Influence of Microstructure on the Mechanical and Corrosion Response of a Friction Stir-Extruded WE43 Magnesium Rod
Abstract
:1. Introduction
2. Materials and Methods
3. Results
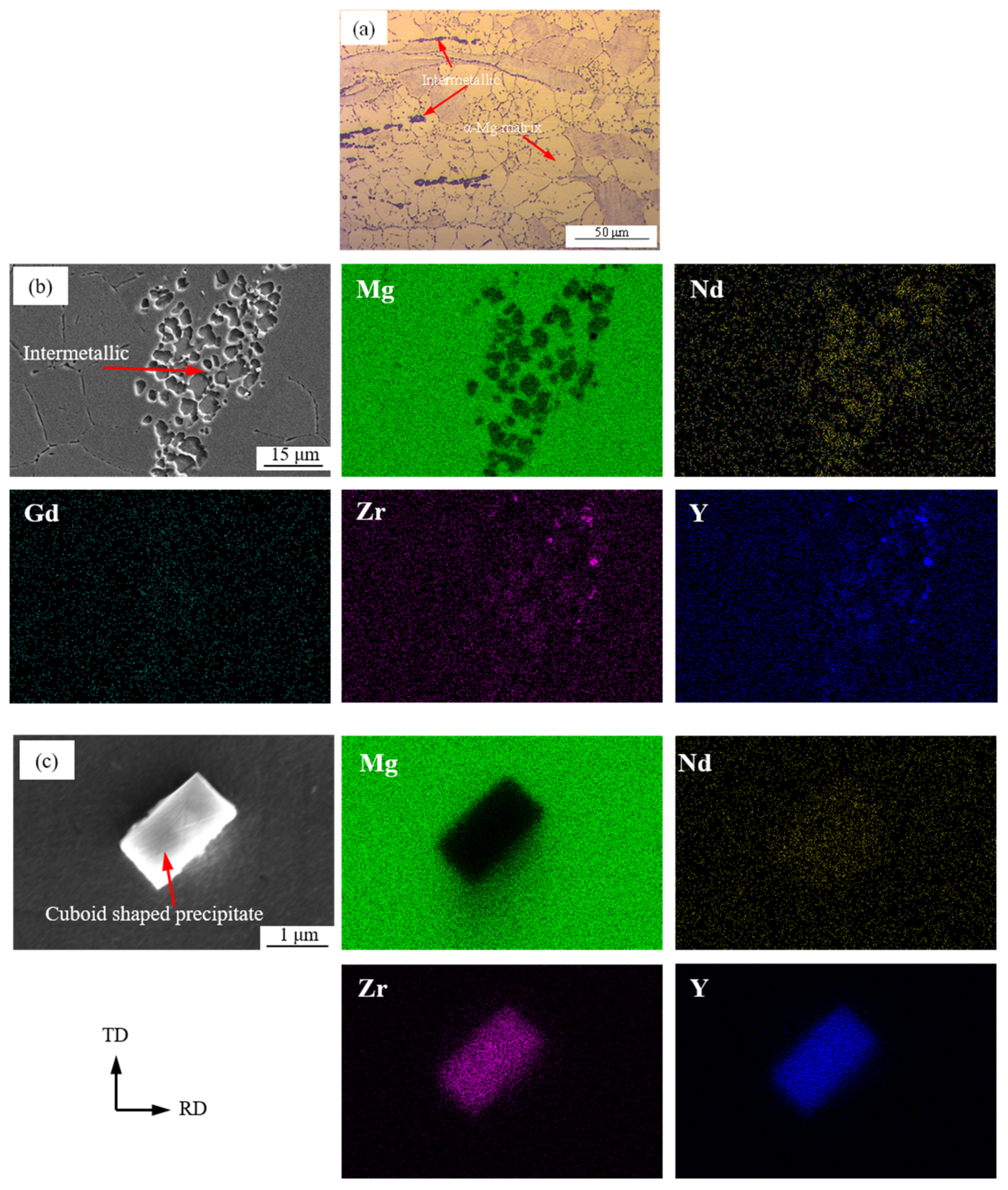
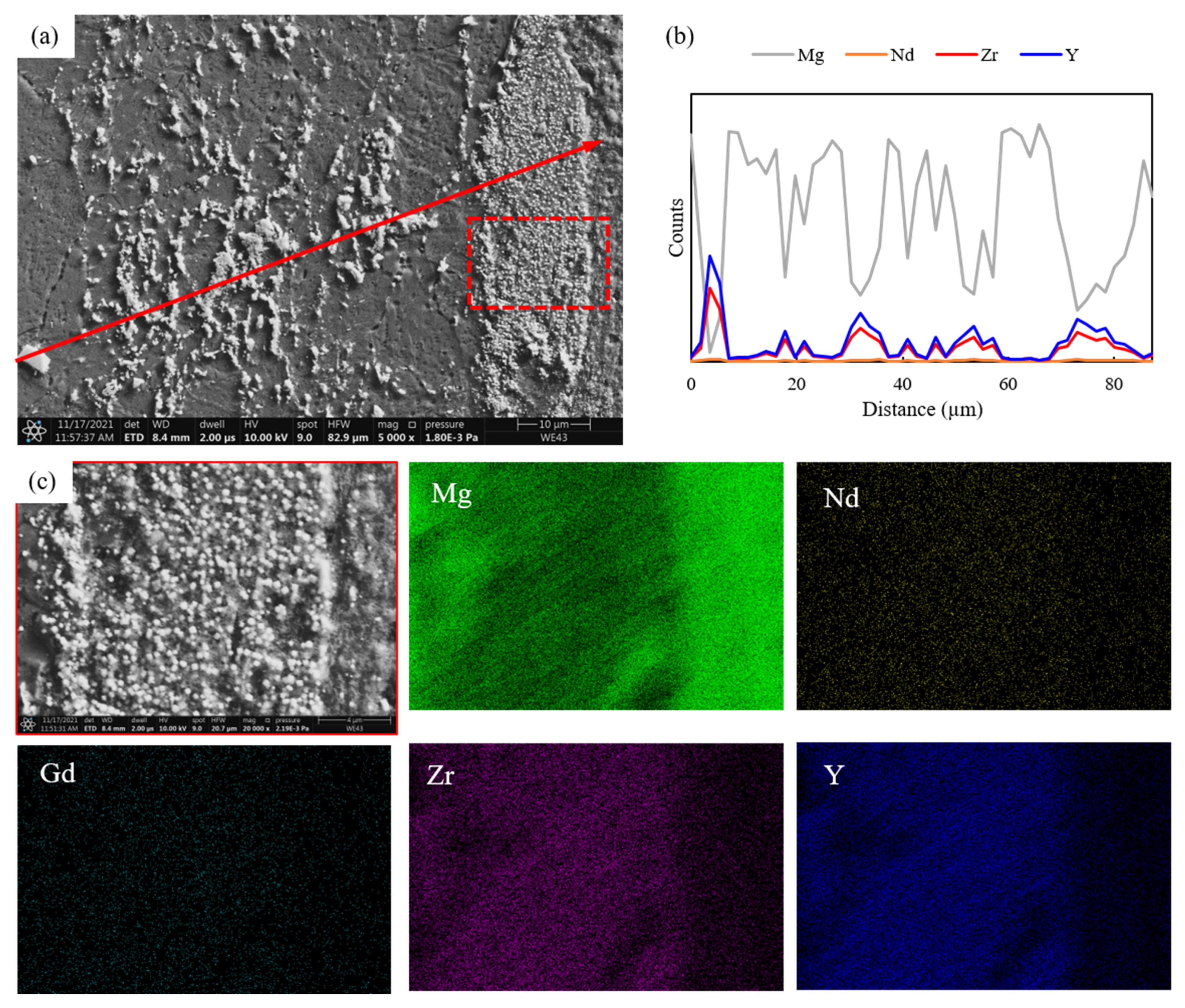
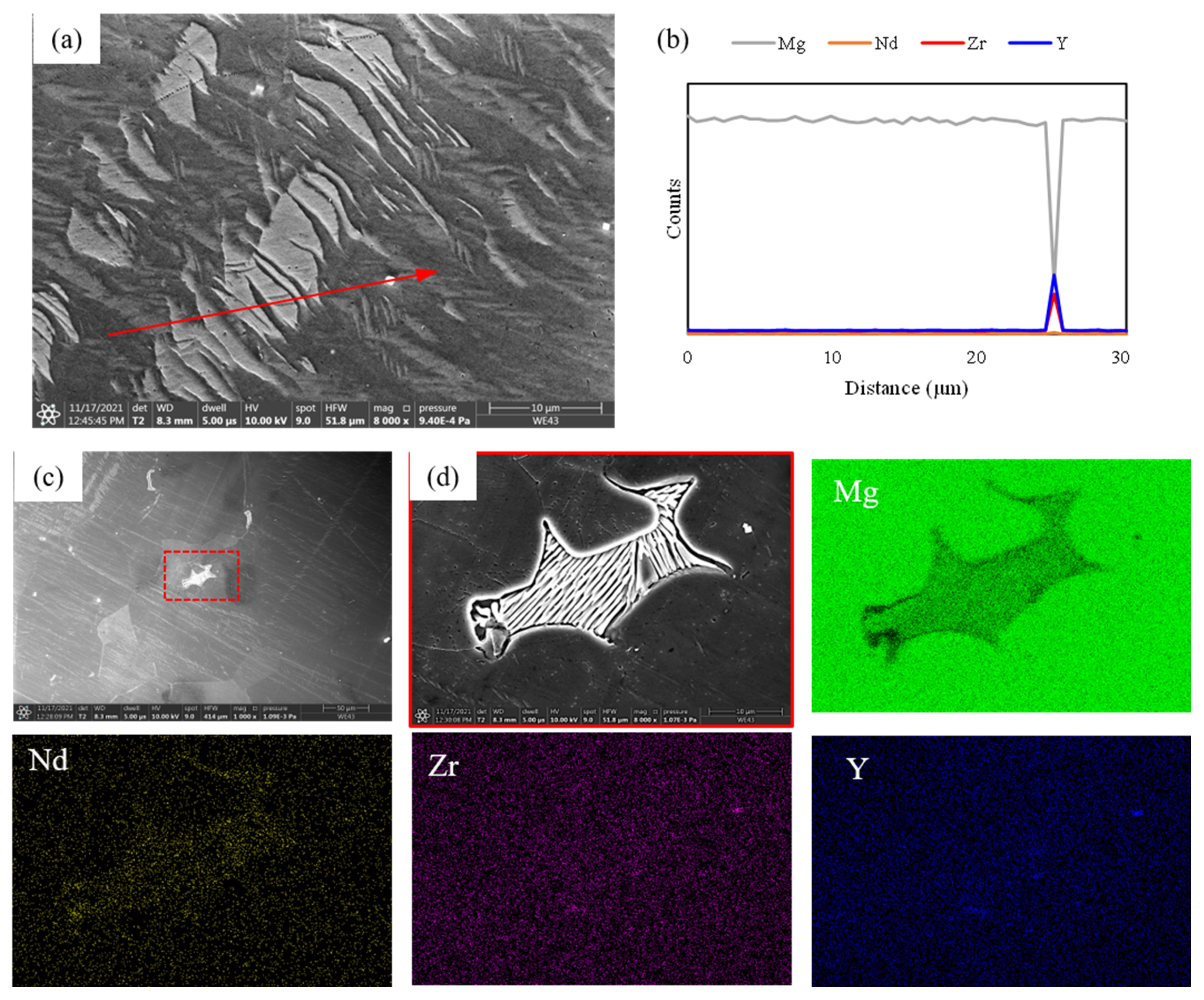
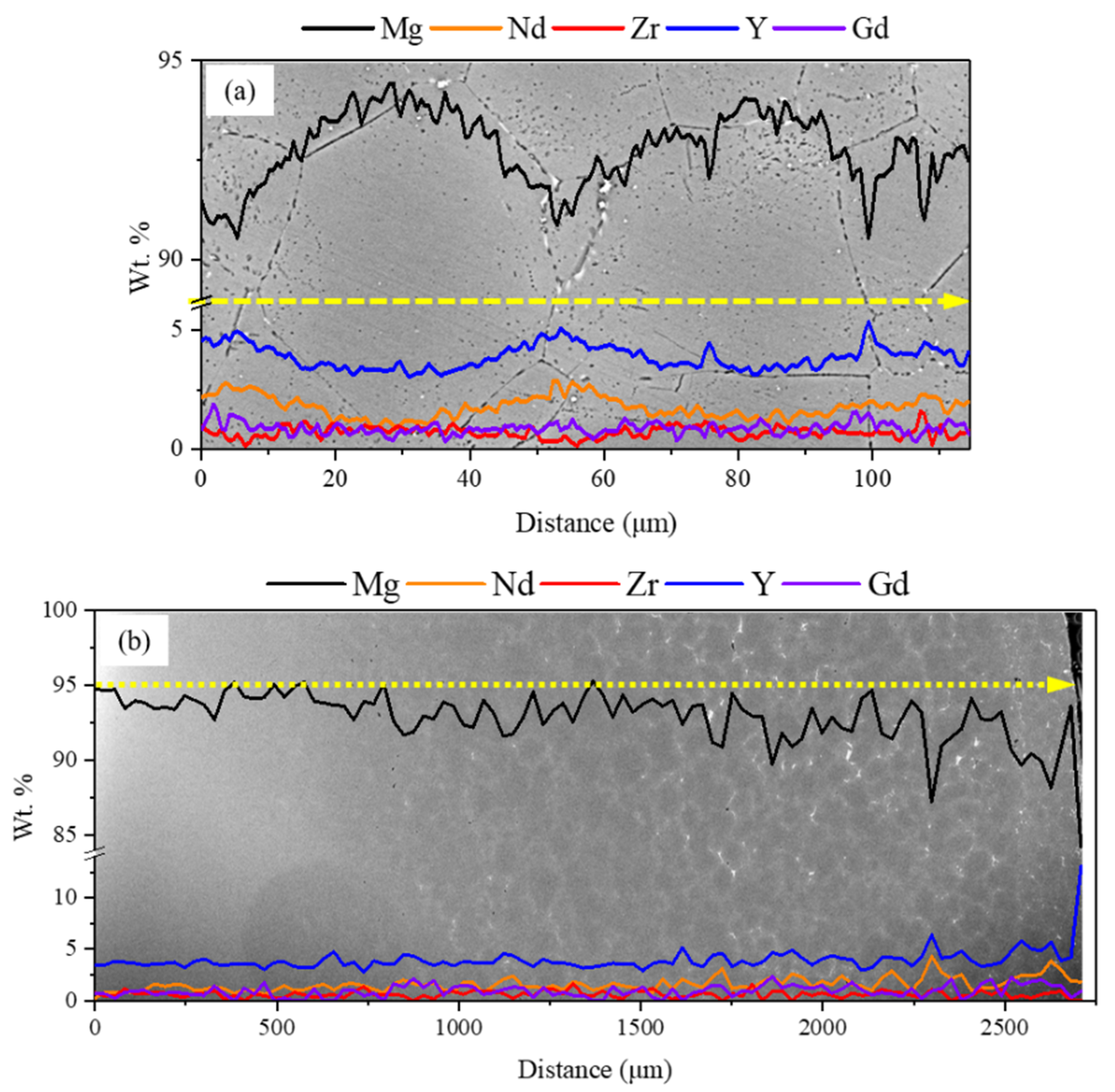
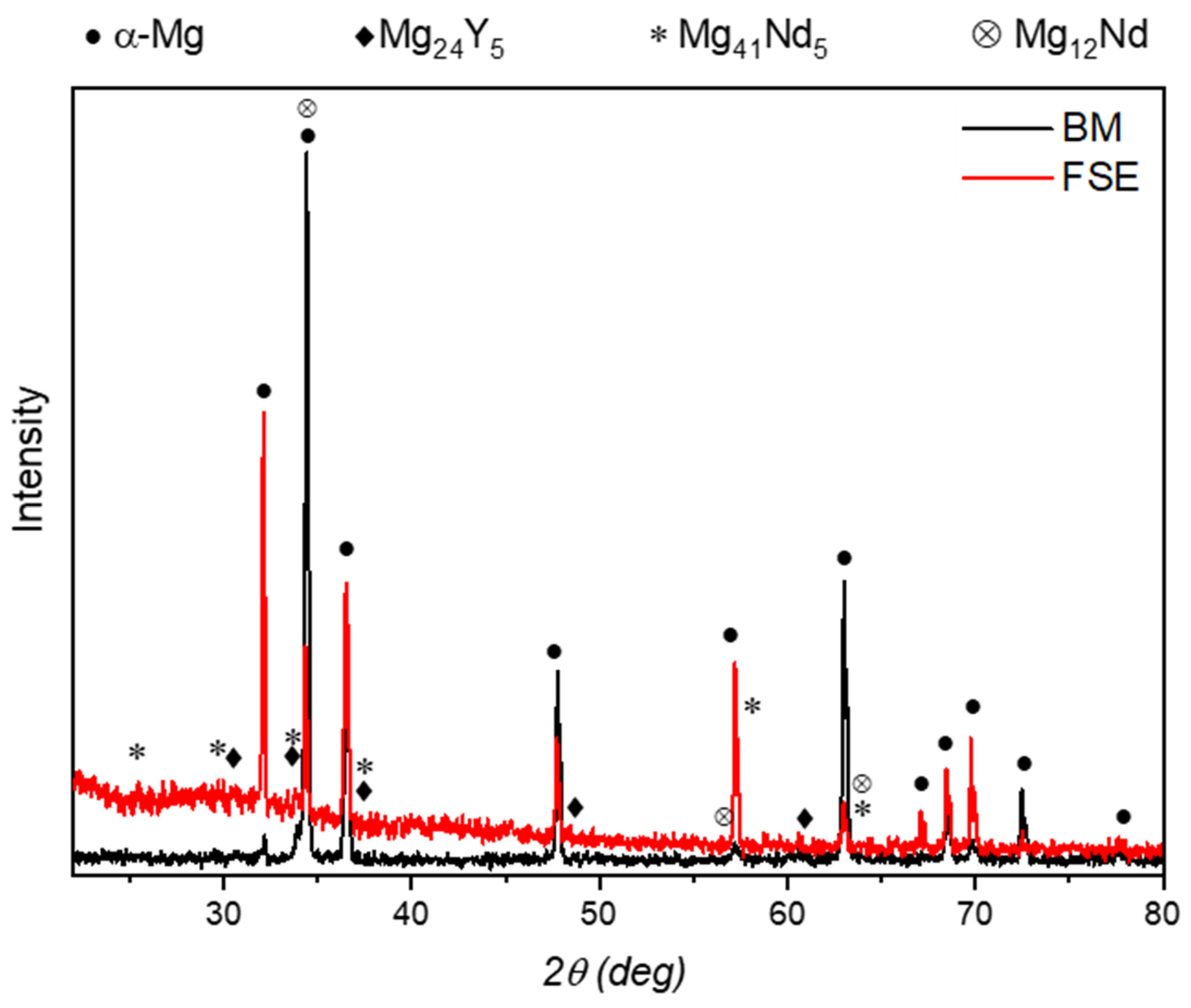
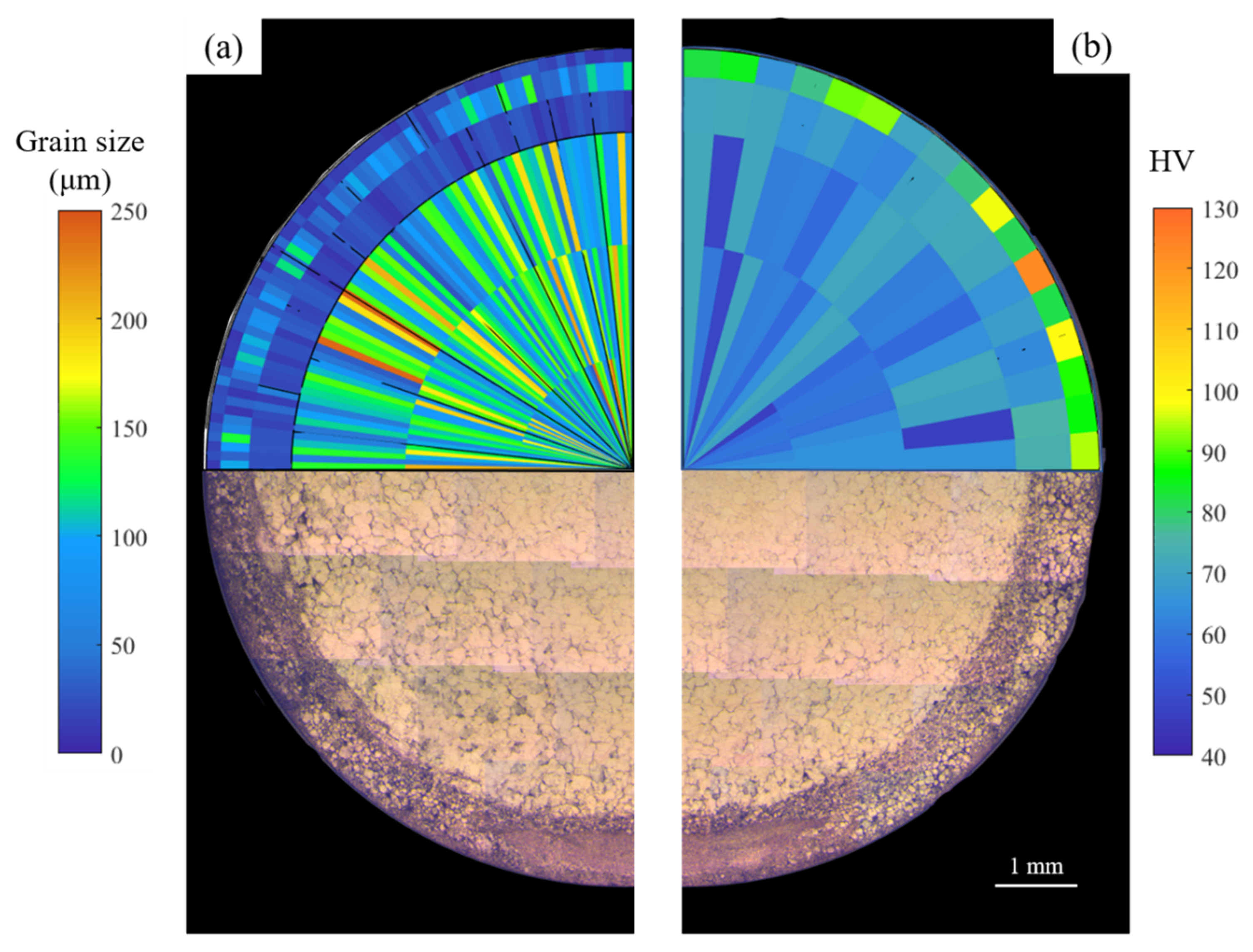
3.1. Microstructure
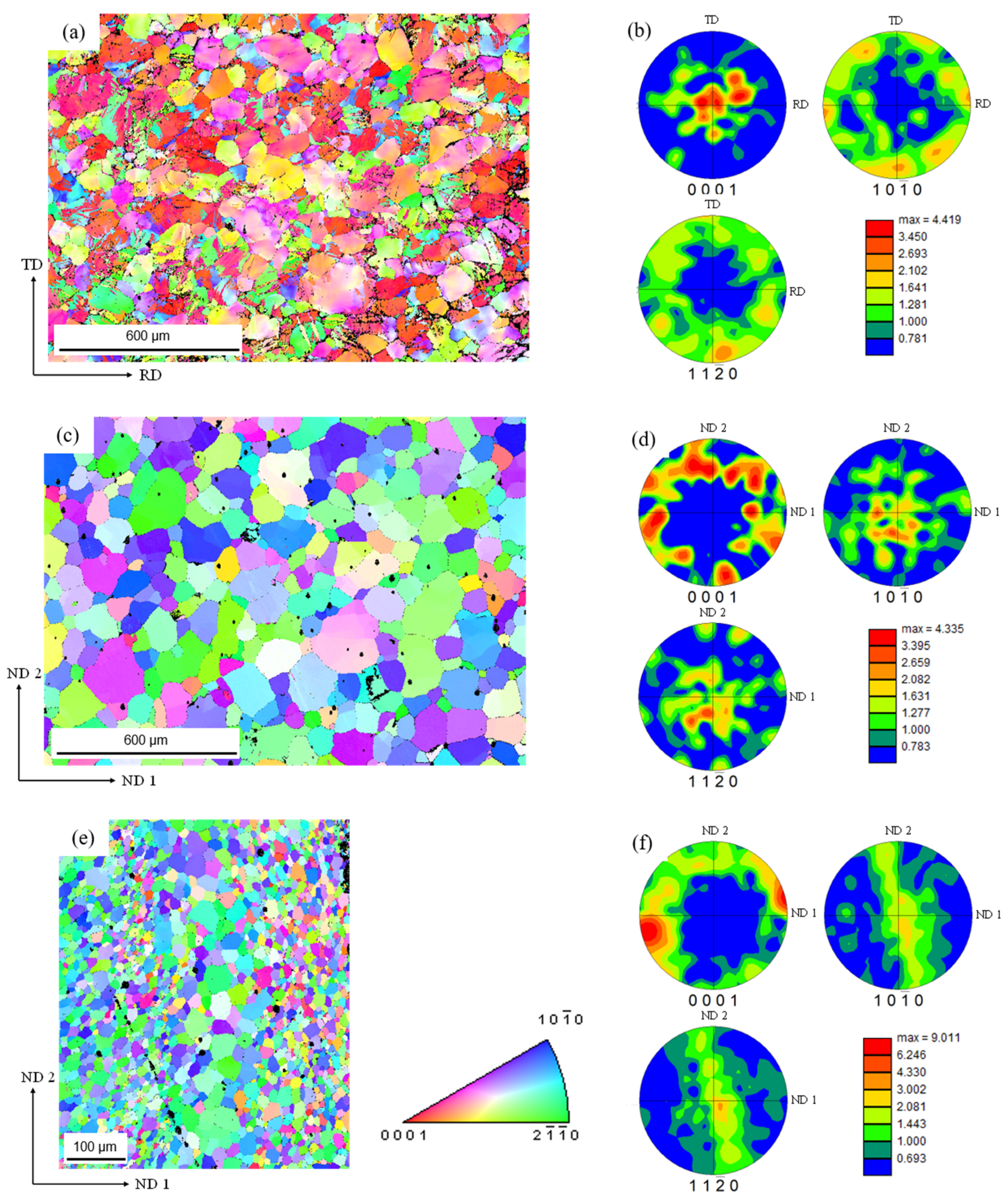
3.2. Texture Analysis
4. Discussion
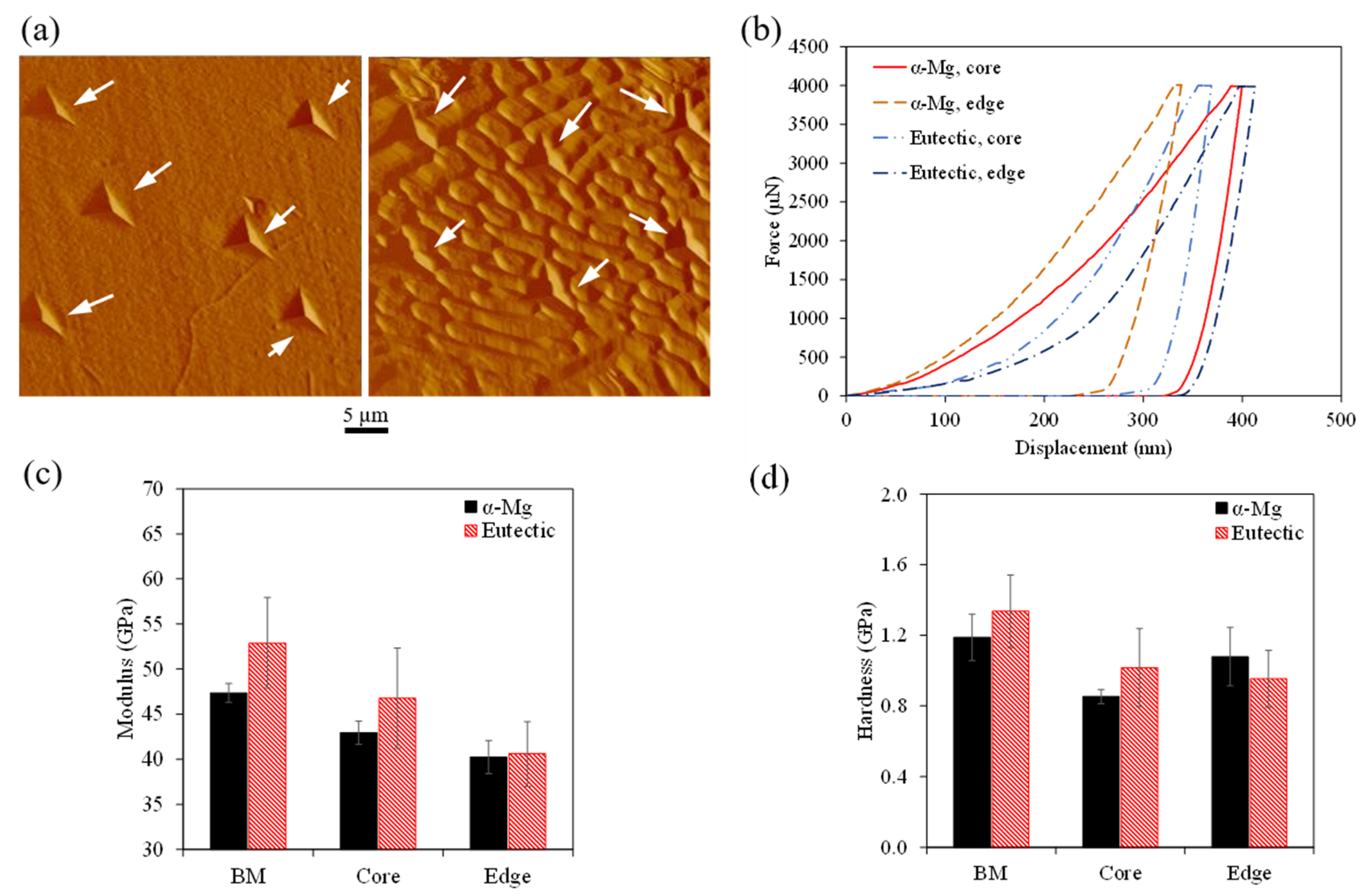
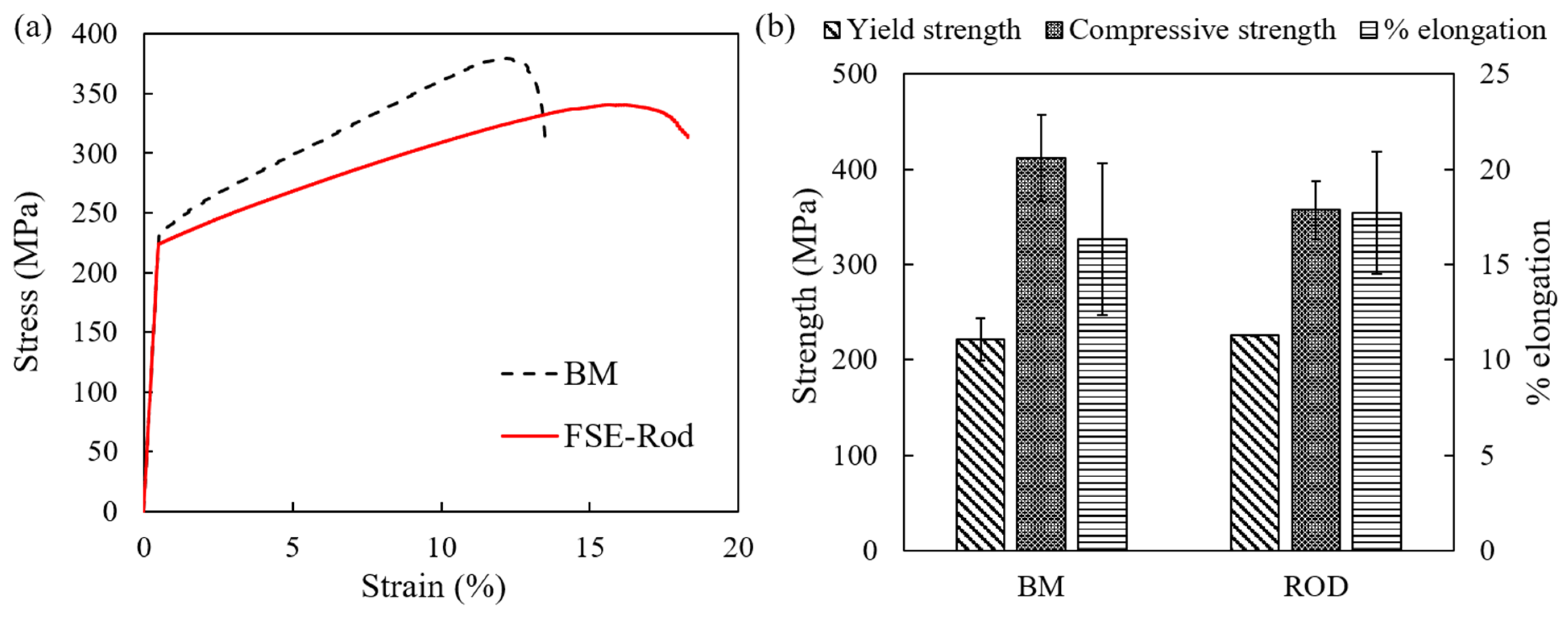
4.1. Mechanical Properties
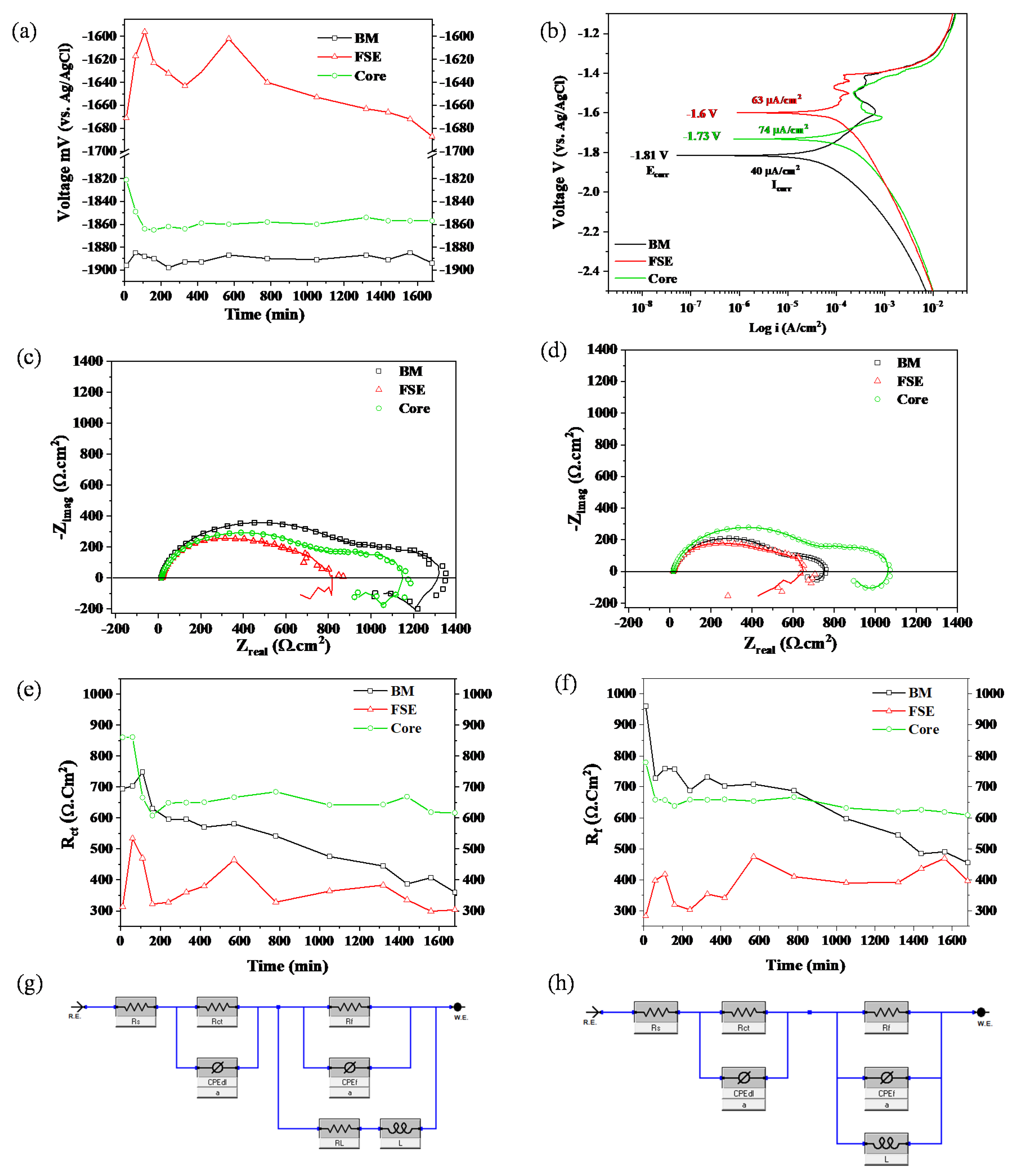
4.2. Electrochemical Analysis
5. Conclusions
- The FSE process resulted in a hybrid microstructure with rod edge regions having smaller grains (82.5% smaller) compared to the rod core. Due to the FSE process, it was observed that higher concentrations of the RE (Nd, Y, Gd) and Zr elements accumulate toward the edge compared to the core. The edge region showed a basal pole orientation perpendicular to the extrusion direction along the rod’s outer surface. The grain size, precipitate distribution, and texture all affected the rod’s mechanical and corrosion response.
- The higher concentration of precipitates observed in the rod edge resulted in a higher observed microhardness (up to 61.6% higher) due to precipitate strengthening. In addition, the α-Mg matrix also showed a higher hardness in nanoscale testing resulting from solid–solution strengthening as the α-Mg matrix showed higher concentrations of RE elements at the edge. The hybrid microstructure resulted in having hybrid mechanical properties by having a strengthened edge and a ductile core.
- Under compression testing, the rod core governed the overall compression response of the rod resulting in an 8.4% increase in ductility. The rod also showed a similar yield strength and 87% of compressive strength, compared to the base material.
