Mechanism of Aluminum Element Segregation in As-Cast Medium-Entropy Alloy CrCoNiAl0.014: A Hybrid MD/MC Simulation and Experimental Study
Abstract
:1. Introduction
2. Method
2.1. Experiments
2.2. Simulation Method
2.3. Post-Process
3. Results and Discussion
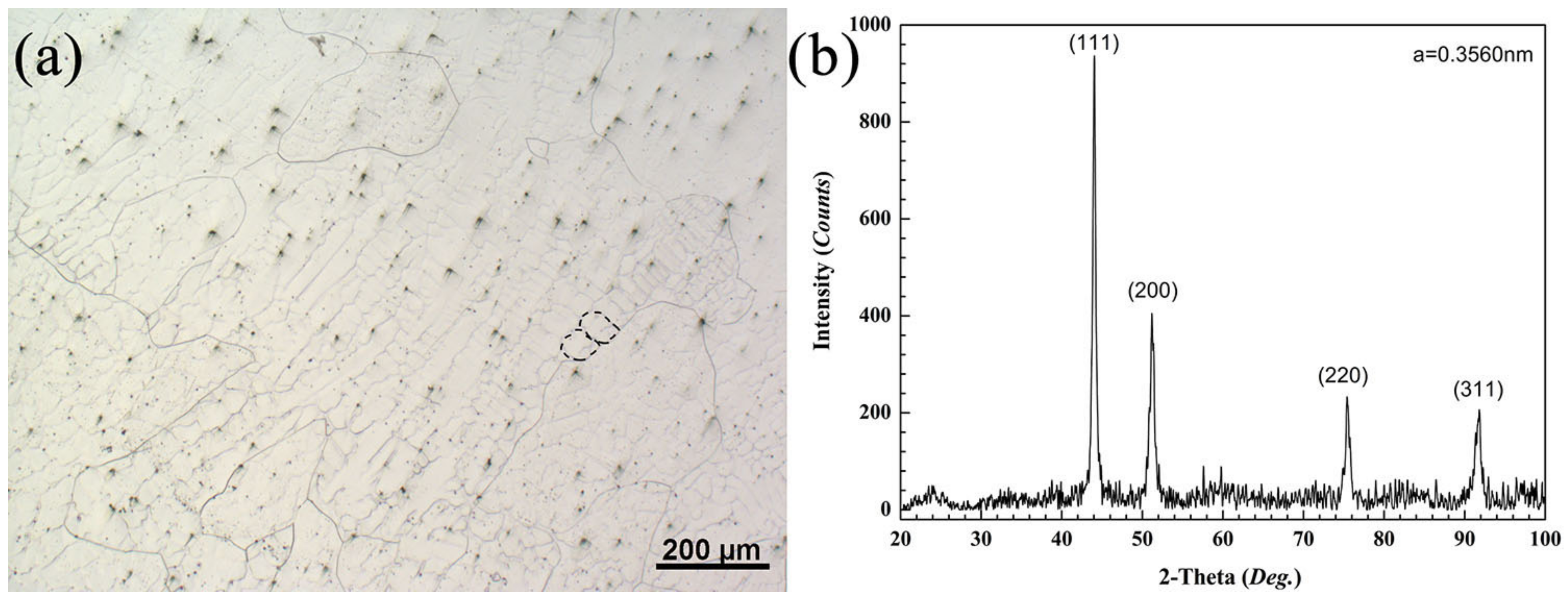
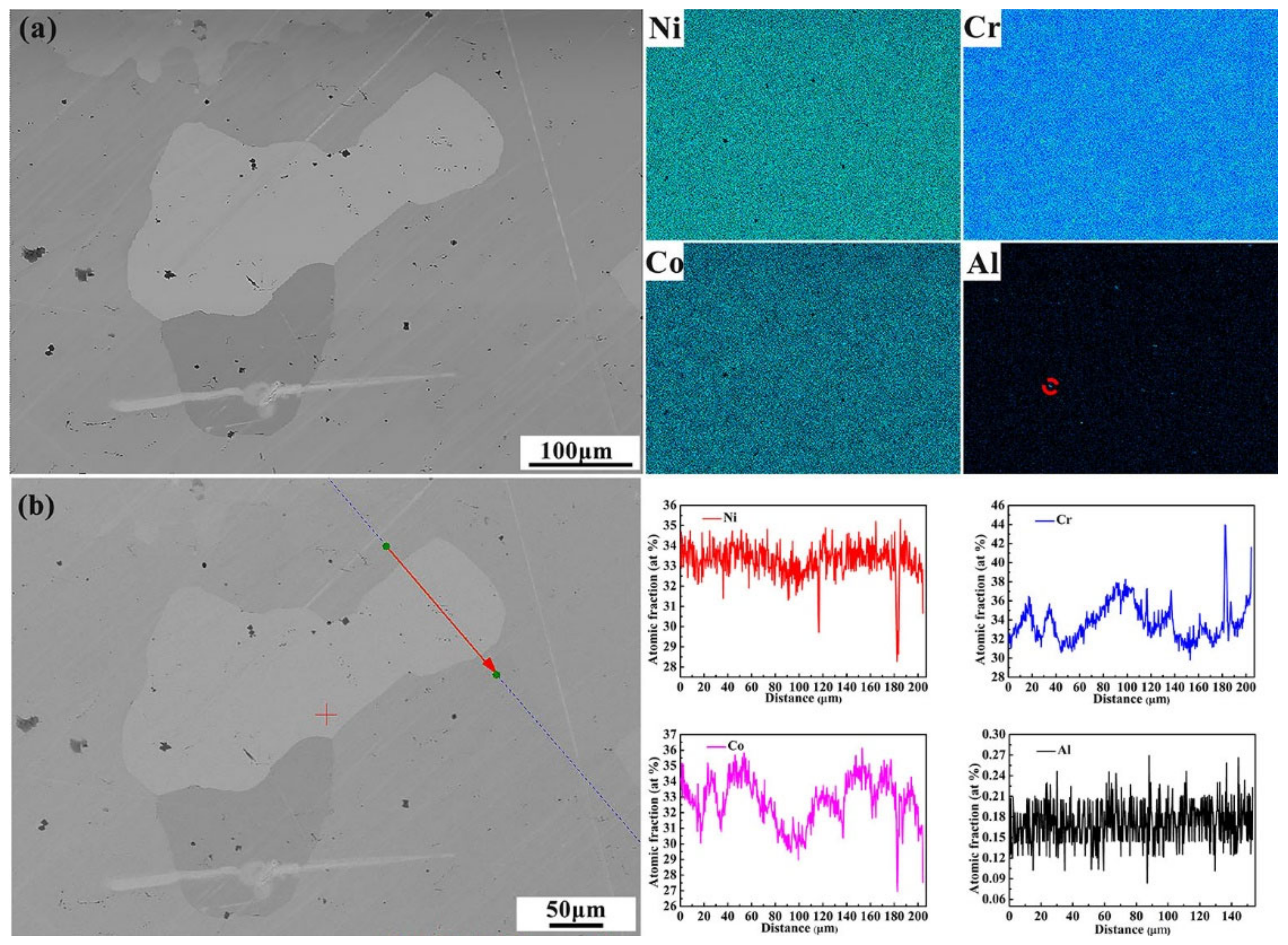
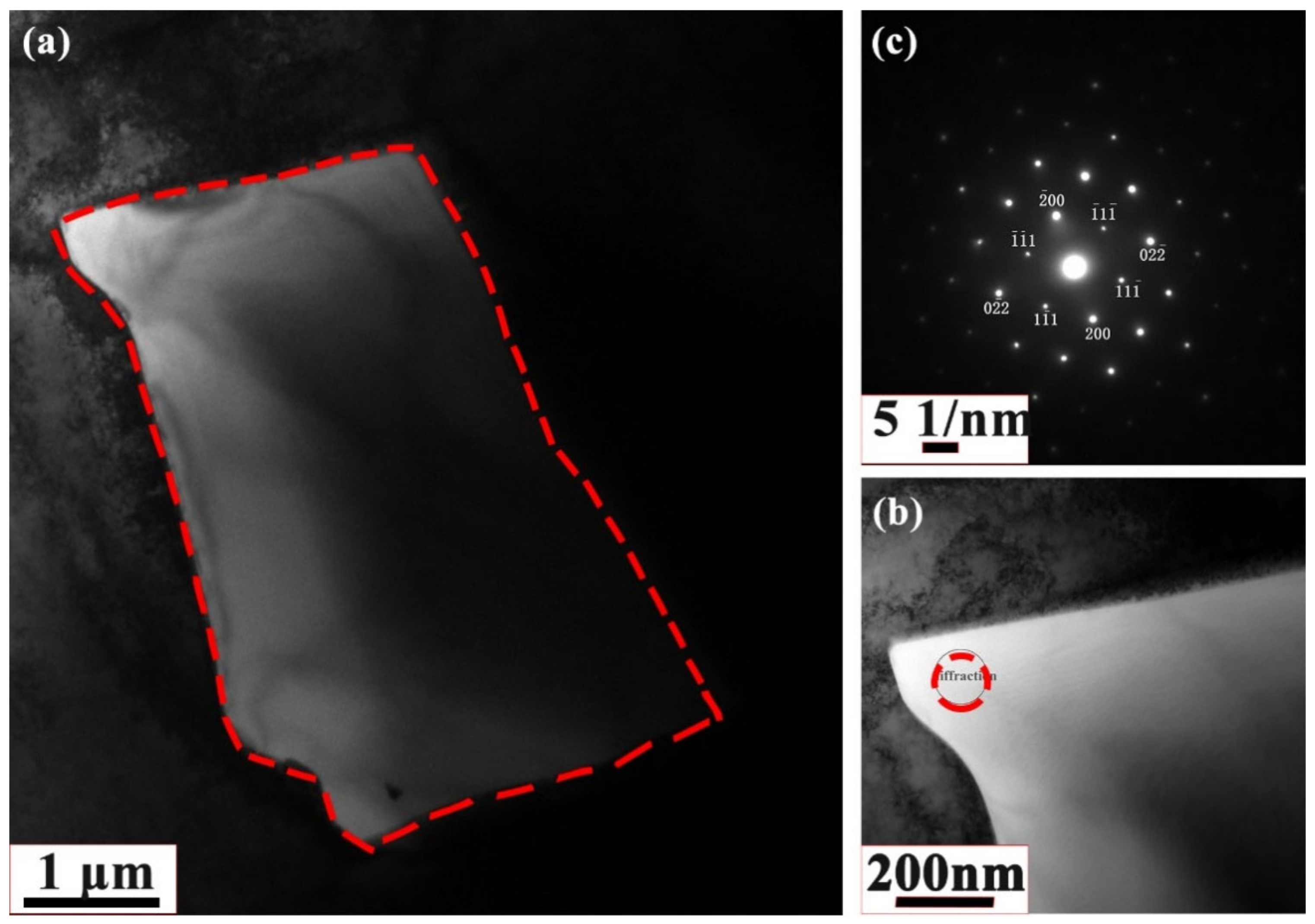
3.1. Experimental
Microstructure and Phase Structure
