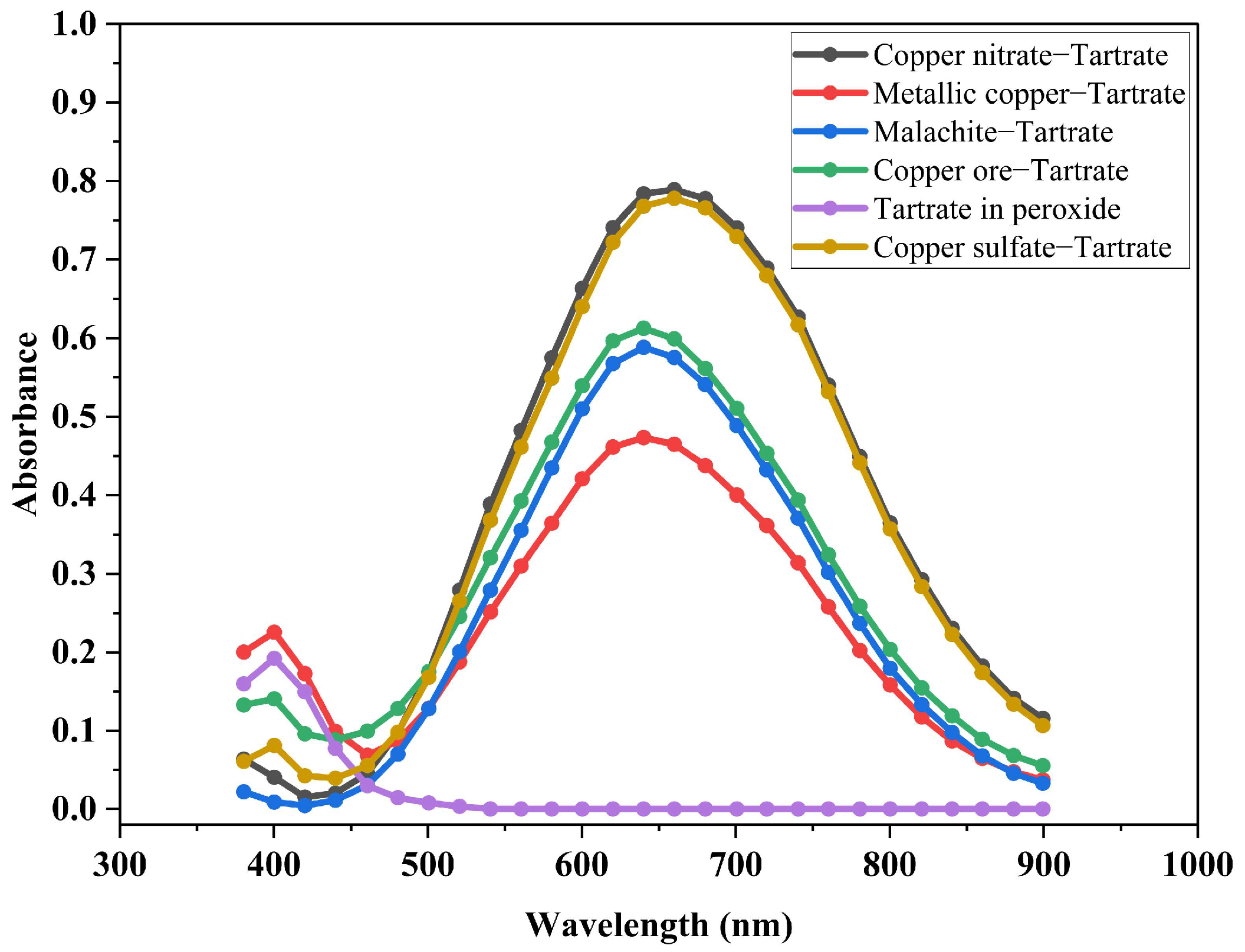
3.2. Identification of the Compound Formed
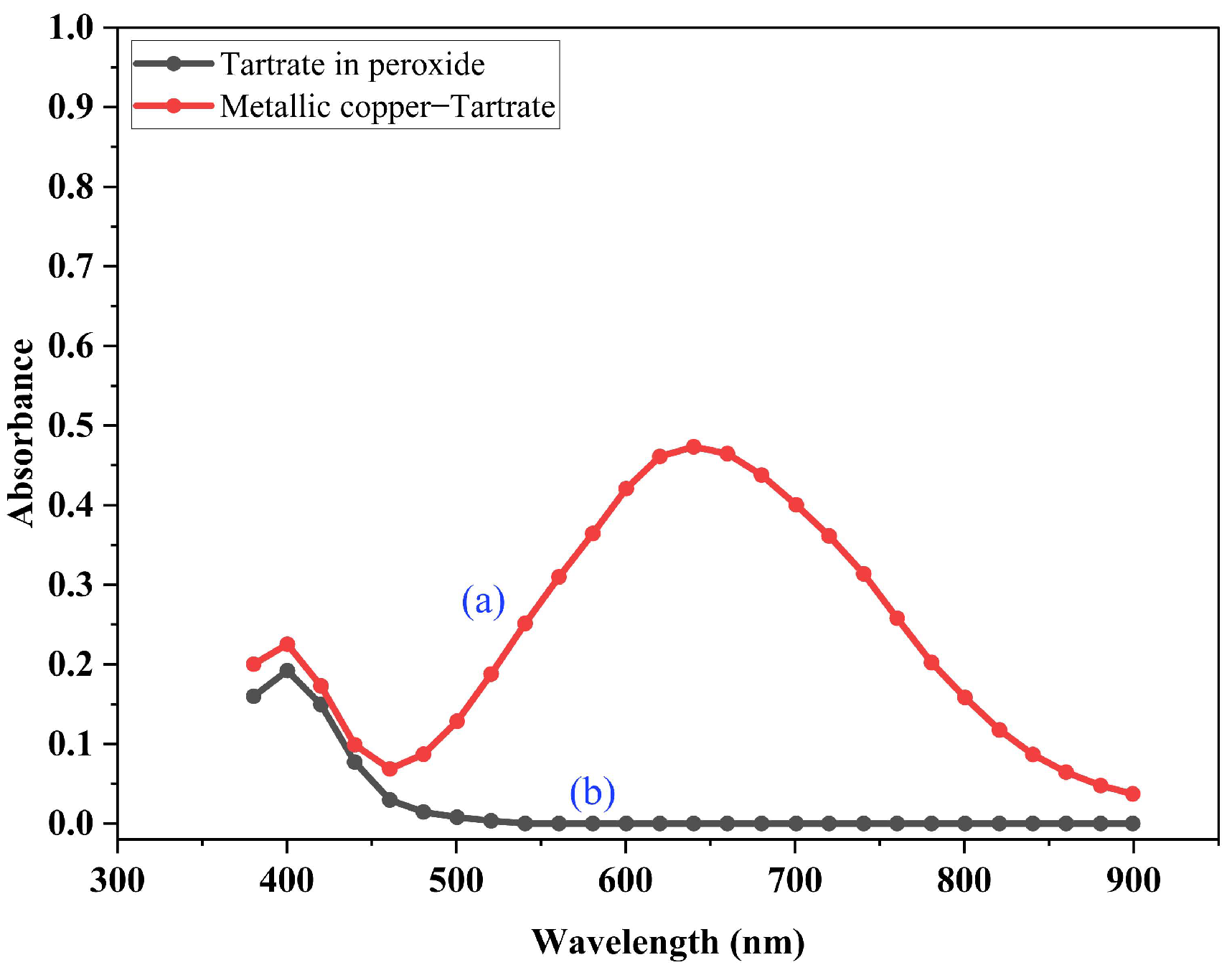
3.3. Dissolution of Metallic Copper in the Presence of an Alkaline Aqueous Solution of Tartrate Ions and Hydrogen Peroxide
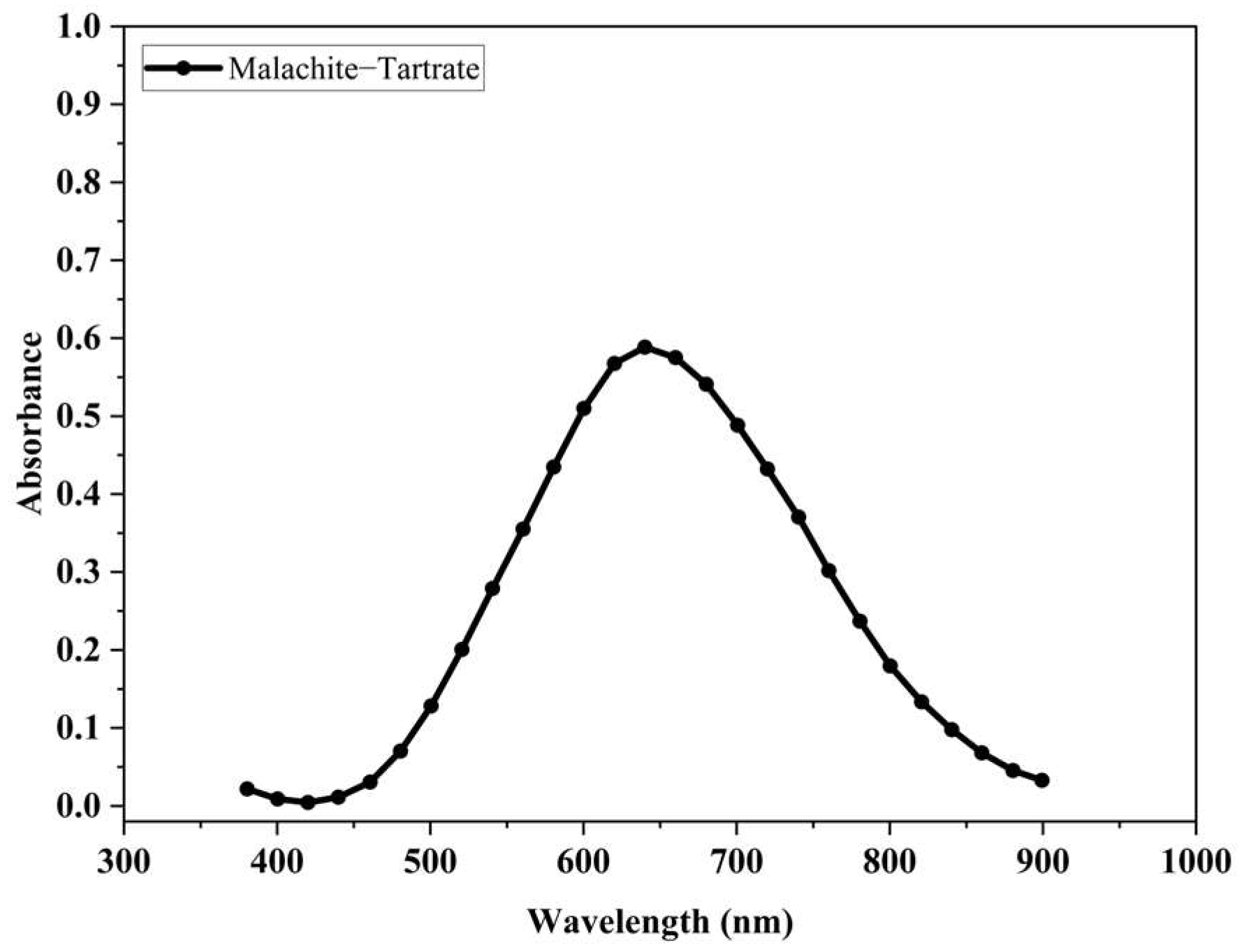
