Effect of Standing Time after Mixing on the Mixture Microstructure of an Al-Si Alloy during Controlled Diffusion Solidification with Simultaneous Mixing
Abstract
:1. Introduction
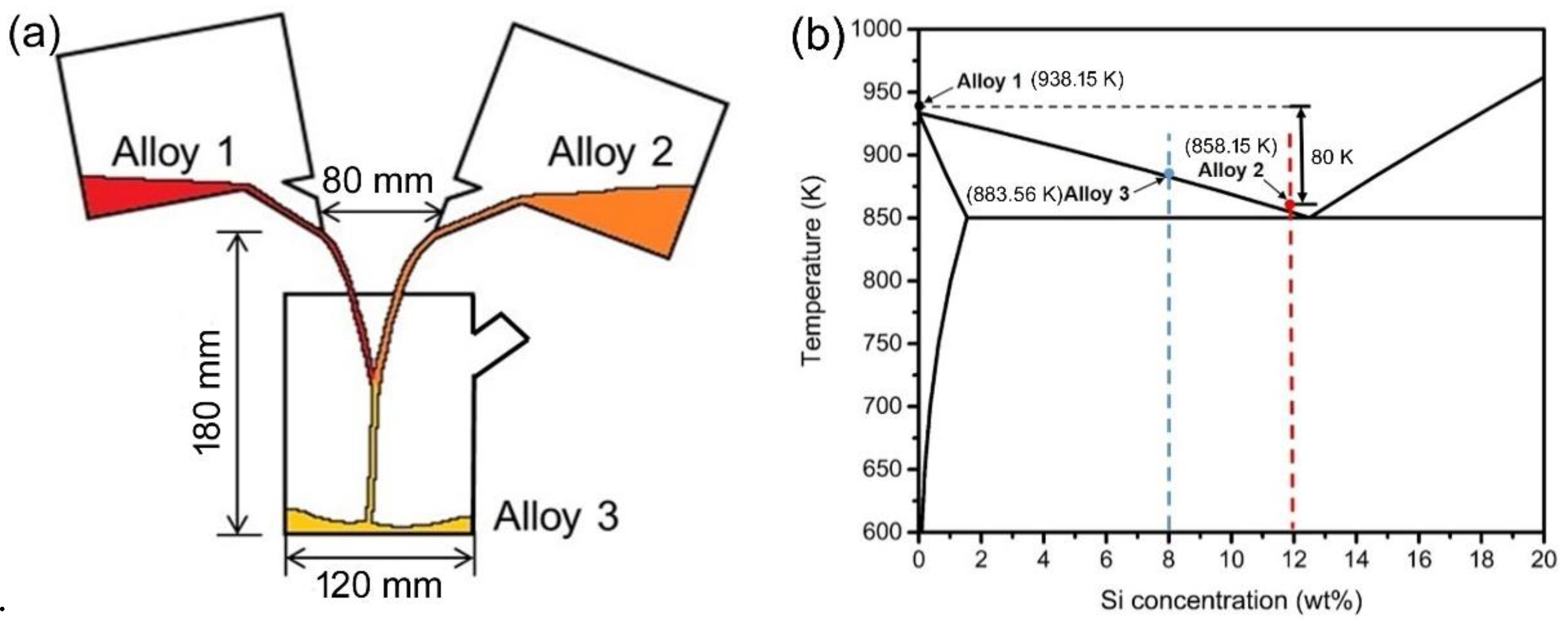
2. Investigation Process
2.1. Simulation and Calculation Processes
2.2. Experimental Process
3. Results and Discussion
3.1. Simulation
3.1.1. Effect on Concentration and Temperature Fields
3.1.2. Effect on Nucleus Number
3.1.3. Effect on Growth Mode and Resultant Morphology of Primary Grains
3.2. Experiment
4. Conclusions
- Air can be entrapped in the mixture along the two precursor melt streams, but it floats upward and overflows from the mixture in the form of bubbles within 1.2 s of standing.
- The blending action still effectively occurs during standing, especially during the initial stage (i.e., 0–0.5 s), due to the extensive convection induced by the inertial force that originates during mixing, which leads to the significant homogenization of the concentration field and, in particular, the temperature field due to the thermal conductivity being much higher than the solute diffusivity.
- It is due to the thermal conductivity being much higher than the solute diffusivity that the melt in the pure Al pockets close to the interface of the pure Al/Al-12Si melts is first rapidly chilled to a supercooling state by the surrounding low-temperature Al-12Si melt, which then causes nuclei to form in this pure Al melt promptly.
- Some nuclei remelt, and the others grow only towards the pure Al melt side in a stable solid/liquid interface during standing, increasing the size of nondendritic grains but decreasing their number. However, all the grown nondendritic grains will finally remelt again due to the superheat characteristics of the mixture.