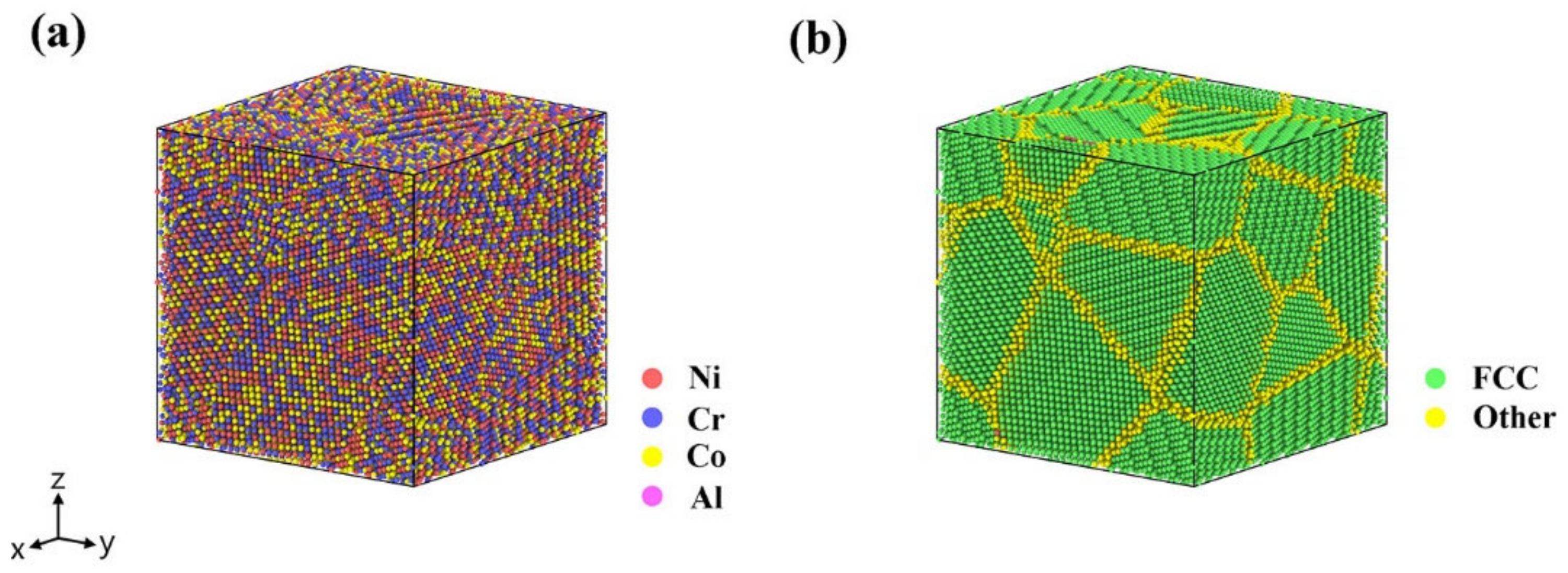
3.2. Simulation
3.2.1. Simulation of the Element Segregation Process
3.2.2. Atomic Trajectories and Element Diffusion
3.2.3. Cohesive Energy and Chemical Affinity
4. Conclusions
- (1)
- Element segregation at the dendrite boundary was demonstrated by the metallographic microstructure of the as-cast medium-entropy alloy CrCoNiAl0.014. Aluminum segregation resulted mainly in dendrite grain boundary segregation of the as-cast medium-entropy alloy CrCoNiAl0.014, and no other new phase was generated.
- (2)
- The hybrid MD and MC method showed that atomic segregation occurred at the grain boundaries of CrCoNiAl0.014 polycrystals owing to the CA and diffusivity between Al–Al. With the prolonged holding time, a small amount of Al can transfer to the grain boundary in the CrCoNiAl0.014 polycrystal.
