Effect of the Deposition Process on High-Temperature Microstructure and Properties of the Direct Energy Deposition Al-Cu Alloy
Abstract
1. Introduction
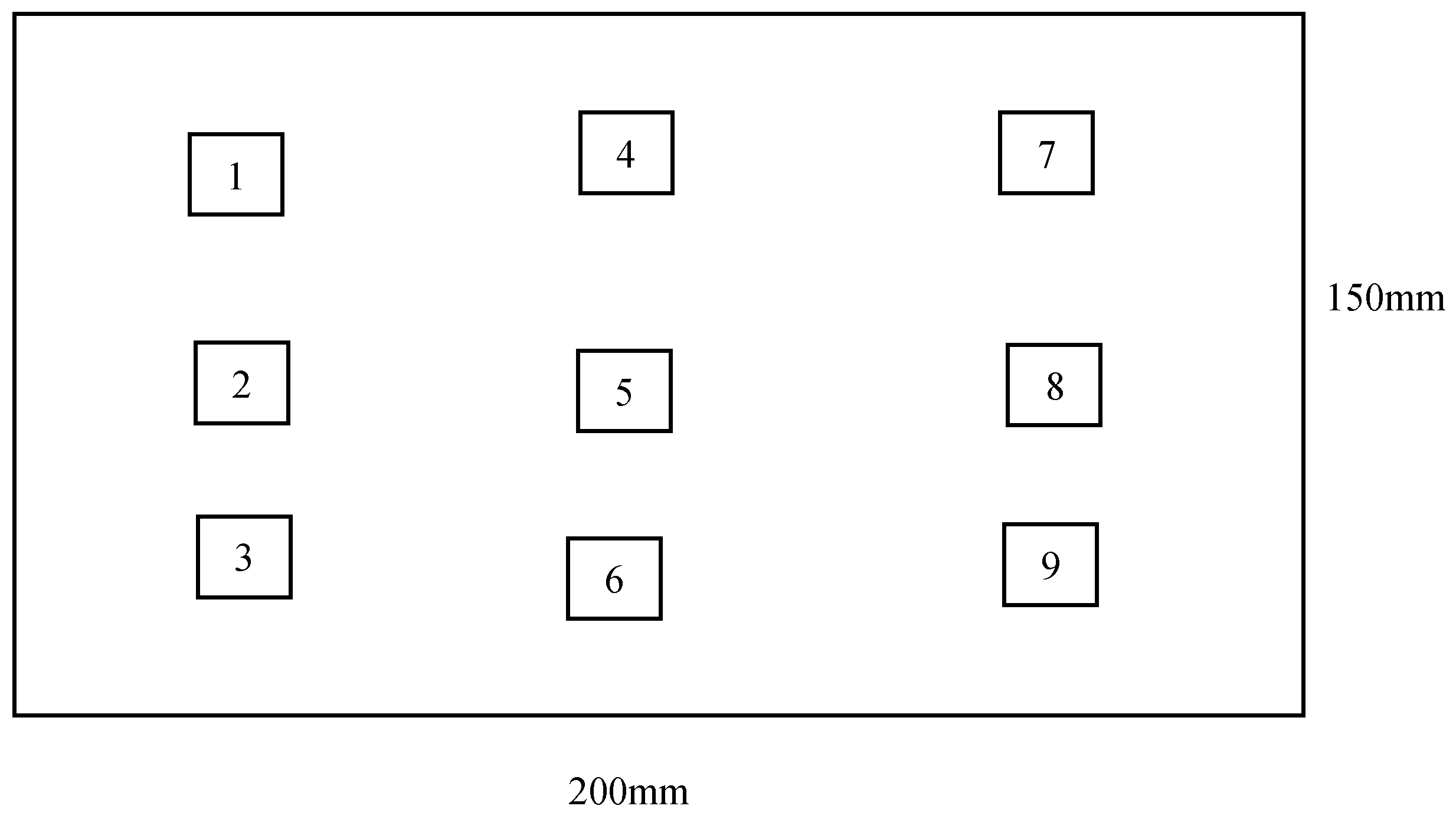
2. Materials and Methods
3. Results and Discussion