- The friction stir-extruded WE43 Mg rod showed lower corrosion resistance compared to the BM, due to the evolved hybrid microstructure. However, removing the edge region from the friction stir-extruded rod improved the corrosion resistance relative to the BM and friction stir-extruded samples. The rod with the edge removed showed the most stable film resistance throughout the immersion time of the study. This is highly beneficial for applications such as frac plugs and biomedical devices, that require a tailored corrosion rate.
- The results show that by utilizing FSE to prepare Mg alloy rods, the multiscale mechanical and corrosion response of the rod can be tailored. The FSE process can be further optimized by tool design and process parameter studies with the goal of minimizing heat input to the extruding material while facilitating intense plastic deformation. This can result in further control of the size and distribution of precipitates in the rod cross-section.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pollock, T.M. Weight Loss with Magnesium Alloys. Science 2010, 328, 986. [Google Scholar] [CrossRef] [PubMed]
- Charyeva, O.; Dakischew, O.; Sommer, U.; Heiss, C.; Schnettler, R.; Lips, K.S. Biocompatibility of Magnesium Implants in Primary Human Reaming Debris-Derived Cells Stem Cells in Vitro. J. Orthop. Traumatol. 2016, 17, 63–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, A.A. Magnesium Casting Technology for Structural Applications. J. Magnes. Alloy. 2013, 1, 2–22. [Google Scholar] [CrossRef] [Green Version]
- Mordike, B.L.; Ebert, T. Magnesium: Properties—Applications—Potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Dunne, T.R.; Yue, W.; Zhao, L.; Nettles, D.; Cheng, P.; Ren, J.; Liu, Y. Addressing Corrosion Stress Cracking Issue of Magnesium Frac Plugs Used in Ultra HPHT Shale Well Development. In Proceedings of the International Petroleum Exhibition & Conference, Abu Dhabi, UAE, 15–18 November 2021; p. D041S107R004. [Google Scholar]
- Fripp, M.; Walton, Z. Degradable Metal for Use in a Fully Dissolvable Frac Plug. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 5 May 2016; p. D021S021R003. [Google Scholar]