- The experiment results, such as the change tendencies of the grain morphology, size and number, are consistent with those from the simulation and calculation, implying that the employed simulation and calculation and the achieved results are reasonable and reliable. The mixture should be poured within 10 s after mixing in order to avoid the obvious remelting of grains.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Apelian, D.; Makhlouf, M.M.; Saha, D. CDS Method for Casting Aluminium-Based Wrought Alloy Compositions: Theoretical Framework. Mater. Sci. Forum. 2006, 519–521, 1771–1776. [Google Scholar] [CrossRef]
- Kumar, G.; Hegde, S.; Prabhu, K.N. Heat transfer and solidification behaviour of modified A357 alloy. J. Mater. Process. Technol. 2007, 182, 152–156. [Google Scholar] [CrossRef]
- Suh, D.W.; Lee, S.Y.; Lee, K.H.; Lim, S.K.; Oh, K.H. Microstructural evolution of Al–Zn–Mg–Cu–(Sc) alloy during hot extrusion and heat treatments. J. Mater. Process. Technol. 2004, 1330, 155–156. [Google Scholar] [CrossRef]
- Saha, D.; Shankar, S.; Apelian, D.; Makhlouf, M.M. Casting of aluminum-based wrought alloys using controlled diffusion solidification. Met. Mater. Trans. A 2004, 35, 2174. [Google Scholar] [CrossRef]
- Ghiaasiaan, R.; Amirkhiz, B.S.; Shankar, S. Quantitative metallography of precipitating and secondary phases after strengthening treatment of net shaped casting of Al-Zn-Mg-Cu (7000) alloys. Mater. Sci. Eng. A 2017, 698, 206. [Google Scholar] [CrossRef]
- Chen, T.J.; Yang, X.K.; Xue, H.; Bi, G.L.; Zhang, X.Z.; Guan, R.G. Mixing process and nucleation of an Al-Si alloy during controlled diffusion solidification with simultaneous mixing and effect of mixing rate. J. Mater. Sci. 2022, 57, 3018. [Google Scholar] [CrossRef]
- Haga, T.; Nakamura, R.; Tago, R.; Watari, H. Effects of casting factors of cooling slope on semisolid condition. Trans. Nonferrous Met. Soc. China 2010, 20, 968. [Google Scholar] [CrossRef]
- Zhang, Z.-X.; Chen, T.-J.; Liu, K.; Xue, H.; Qi, J.-C.; Bi, G.-L.; Ma, Y. Efficient fabrication of semisolid nondendritic Al alloy slurry with high quality. China Foundry 2022, 19, 117–130. [Google Scholar] [CrossRef]
- Birol, Y. A357 thixoforming feedstock produced by cooling slope casting. J. Mater. Process. Technol. 2007, 186, 94–101. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.-D.; Luo, X.-M.; Zhou, H.-W.; Cai, Q.-Y.; Li, M.; Ma, Y. Microstructural evaluation and mechanical properties of 7075 aluminum alloy prepared by controlled diffusion solidification. China Foundry 2019, 16, 238–247. [Google Scholar] [CrossRef]
- Ghiaasiaan, R.; Zeng, X.; Shankar, S. Controlled Diffusion Solidification (CDS) of Al-Zn-Mg-Cu (7050): Microstructure, heat treatment and mechanical properties. Mater. Sci. Eng. A 2014, 594, 260–277. [Google Scholar] [CrossRef]
- Cini, E.; Vinet, B.; Desré, P.J. A thermodynamic approach to homogeneous nucleation via fluctuations of concentration in binary liquid alloys. Philos. Mag. A 2009, 80, 955–966. [Google Scholar] [CrossRef]
- Ghiaasiaan, R.; Shankar, S. Microstructure, Intermetallic Phases, and Fractography of the Cast Al-5.8Zn-2.2Mg-2.5Cu Alloy by Controlled Diffusion Solidification. Met. Mater. Trans. A 2020, 51, 4711–4726. [Google Scholar] [CrossRef]
- Khalaf, A.A. Controlled Diffusion Solidification: Process Mechanism and Parameter Study. Ph.D. Thesis, McMaster University, Hamilton, ON, Canada, 2010. [Google Scholar]
- Birsan, G.; Ashtari, P.; Shankar, S. Valid mould and process design to cast tensile and fatigue test bars in tilt pour casting process. Int. J. Cast Met. Res. 2013, 24, 284–378. [Google Scholar] [CrossRef]
- Pourgharibshahi, M.; Saghafian, H.; Divandari, M.; Timelli, G. Controlled Diffusion Solidification Pathway of an AA 7xxx Series Aluminum Alloy. Met. Mater. Trans. A 2018, 50, 326–335. [Google Scholar] [CrossRef]
