Determination of Corrosion Resistance of High-Silicon Ductile Iron Alloyed with Nb
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Chemical Composition
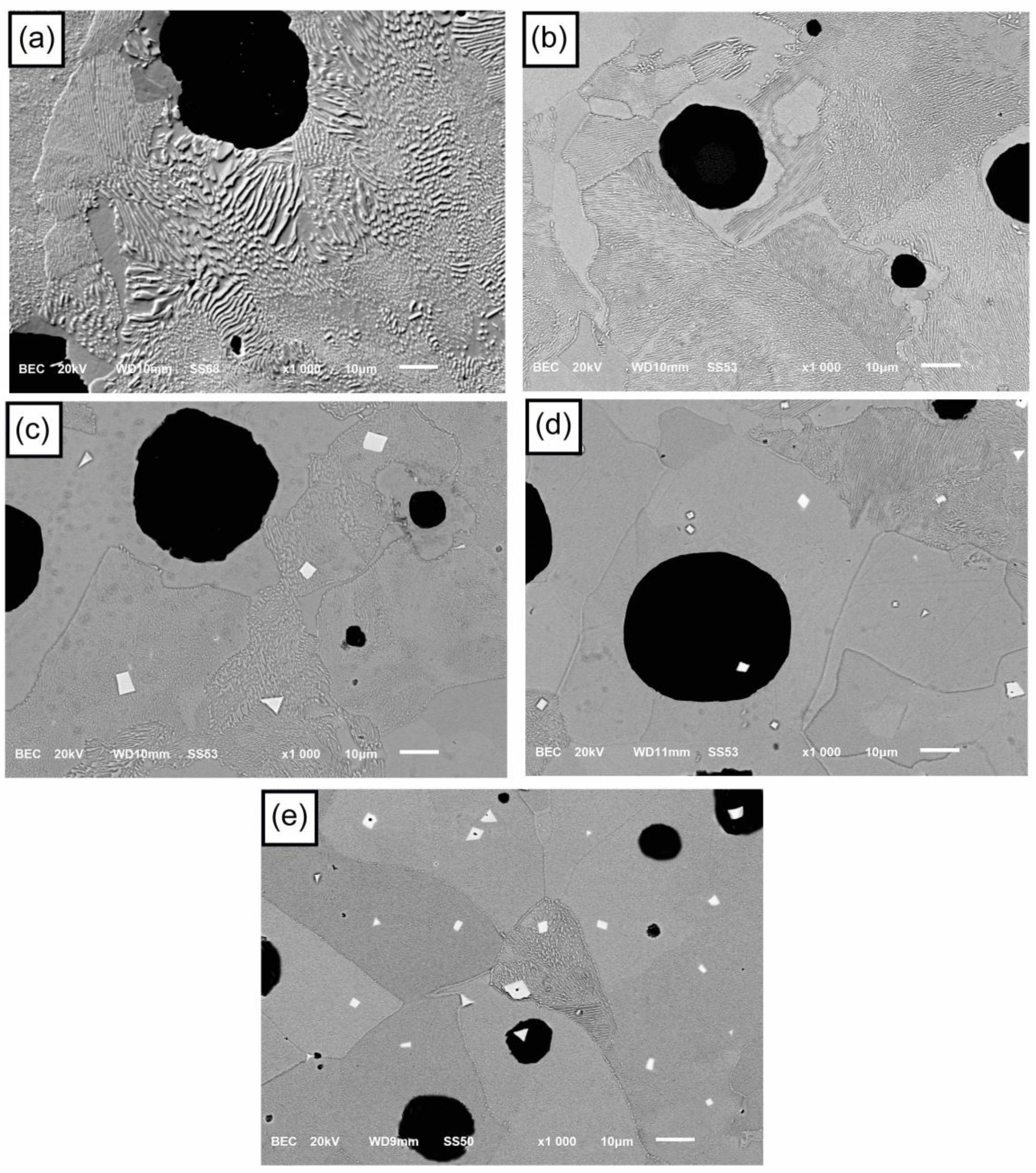
