Abstract
Chromium slag and copper smelting waste slag are solid wastes generated in the process of industrial production of chromium salt and copper metal, respectively. In this study, chromium slag and copper smelting waste slag were used as raw materials to produce black ceramic tiles. It can not only reduce environmental pollution but also increase their utilization value. The chromaticity values of ceramic tiles (L*, a*, and b*), which are color models developed by the International Commission on Illumination, were measured using a colorimeter. The phases and microstructure of the ceramic tile were analyzed by X-ray diffractometer (XRD), scanning electron microscope and energy-dispersive X-ray spectrometry (SEM-EDS), respectively. The effects of different process parameters on the coloring performance of ceramic tiles were also investigated. The results show that the color of ceramic tile is the best when the Fe/Cr ratio is 1.5, the sintering temperature is 1200 °C, the holding time is 30 min and the ceramic tile is cooled in the furnace. The values of L*, a*, and b* are, respectively, 22.5, 0, and −1.6. The compressive strength of ceramic tile and the leaching concentration of Cr(VI) are 127.2 MPa and 3.31 mg/L, respectively, which can meet the relevant national standards.
1. Introduction
Chromium slag is a solid waste produced in the production of chromium salt from chromite, which is a hazardous waste of heavy metals with strong toxicity and carcinogenicity [1]. Up until now, the total amount of chromium slag historically stockpiled in China has exceeded 6 million tons, and the disposal rate of chromium slag yards is only 15% [2]. Due to its long-term stockpiling, Cr-containing compounds in waste slag enter the soil and groundwater and, therefore, damage the ecological environment and endanger human health [3]. Compared with the traditional chromium salts production process, the calcium-free roasting and liquid-phase oxidation methods produce less slag, contain significant metal elements and are worth recycling [4,5]. On the other hand, copper smelting slag is another solid waste slag after copper extraction in the copper smelting process. About 2–3 tons of copper smelting waste slag is discharged for each ton of refined copper produced [6]. The historical stockpiling of copper smelting waste slag has exceeded 100 million tons, and the annual copper smelting waste slag production is about 15 million tons. Copper smelting waste slag usually contains 0.2–0.4% Cu, 35–45% Fe, 25–35% Si, 3–10% Al, 2–8% Ca, 1–2% Zn, 0.5–1% Pb, 0.3–0.5% S and other recoverable valuable metals [7]. Therefore, the research and development of efficient and low-cost comprehensive utilization technology of chromium slag and copper smelting waste slag are of great significance to promote the construction of ecological civilization. Due to the complex composition of slag generated in the production process of chromium slag and copper smelting waste slag, the resource utilization of these waste slags may cause secondary pollution, and increase the cost of the treatment process. Therefore, completely utilizing these waste slags to prepare value-added products could attain resource utilization efficiently and decrease the secondary pollution of recycling process simultaneously.
Black ceramic tiles are frequently utilized in the building and decorating sectors. In the traditional production process, the main raw materials for black ceramic tiles are oxides of transition metal elements such as Fe, Cr and Co. As Co2O3 is expensive, this has hampered its massive addition in black ceramics due to the cost consideration. Consequently, the development of low-cost cobalt-free black ceramic pigments has attracted the attention of many scholars. However, the use of industrial solid waste or natural mineral raw materials to produce Co-free black ceramic pigments to achieve the purpose of resource utilization of waste has become the hotspot of research [8,9]. He et al. [10] prepared blue-green and black ceramic pigments by solid-phase reaction method using calcined leather waste and industrial-grade metal oxides as raw materials. It was found that the black pigment showed the best color development with an L* value of 9.95 when the content of calcined leather reached 45 wt%. Du et al. [11] used wastes rich in Fe and Cr, vanadium tailings and leather sludge as raw materials to prepare Fe-Cr black hematite (FexCr1-x)2O3 pigment. The pigment could be used to color ceramic tile bodies with remarkable effect. Yang et al. [12] prepared V-Ti black ceramics using vanadium slag, kaolin and clay as raw materials. It can be found that the preparation of cobalt-free black ceramic pigments or tiles from industrial solid waste rich in transition metal elements not only has good performance, but also effectively solves the environmental problem of waste slags to the surrounding area, which provides a solution for the recovery of industrial solid wastes. The traditional process for treating chromium slag and copper slag also has high temperature, high energy consumption, need to add large equipment and complex process, which leads to high operating costs and is not conducive to the current economic development. In this study, using chromium slag and copper slag as raw materials to prepare black ceramic tiles can solve the above problems, reduce the production cost of enterprises, and realize the harmless, resourceful and economic utilization of chromium slag and copper slag.
Combined with the above practice, the oxides of Al2O3, SiO2, and CaO rich in chromium slag and copper smelting waste slag are the main components of the ceramic matrix [13]. In addition, the Fe3+, Fe2+ and Cr3+ contained in the chromium slag and copper smelting waste slag during high-temperature roasting will undergo a solid solution reaction to produce the black spinel phase that will exist in the tile matrix as the base phase. Moreover, the spinel phase will enhance the performance of the tiles due to its excellent thermal stability, heat resistance, and chemical corrosion resistance [14]. Therefore, chromium slag and copper smelting waste slag sintered into ceramic tiles with a certain ratio of ingredients can not only effectively reduce the energy consumption and process cost but also utilize the valuable metals in the waste slag. In this study, Fe-Cr black ceramic tiles were prepared using chromium slag and copper smelting waste slag as raw materials. The influence of Fe/Cr molar ratio, sintering temperature, holding time, cooling methods and sintering atmosphere on the phases and color of the sintered ceramic tiles were investigated to provide theoretical guidance for industrial applications.
2. Experiment
2.1. Raw Materials
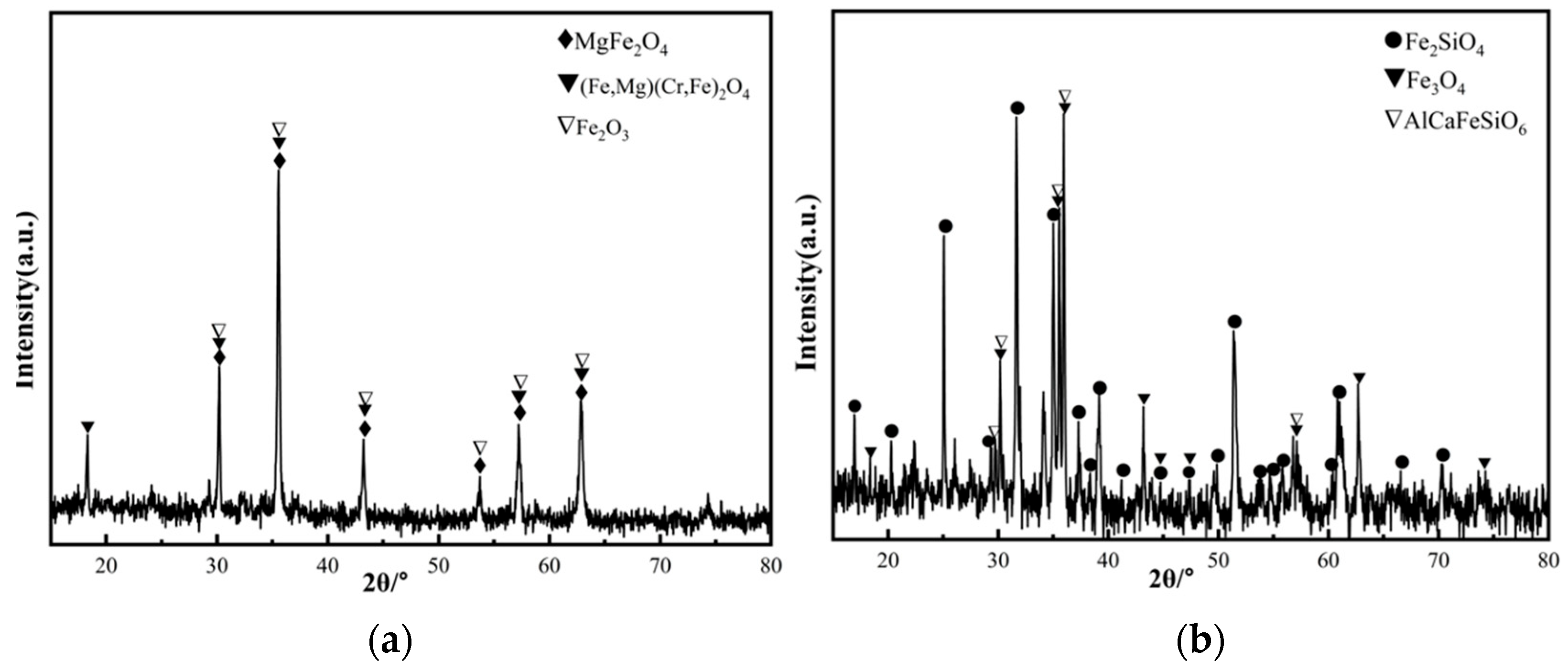
Chromium slag and copper smelting waste slag were used as raw materials in this study, which were obtained from a Chinese chromium salt plant and copper production plant, respectively. Their chemical compositions are measured by inductive coupled plasma emission spectrometer (ICP-AES, Thermo Elemental IRIS Advantage Radial, Philadelphia, PA, USA) shown in Table 1. Due to the burning loss during composition test process, the measured total contents component are less than 100 wt%. From Table 1, it can be seen that the chromium slag and copper smelting waste slag contain a large number of iron oxide (more than 40 wt%) and a small number of Cr2O3 (8.59 wt% and 0.24 wt%) which can be used as coloring elements, as well as some amount of SiO2 (7.10 wt% and 33.44 wt%) and Al2O3 (10.73 wt% and 5.68 wt%), which are the main components of the ceramic matrix. The identified XRD patterns of chromium slag and copper smelting waste slag are shown in Figure 1. It indicates that the main phases of chromium slag are MgFe2O4, (Fe, Mg)(Cr, Fe)2O4 and Fe2O3, while the main phases in copper smelting waste slag are Fe2SiO4 and Fe3O4.

Table 1.
Chemical compositions of chromium slag and copper smelting waste slag (wt%).
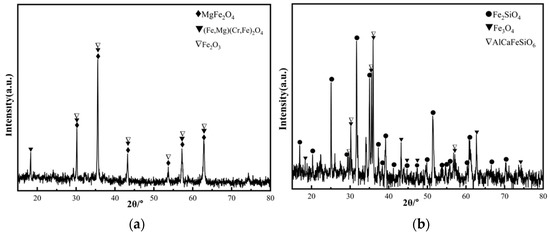
Figure 1.
XRD patterns of slag. (a) Chromium slag. (b) Copper smelting waste slag.
2.2. Preparation of Black Ceramic Tiles
Firstly, the dried chromium slag and copper smelting waste slag were crushed to below 48 μm, and the Fe/Cr molar ratio in the raw material was adjusted (0.5, 1.0, 1.5, 2.0, 2.5) by modifying the ratio of chromium slag and copper slag. Then, the mixture material was further mixed for 30 min using a flip shaker (DR-MIX, Beijing Haonos Technology Co., Ltd., Beijing, China). About 5 g of thoroughly mixed raw materials were pressed in a cylindric mold with the diameter of 15 mm under the pressure of 10 MPa and held for 60 s. Then, the cylindric samples were heating in a muffle furnace (SX2-10-13, Shanghai Shi Yan Electric Furnace Co., Ltd, Shanghai, China) at a heating rate of 7 °C/min and held at various target sintering temperature (1100, 1125, 1150, 1175, 1200 or 1225 °C) for different holding time (15, 30, 45, 60, 90 min). Table 2 shows the mass fractions of chromium slag and copper smelting waste slag in the samples with different Fe/Cr molar ratio. Table 3 shows the parameters for producing black ceramic tiles.

Table 2.
Mass fraction of chromium slag and copper smelting waste slag in the samples with different Fe/Cr molar ratio (wt%).

Table 3.
Parameters of producing ceramic tile.
2.3. Sample Characterization
The phases in the chromium slag, copper smelting waste slag and produced ceramic tiles were identified by X-ray diffractometer (XRD) (PANalytical X’Pert PRO MPD, Netherlands, Almelo) with 2θ scanning ranging from 15° to 80° and Cu Kα radiation at a tube voltage of 40 kV, current of 40 mA. The morphology of phase in the ceramic tiles were observed by a field-emission scanning electron microscope (SEM), coupled with an energy-dispersive X-ray spectrometry (EDS) (SEM-E, Nova NanoSEM400, FEI USA, EVO 010, Zeiss, X-MaxN 79416, Oxford).
The chromaticity values (L*, a*, and b*) of ceramic tiles were measured using a colorimeter (3nh, TS7010). L*, a*, and b* are color models developed by the International Commission on Illumination (CIE). L* value represents the lightness of color and ranges from 0 (black) to 100 (white) (L* = 100); a* and b* values represent the green (−a*) to red (+a*) and blue (−b*) to yellow (+b*) scale, respectively.