3.4. Evaluation of the Dissolution of Basic Cupric Carbonate (Malachite) by the Action of an Aqueous Solution, Consisting of Tartrate Ions in an Alkaline Medium
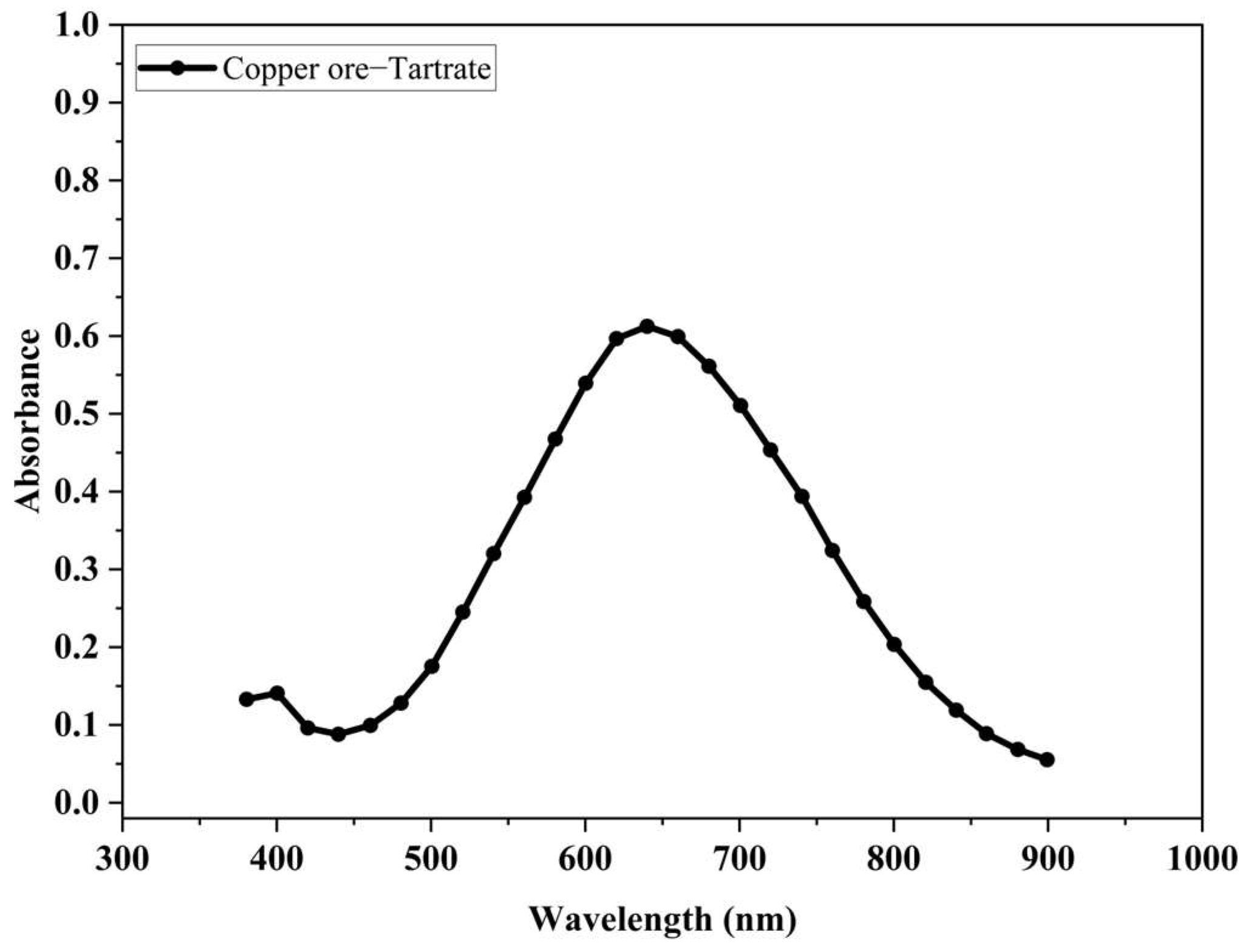
3.5. Ability of the Tartrate Ion to Remove Copper Ions in the Oxidized Ore Leaching Process
4. Conclusions
- (i)
- A new leaching methodology, which consists in replacing the use of an aqueous solution of sulfuric acid with an alkaline solution enriched by the presence of tartrate ions as chelating agent for the release of copper from the oxidized mineral, has been successfully developed. This statement is supported by the observed enhancement of the capacity of the tartrate ion to dissolve copper from ores in the presence of hydrogen peroxide.
- (ii)
- Basic cupric carbonate (malachite) is soluble in an alkaline solution, due to the complexing ability of tartrate ions.