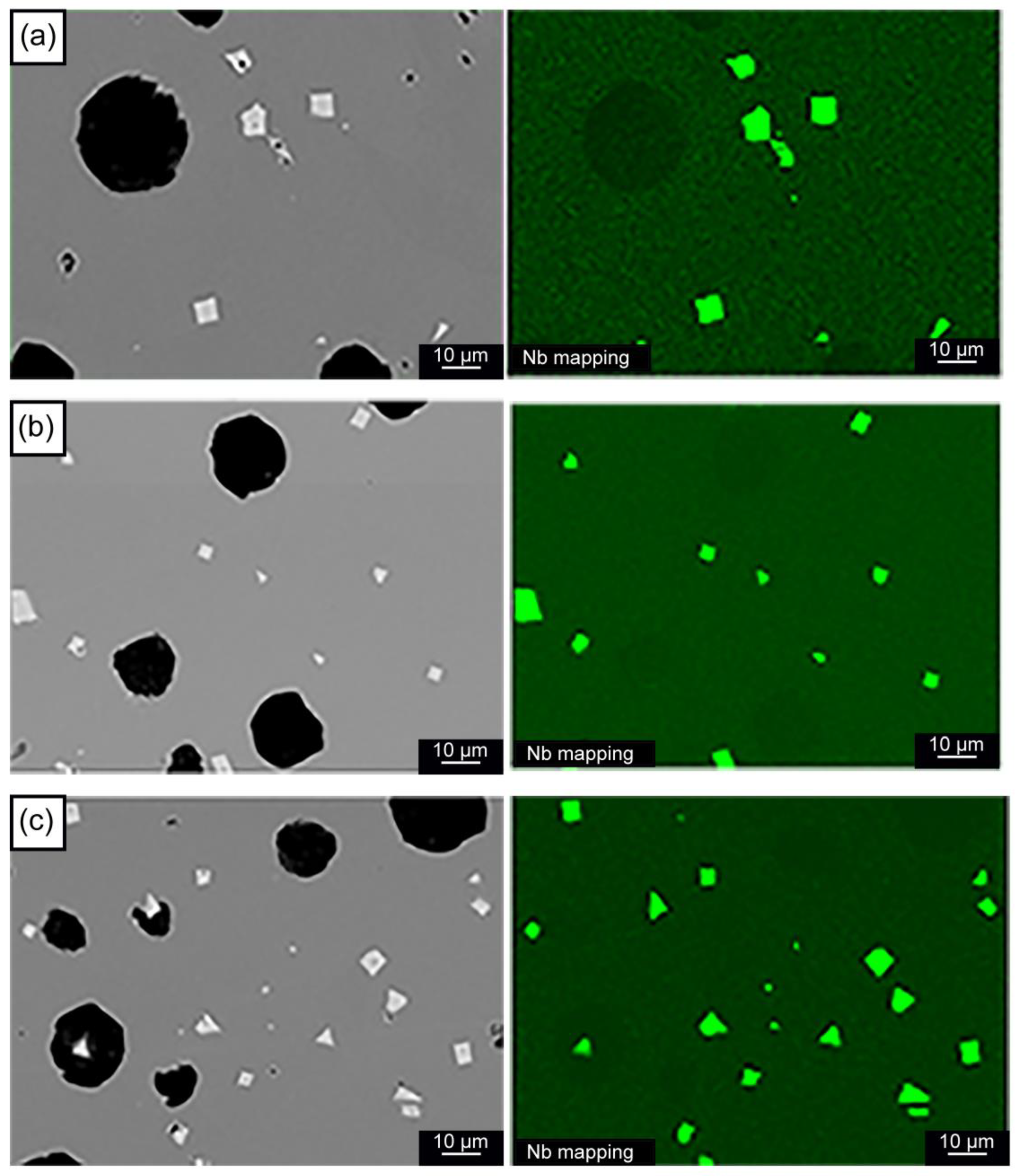
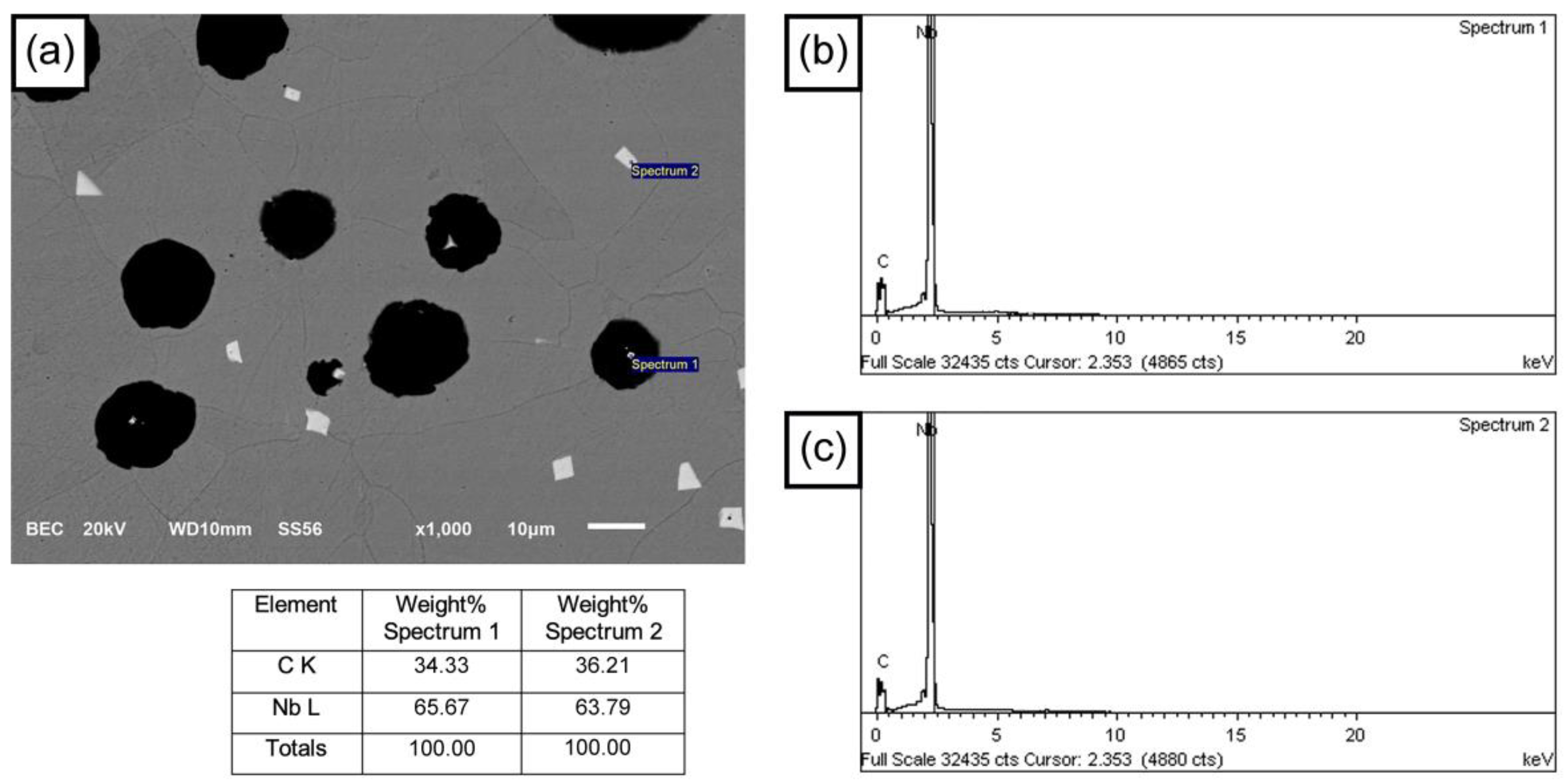