The leaching test for Cr6+ concentration in the chromium slag, copper smelting waste slag and ceramic tile was carried out based on the Chinese environmental protection industry standard HJ/T299-2007 [15]. The extraction agent with the pH of 3.2 ± 0.5 was prepared by using concentrated nitric acid, concentrated sulfuric acid and deionized water. The mass ratio of concentrated nitric acid to concentrated sulfuric acid was 1:2. The prepared extraction agent was loaded into a 1000 mL volume bottle and then 100 g powdery sample was added into the volume bottle. The overturning shaker was employed to mix the agent for 20 h at the temperature of 23 ± 2 °C with the speed of 30 ± 2 r/min. The Cr6+ concentration in the leaching solution was measured by spectrophotometry (UV-6100S ultraviolet visible spectrophotometer, Shanghai Yuanxi Instrument Co., Ltd., Shanghai, China).
The compressive strength of ceramic tiles was measured by using a hydraulic universal test machine (WE-30, Tai Tian machinery Jiangsu Co., Ltd., Jiangyin, China). Three measurements for each sample were conducted in order to ensure the reliability of the test results.
3. Results and Discussion
3.1. Effect of Fe/Cr Molar Ratio on the Chromatic Performance of Ceramic Tiles
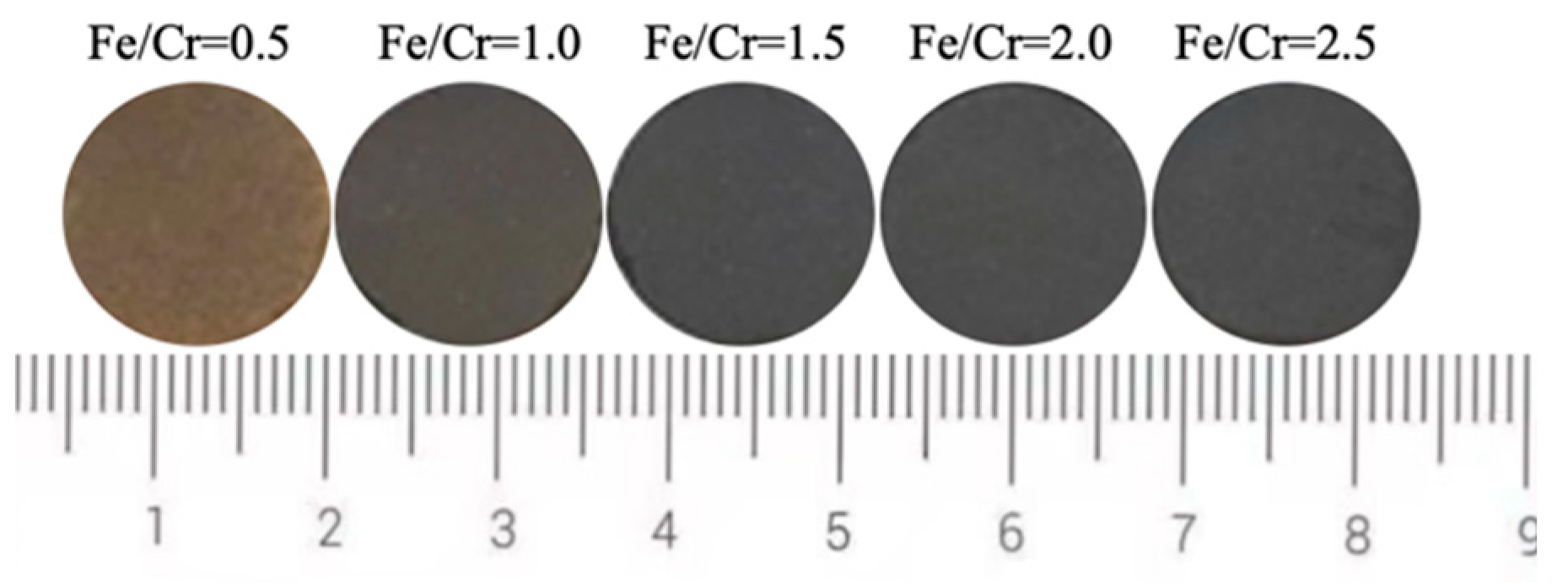
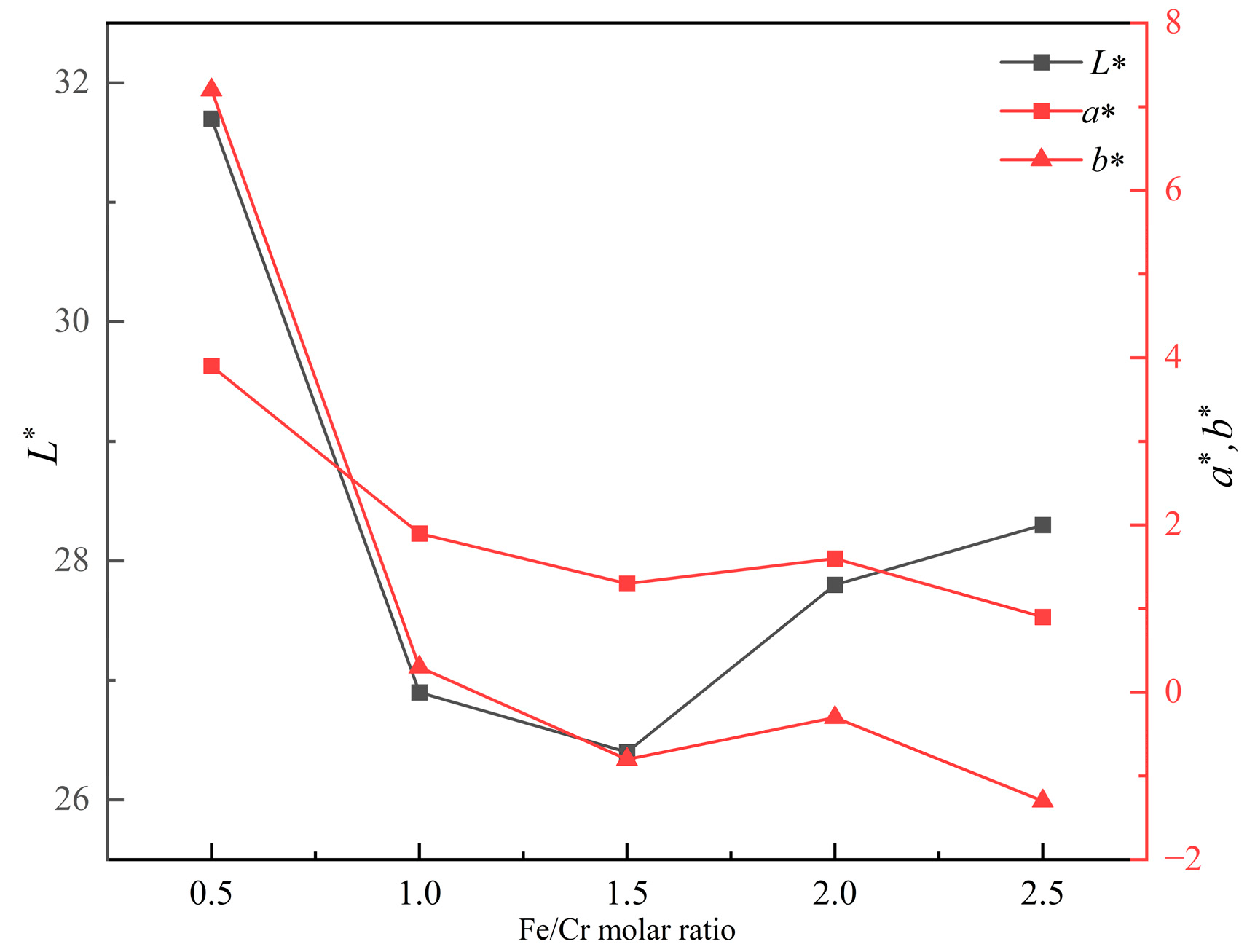
Figure 2 shows the photos of produced ceramic tiles with various Fe/Cr molar ratios and held at 1150 °C for 30 min followed by furnace cooling. It can be seen from Figure 2 that the ceramic tile presents a brownish yellow color when the Fe/Cr molar ratio is 0.5. The ceramic tile has a smooth surface and shows black when Fe/Cr molar ratio is 1.5. The chromaticity values of the ceramic tiles in Figure 3 present that the L* value of ceramic tile decreases from 31.7 to 26.4 with increasing the Fe/Cr molar ratio from 0.5 to 1.5 and then increases to 28.3 with further increasing the Fe/Cr molar ratio to 2.5. When Fe/Cr molar ratio is 0.5, the a* and b* values are significantly higher than those of other ceramic tiles.
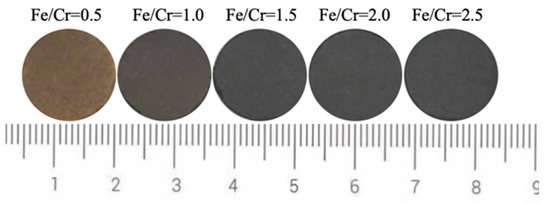
Figure 2.
Photos of ceramic tiles with different Fe/Cr molar ratio.
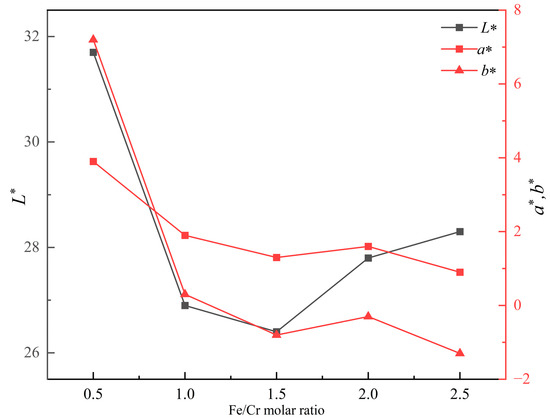
Figure 3.
Effects of Fe/Cr molar ratio on the coloration of ceramic tiles.
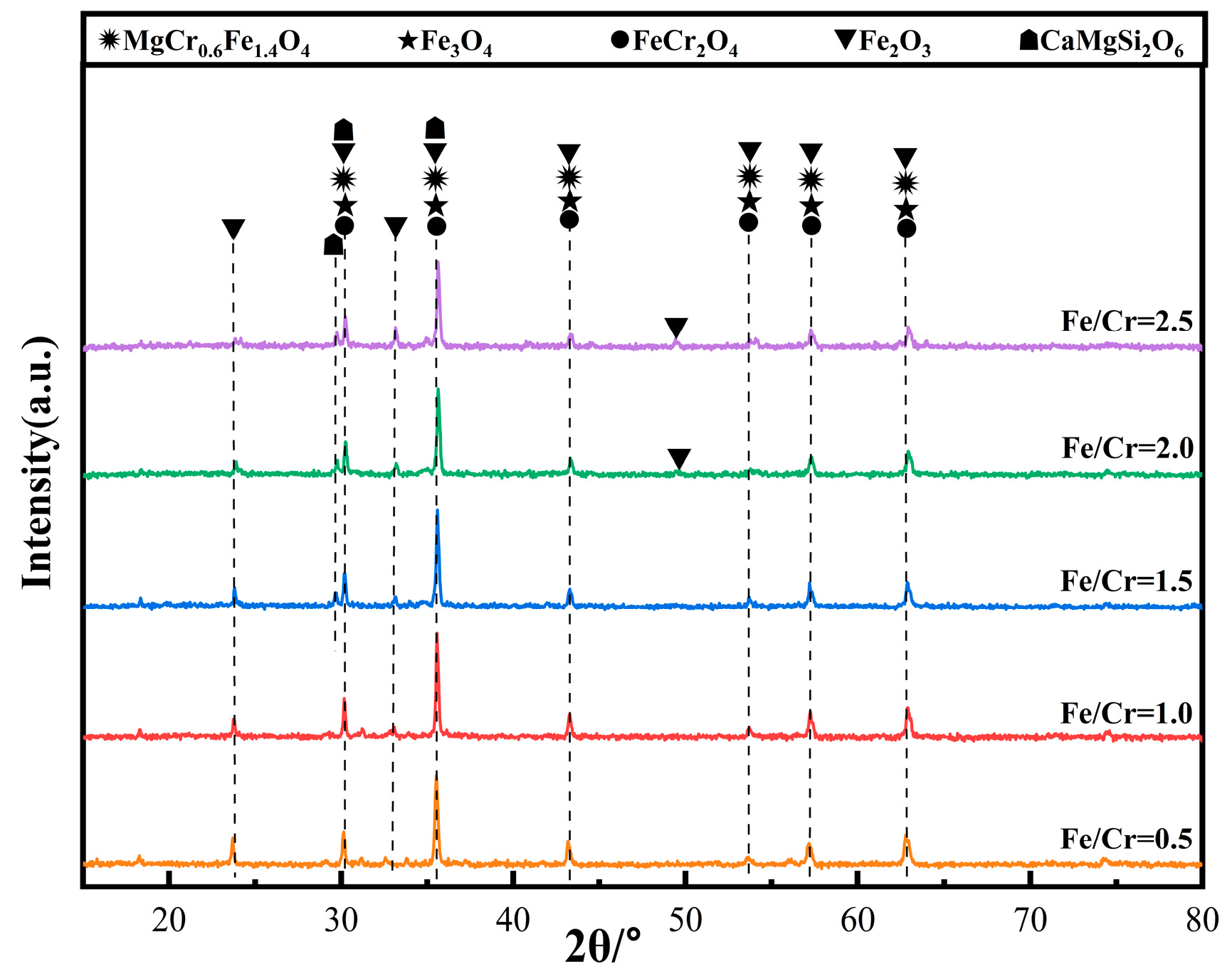
Figure 4 shows the XRD patterns of the ceramic tiles with different Fe/Cr molar ratio. The main coloring phases in the ceramic tiles are MgCr0.4Fe1.6O4, Fe3O4, FeCr2O4, and Fe2O3. Fe3O4 was generated from the solid solution reaction between FeO and Fe2O3, and the formation of FeCr2O4 resulted from the solid solution reaction between FeO and Cr2O3 in the raw material [16]. The generation of MgCr0.4Fe1.6O4 was attributed to the solid solution behavior of Cr3+ in MgFe2O4 at elevated temperature. It resulted in the transformation of brick red MgFe2O4 to black MgCr0.4Fe1.6O4, which was because the Cr3+ in the MgCr0.4Fe1.6O4 increased the absorption range of visible light in the band from 550 nm to 700 nm [17]. Thus, the ceramic tile had the ability to absorb the full spectrum ranging in the visible light, which enhanced the black chromaticity value of the ceramic tiles. The ceramic tiles with the Fe/Cr molar ratio of 0.5 present brownish-yellow, resulting from a large number of unreacted Fe2O3 in the mixed raw material. With the increase of Fe/Cr molar ratio, more Fe2+ participated in the solid solution reaction between FeO and Fe2O3, as well as Cr2O3, resulting blacker of the ceramic tiles.