- (iii)
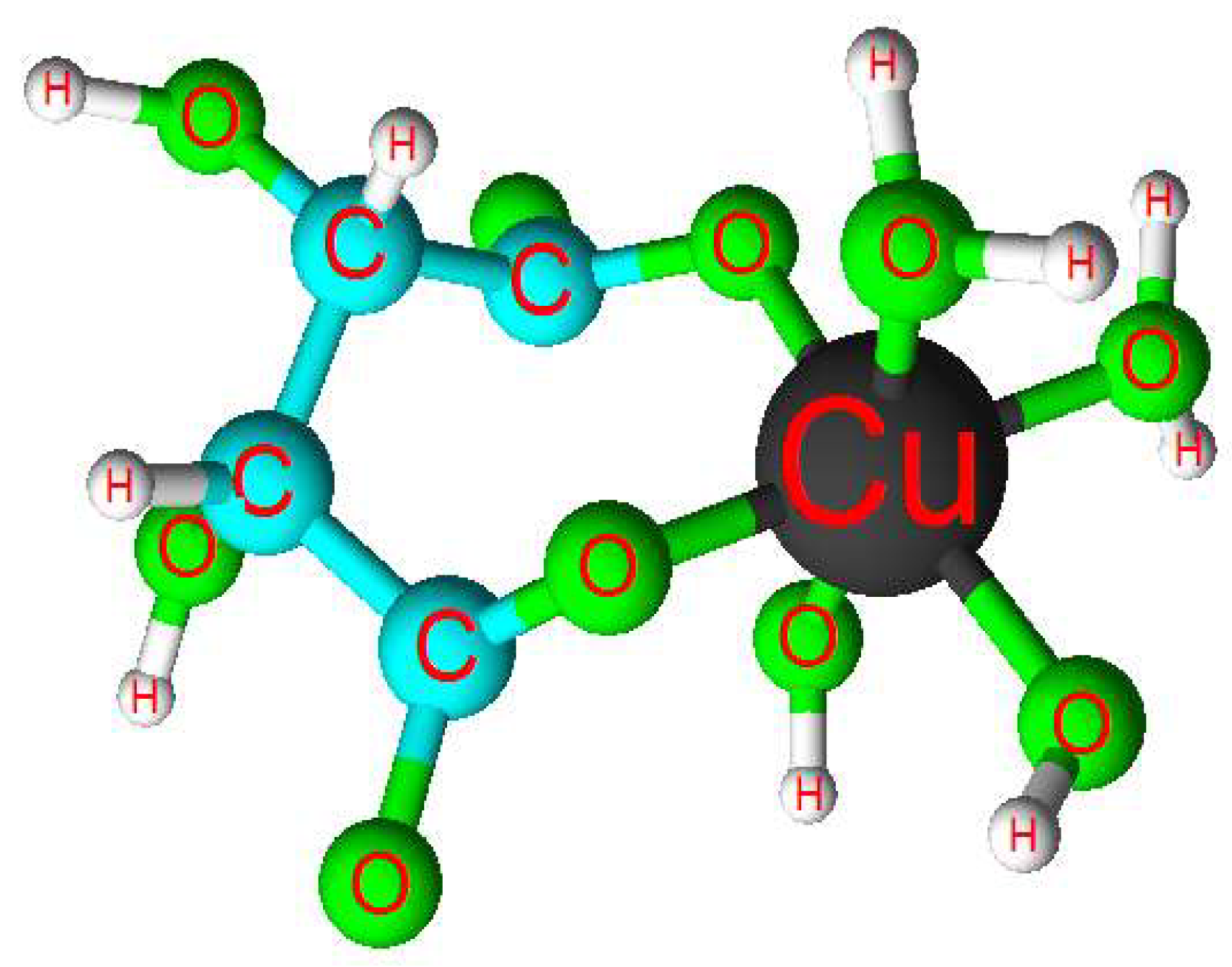
- Copper is released from the oxidized mineral in the leachate solution forming the [Cu(OH)2(C4H4O6]2− complex.
- (iv)
- The concentration of copper ions in the solution resulting from the leaching process is strongly dependent on the concentration of tartrate ions in the leachate solution. This behavior confirms the chelating capacity of the tartrate ions in the release of copper ions from the oxidized mineral.