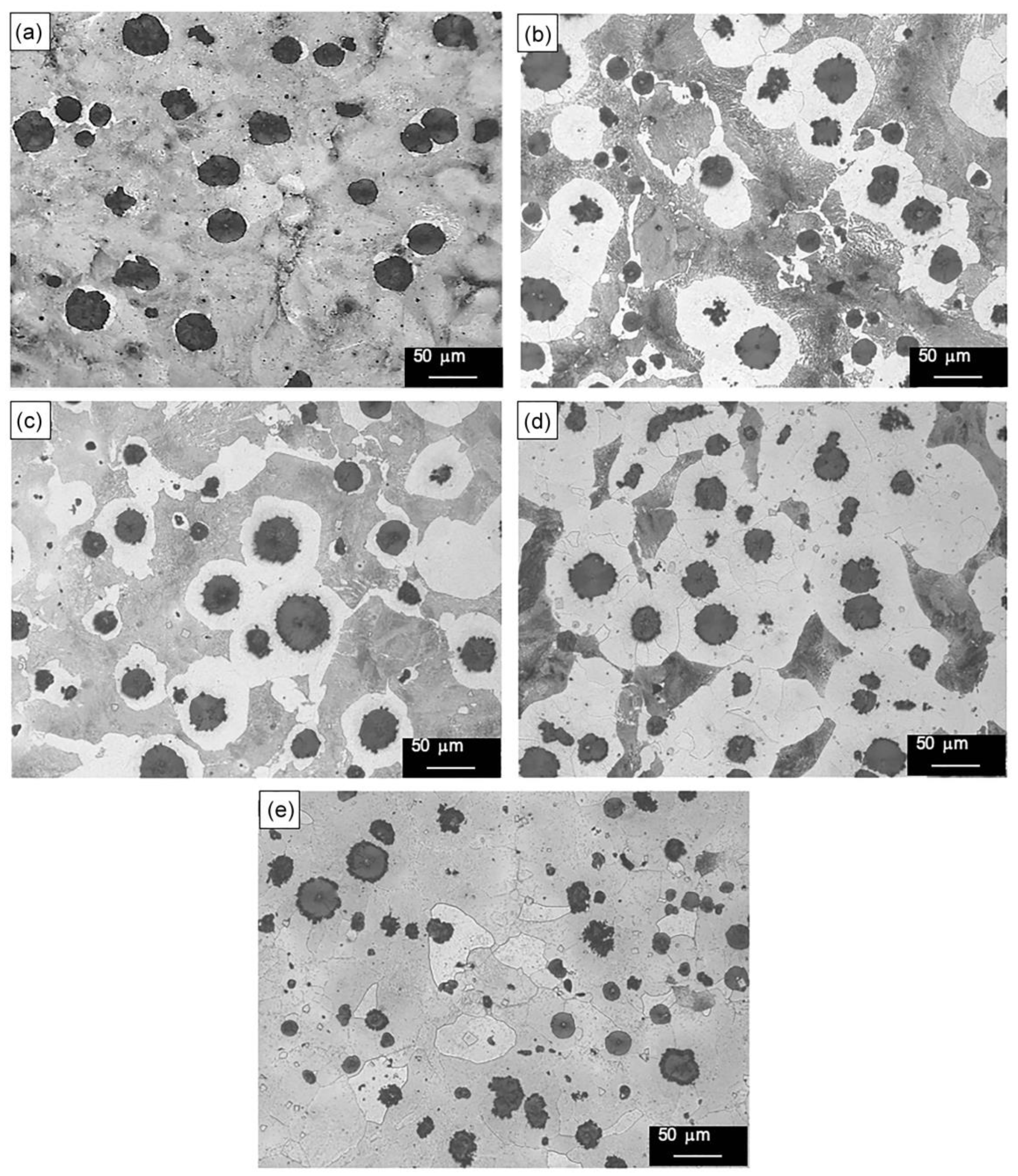
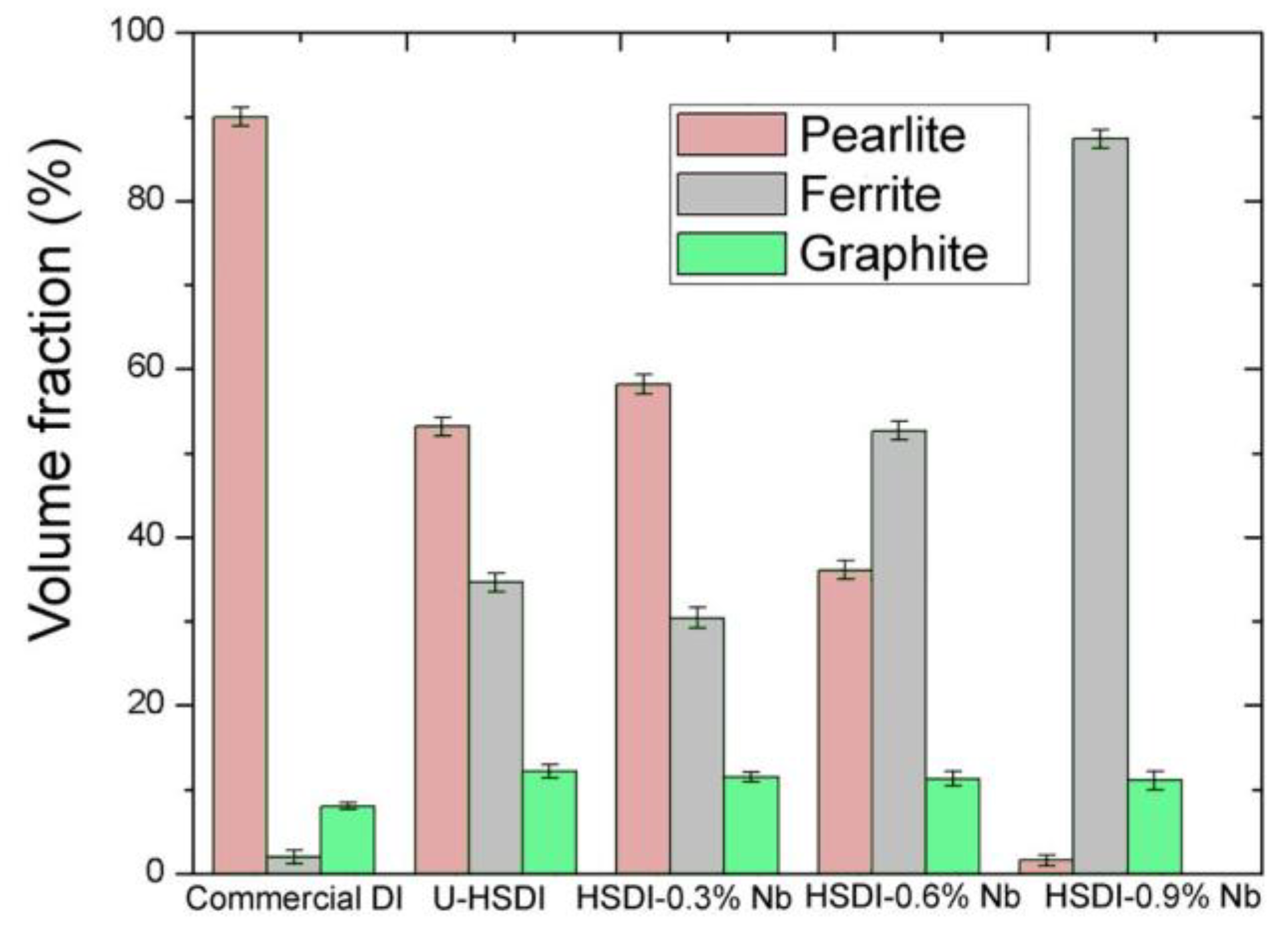