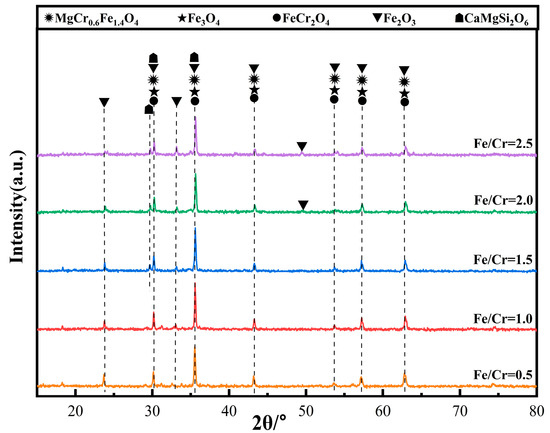
Figure 4.
XRD patterns of ceramic tiles with different Fe/Cr mole ratio.
3.2. Effect of Sintering Temperatures on the Chromatic Performance of Ceramic Tiles
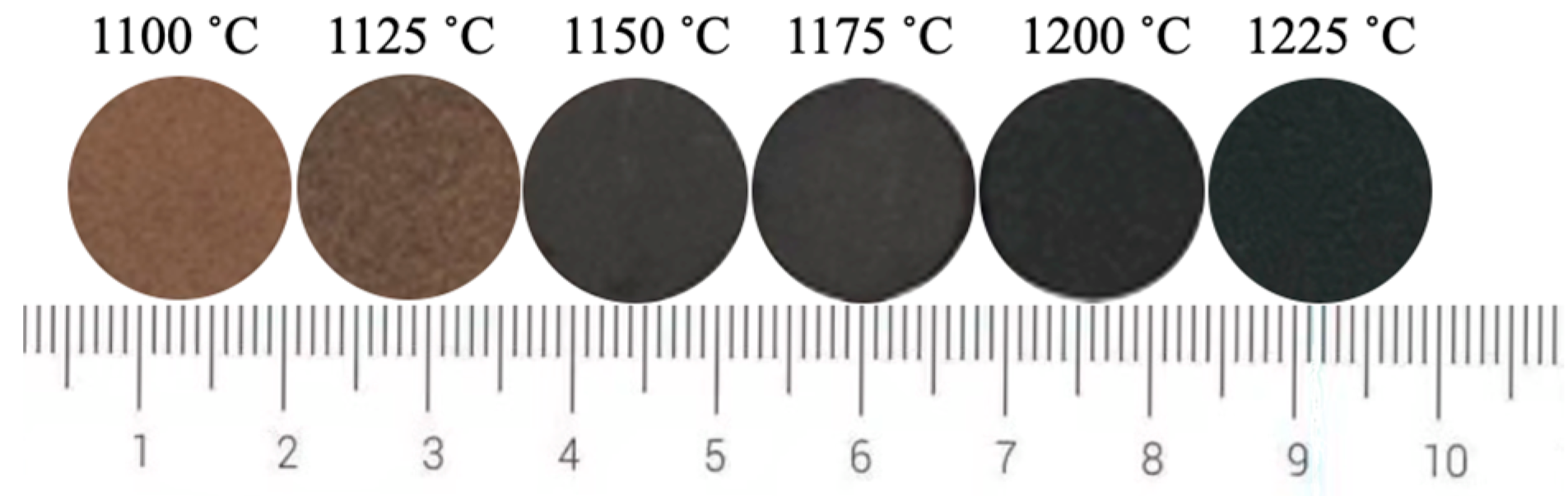
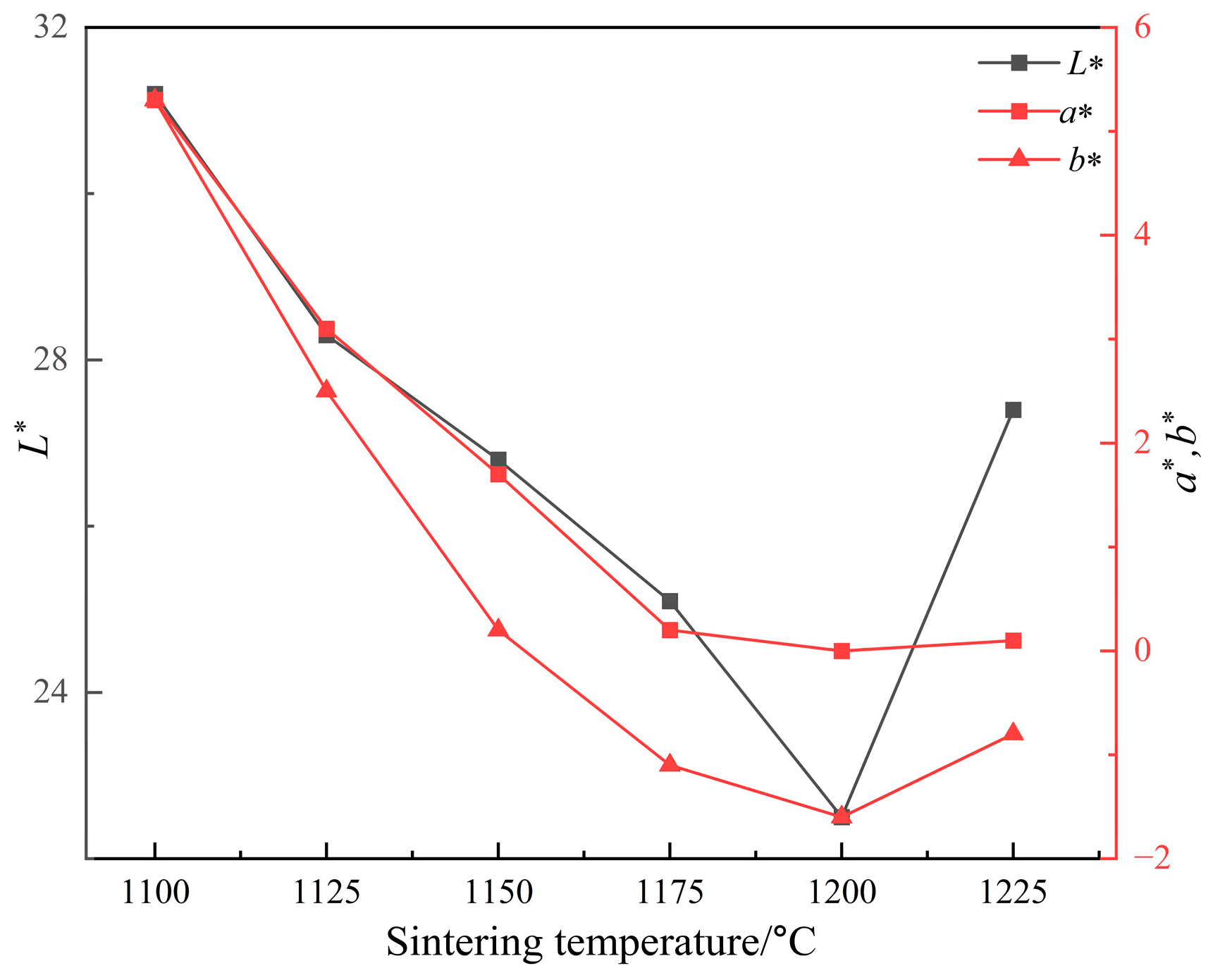
Since the L* value has the greatest effect on the black color of ceramic tile, Fe/Cr ratio of 1.5 was selected to explore the influence of sintering temperature on the L* value of ceramic tile for its lowest L* value of 26.4. Figure 5 shows the photos of produced ceramic tiles with the Fe/Cr molar ratio of 1.5 and held at various sintering temperatures for 30 min followed by furnace cooling. It is clear that the ceramic tiles present brownish yellow when the sintering temperature is 1100 °C. When the sintering temperature increases to 1125 °C, the color of ceramic tiles becomes darker and not uniform. When the sintering temperatures are 1150 °C and 1200 °C, the surfaces of the ceramic tiles are smooth and present black. When the sintering temperature is 1225 °C, the bottom of the ceramic tile is bonded to the crucible, indicating the formation of liquid phase in the ceramic tile. Figure 6 shows the chromaticity values of produced ceramic tiles. It is clear that the L* value of ceramic tile decreases from 31.2 to 22.5 with increasing the sintering temperatures from 1100 °C to 1200 °C and then increases to 27.4 with further increasing the sintering temperatures to 1225 °C. With increasing the sintering temperatures, the a* value first presents a decreasing tendency and then unchanged. The change of b* value exhibits a similar tendency with that of L* value.
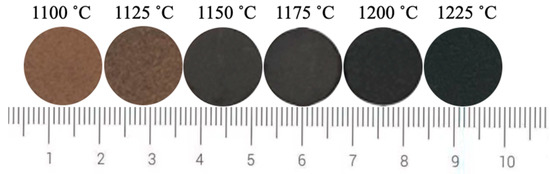
Figure 5.
Photos of ceramic tiles sintered at different temperatures.
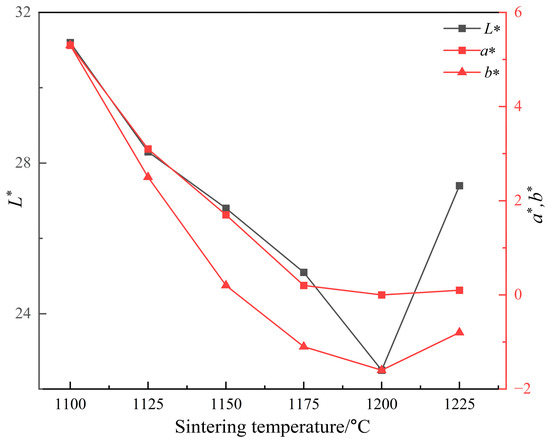
Figure 6.
Chromaticity values of ceramic tiles sintered at different temperatures.