- (v)
- In addition, the results of this investigation provide the basis of a new environmentally friendly hydrometallurgical process of oxidized copper minerals, in which the use of sulfuric acid solutions is eliminated.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hocking, M.B. Ore Enrichment and Smelting of Copper. In Handbook of Chemical Technology and Pollution Control; Academic Press: Cambridge, MA, USA, 2005; pp. 391–420. [Google Scholar] [CrossRef]
- Joaquín Jara, J.; Pérez, P.; Villalobos, P. Good deposits are not enough: Mining labor productivity analysis in the copper industry in Chile and Peru 1992–2009. Resour. Policy 2010, 35, 247–256. [Google Scholar] [CrossRef]
- Leon Mendoza, J.C. Determinantes Económicos y Sociopolíticos de Los Conflictos Socioambientales en el Peru. Rev. De Investig. Altoandinas J. High Andean Res. 2019, 21, 122–138. [Google Scholar] [CrossRef]
- Terrones-Saeta, J.M.; Suárez-Macías, J.; Río, F.J.L.D.; Corpas-Iglesias, F.A. Study of Copper Leaching from Mining Waste in Acidic Media, at Ambient Temperature and Atmospheric Pressure. Minerals 2020, 10, 873. [Google Scholar] [CrossRef]
- Stevanović, Z.; Antonijević, M.; Jonović, R.; Avramović, L.; Marković, R.; Bugarin, M.; Trujić, V. Leach-SX-EW Copper revalorization from overburden of abandoned copper mine Cerovo, Eastern Serbia. J. Min. Metall. 2009, 45, 45–57. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, B.; Yi, Y.; Fang, Q. Synergistic activation of smithsonite with copper-ammonium species for enhancing surface activity and xanthate adsorption. Int. J. Min. Sci. Technol. 2023, 33, 519–527. [Google Scholar] [CrossRef]
- Shen, Z.; Wen, S.; Wang, H.; Meng, S.; Feng, Q. Effect of dissolved components of malachite and calcite on surface properties and flotation behavior. Int. J. Miner. Miner. Metall. Mater. 2023, 30, 1297–1309. [Google Scholar] [CrossRef]
- Balasubramaniam, B.; Singh, N.; Verma, S.; Gupta, R.K. Recycling of Lithium from Li-ion Batteries. In Encyclopedia of Renewable and Sustainable Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 546–554. [Google Scholar] [CrossRef]
- Lin, F.H.-J.; Horváth, C. Measurements of stability constants for complexes of divalent cations with tartaric acid by ion chromatography. J. Chromatogr. A 1992, 589, 185–195. [Google Scholar] [CrossRef]
- Zabiszak, M.; Nowak, M.; Hnatejko, Z.; Grajewski, J.; Ogawa, K.; Kaczmarek, M.T.; Jastrzab, R. Thermodynamic and Spectroscopic Studies of the complexes formed in tartaric acid and lanthanide (III) ions binary systems. Molecules 2020, 25, 1121. [Google Scholar] [CrossRef]