3.1. Chemical Composition
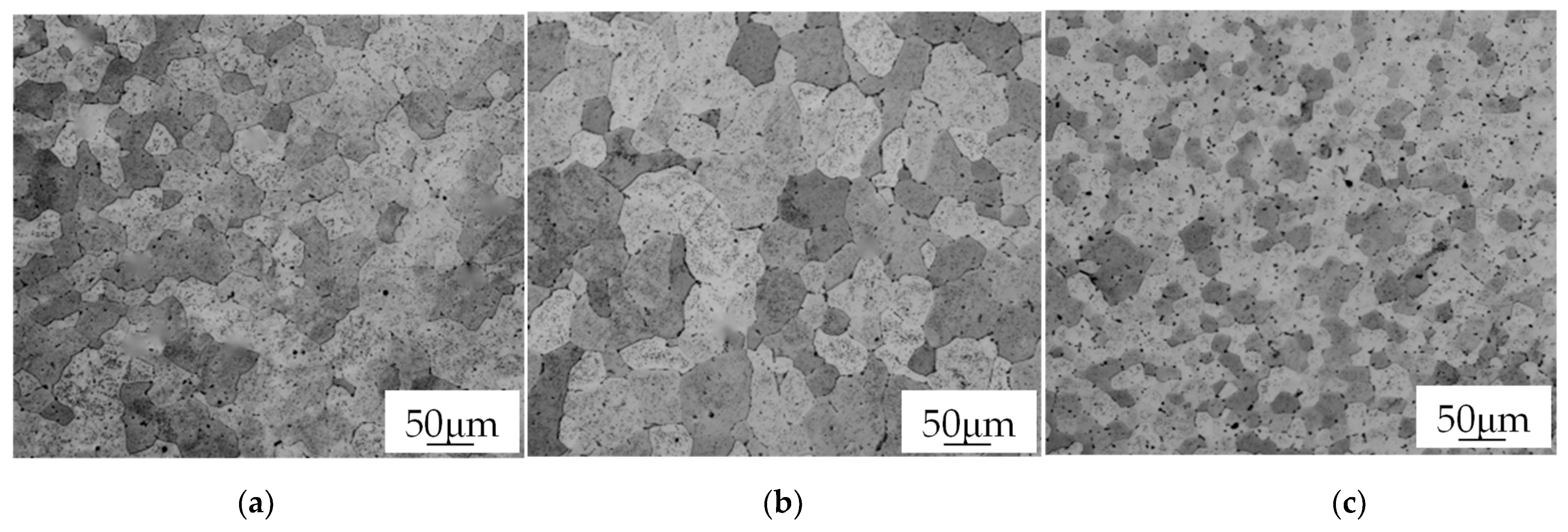
3.2. Grain Size
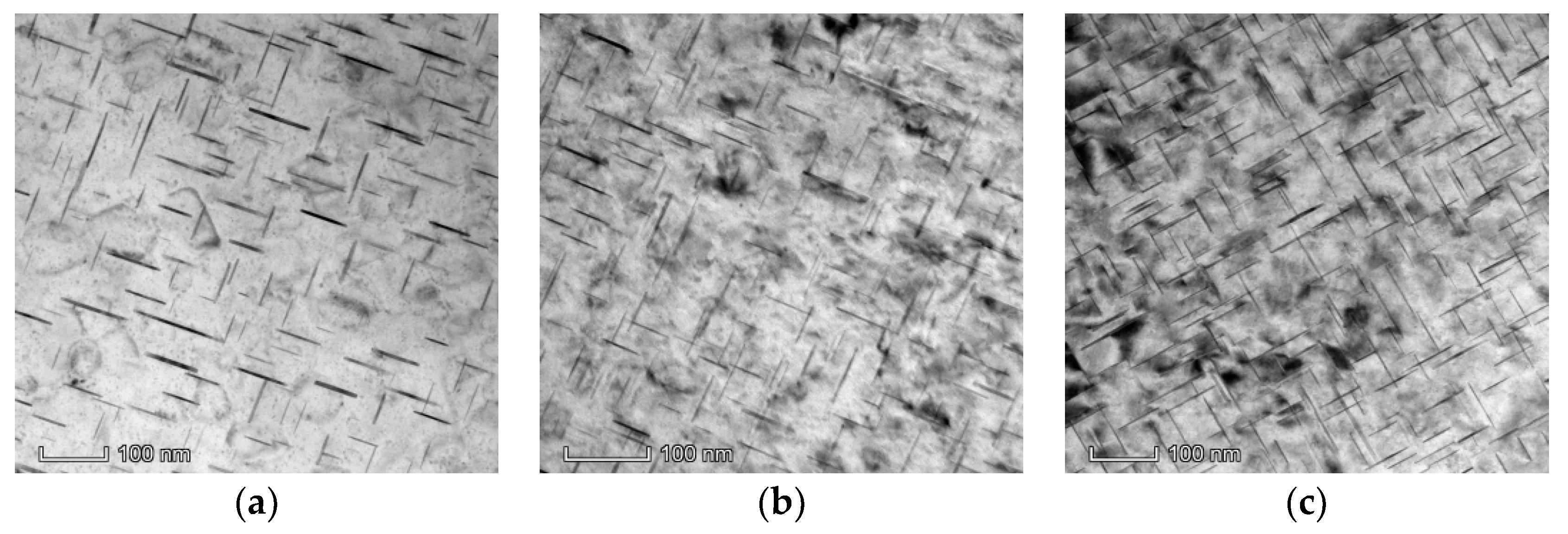
3.3. Precipitated Phases
3.4. Strengthening Phases
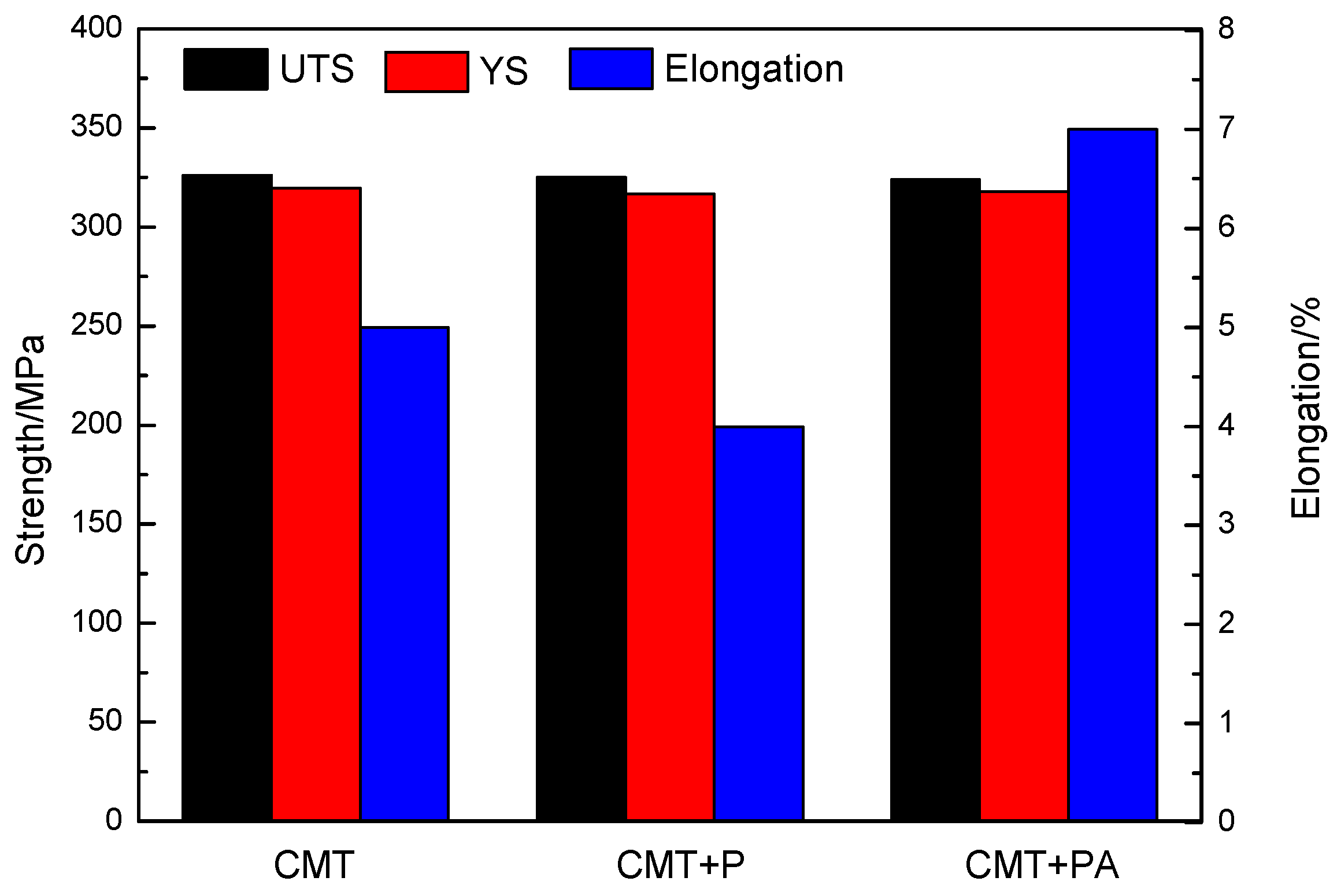
3.5. Mechanical Properties
3.6. Fracture Morphology
4. Conclusions
- The grain size of ZCL205A alloy deposits manufactured using different processes at high temperatures is quite different, and the order for grain size is CMT Pulse and Advanced process < CMT process < CMT Pulse process.