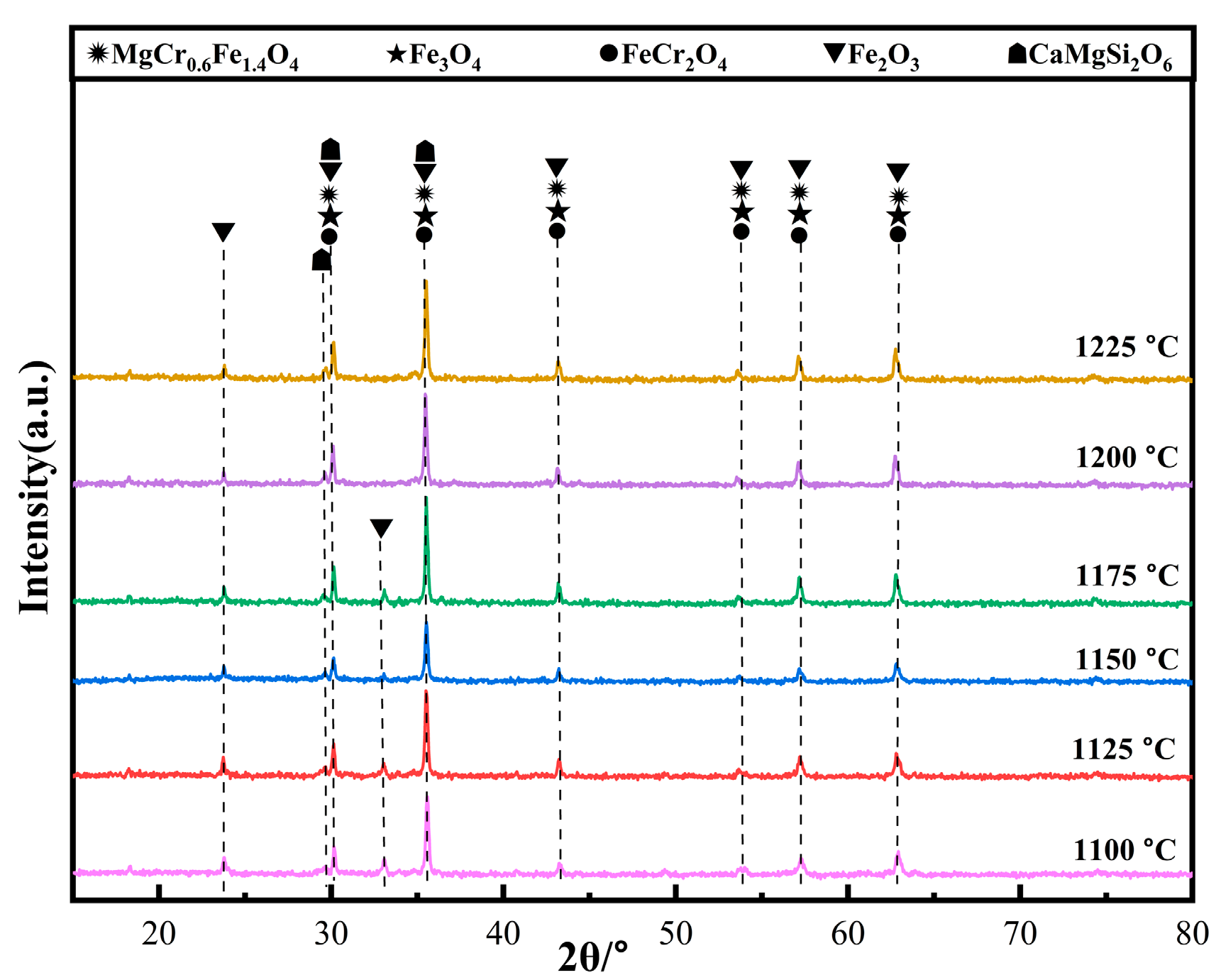
Figure 7 shows the XRD patterns of the ceramic tiles sintered at different temperatures. From Figure 7, it can be seen that the mixture material has reacted at 1100 °C and the main phases in the ceramic tiles sintered at 1100~1175 °C are MgCr0.4Fe1.6O4, Fe3O4, FeCr2O4, Fe2O3, and CaMg2Si2O6. When the temperatures are 1100 °C and 1125 °C, Fe2SiO4 in the raw material decomposes into Fe2O3 and SiO2 [18,19], which increases the Fe2O3 content in the ceramic tiles, resulting in stronger diffraction peaks corresponding to Fe2O3 in Figure 7. However, the lower temperature is not sufficient to completely react for a large amount of Fe2O3 present in the matrix with FeO to form spinel, resulting in a brownish-yellow color of the tiles. As the temperature increases to 1200 °C, the reaction rate between solid phases is enhanced, which is favorable to the formation of Fe3O4 and FeCr2O4 black spinel. Thus, Cr3+ has enough kinetic energy to diffuse into MgFe2O4, which causes the ceramic tiles to present black. When the sintering temperature increases to 1225 °C, the amount of liquid phase CaMgSi2O6 in the ceramic tiles increases, resulting in the bonding of the ceramic tiles with the crucible.
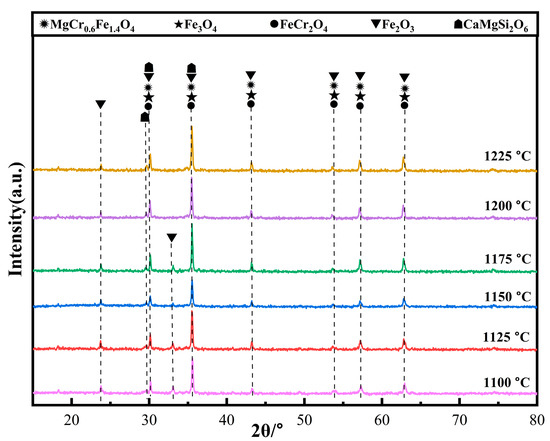
Figure 7.
XRD patterns of ceramic tiles sintered at different temperatures.
3.3. Effect of Holding Time on the Chromatic Performance of Ceramic Tiles
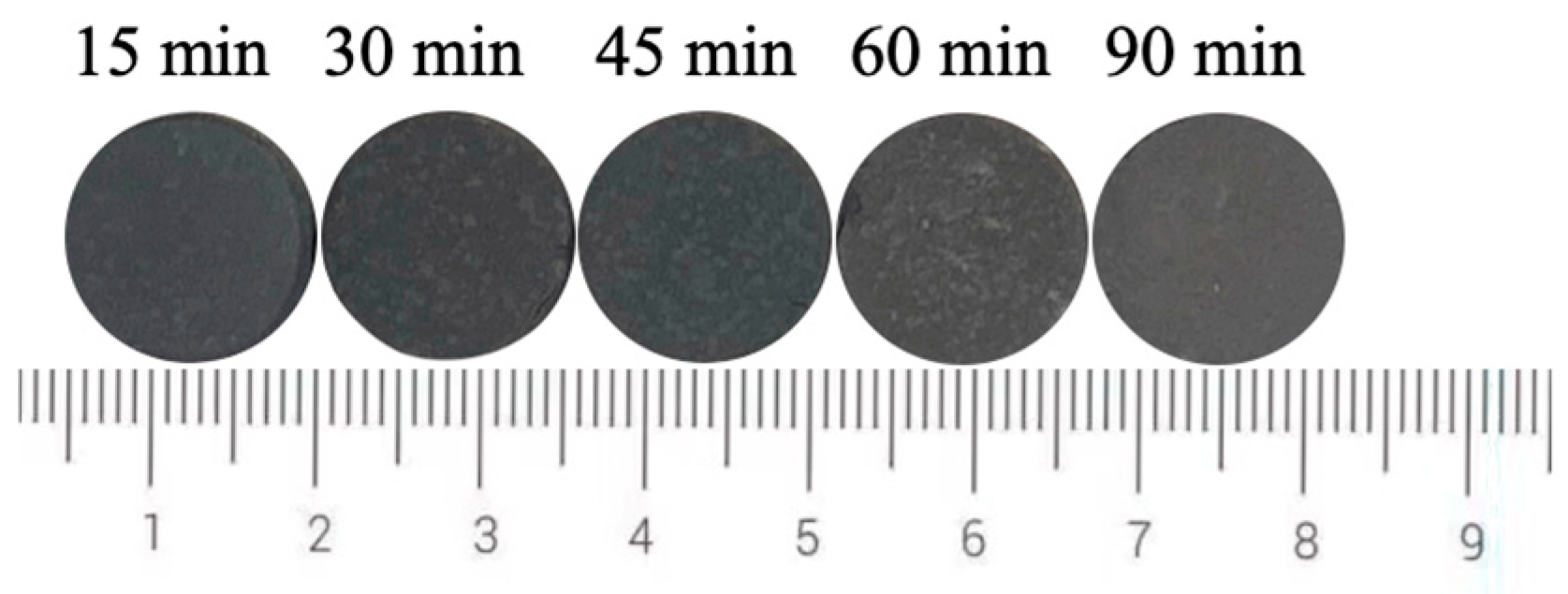
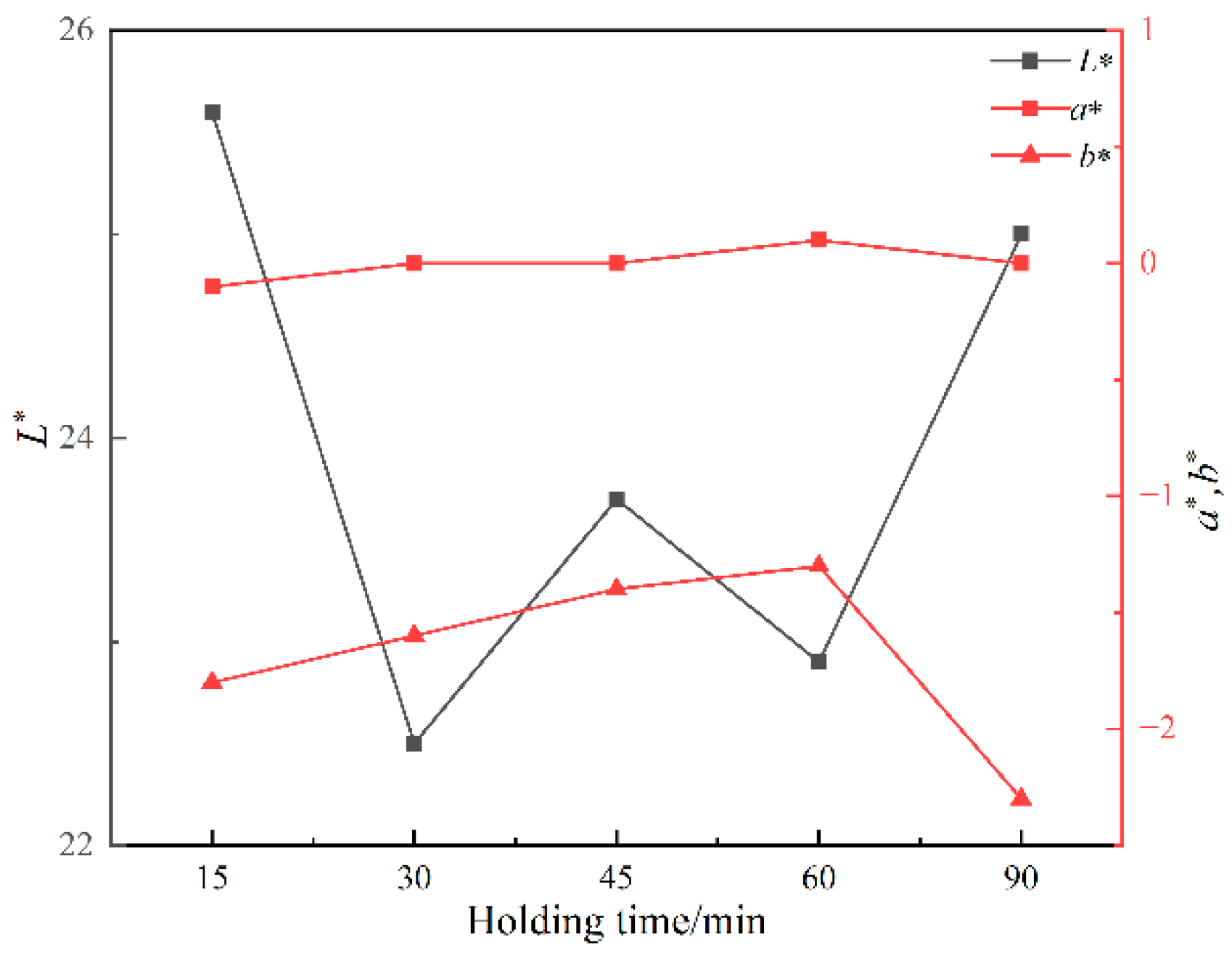
Figure 8 shows the photos of ceramic tiles with the Fe/Cr molar ratio of 1.5 and sintered at 1200 °C for various holding times followed by furnace cooling. When held for 15~45 min, the produced ceramic tiles present wholly black. As the holding time increases to 60~90 min, the ceramic tiles present an unorthodox black color. Figure 9 shows the chromaticity values of produced ceramic tiles. The minimum L* value of the ceramic tiles is 22.5 when the holding time is 30 min. The a* value fluctuates around 0 with the increase in holding time, and its change is not significant. The b* value of ceramic tile increases from −1.78 to −1.32 with increasing the holding time from 15 min to 60 min and then decreases to −2.32 with further increasing the holding time to 90 min. It indicates that excessive holding time deteriorates the chromatic performance of ceramic tiles.
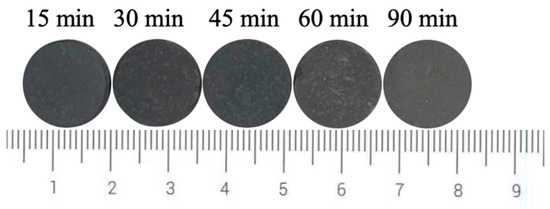
Figure 8.
Photos of ceramic tiles with different holding time.