- (3)
- Based on the element segregation rule of the cast entropy alloy CrCoNiAl0.014, the hybrid MD and MC methods, a comprehensive explanation was provided concerning the simulation results and influencing factors of the element segregation of the as-cast entropy alloy CrCoNiAl0.014. In the process of its unbalanced crystallization, although the as-cast entropy alloy CrCoNiAl0.014 with regard to the diffusion rate and CA of Cr, Co, Ni, and Al would cause element segregation in the microstructure, these elements could also be affected by other factors, such as the cooling rate. The MD simulation considered the influence of other factors on the element segregation of the medium-entropy alloy CrCoNiAl0.014, which is the future scope of this study. Therefore, the combination of MD and MC simulation with the element segregation experiment of the as-cast entropy alloy CrCoNiAl0.014 provided a reference for the subsequent improvement in the mechanical properties of the as-cast entropy alloy CrCoNiAl0.014 in the as-cast state.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Coury, F.G.; Zepon, G.; Bolfarini, C. Multi-principal element alloys from the CrCoNi family: Outlook and perspectives. J. Mater. Res. Technol. 2021, 15, 3461–3480. [Google Scholar] [CrossRef]
- George, E.P.; Curtin, W.; Tasan, C. High entropy alloys: A focused review of mechanical properties and deformation mechanisms. Acta Mater. 2020, 188, 435–474. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Wu, Z.; Bei, H.; Otto, F.; Pharr, G.; George, E. George, Recovery, recrystallization, grain growth and phase stability of a family of FCC-structured multi-component equiatomic solid solution alloys. Intermetallics 2014, 46, 131–140. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Thurston, K.V.S.; Bei, H.; Wu, Z.; George, E.P.; Ritchie, R.O. Exceptional damage-tolerance of a medium-entropy alloy CrCoNi at cryogenic temperatures. Nat. Commun. 2016, 7, 10602. [Google Scholar] [CrossRef]
- Zhang, Z.; Sheng, H.; Wang, Z.; Gludovatz, B.; Zhang, Z.; George, E.P.; Yu, Q.; Mao, S.X.; Ritchie, R.O. Dislocation mechanisms and 3D twin architectures generate exceptional strength-ductility-toughness combination in CrCoNi medium-entropy alloy. Nat. Commun. 2017, 8, 14390. [Google Scholar] [CrossRef]