- Doege, E.; Droder, K. Sheet Metal Forming of Magnesium Wrought Alloys—Formability and Process Technology. J. Mater. Process. Technol. 2001, 115, 14–19. [Google Scholar] [CrossRef]
- Di Mario, C.; Griffiths, H.; Goktekin, O.; Peeters, N.; Verbist, J.; Bosiers, M.; Deloose, K.; Heublein, B.; Rohde, R.; Kasese, V.; et al. Drug-Eluting Bioabsorbable Magnesium Stent. J. Interv. Cardiol. 2004, 17, 391–395. [Google Scholar] [CrossRef]
- Levy, G.; Aghion, E. Effect of Diffusion Coating of Nd on the Corrosion Resistance of Biodegradable Mg Implants in Simulated Physiological Electrolyte. Acta Biomater. 2013, 9, 8624–8630. [Google Scholar] [CrossRef]
- Rapetto, C.; Leoncini, M. Magmaris: A New Generation Metallic Sirolimus-Eluting Fully Bioresorbable Scaffold: Present Status and Future Perspectives. J. Thorac. Dis. 2017, 9, S903–S913. [Google Scholar] [CrossRef] [Green Version]
- Kulekci, M.K. Magnesium and Its Alloys Applications in Automotive Industry. Int. J. Adv. Manuf. Technol. 2008, 39, 851–865. [Google Scholar] [CrossRef]
- Alaneme, K.K.; Okotete, E.A. Enhancing Plastic Deformability of Mg and Its Alloys—A Review of Traditional and Nascent Developments. J. Magnes. Alloy. 2017, 5, 460–475. [Google Scholar] [CrossRef]
- Langelier, B.; Esmaeili, S. Effects of Ce Additions on the Age Hardening Response of Mg–Zn Alloys. Mater. Charact. 2015, 101, 1–8. [Google Scholar] [CrossRef]
- Renna, G.; Leo, P.; Casavola, C. Effect of ElectroSpark Process Parameters on the WE43 Magnesium Alloy Deposition Quality. Appl. Sci. 2019, 9, 4383. [Google Scholar] [CrossRef] [Green Version]
- Eivani, A.R.; Mehdizade, M.; Chabok, S.; Zhou, J. Applying Multi-Pass Friction Stir Processing to Refine the Microstructure and Enhance the Strength, Ductility and Corrosion Resistance of WE43 Magnesium Alloy. J. Mater. Res. Technol. 2021, 12, 1946–1957. [Google Scholar] [CrossRef]
- Cao, G.; Zhang, D.; Zhang, W.; Zhang, W. In Vitro Corrosion Study of Friction Stir Processed WE43 Magnesium Alloy in a Simulated Body Fluid. Materials 2016, 9, 542. [Google Scholar] [CrossRef] [Green Version]