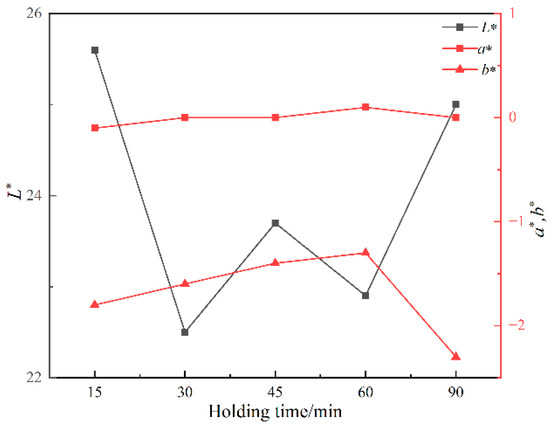
Figure 9.
Chromaticity values of ceramic tiles at different holding times.
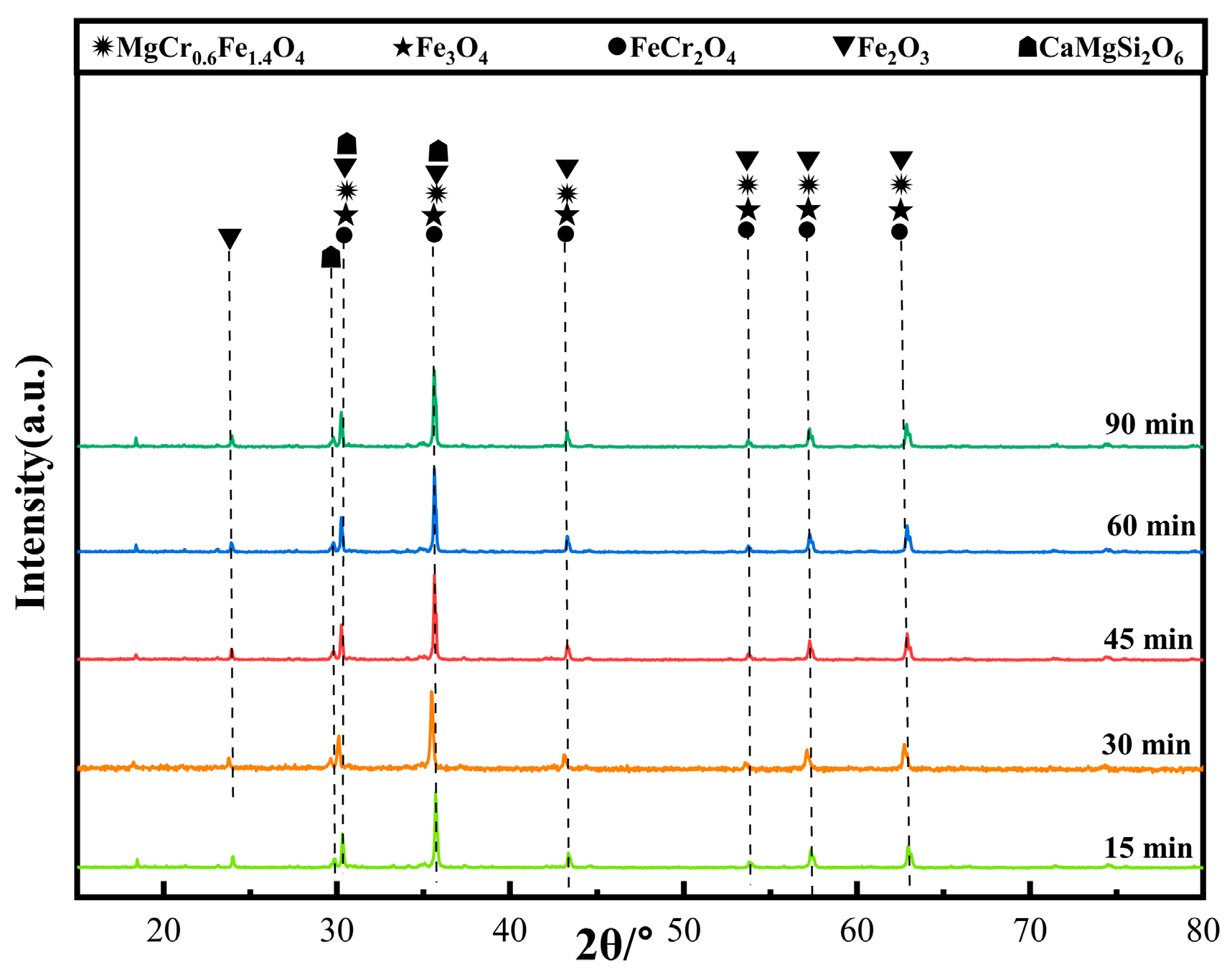
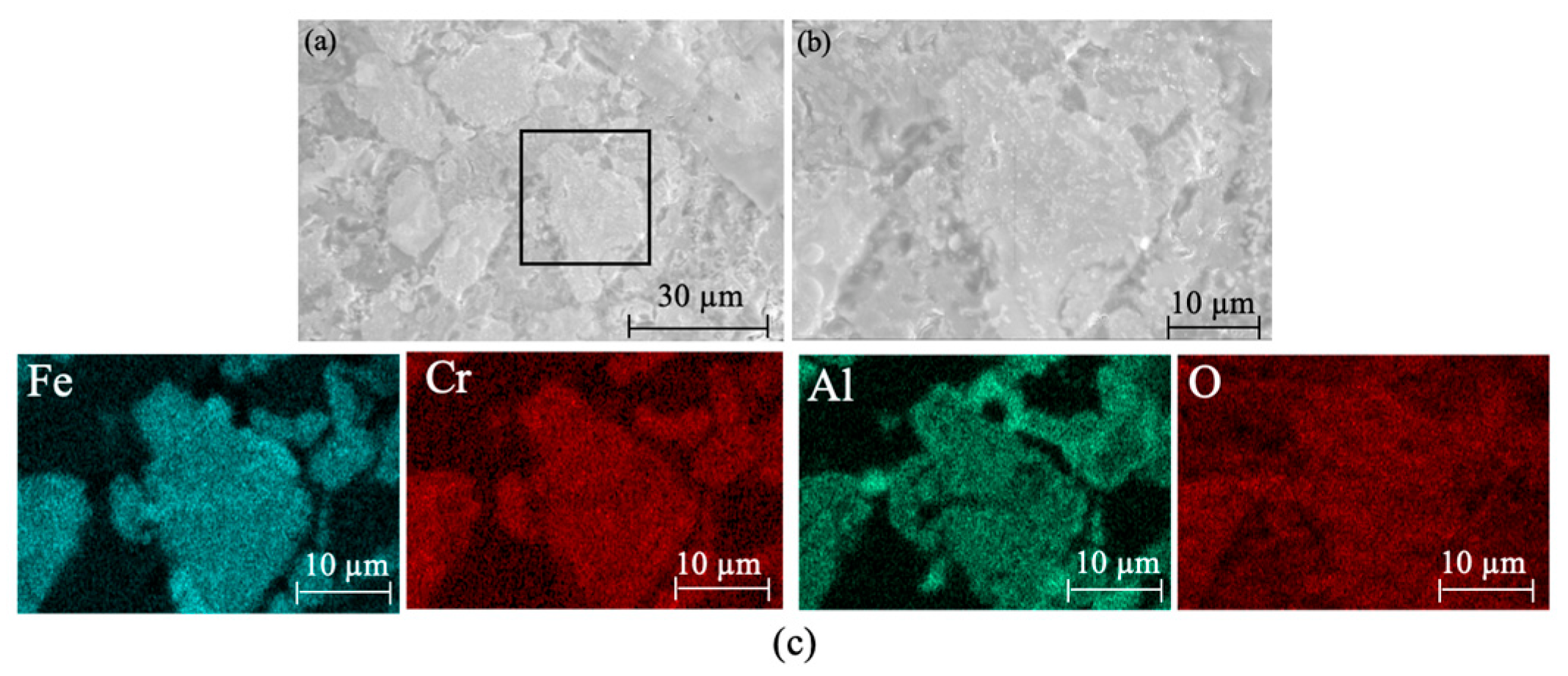
Figure 10 shows the XRD patterns of ceramic tiles at different holding time. It is clear that the main phases in the produced ceramic tiles are MgCr0.4Fe1.6O4, Fe3O4, FeCr2O4, Fe2O3, and CaMgSi2O6. Extending the holding time can enhance the reaction between the compounds in the samples. However, excessive holding time deteriorates the coloring ability of Fe3+ at elevated temperatures. Figure 11 is the SEM-EDS results of ceramic title holding for 30 min. Figure 11a shows that the surface of the ceramic tile sample has defects and is not smooth, and its phase distribution presents irregular polygons of different sizes embedded in the ceramic matrix. Figure 11b is an enlarged view of the marked area in Figure 11a. The element mapping results in Figure 11c show that the phase in the ceramic tiles is enriched in the elements of Fe, Cr, Mg and O. MgCr0.4Fe1.6O4 with mosaic structure is formed; there are similar reports in literature [20].
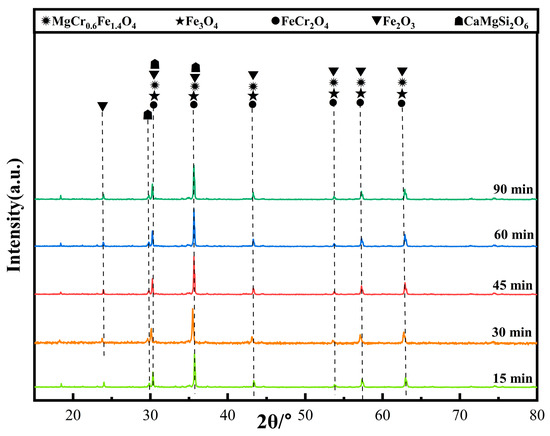
Figure 10.
XRD patterns of ceramic tiles at different holding time.
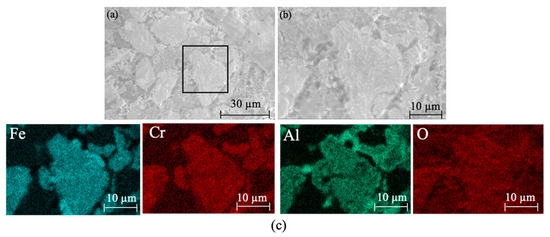
Figure 11.
SEM images and element mapping of the black ceramic tiles holding for 30 min. (a) SEM image of the black ceramic tile holding for 30 min; (b) Enlarged view of the marked area in figure (a); (c) Element mapping of figure (b).