- The order for the number and size of the undissolved θ phases in the ZCL205A alloy deposits manufactured using different Direct Energy Deposition processes at high temperatures is CMT Pulse and Advanced process < CMT process < CMT Pulse process.
- After the high-temperature process, the transition θ′ phase transforms into the equilibrium θ phase, and the order for the number of equilibrium θ phase is CMT Pulse process < CMT process < CMT Pulse and Advanced process.
- The tensile strength and yield strength of the ZCL205A alloy deposits prepared using different processes are consistent at the high temperature, and the elongation varies with the deposition processes (CMT Pulse process < CMT process < CMT Pulse and Advanced process), and the deposition process can be selected according to the part’s structure.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wojciechowski, S. New trends in the development of mechanical engineering materials. J. Mater. Process. Technol. 2000, 106, 230–235. [Google Scholar] [CrossRef]
- Warner, T. Recently-developed aluminum solutions for aerospace applications. Mater. Sci. 2006, 519, 1271–1278. [Google Scholar]
- Heinz, A.; Haszler, A.; Keidel, C.; Moldenhauer, S.; Benedictus, R.; Miller, W.S. Recent development in aluminum alloys for aerospace applications. Mater. Sci. Eng. 2000, 280, 102–107. [Google Scholar] [CrossRef]
- Williams, S.W.; Martina, F.; Addison, A.C.; Ding, J.; Pardal, G.; Colegrove, P. Wire + Arc Additive Manufacturing. Mater. Sci. Technol. 2016, 32, 641–647. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Q.; Wang, K.; Peng, Y.; Ding, J.; Kong, J.; Williams, S. Study on microstructure and tensile properties of high nitrogen Cr-Mn steel processed by CMT wire and arc additive manufacturing. Mater. Des. 2019, 166, 107611. [Google Scholar] [CrossRef]
- Lin, J.J.; Lv, Y.H.; Liu, Y.X.; Xu, B.S.; Sun, Z.; Li, Z.G.; Wu, Y.X. Microstructural evolution and mechanical properties of Ti-6Al-4V wall deposited by pulsed plasma arc additive manufacturing. Mater. Des. 2016, 102, 30–40. [Google Scholar] [CrossRef]
- Meza, E.S.; Bertelli, F.; Goulart, P.R.; Cheung, N.; Garcia, A. The effect of the growth rate on microsegregation: Experimental investigation in hypoeutectic Al–Fe and Al–Cu alloys directionally solidified. Alloy Compd. 2013, 561, 193–200. [Google Scholar] [CrossRef]
- Suyitno, D.G.; Eskin, L.K. Structure observations related to hot tearing of Al-Cu billets produced by direct-chill casting. Mater. Sci. Eng. A 2006, 420, 1–7. [Google Scholar] [CrossRef]
- Li, B.; Shen, Y.; Hu, W. Casting defects induced fatigue damage in aircraft frames of ZL205A aluminum alloy—A failure analysis. Mater. Des. 2011, 32, 2570. [Google Scholar] [CrossRef]
- Rao, S.R.K.; Reddy, G.M.; Rao, K.S.; Kamaraj, M.; Rao, K.P. Reasons for superior mechanical and corrosion properties of 2219 aluminum alloy electron beam welds. Mater. Charact. 2005, 55, 345. [Google Scholar]
- Zhou, Y.; Qi, G. Microstructure and mechanical properties of Ti-6Al-4V deposited by wire and arc additive manufacturing with different deposition paths. Mater. Sci. Eng. A. 2019, 9, 138654. [Google Scholar] [CrossRef]