- Marwa, A.; Usman, C.A.; Shunmugasamy, V.C.; Karaman, I.; Mansoor, B. Corrosion Behavior of Mg-Zn-Zr-RE Alloys under Physiological Environment—Impact on Mechanical Integrity and Biocompatibility. J. Magnes. Alloy. 2021, 10, 1542–1572. [Google Scholar]
- Kalb, H.; Rzany, A.; Hensel, B. Impact of Microgalvanic Corrosion on the Degradation Morphology of WE43 and Pure Magnesium under Exposure to Simulated Body Fluid. Corros. Sci. 2012, 57, 122–130. [Google Scholar] [CrossRef]
- Song, G.; St John, D. The Effect of Zirconium Grain Refinement on the Corrosion Behaviour of Magnesium-Rare Earth Alloy MEZ. J. Light Met. 2002, 2, 1–16. [Google Scholar] [CrossRef]
- Ramesh, S.; Lakshminarayanan, A.K.; Raghavan, Y.; Harathi, Y. Fabrication and Numerical Analysis of Friction Stir Back Extruded Lightweight Magnesium Alloy Heat Pipes. Mater. Sci. Forum 2020, 979, 129–134. [Google Scholar] [CrossRef]
- Abu-Farha, F. A Preliminary Study on the Feasibility of Friction Stir Back Extrusion. Scr. Mater. 2012, 66, 615–618. [Google Scholar] [CrossRef]
- Shunmugasamy, V.C.; Khalid, E.; Mansoor, B. Friction Stir Extrusion of Ultra-Thin Wall Biodegradable Magnesium Alloy Tubes—Microstructure and Corrosion Response. Mater. Today Commun. 2021, 26, 102129. [Google Scholar] [CrossRef]
- Al-Buainain, M.; Shunmugasamy, V.C.; Mansoor, B. Friction Stir Extrusion of AZ31 Magnesium Alloy Rod BT—Magnesium Technology 2022; Maier, P., Barela, S., Miller, V.M., Neelameggham, N.R., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 207–212. [Google Scholar]
- Buffa, G.; Campanella, D.; Fratini, L.; Micari, F. AZ31 Magnesium Alloy Recycling through Friction Stir Extrusion Process. Int. J. Mater. Form. 2016, 9, 613–618. [Google Scholar] [CrossRef]
- Rokhlin, L.L. Dependence of the Rare Earth Metal Solubility in Solid Magnesium on Its Atomic Number. J. Phase Equilibria 1998, 19, 142–145. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent Advances on the Development of Magnesium Alloys for Biodegradable Implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef] [PubMed]