3.4. Effect of Cooling Methods on the Chromatic Performance of Ceramic Tiles
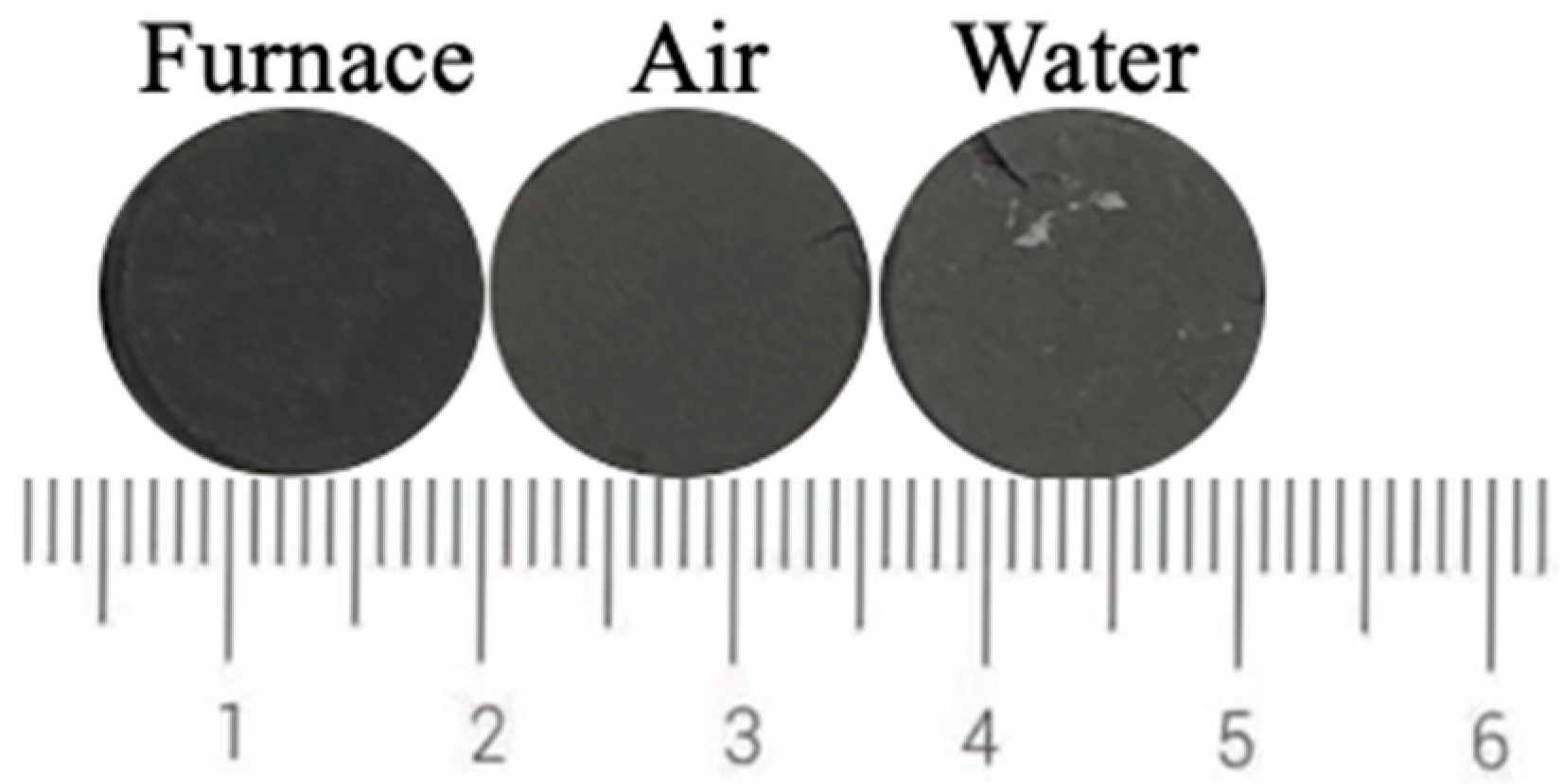
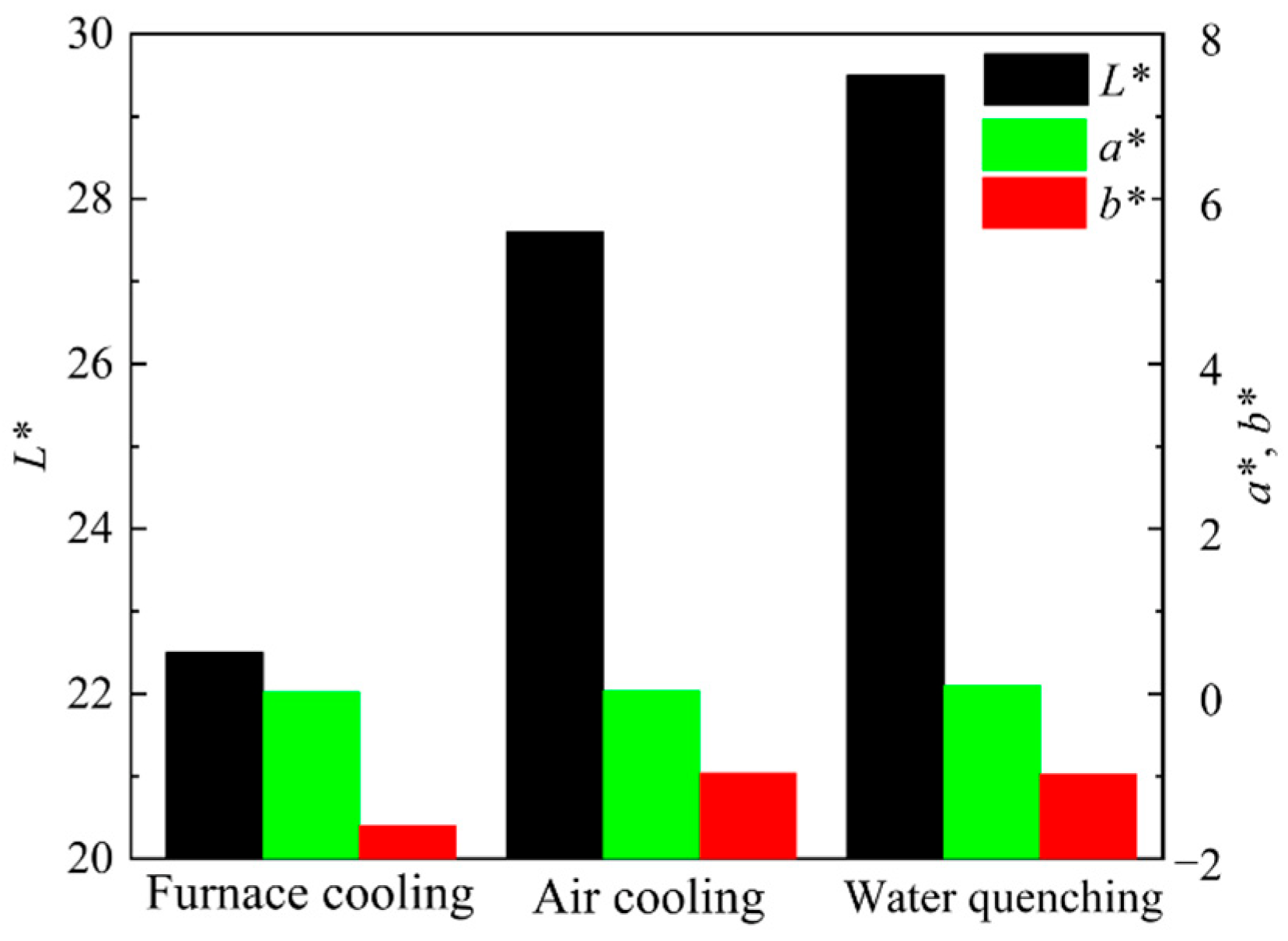
Figure 12 shows the photos of ceramic tiles with the Fe/Cr molar ratio of 1.5 and sintered at 1200 °C for 30 min followed by furnace cooling, air cooling and water cooling, respectively. Ceramic tiles break after water quenching, and obvious cracks appear on the surface of air cooling. The chromaticity values of the ceramic tiles in Figure 13 show that the L* value increases as the cooling rate increases.
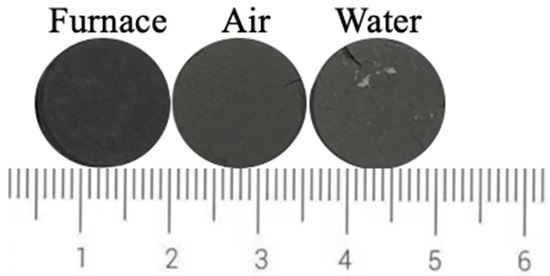
Figure 12.
Photos of ceramic tiles cooled by different methods.
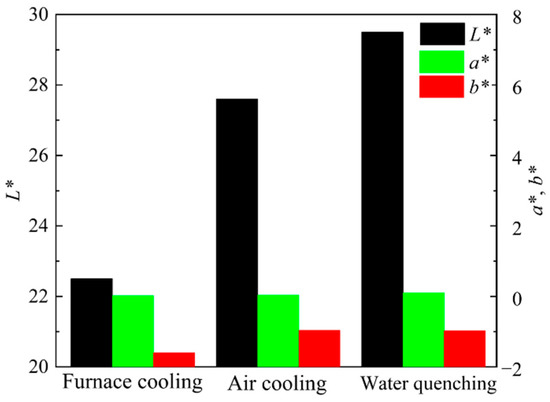
Figure 13.
Chromaticity values of ceramic tiles cooled by different methods.
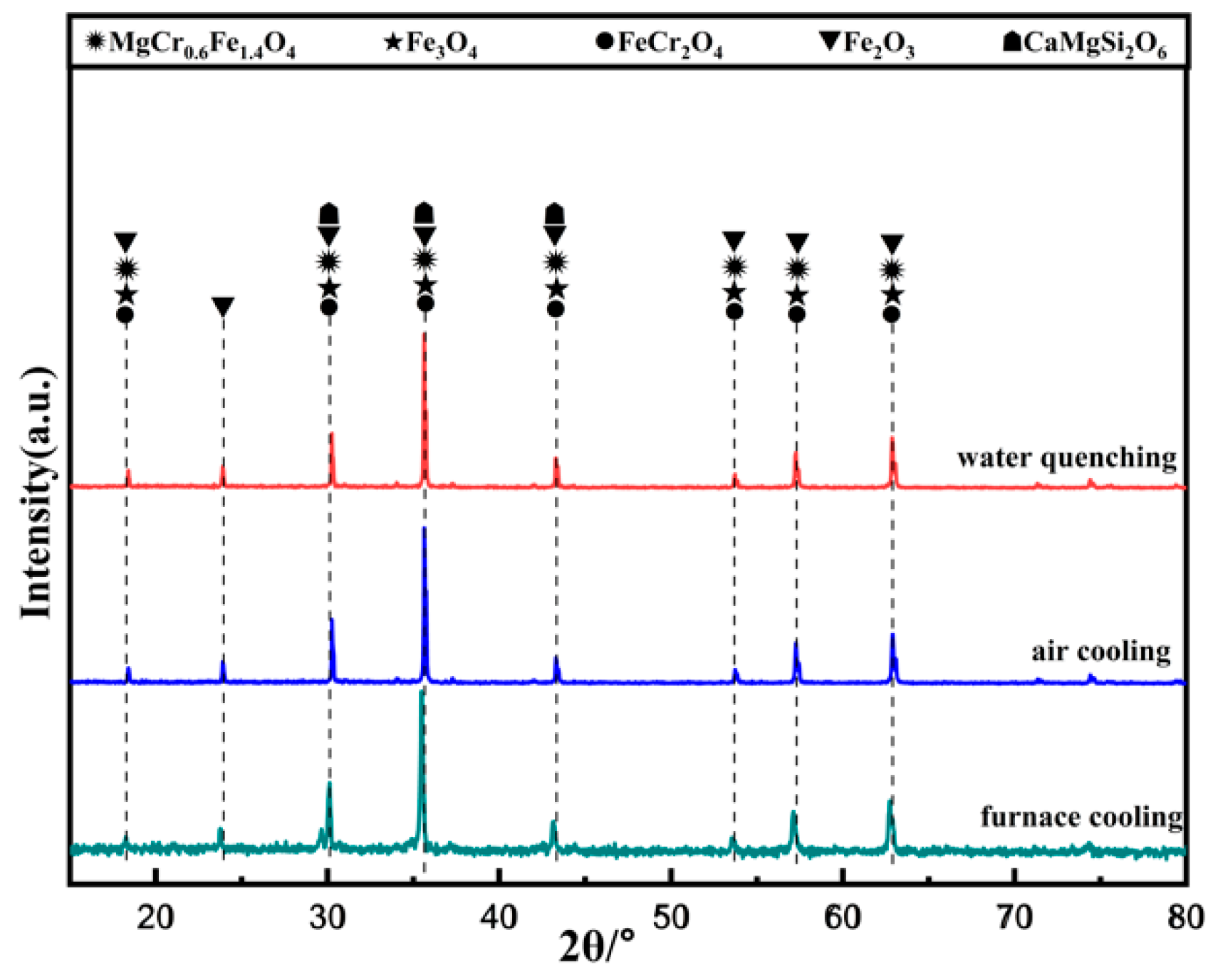
As seen in Figure 14, the main phases in the ceramic tiles are MgCr0.4Fe1.6O4, Fe3O4, FeCr2O4, Fe2O3, and CaMgSi2O6. Cooling rate has an obvious influence on the crystallinity changes of the black phase in ceramic tiles. When the cooling rate is fast, the supercooling degree of the material is large, which lead to short crystallization time, fast crystal nucleus formation, and incomplete crystal growth. Therefore, when the cooling rate is fast, the crystal particles of the black phase are small, reducing the chromaticity value of the ceramic tile [21].
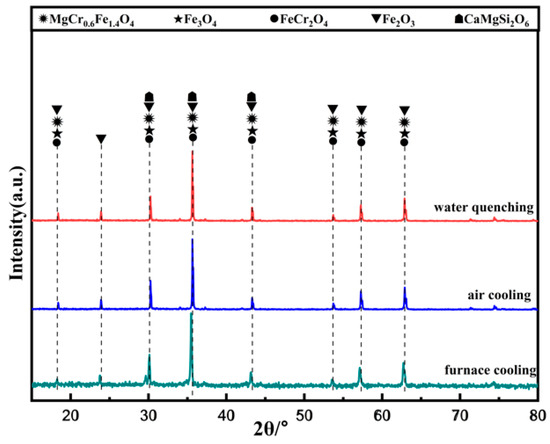
Figure 14.
XRD patterns of ceramic tiles with different cooling methods.