- Laplanche, G.; Kostka, A.; Reinhart, C.; Hunfeld, J.; Eggeler, G.; George, E.P. Reasons for the superior mechanical properties of medium-entropy CrCoNi compared to high-entropy CrMnFeCoNi. Acta Mater. 2017, 128, 292–303. [Google Scholar] [CrossRef]
- Tsai, K.-Y.; Tsai, M.-H.; Yeh, J.-W. Sluggish diffusion in Co–Cr–Fe–Mn–Ni high-entropy alloys. Acta Mater. 2013, 61, 4887–4897. [Google Scholar] [CrossRef]
- Yeh, J.-W. Alloy design strategies and future trends in high-entropy alloys. JOM 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Miracle, D.B.; Miller, J.D.; Senkov, O.N.; Woodward, C.; Uchic, M.D.; Tiley, J. Exploration and development of high entropy alloys for structural applications. Entropy 2014, 16, 494–525. [Google Scholar] [CrossRef]
- Lim, K.R.; Lee, K.S.; Lee, J.S.; Kim, J.Y.; Chang, H.J.; Na, Y.S. Dual-phase high-entropy alloys for high-temperature structural applications. J. Alloys Compd. 2017, 728, 1235–1238. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, X.; Li, J. Microstructure and mechanical properties of a refractory CoCrMoNbTi high-entropy alloy. J. Mater. Eng. Perform. 2017, 26, 3657–3665. [Google Scholar] [CrossRef]
- Huo, W.; Zhou, H.; Fang, F.; Zhou, X.; Xie, Z.; Jiang, J. Microstructure and properties of novel CoCrFeNiTax eutectic high-entropy alloys. J. Alloys Compd. 2018, 735, 897–904. [Google Scholar] [CrossRef]
- Yang, Q.; Tang, Y.; Wen, Y.; Zhang, Q.; Deng, D.; Nai, X. Microstructures and properties of CoCrCuFeNiMox high-entropy alloys fabricated by mechanical alloying and spark plasma sintering. Powder Met. 2017, 61, 115–122. [Google Scholar] [CrossRef]
- Tan, Y.; Li, J.; Wang, J.; Kolbe, M.; Kou, H. Microstructure characterization of CoCrFeNiMnPd eutectic high-entropy alloys. J. Alloys Compd. 2018, 731, 600–611. [Google Scholar] [CrossRef]
- Jiang, H.; Han, K.; Qiao, D.; Lu, Y.; Cao, Z.; Li, T. Effects of Ta addition on the microstructures and mechanical properties of CoCrFeNi high entropy alloy. Mater. Chem. Phys. 2018, 210, 43–48. [Google Scholar] [CrossRef]
- Gao, X.; Lu, Y.; Zhang, B.; Liang, N.; Wu, G.; Sha, G.; Liu, J.; Zhao, Y. Microstructural origins of high strength and high ductility in an AlCoCrFeNi2.1 eutectic high-entropy alloy. Acta Mater. 2017, 141, 59–66. [Google Scholar] [CrossRef]