- Hort, N.; Huong, Y.; Kainer, K.U. Intermetallics in Magnesium Alloys. Adv. Eng. Mater. 2006, 8, 235–240. [Google Scholar] [CrossRef]
- Rzychoń, T.; Kiełbus, A. Microstructure of WE43 Casting Magnesium Alloy. J. Achiev. Mater. Manuf. Eng. 2007, 21, 31–34. [Google Scholar]
- Mengucci, P.; Barucca, G.; Riontino, G.; Lussana, D.; Massazza, M.; Ferragut, R.; Aly, E.H. Structure Evolution of a WE43 Mg Alloy Submitted to Different Thermal Treatments. Mater. Sci. Eng. A 2008, 479, 37–44. [Google Scholar] [CrossRef]
- Cai, Y.; Wei, J.; Yan, H.; Chen, Y.; Chen, R. Low-Cycle Fatigue Behavior of Solutionized and Aged WE43 Magnesium Alloys at Room Temperature. J. Magnes. Alloy. 2022. [Google Scholar] [CrossRef]
- Kang, Y.; Huang, Z.; Zhao, H.; Gan, C.; Zhou, N.; Zheng, K.; Zhang, J.; Pan, F.; Huang, J.C.; Wang, S. Comparative Study of Hot Deformation Behavior and Microstructure Evolution of As-Cast and Extruded WE43 Magnesium Alloy. Metals 2020, 10, 429. [Google Scholar] [CrossRef] [Green Version]
- Gladman, T. On the Theory of the Effect of Precipitate Particles on Grain Growth in Metals. Proc. R. Soc. London. Ser. A. Math. Phys. Sci. 1966, 294, 298–309. [Google Scholar]
- Lee, H.J.; Lee, S.K.; Jung, K.H.; Lee, G.A.; Ahn, B.; Kawasaki, M.; Langdon, T.G. Evolution in Hardness and Texture of a ZK60A Magnesium Alloy Processed by High-Pressure Torsion. Mater. Sci. Eng. A 2015, 630, 90–98. [Google Scholar] [CrossRef]
- Kulakov, M.; Mail, M.; Kulagin, R. The Effect of the Die Rotation During Extrusion on the Shape of Embedded Markers. Mater. Lett. 2022, 322, 132486. [Google Scholar] [CrossRef]
- Baffari, D.; Buffa, G.; Fratini, L. A Numerical Model for Wire Integrity Prediction in Friction Stir Extrusion of Magnesium Alloys. J. Mater. Process. Technol. 2017, 247, 1–10. [Google Scholar] [CrossRef]
- Wang, Y.N.; Huang, J.C. Texture Analysis in Hexagonal Materials. Mater. Chem. Phys. 2003, 81, 11–26. [Google Scholar] [CrossRef]