3.5. Effect of Sintering Atmosphere on the Chromatic Performance of Ceramic Tiles
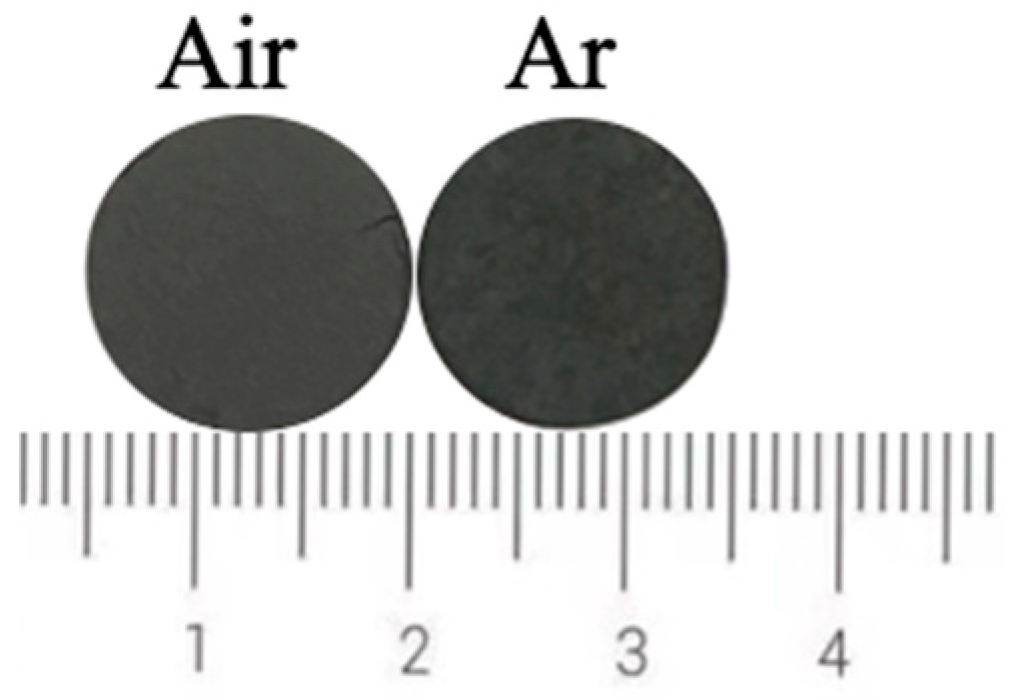
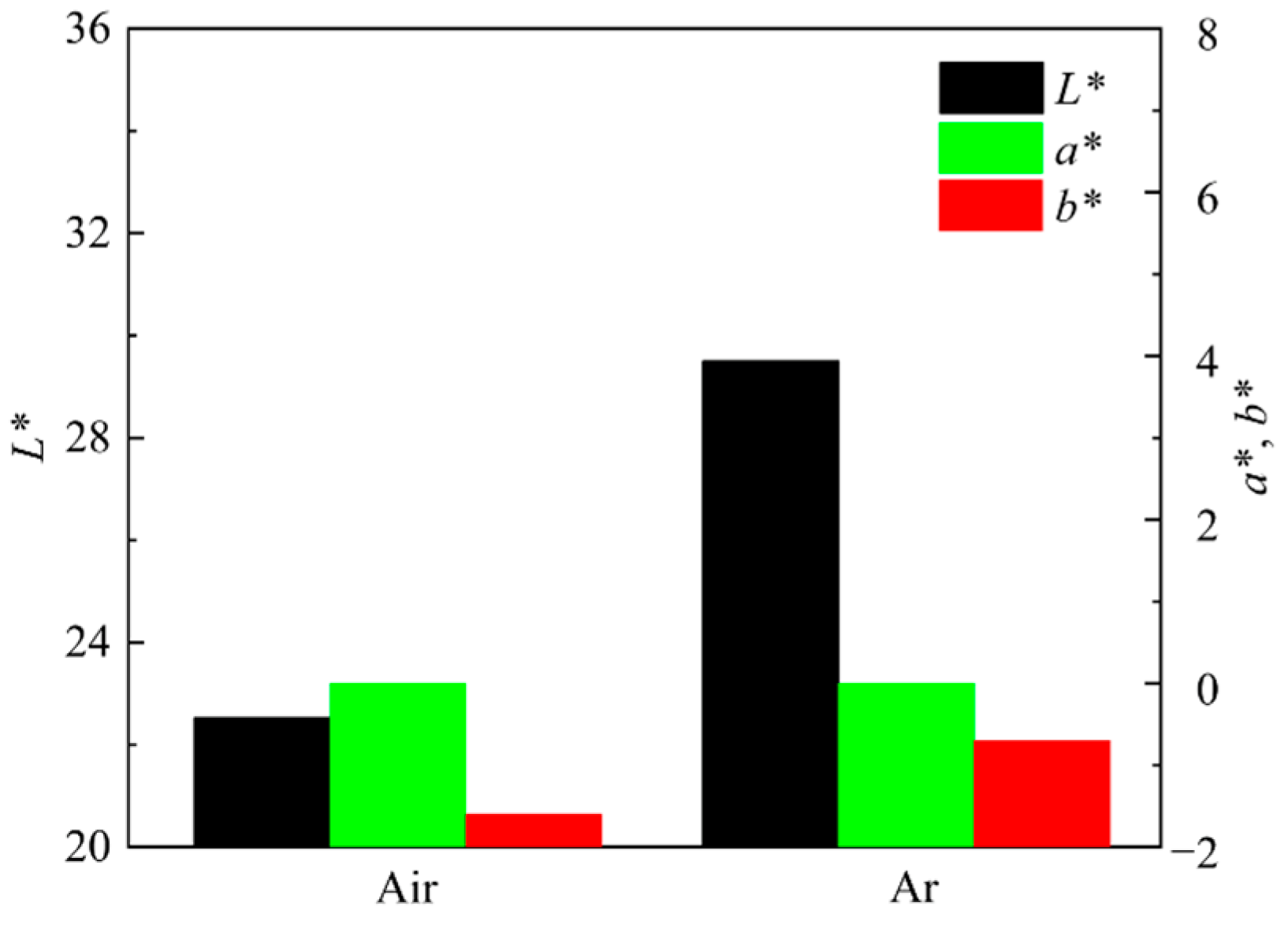
Figure 15 shows the photos of ceramic tiles with the Fe/Cr molar ration of 1.5 and sintered under the air and argon atmosphere at 1200 °C for 30min followed by furnace cooling. It can be seen from Figure 15 that there is no obvious difference between the surface of these two samples. The chromaticity values of the ceramic tiles in Figure 16 show that the L* and b* values of the ceramic tile are higher under argon atmosphere, and the a* values have ignorable difference.
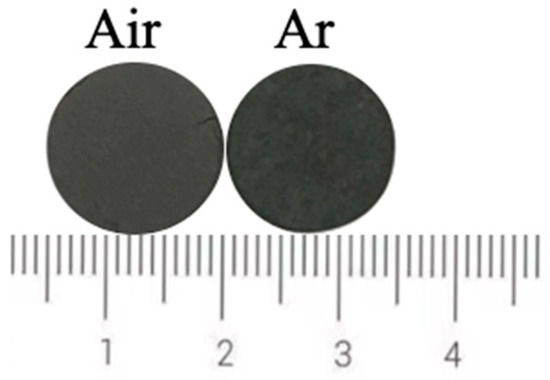
Figure 15.
Photos of ceramic tiles sintered under different atmosphere.
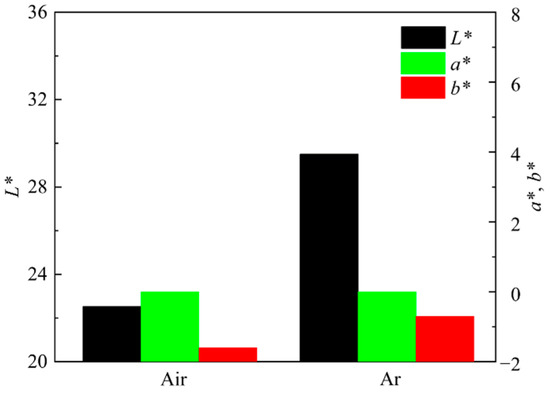
Figure 16.
Chromaticity values of ceramic tiles sintered under different atmosphere.
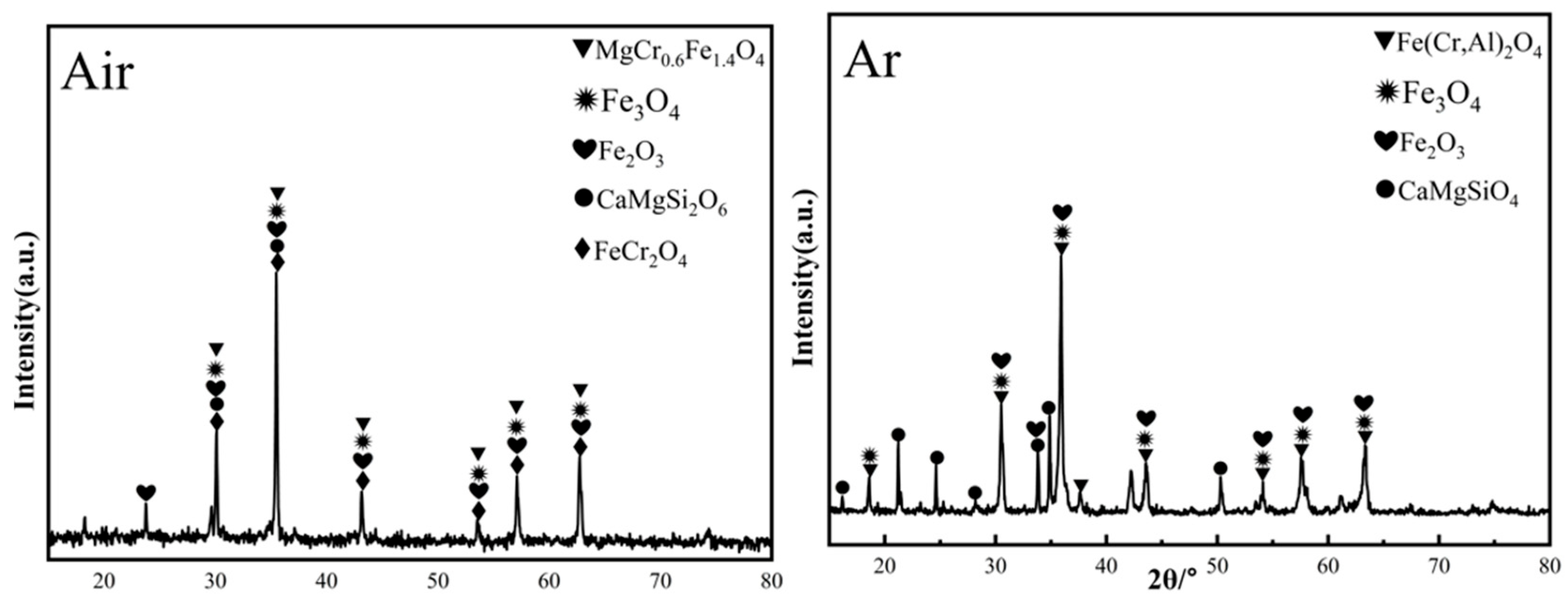
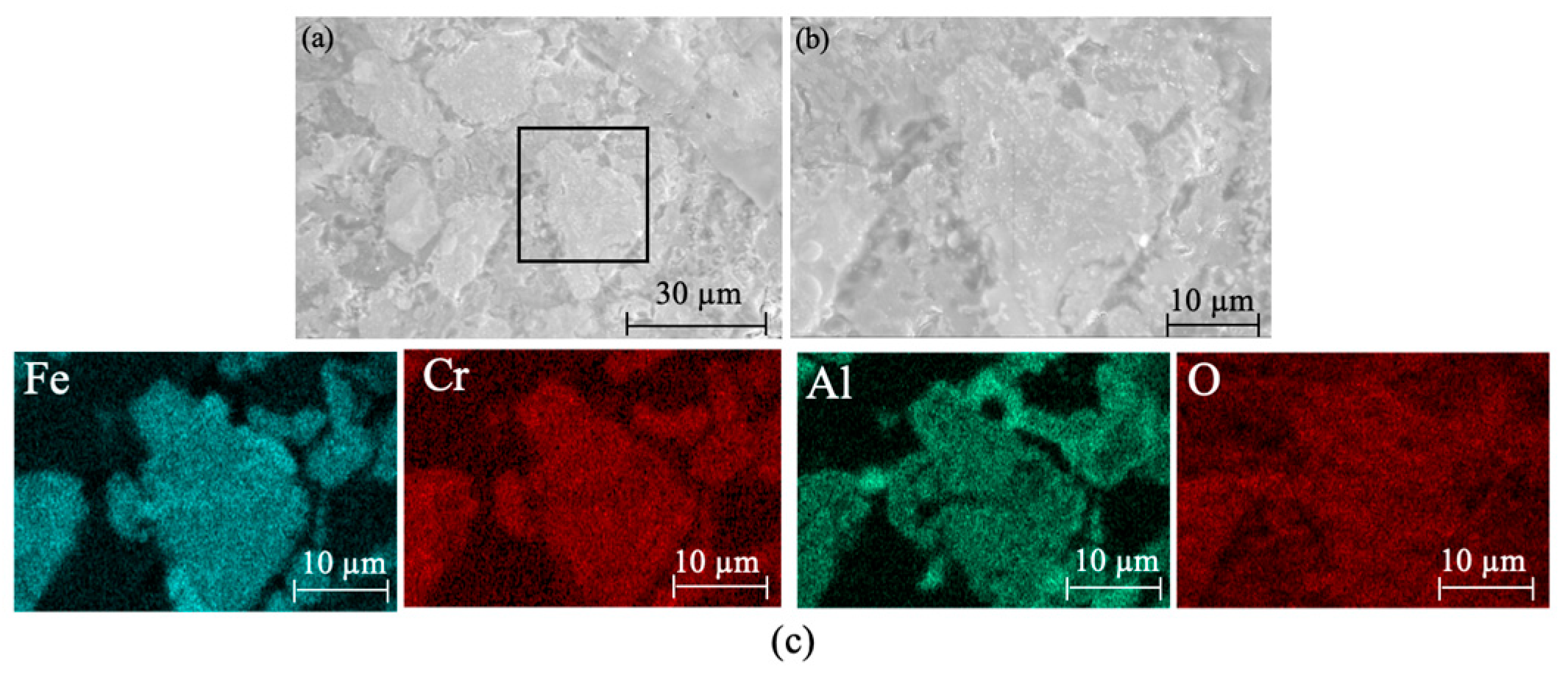
Figure 17 shows the XRD patterns of ceramic black tiles sintered under air atmosphere and argon atmospheres. The phases in the ceramic tile sintered under air atmosphere are MgCr0.4Fe1.6O4, Fe3O4, Fe(Cr,Al)2O4, Fe2O3 and CaMgSi2O6. The phases in the ceramic tile sintered under argon atmosphere are Fe(Cr,Al)2O4, Fe3O4, Fe2O3, and CaMgSiO4, including the spinel phases Fe(Cr,Al)2O4 and Fe3O4. MgCr0.4Fe1.6O4 is undetectable in the ceramic tiles. Fe(Cr,Al)2O4 was formed because FeO was more stable under argon atmosphere and it was easier to react with Cr2O3 to generate FeCr2O4. Simultaneously, the isomorphism of Al3+ in the matrix replaces Cr3+ in the spinel octahedron [22], resulting in the formation of composite spinel Fe(Cr,Al)2O4. Figure 18 presents the SEM-EDS results of ceramic title sintered under argon atmosphere. As shown in Figure 18a–c, some defects can be observed in the ceramic tiles. The element mapping results show that the phases in the ceramic tiles are enriched in the elements of Fe, Cr, Al and O, which correspond to Fe(Cr,Al)2O4 and Fe3O4.