- Gu, J.L. Study on Microstructure and Mechanical Properties of Additively Manufactured Al-Cu-(Mg) Alloys with the CMT Process; Northeastern University: Shenyang, China, 2016. [Google Scholar]
- Wang, S. Mechanical Property and Microstructure of WAAM High Strength Al-Cu Alloy; Northeastern University: Shenyang, China, 2019. [Google Scholar]
- Cook, G.E.; Eassa, E.H. The Effect of High-Frequency Pulsing of a Welding Arc. IEEE Trans. Ind. Appl. 1985, 1, 1294–1299. [Google Scholar] [CrossRef]
- Cong, B.; Ouyang, R.; Qi, B.; Ding, J. Influence of Cold Metal Transfer Process and Its Heat Input on Weld Bead Geometry and Porosity of Aluminum-Copper Alloy Welds. Rare Met. Mater. Eng. 2016, 45, 606–611. [Google Scholar] [CrossRef]
- Lin, R.Y.; Fan, J. Solubility of hydrogen in aluminum alloy melt. Light Alloy Fabr. Technol. 1991, 4, 17–20. [Google Scholar]
- Song, W. Metallurgy; University of Science and Technology Beijing, Metallurgical Industry Press: Beijing, China, 2008; pp. 85–87. [Google Scholar]
- Wang, F.X.; Luo, L.S.; Wang, L.; Zhang, D.H.; Li, X.Z.; Su, Y.Q.; Guo, J.J.; Fu, Z.J. Effect of alloy composition and cooling rate on the growth morphology of primary Al2Cu phase in Al-Cu alloy during solidification. Acta Metall. Sincia 2016, 3, 361–368. [Google Scholar]







| Alloy | Cu | Mn | Ti | Cd | Zr | B | V | Al |
|---|---|---|---|---|---|---|---|---|
| Content/% | 5.15 | 0.42 | 0.28 | 0.22 | 0.16 | 0.03 | 0.12 | Rem |
| Process | I [A] | U [V] | Vwfs [m·min−1] | Vts [m·min−1] | Cooling Time [s] | Gas Flow [mL·min−1] |
|---|---|---|---|---|---|---|
| CMT | 144 | 16.4 | 6.5 | 8 | 60 | 25 |
| CMT Pules | 135 | 18.2 | 6.5 | 8 | 60 | 25 |
| CMT Pules Advanced | 98 | 11.2 | 6.5 | 8 | 60 | 25 |
| Processes | Elements | Cu | Mn | Ti | Cd | Zr | B | V | Al |
|---|---|---|---|---|---|---|---|---|---|
| CMT | Burn out rate/% | 5.2 | 4.9 | 10.7 | 54.6 | 3.8 | 61.8 | 0 | Rem |
| CMT + P | 5.3 | 4.9 | 11.2 | 55.3 | 3.8 | 63.2 | 0 | Rem | |
| CMT + P + A | 5.2 | 4.7 | 9.3 | 50.2 | 3.6 | 59.8 | 0 | Rem |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, S.; Ren, L.; Li, C.; Wang, W.; Ming, Z.; Zhai, Y. Effect of the Deposition Process on High-Temperature Microstructure and Properties of the Direct Energy Deposition Al-Cu Alloy. Metals 2023, 13, 765. https://doi.org/10.3390/met13040765
Wang Z, Wang S, Ren L, Li C, Wang W, Ming Z, Zhai Y. Effect of the Deposition Process on High-Temperature Microstructure and Properties of the Direct Energy Deposition Al-Cu Alloy. Metals. 2023; 13(4):765. https://doi.org/10.3390/met13040765
Chicago/Turabian StyleWang, Zhenbiao, Shuai Wang, Lingling Ren, Chengde Li, Wei Wang, Zhu Ming, and Yuchun Zhai. 2023. "Effect of the Deposition Process on High-Temperature Microstructure and Properties of the Direct Energy Deposition Al-Cu Alloy" Metals 13, no. 4: 765. https://doi.org/10.3390/met13040765
APA StyleWang, Z., Wang, S., Ren, L., Li, C., Wang, W., Ming, Z., & Zhai, Y. (2023). Effect of the Deposition Process on High-Temperature Microstructure and Properties of the Direct Energy Deposition Al-Cu Alloy. Metals, 13(4), 765. https://doi.org/10.3390/met13040765