- Rodriguez, A.K.; Ayoub, G.A.; Mansoor, B.; Benzerga, A.A. Effect of Strain Rate and Temperature on Fracture of Magnesium Alloy AZ31B. Acta Mater. 2016, 112, 194–208. [Google Scholar] [CrossRef]
- Milner, J.L.; Abu-Farha, F. Friction Stir Back Extrusion of Mg AZ31B-F: A Preliminary Investigation. In Magnesium Technology 2014; Alderman, M., Manuel, M.V., Hort, N., Neelameggham, N.R., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 497–503. ISBN 978-3-319-48231-6. [Google Scholar]
- Afrin, N.; Chen, D.L.; Cao, X.; Jahazi, M. Strain Hardening Behavior of a Friction Stir Welded Magnesium Alloy. Scr. Mater. 2007, 57, 1004–1007. [Google Scholar] [CrossRef]
- Dorbane, A.; Ayoub, G.; Mansoor, B.; Hamade, R.F.; Kridli, G.; Shabadi, R.; Imad, A. Microstructural Observations and Tensile Fracture Behavior of FSW Twin Roll Cast AZ31 Mg Sheets. Mater. Sci. Eng. A 2016, 649, 190–200. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Zhang, N.; Liu, Q.; Ma, Q.; Han, Z. Study on the Evolution of the Microstructure, Phase Composition, Three-Dimensional Morphology, and Hardness in the Solution Treatment of Mg−7.80Gd−2.43Y−0.38Zr Alloy. Materwiss. Werksttech. 2021, 52, 847–859. [Google Scholar] [CrossRef]
- Xiang, C. Magnesium Alloy WE43 and WE43-T5—Mechanical and Thermal Properties. Ph.D. Thesis, New York University, New York, NY, USA, 2016. [Google Scholar]
- Gao, L.; Chen, R.S.; Han, E.H. Solid Solution Strengthening Behaviors in Binary Mg–Y Single Phase Alloys. J. Alloy. Compd. 2009, 472, 234–240. [Google Scholar] [CrossRef]
- Li, J.; Meng, X.; Li, Y.; Wan, L.; Huang, Y. Friction Stir Extrusion for Fabricating Mg-RE Alloys with High Strength and Ductility. Mater. Lett. 2021, 289, 129414. [Google Scholar] [CrossRef]
- Yang, C.; Gupta, N.; Ding, H.; Xiang, C. Effect of Microstructure on Corrosion Behavior of WE43 Magnesium Alloy in as Cast and Heat-Treated Conditions. Metals 2020, 10, 1552. [Google Scholar] [CrossRef]
- Feng, B.; Liu, G.; Yang, P.; Huang, S.; Qi, D.; Chen, P.; Wang, C.; Du, J.; Zhang, S.; Liu, J. Different Role of Second Phase in the Micro-Galvanic Corrosion of WE43 Mg Alloy in NaCl and Na2SO4 Solution. J. Magnes. Alloy. 2022, 10, 1598–1608. [Google Scholar] [CrossRef]