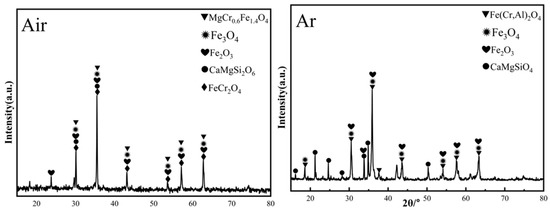
Figure 17.
XRD patterns of ceramic tiles sintered under air atmosphere and argon atmosphere.
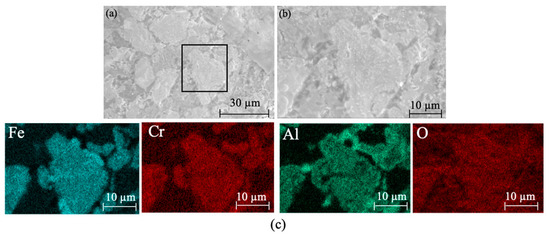
Figure 18.
SEM images and element mapping of the ceramic tiles sintered under argon atmosphere. (a) SEM image of the black ceramic tile under argon atmosphere. (b) Enlarged view of the marked area in figure (a). (c) Element mapping of figure (b).
Therefore, the optimal process parameter is as followed: Fe/Cr molar ratio is 1.5; sintering temperature is 1200 °C; holding time is 30 min; sintered under air atmosphere and cooled in the furnace.
3.6. Compression and Leaching Tests of Ceramic Tiles
The compressive strength and Cr6+ leaching concentration of the ceramic tiles produced with the optimal parameter were detected. The value of measured compressive strength is 127.27 MPa. It is much higher than 27 MPa which is the minimum value in the national standard (GB/T4100-2006) [23] for standard polished tiles. Table 4 shows that the concentrations of Cr6+ in mixed raw material and produced ceramic tiles are 16.65 mg/L and 3.31 mg/L, respectively, as well as the national standards (GB 5085.3-2007) [24] and the standards of the U.S. Environmental Protection Agency (EPA) [25] is 5 mg/L, using chromium slag and copper smelting waste slag to prepare black ceramic tiles can not only achieve harmless treatment but also increase its value.

Table 4.
Leaching concentration of Cr6+ in mixed raw materials and ceramic tile (mg/L), GB 5085.3-2007 and U.S. EPA.
4. Conclusions
- The optimum parameters of producing ceramic tile using chromium slag and copper smelting waste slag are the Fe/Cr molar ratio of 1.5, the sintering temperature of 1200 °C, holding time of 30 min and cooling in the furnace. The values of L*, a*, and b* of produced ceramic tile are 22.5, 0, and −1.6, respectively.
- Although extending holding time can enhance the reaction between the compounds, it restricts the coloring ability of Fe3+ at elevated temperatures, which deteriorates the chromatic performance of ceramic tiles.
- Increasing the cooling rate of produced ceramic tile promotes the formation of cracking and fragile in the ceramic tile and reduces the chromaticity value of the ceramic tile.
- The compressive strength of produced ceramic tile is 127.2 MPa, and the leaching concentration of Cr6+ is 3.31 mg/L, both of which meet the relevant national standards.
Author Contributions
Conceptualization, Y.H. (Yanglai Hou); methodology, J.Y.; formal analysis, J.Y. and Z.L.; investigation, Y.H. (Yuanhao Hai), J.X. and D.Z.; resources, Y.H. (YuanHao Hai), Z.L. and D.Z.; data curation, J.Y. and J.X.; writing—original draft preparation, J.Y.; writing—review and editing, Y.H. (Yanglai Hou); visualization, J.Y.; supervision, D.Z.; project administration, Y.H. (Yanglai Hou); funding acquisition, Y.H. (Yanglai Hou) All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Hubei Provincial Key Laboratory for New Processes of Ironmaking and Steelmaking (Grant No. KF-20-3) and the State Key Laboratory Youth Foundation project (Grant No. 2018QN10).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qing, P.H.; Xu, H.S.; Dong, Y.M.; Wang, X.R.; Zhu, K.S.; Niu, R.J.; Meng, J.J.; Chen, X.X.; Chen, H.X.; Zhang, H.L. Chromium extraction and detoxification of processing residue from lime-free roasting process of chromite ore. Inorg. Chem. Ind. 2020, 52, 6. [Google Scholar]
- Bai, Z.P.; Han, Y.; Xi, Z.G. Indoor Air Pollution and Prevention; Chemical Industry Press: Beijing, China, 2006. [Google Scholar]
- Liu, Z.B.; Zheng, J.Y.; Liu, W.Z.; Liu, X.M.; Chen, Y.X.; Ren, X.Q.; Ning, P.; Lin, Z. Identification of the key host phases of Cr in fresh chromite ore processing residue (COPR). Sci. Total Environ. 2020, 703, 135075. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.X.; Jiang, L.Y.; Zhang, S.; Zhang, Y.H. New calcareless roasting process of chromite and utilization of chromium residue. Chromium Salt Ind. 2006, 1, 46–50. [Google Scholar]
- Liang, H.Q.; Yu, S.H. Recycling of precious metal secondary resources. Jiangsu Metall. 1989, 000, 53–54. [Google Scholar]
- Zhang, T.A.; Niu, L.P.; Dou, Z.H.; Wang, C.; Zhang, X.H.; Zhang, Z.M.; Liu, Y.; He, Y.C.; Jiang, X.L. A Method of Smelting Antibacterial Stainless Steel Containing Copper Directly by Reducing Hot Metal with Copper Slag: CN104120351B. 2016. Available online: https://dr2am.wust.edu.cn/--/cn/com/wanfangdata/d/hs/_/patent/ChJQYXRlbnROZXdTMjAyMjEyMDcSEENOMjAxNDEwMzQ1MTk3LjIaCHp3c2s5cm44.2014-10-29 (accessed on 2 March 2023).
- Kang, J.X.; Yu, C.B.; Song, L.; Guo, S.H.; Wang, C.L.; Liu, Z.G.; Wang, X.; Wang, Y.Y. Recovery of Valuable Metals from Copper slag: CN202010198102. 2020. Available online: https://dr2am.wust.edu.cn/--/cn/com/wanfangdata/d/hs/_/patent/ChJQYXRlbnROZXdTMjAyMjEyMDcSEENOMjAyMDEwMTk4MTAyLjQaCDJjbXEyZnJh.2020-06-12 (accessed on 2 March 2023).
- Zheng, K.P.; Jiang, X.P.; Chen, C.; Li, X.H.; Liao, Y.Q. Effect of Co2O3 doping on the structure and electrical properties of (Na0.8K0.2)0.5Bi0.5TiO3 ceramics. Chin. Ceram. 2013, 49, 5-8+20. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.Q.; Ma, G.J.; Wang, Q.; Liu, M.K. Research progress of spinel-type cobalt-free black ceramic pigments. Bull. Chin. Ceram. Soc. 2021, 40, 1318–1329. [Google Scholar] [CrossRef]
- He, B.; Du, Y.; Xu, H.X.; Ma, J.C.; Cheng, C.B.; Du, M.X. Synthesis of ceramic pigments with chromium content from leather waste. T Indian Ceram Soc. 2021, 80, 103–109. [Google Scholar] [CrossRef]
- Du, M.X.; Du, Y.; Chen, Z.T.; Li, Z.F.; Yang, K.; Lv, X.J.; Feng, Y.B. Synthesis and characterization of black ceramic pigments by recycling of two hazardous wastes. Mater. Sci. Process. 2017, 123, 568–575. [Google Scholar] [CrossRef]
- Yang, Y.G.; Xu, J.H.; Cai, B.; Wang, Q.C.; Xiu, D.P.; Zhao, Z.B.; Sun, Q.Z.; Cao, S.L. Synthesis and applications of black ceramic from recycled industrial wastes. Adv. Appl. Ceram. 2013, 112, 146–148. [Google Scholar] [CrossRef]
- Zhang, L.F.; Kang, Z.S.; Liu, Z.K.; Yan, K.; Li, S.; Lian, Y.C.; Cao, R.X. Aluminum Based Solid Waste Porous Material and Its Preparation Method: CN114105611A. 2022. Available online: https://dr2am.wust.edu.cn/--/cn/com/wanfangdata/d/hs/_/patent/ChJQYXRlbnROZXdTMjAyMjEyMDcSEENOMjAyMTExNTQ5NDkwLjcaCDdxaWUxeTRx.2022-03-01 (accessed on 2 March 2023).
- Ma, L. Inverstigation of Fabrication, Microstructure and Hot Corrosion Properties of Ni-Fe Spinel Composite Coating; Central South University: Changsha, China, 2012. [Google Scholar]
- HJ/T299-2007; Solid Waste-Extraction Procedure for Leaching Toxicity-Sulphuric Acid Nitric Acid Method. China Environmental Press: Beijing, China, 2007.
- Chang, G.Y. Preparation of Several Chromium Containing Spinel Catalysts and Their Methane Combustion Performance; Inner Mongolia University: Hohhot, China, 2006. [Google Scholar]
- Li, Z.Q.; Zhang, X.; Ma, G.J.; Zheng, D.L.; Du, T.Y.; He, R.X. Effect of the nickel molar content on the preparation and properties of spinel-type black ceramic pigment by microwave processing from stainless steelmaking dust. Mater. Today Commun. 2022, 32, 104–151. [Google Scholar] [CrossRef]
- Gorai, B.; Jana, R.K.; Premchand. Characteristics and utilisation of copper slag—A review. Resour. Conserv. Recycl. 2003, 39, 299–313. [Google Scholar] [CrossRef]
- Fan, Y.; Shibata, E.; Iizuka, A.; Nakamura, T. Crystallization behavior of copper smelter slag during molten oxidation. Metall. Mater. Trans. B 2015, 46, 2158–2164. [Google Scholar] [CrossRef]
- Li, Z.Q.; Zhang, X.; Ma, G.J.; Liu, M.K.; Wang, Q. Preparation and coloring performance analysis of Fe-Cr-Ni-Mn system black ceramic pigment. Bull. Chin. Ceram. Soc. 2021, 40, 4092–4101. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Zhang, J.S. Crystallization behaviors of molten ash slag under different temperatures and colling rates. J. Chem. Ind. Eng. 2018, 69, 8. [Google Scholar] [CrossRef]
- Ye, D. Mineral Gemmological Characteristics and Heat Treatment of Ruby from Yuanjiang, Yunnan; Kunming University of Science and Technology: Kunming, China, 2007. [Google Scholar]
- GB/T4100-2006; Ceramic Tiles. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2006.
- GB 5085.3-2007; Identification Standards for Hazardous Wastes-Identification for Extraction Toxicity. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2007.
- Laforest, G.; Duchesne, J. Characterization and leachability of electric arc furnace dust made from remelting of stainless steel. J. Hazard. Mater. 2006, 135, 156–164. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).