- Shun, T.-T.; Chang, L.-Y.; Shiu, M.-H. Microstructure and mechanical properties of multiprincipal component CoCrFeNiMox alloys. Mater. Charact. 2012, 70, 63–67. [Google Scholar] [CrossRef]
- Jiang, H.; Jiang, L.; Qiao, D.; Lu, Y.; Wang, T.; Cao, Z.; Li, T. Effect of niobium on microstructure and properties of the CoCrFeNbx Ni high entropy alloys. J. Mater. Sci. Technol. 2017, 33, 712–717. [Google Scholar] [CrossRef]
- Lu, W.; Luo, X.; Yang, Y.; Zhang, J.; Huang, B. Effects of Al addition on structural evolution and mechanical properties of the CrCoNi medium-entropy alloy. Mater. Chem. Phys. 2019, 238, 121841. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, Y.; Chen, X.; Fu, X.; Chen, D.; Chen, S.; Gu, L.; Wei, F.; Bei, H.; Gao, Y.; et al. Tuning element distribution, structure and properties by composition in high-entropy alloys. Nature 2019, 574, 223–227. [Google Scholar] [CrossRef]
- Jian, W.-R.; Xie, Z.; Xu, S.; Su, Y.; Yao, X.; Beyerlein, I.J. Effects of lattice distortion and chemical short-range order on the mechanisms of deformation in medium entropy alloy CoCrNi. Acta Mater. 2020, 199, 352–369. [Google Scholar] [CrossRef]
- Li, Q.-J.; Sheng, H.; Ma, E. Strengthening in multi-principal element alloys with local-chemical-order roughened dislocation pathways. Nat. Commun. 2019, 10, 3563. [Google Scholar] [CrossRef]
- Chen, S.; Aitken, Z.H.; Pattamatta, S.; Wu, Z.; Yu, Z.G.; Banerjee, R.; Srolovitz, D.J.; Liaw, P.K.; Zhang, Y.-W. Chemical-affinity disparity and exclusivity drive atomic segregation, short-range ordering, and cluster formation in high-entropy alloys. Acta Mater. 2021, 206, 116638. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Farkas, D.; Caro, A. Model interatomic potentials for Fe–Ni–Cr–Co–Al high-entropy alloys. J. Mater. Res. 2020, 35, 3031–3040. [Google Scholar] [CrossRef]
- Jarlöv, A.; Ji, W.; Zhu, Z.; Tian, Y.; Babicheva, R.; An, R.; Seet, H.L.; Nai, M.L.S.; Zhou, K. Molecular dynamics study on the strengthening mechanisms of Cr–Fe–Co–Ni high-entropy alloys based on the generalized stacking fault energy. J. Alloys Compd. 2022, 905, 164137. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.; Wang, P.; An, Q. Dynamic interactions between low-angle grain boundary and stacking fault tetrahedron in Ni-Fe solid solution alloys. J. Alloys Compd. 2022, 907, 164572. [Google Scholar] [CrossRef]