- Brady, M.P.; Rother, G.; Anovitz, L.M.; Littrell, K.C.; Unocic, K.A.; Elsentriecy, H.H.; Song, G.-L.; Thomson, J.K.; Gallego, N.C.; Davis, B. Film Breakdown and Nano-Porous Mg(OH)2 Formation from Corrosion of Magnesium Alloys in Salt Solutions. J. Electrochem. Soc. 2015, 162, 140–149. [Google Scholar] [CrossRef]
- Song, D.; Ma, A.; Jiang, J.; Lin, P.; Yang, D.; Fan, J. Corrosion Behavior of Equal-Channel-Angular-Pressed Pure Magnesium in NaCl Aqueous Solution. Corros. Sci. 2010, 52, 481–490. [Google Scholar] [CrossRef]
- Coy, A.E.; Viejo, F.; Skeldon, P.; Thompson, G.E. Susceptibility of Rare-Earth-Magnesium Alloys to Micro-Galvanic Corrosion. Corros. Sci. 2010, 52, 3896–3906. [Google Scholar] [CrossRef]
- Chu, P.W.; Marquis, E.A. Linking the Microstructure of a Heat-Treated WE43 Mg Alloy with Its Corrosion Behavior. Corros. Sci. 2015, 101, 94–104. [Google Scholar] [CrossRef]
- Gerashi, E.; Alizadeh, R.; Langdon, T.G. Effect of Crystallographic Texture and Twinning on the Corrosion Behavior of Mg Alloys: A Review. J. Magnes. Alloy. 2022, 10, 313–325. [Google Scholar] [CrossRef]
- Torbati-Sarraf, H.; Torbati-Sarraf, S.A.; Poursaee, A.; Langdon, T.G. Electrochemical Behavior of a Magnesium ZK60 Alloy Processed by High-Pressure Torsion. Corros. Sci. 2019, 154, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Song, D.; Ma, A.B.; Jiang, J.H.; Lin, P.H.; Yang, D.H.; Fan, J.F. Corrosion Behaviour of Bulk Ultra-Fine Grained AZ91D Magnesium Alloy Fabricated by Equal-Channel Angular Pressing. Corros. Sci. 2011, 53, 362–373. [Google Scholar] [CrossRef]
- Op’t Hoog, C.; Birbilis, N.; Estrin, Y. Corrosion of Pure Mg as a Function of Grain Size and Processing Route. Adv. Eng. Mater. 2008, 10, 579–582. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, A.; Xu, B.; Jiang, J.; Sun, J. Corrosion Behavior of AZ91 Mg Alloy with a Heterogeneous Structure Produced by ECAP. Corros. Sci. 2021, 187, 109517. [Google Scholar] [CrossRef]