- Han, M.J.; He, J.I.; Sun, W.; Li, S.; Yu, H.; Yue, T.; Wei, X.; Zhang, C.-Y. Coordination configurations of cupric tartrate in electronic industry wastewater. Trans. Nonferrous Met. Soc. China 2022, 32, 3753–3766. [Google Scholar] [CrossRef]
- Da Silva Júnior, W.F.; de Oliveira Pinheiro, J.G.; Moreira, C.D.; de Souza, F.J.; de Lima, D.A. Alternative Technologies to Improve Solubility and Stability of Poorly Water-Soluble Drugs. In Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 281–305. [Google Scholar] [CrossRef]
- Renny, J.S.; Tomasevich, L.L.; Tallmadge, E.H.; Collum, D.B. Method of continuous variations: Applications of Job plots to the study of molecular associations in organometallic chemistry. J. Angew. Chem. Int. Ed. 2013, 52, 11998–12013. [Google Scholar] [CrossRef]
- James, A.M.; Prichard, F.E. Practical Physical Chemistry, 3rd ed.; Longman: London, UK, 1974. [Google Scholar]
- Baker, A.T. The Ligand Field Spectra of Copper(II) Complexes. J. Chem. Educ. 1998, 75, 98. [Google Scholar] [CrossRef]
- Tanda, B.C.; Eksteen, J.J.; Oraby, E.A. An investigation into the leaching behaviour of copper oxide minerals in aqueous alkaline glycine solutions. Hydrometallurgy 2017, 167, 153–162. [Google Scholar] [CrossRef]
- Wasim, A.A.; Khan, M.N.; Wahab, M.F.; Rehman, S.F.U. Construction of Job’s plot in case of high spectral overlap between the reactants and the complex: Application of multiwavelength linear regression analysis using the Cu-EDTA system. Chem. Bulg. J. Sci. Educ. 2019, 28, 475–484. [Google Scholar]
- Schoenberg, L.N. The structure of the complexed copper species in electroless copper plating solutions. J. Electrochem. Soc. Electrochem. Sci. 1971, 118, 1571–1576. [Google Scholar] [CrossRef]
- Thomsen, M.W. Determination of the solubility product of copper(II) tartrate. J. Chem. Educ. 1992, 69, 328. [Google Scholar] [CrossRef]
- Hörner, T.G.; Klüfers, P. The Species of Fehling’s Solution. Eur. J. Inorg. Chem. 2016, 2016, 1798–1807. [Google Scholar] [CrossRef]
- Logunov, L.S.; Panov, M.S.; Myund, L.A.; Tumkin, I.I.; Khairullina, E.M.; Ryazantsev, M.N.; Balova, I.A.; Kochemirovsky, V.A. Influence of the ligand nature on the in situ laser-induced synthesis of the electrocatalytically active copper microstructures. Arab. J. Chem. 2018, 11, 624–634. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Handbook of Chemistry, 15th ed.; McGraw-Hill, Inc.: New York, NY, USA; St. Louis, MO, USA; San Francisco, CA, USA, 1999. [Google Scholar]