- Al Hasan, M.A.; Wang, J.; Shin, S.; Gilbert, D.A.; Liaw, P.K.; Tang, N.; Liyanage, W.N.C.; Santodonato, L.; DeBeer-Schmitt, L.; Butch, N.P. Effects of aluminum content on thermoelectric performance of Al CoCrFeNi high-entropy alloys. J. Alloys Compd. 2022, 883, 160811. [Google Scholar] [CrossRef]
- Lu, Y.-S.; Chang, M.-P.; Fang, T.-H. Phase transformation and microstructure evolution of nanoimprinted NiCoCr medium entropy alloys. J. Alloys Compd. 2022, 892, 162138. [Google Scholar] [CrossRef]
- Sun, Z.; Shi, C.; Gao, L.; Lin, S.; Li, W. Thermal physical properties of high entropy alloy Al0.3CoCrFeNi at elevated temperatures. J. Alloys Compd. 2022, 901, 163554. [Google Scholar] [CrossRef]
- Zhang, N.; Gan, K.; Li, Z. Atomistic insights on the deformation mechanisms of Cox(CrNi)100-x multicomponent alloys: The effect of Co content. Comput. Mater. Sci. 2022, 211, 111559. [Google Scholar] [CrossRef]
- Abere, M.J.; Ziade, E.; Lu, P.; Saltonstall, C.B.; Gu, X.; Wright, W.J.; Argibay, N.; Kustas, A.B. A predictive analytical model of thermal conductivity for aluminum/transition metal high-entropy alloys. Scr. Mater. 2022, 208, 114330. [Google Scholar] [CrossRef]
- Hirel, P. Atomsk: A tool for manipulating and converting atomic data files. Comput. Phys. Commun. 2015, 197, 212–219. [Google Scholar] [CrossRef]
- Sadigh, B.; Erhart, P.; Stukowski, A.; Caro, A.; Martinez, E.; Zepeda-Ruiz, L. Scalable parallel Monte Carlo algorithm for atomistic simulations of precipitation in alloys. Phys. Rev. B 2012, 85, 184203. [Google Scholar] [CrossRef]
- Utt, D.; Stukowski, A.; Albe, K. Grain boundary structure and mobility in high-entropy alloys: A comparative molecular dynamics study on a Σ11 symmetrical tilt grain boundary in face-centered cubic CuNiCoFe. Acta Mater. 2019, 186, 11–19. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, J.; Xu, B.; Xiong, Y.; Shao, W.; Zhao, S. Chemical short-range ordering regulated dislocation cross slip in high-entropy alloys. J. Alloys Compd. 2022, 911, 165144. [Google Scholar] [CrossRef]