- Khalaf, A.A.; Ashtari, P.; Shankar, S. Formation of Nondendritic Primary Aluminum Phase in Hypoeutectic Alloys in Controlled Diffusion Solidification (CDS): A Hypothesis. Met. Mater. Trans. B 2009, 40, 843–849. [Google Scholar] [CrossRef]
- Khalaf, A.A.; Shankar, S. Mechanism of Anomalous Grain Formation during Controlled Diffusion Solidification. JOM 2020, 72, 3733–3743. [Google Scholar] [CrossRef]
- Khalaf, A.A.; Shankar, S. Effect of mixing rate on the morphology of primary Al phase in the controlled diffusion solidification (CDS) process. J. Mater. Sci. 2012, 47, 8153–8166. [Google Scholar] [CrossRef]
- Ghiaasiaan, R. Controlled Diffusion Solidification Process (CDS) of Al-7xxx Wrought Alloys: Heat Treatments, Microstructures and Mechanical Properties. Ph.D. Thesis, McMaster University, Hamilton, ON, Canada, 2015. [Google Scholar]
- Li, Y.D.; Zang, X.L.; Ma, Y.; Apelian, D.; Zhou, H.W. Effect of mixing rate and temperature on primary Si phase of hypereutectic Al-20Si alloy during controlled diffusion solidification (CDS) process. China Foundry 2015, 12, 173. [Google Scholar]
- Khalaf, A.A. Mechanism of controlled diffusion solidification: Mixing, nucleation and growth. Acta Mater. 2016, 103, 301–310. [Google Scholar] [CrossRef]
- Kund, N.K.; Dutta, P. Numerical simulation of solidification of liquid aluminum alloy flowing on cooling slope. Trans. Indian Inst. Met. 2012, 20, s898–s905. [Google Scholar] [CrossRef]
- Ji, S.; Fan, Z. Solidification Behavior of Sn-15 Wt Pct Pb Alloy under a High Shear Rate and High Intensity of Turbulence during Semisolid Processing. Metall. Mater. Tran. A Phys. Metall. Mater. Sci. 2002, 33A, 3511–3520. [Google Scholar] [CrossRef]
- Gumulya, M.; Utikar, R.; Evans, G.; Joshi, J.; Pareek, V. Interaction of bubbles rising inline in quiescent liquid. Chem. Eng. Sci. 2017, 166, 1–10. [Google Scholar] [CrossRef]
- Khalaf, A.A. Microstructure evolution of Al-Si hypoeutectic alloys prepared by controlled diffusion solidification. Int. J. Adv. Manuf. Technol. 2022, 120, 5003–5014. [Google Scholar] [CrossRef]
- Khalaf, A.A.; Shankar, S. Favorable Environment for a Nondendritic Morphology in Controlled Diffusion Solidification. Metall. Mater. Trans. A 2011, 42, 2456–2465. [Google Scholar] [CrossRef]
- Das, A.; Fan, Z. Morphological development of solidification structures under forced fluid flow: Experimental observation. Mater. Sci. Technol. 2003, 19, 573–580. [Google Scholar] [CrossRef]
- Hariharan, A.; Lu, L.; Risse, J.; Kostka, A.; Gault, B.; Jägle, E.A.; Raabe, D. Misorientation-dependent solute enrichment at interfaces and its contribution to defect formation mechanisms during laser additive manufacturing of superalloys. Phys. Rev. Mater. 2019, 3, 123602. [Google Scholar] [CrossRef]
- Kontis, P.; Chauvet, E.; Peng, Z.; He, J.; da Silva, A.K.; Raabe, D.; Tassin, C.; Blandin, J.-J.; Abed, S.; Dendievel, R.; et al. Atomic-scale grain boundary engineering to overcome hot-cracking in additively-manufactured superalloys. Acta Mater. 2019, 177, 209–221. [Google Scholar] [CrossRef]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, T. Effect of Standing Time after Mixing on the Mixture Microstructure of an Al-Si Alloy during Controlled Diffusion Solidification with Simultaneous Mixing. Metals 2023, 13, 733. https://doi.org/10.3390/met13040733
Liu Y, Chen T. Effect of Standing Time after Mixing on the Mixture Microstructure of an Al-Si Alloy during Controlled Diffusion Solidification with Simultaneous Mixing. Metals. 2023; 13(4):733. https://doi.org/10.3390/met13040733
Chicago/Turabian StyleLiu, Yanghua, and Tijun Chen. 2023. "Effect of Standing Time after Mixing on the Mixture Microstructure of an Al-Si Alloy during Controlled Diffusion Solidification with Simultaneous Mixing" Metals 13, no. 4: 733. https://doi.org/10.3390/met13040733