- Wright, S.W.; Silverstein, T.P. Improvements upon the “Colorful Cobalt Catalysis” Demonstration and Evidence for the Presence of an Autocatalytic Mechanism. J. Chem. Educ. 2010, 87, 1064–1069. [Google Scholar] [CrossRef]
- Tianbao, D.; Vijayakumar, A.; Desai, V. Effect of hydrogen peroxide on oxidation of copper in CMP slurries containing glycine and Cu ions. Electrochim. Acta 2004, 49, 4505–4512. [Google Scholar]
- Bingöl, D.; Canbazoğlu, M.; Aydoğan, S. Dissolution kinetics of malachite in ammonia/ammonium carbonate leaching. Hydrometallurgy 2005, 76, 55–62. [Google Scholar] [CrossRef]
- D’Aloya, A.; Nikoloski, A. The passivation of iron in ammoniacal solutions containing copper (II) ions. Hydrometallurgy 2012, 111–112, 58–64. [Google Scholar] [CrossRef]
- Liu, Z.X.; Yin, Z.L.; Hu, H.P.; Chen, Q.Y. Dissolution kinetics of malachite in ammonia/ammonium sulphate solution. J. Cent. South Univ. 2012, 19, 903–910. [Google Scholar] [CrossRef]
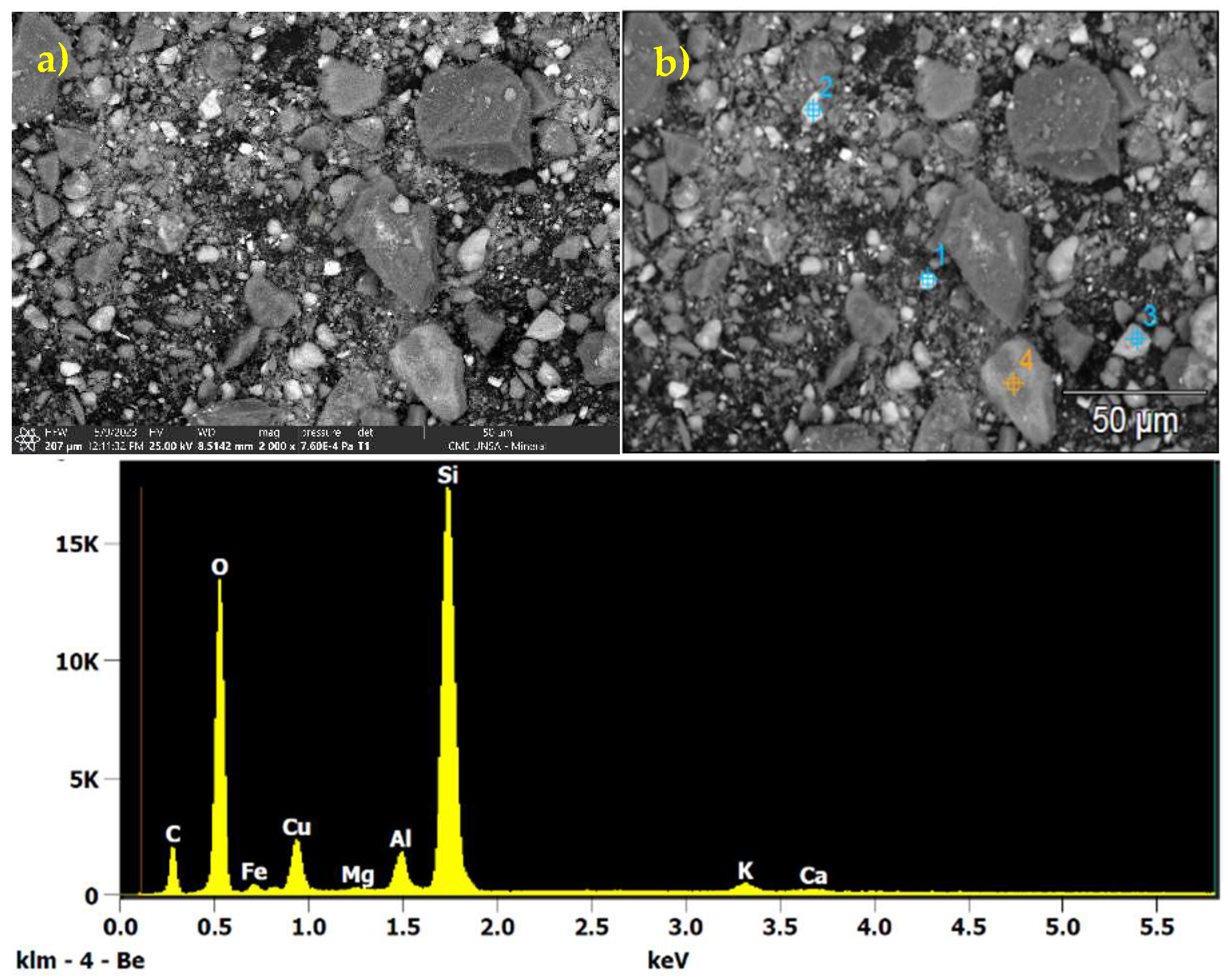




| Element | % |
|---|---|
| C | 6.16 |
| O | 49.79 |
| Mg | 0.20 |
| Al | 2.07 |
| Si | 23.81 |
| K | 0.78 |
| Ca | 0.17 |
| Fe | 6.31 |
| Cu | 10.72 |
| Element | % |
|---|---|
| C | 18.16 |
| O | 63.95 |
| Na | 1.03 |
| Al | 0.73 |
| Si | 11.73 |
| K | 0.64 |
| Fe | 1.97 |
| Cu | 1.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velarde, F.J.S.; Ortiz, J.A.Q.; Apaza, A.A.H.; de Namor, A.F.D. A New Approach for Increasing the Chelating Capacity of the Tartrate Ion in the Extraction of Copper from Ores. Metals 2023, 13, 1672. https://doi.org/10.3390/met13101672
Velarde FJS, Ortiz JAQ, Apaza AAH, de Namor AFD. A New Approach for Increasing the Chelating Capacity of the Tartrate Ion in the Extraction of Copper from Ores. Metals. 2023; 13(10):1672. https://doi.org/10.3390/met13101672
Chicago/Turabian StyleVelarde, Félix J. Sueros, Jhon A. Quispe Ortiz, Angie A. Hidalgo Apaza, and Angela F. Danil de Namor. 2023. "A New Approach for Increasing the Chelating Capacity of the Tartrate Ion in the Extraction of Copper from Ores" Metals 13, no. 10: 1672. https://doi.org/10.3390/met13101672
APA StyleVelarde, F. J. S., Ortiz, J. A. Q., Apaza, A. A. H., & de Namor, A. F. D. (2023). A New Approach for Increasing the Chelating Capacity of the Tartrate Ion in the Extraction of Copper from Ores. Metals, 13(10), 1672. https://doi.org/10.3390/met13101672