- Campos, R.P.; Shinzato, S.; Ishii, A.; Nakamura, S.; Ogata, S. Database-driven semigrand canonical Monte Carlo method: Application to segregation isotherm on defects in alloys. Phys. Rev. E 2021, 104, 025310. [Google Scholar] [CrossRef] [PubMed]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO—The Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2010, 18, 015012. [Google Scholar] [CrossRef]
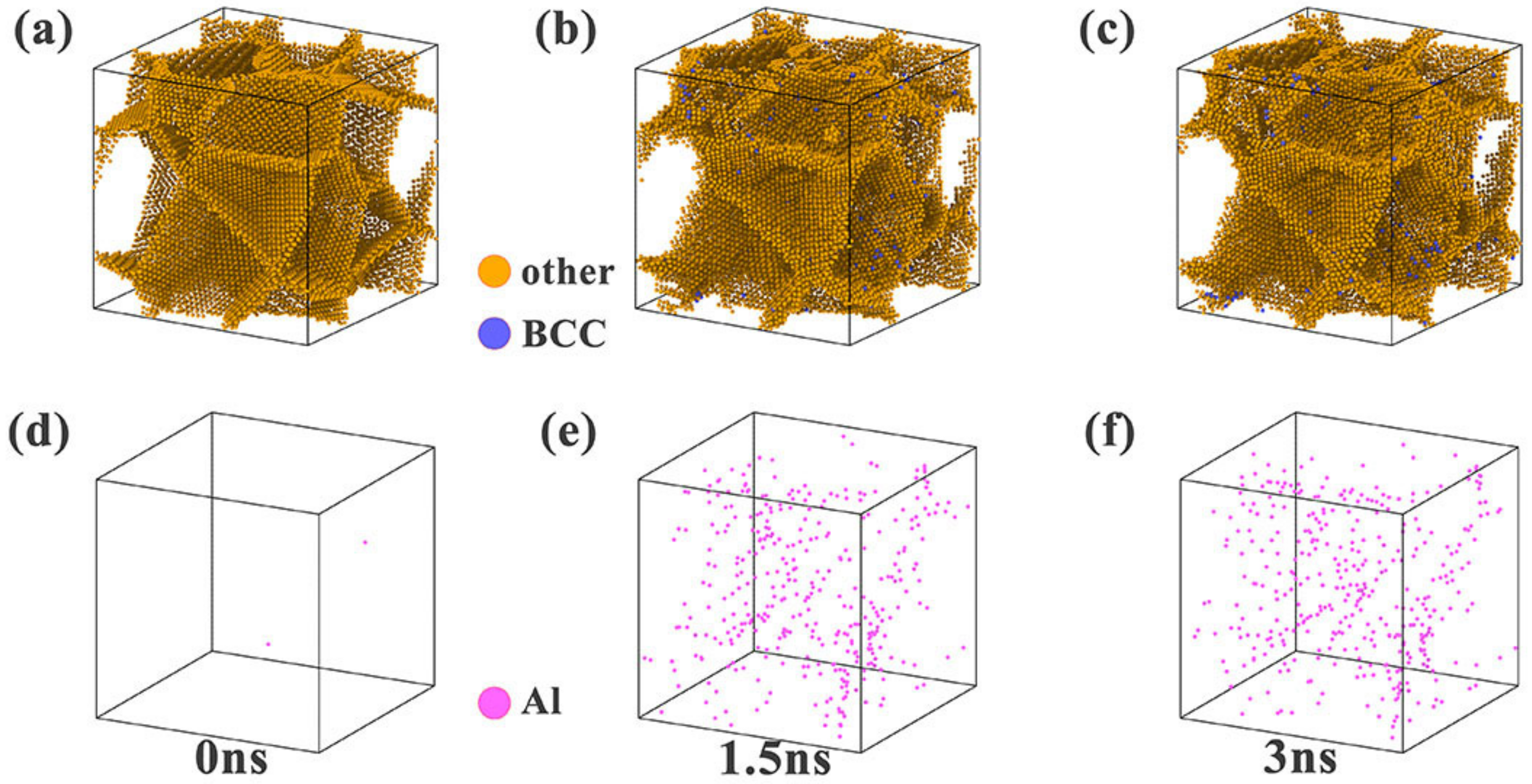
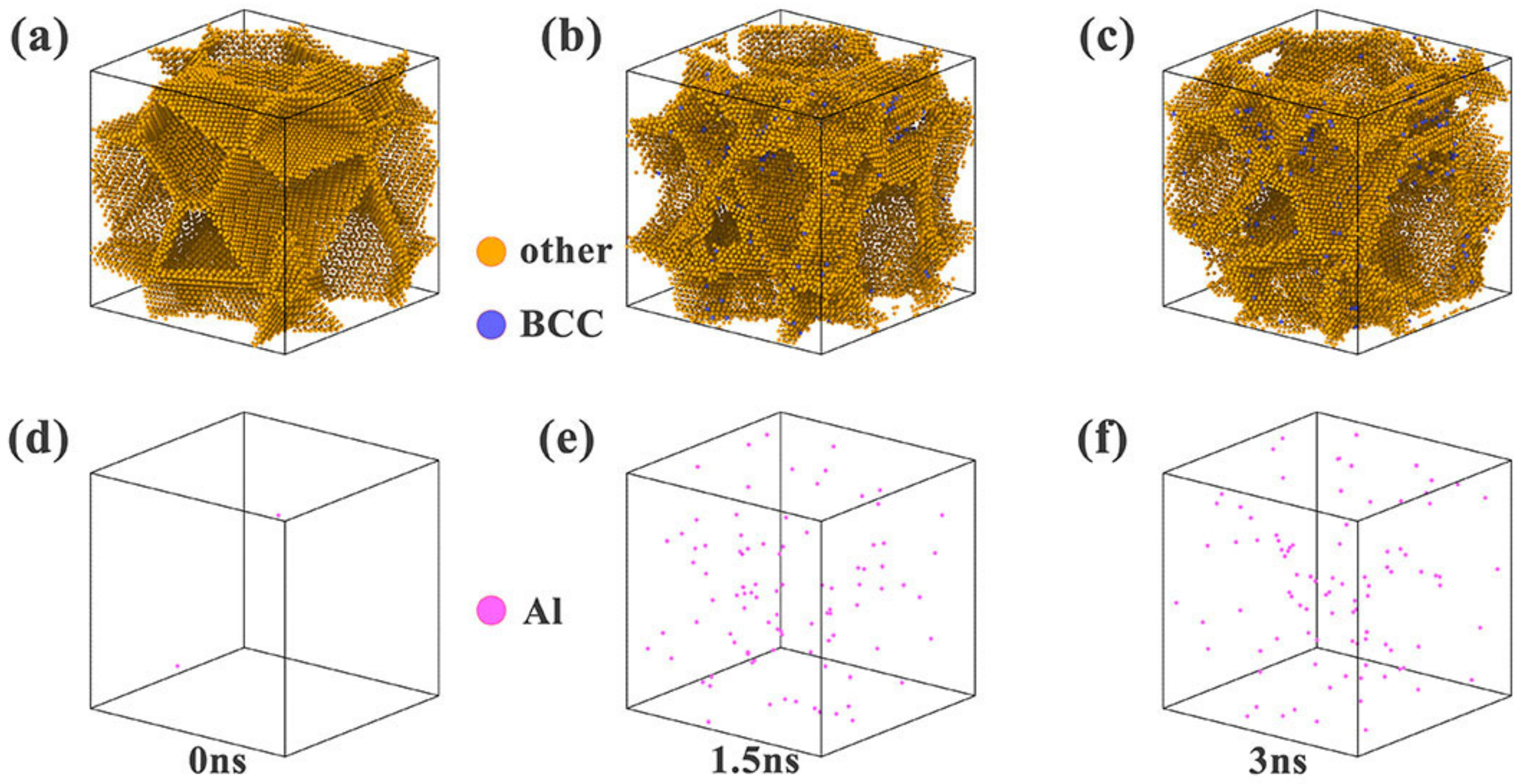
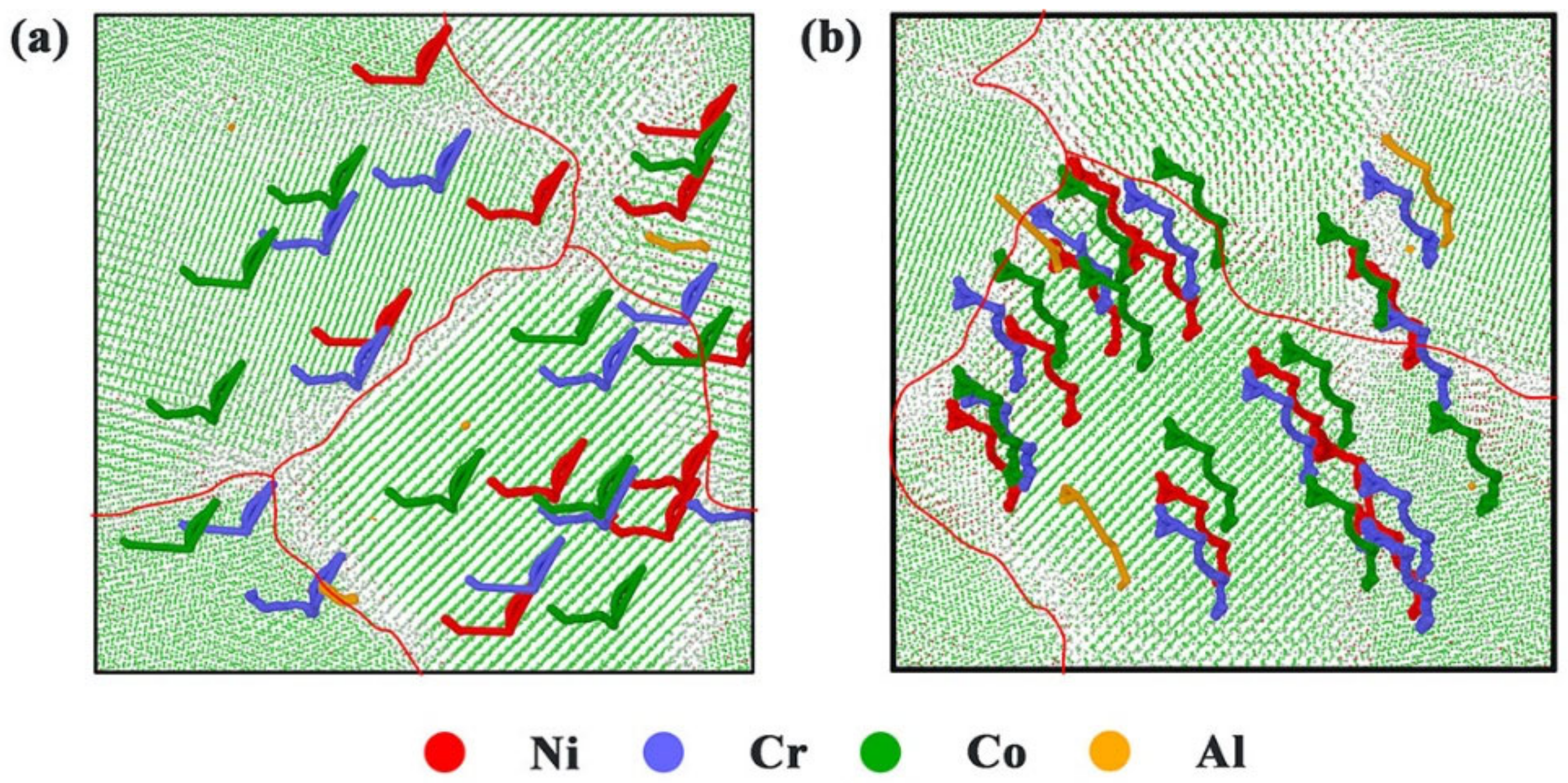
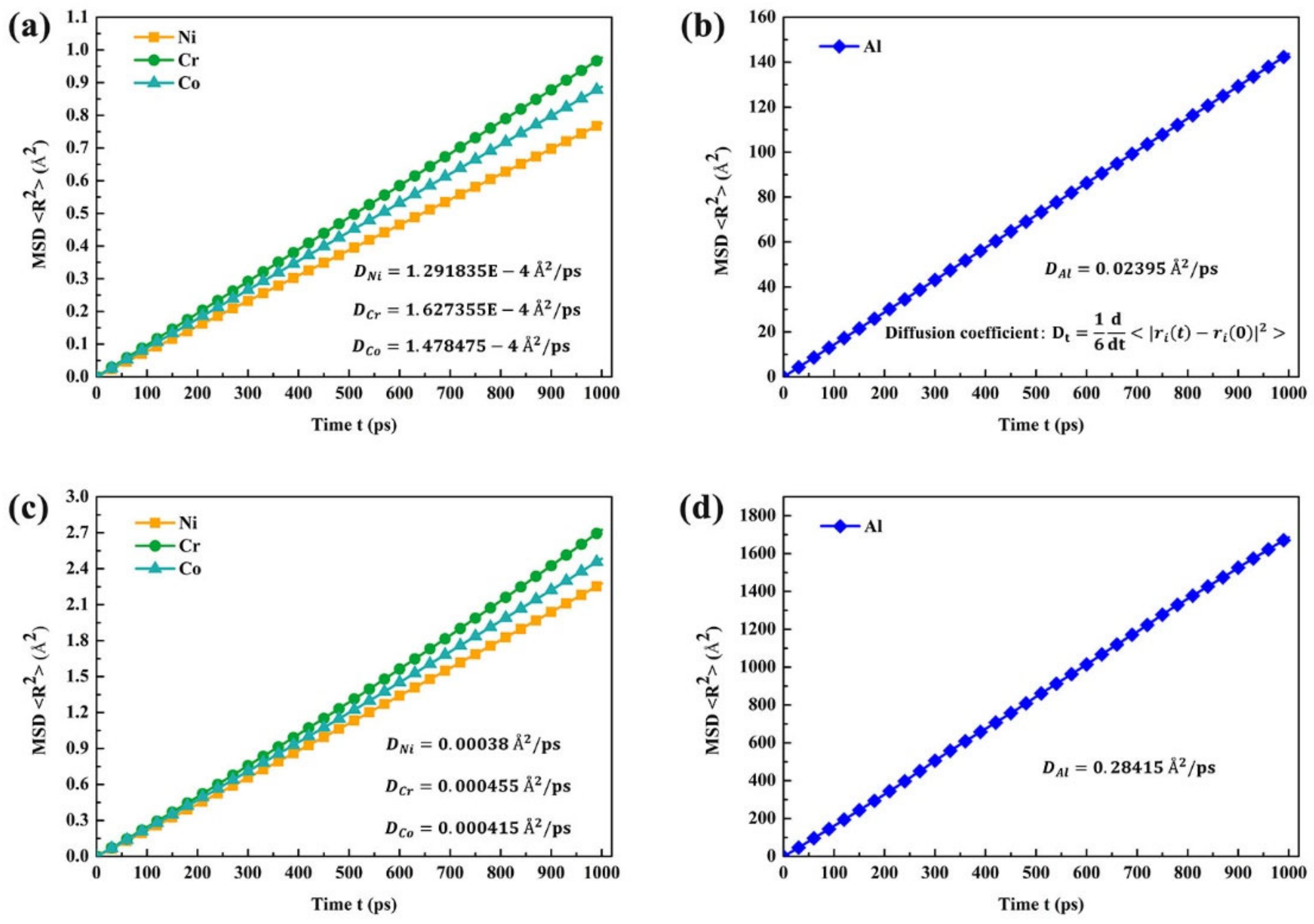

| Elements | Ni | Cr | Co | Al |
|---|---|---|---|---|
| CrCoNiAl0.014 | 32.96 | 34.58 | 32.45 | 0.014 |
| Time (ns) | Element Concentration (at. %) | |||
|---|---|---|---|---|
| Cr | Co | Ni | Al | |
| 0 | 34.7 | 32.3 | 33.1 | 0.0 |
| 1.5 | 52.5 | 31.2 | 15.1 | 1.2 |
| 3 | 52.5 | 31.4 | 14.7 | 1.3 |
| Time (ns) | Element Concentration (at. %) | |||
|---|---|---|---|---|
| Cr | Co | Cr | Al | |
| 0 | 34.7 | 32.3 | 33.1 | 0.0 |
| 1.5 | 42.5 | 31.6 | 25.5 | 0.3 |
| 3 | 42.3 | 31.7 | 25.7 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, B.; Feng, Z.; Chen, J.; Zhang, C.; Li, T.; Tan, J.; Li, C.; Yi, J. Mechanism of Aluminum Element Segregation in As-Cast Medium-Entropy Alloy CrCoNiAl0.014: A Hybrid MD/MC Simulation and Experimental Study. Metals 2023, 13, 331. https://doi.org/10.3390/met13020331
Xue B, Feng Z, Chen J, Zhang C, Li T, Tan J, Li C, Yi J. Mechanism of Aluminum Element Segregation in As-Cast Medium-Entropy Alloy CrCoNiAl0.014: A Hybrid MD/MC Simulation and Experimental Study. Metals. 2023; 13(2):331. https://doi.org/10.3390/met13020331
Chicago/Turabian StyleXue, Baoshuai, Zhongxue Feng, Jinliang Chen, Chao Zhang, Tongman Li, Jun Tan, Caiju Li, and Jianhong Yi. 2023. "Mechanism of Aluminum Element Segregation in As-Cast Medium-Entropy Alloy CrCoNiAl0.014: A Hybrid MD/MC Simulation and Experimental Study" Metals 13, no. 2: 331. https://doi.org/10.3390/met13020331
APA StyleXue, B., Feng, Z., Chen, J., Zhang, C., Li, T., Tan, J., Li, C., & Yi, J. (2023). Mechanism of Aluminum Element Segregation in As-Cast Medium-Entropy Alloy CrCoNiAl0.014: A Hybrid MD/MC Simulation and Experimental Study. Metals, 13(2), 331. https://doi.org/10.3390/met13020331