- Bender, S.; Goellner, J.; Heyn, A.; Schmigalla, S. A New Theory for the Negative Difference Effect in Magnesium Corrosion. Mater. Corros. 2012, 63, 707–712. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, X.; Deng, X.; Li, X.; Ma, M. Relationship between Extrusion, Y and Corrosion Behavior of Mg-Y Alloy in NaCl Aqueous Solution. J. Magnes. Alloy. 2013, 1, 134–138. [Google Scholar] [CrossRef] [Green Version]
- Jamesh, M.I.; Wu, G.; Zhao, Y.; McKenzie, D.R.; Bilek, M.M.M.; Chu, P.K. Electrochemical Corrosion Behavior of Biodegradable Mg-Y-RE and Mg-Zn-Zr Alloys in Ringer’s Solution and Simulated Body Fluid. Corros. Sci. 2015, 91, 160–184. [Google Scholar] [CrossRef]
- Müller, W.D.; Nascimento, M.L.; Zeddies, M.; Córsico, M.; Gassa, L.M.; de Mele, M.A.F.L. Magnesium and Its Alloys as Degradable Biomaterials. Corrosion Studies Using Potentiodynamic and EIS Electrochemical Techniques. Mater. Res. 2007, 10, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Baril, G.; Galicia, G.; Deslouis, C.; Pébère, N.; Vivier, V.; Baril, G.; Galicia, G.; Deslouis, C.; Pébère, N.; Tribollet, B. An Impedance Investigation of the Mechanism of Pure Magnesium Corrosion in Sodium Sulfate Solutions. J. Electrochem. Soc. 2013, 154, 108–113. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Buainain, M.; Shunmugasamy, V.C.; Usman, C.A.; Mansoor, B. Influence of Microstructure on the Mechanical and Corrosion Response of a Friction Stir-Extruded WE43 Magnesium Rod. Metals 2023, 13, 191. https://doi.org/10.3390/met13020191
Al-Buainain M, Shunmugasamy VC, Usman CA, Mansoor B. Influence of Microstructure on the Mechanical and Corrosion Response of a Friction Stir-Extruded WE43 Magnesium Rod. Metals. 2023; 13(2):191. https://doi.org/10.3390/met13020191
Chicago/Turabian StyleAl-Buainain, Maryam, Vasanth C. Shunmugasamy, Chaudhry A. Usman, and Bilal Mansoor. 2023. "Influence of Microstructure on the Mechanical and Corrosion Response of a Friction Stir-Extruded WE43 Magnesium Rod" Metals 13, no. 2: 191. https://doi.org/10.3390/met13020191