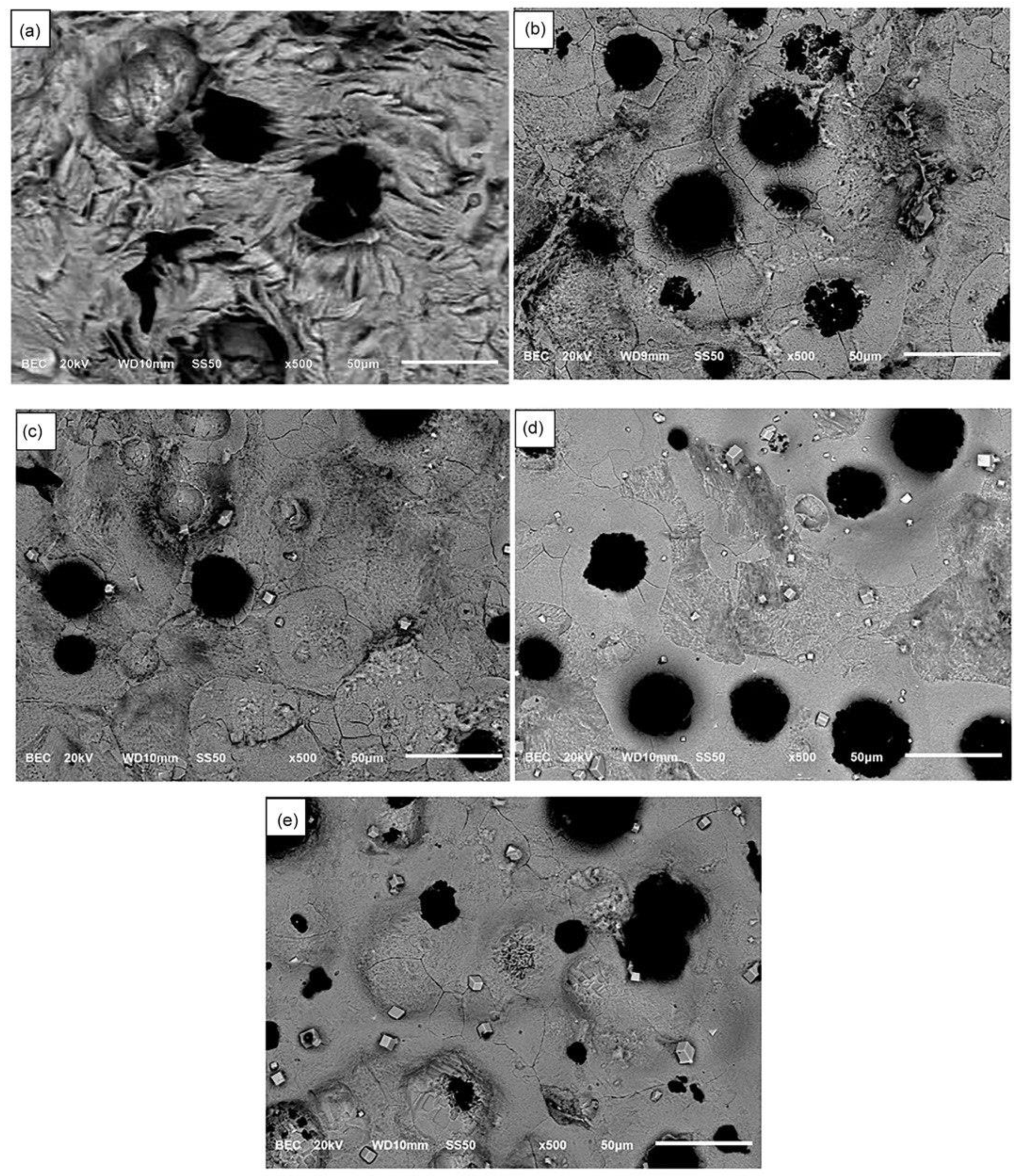
3.2. Microstructural Analysis
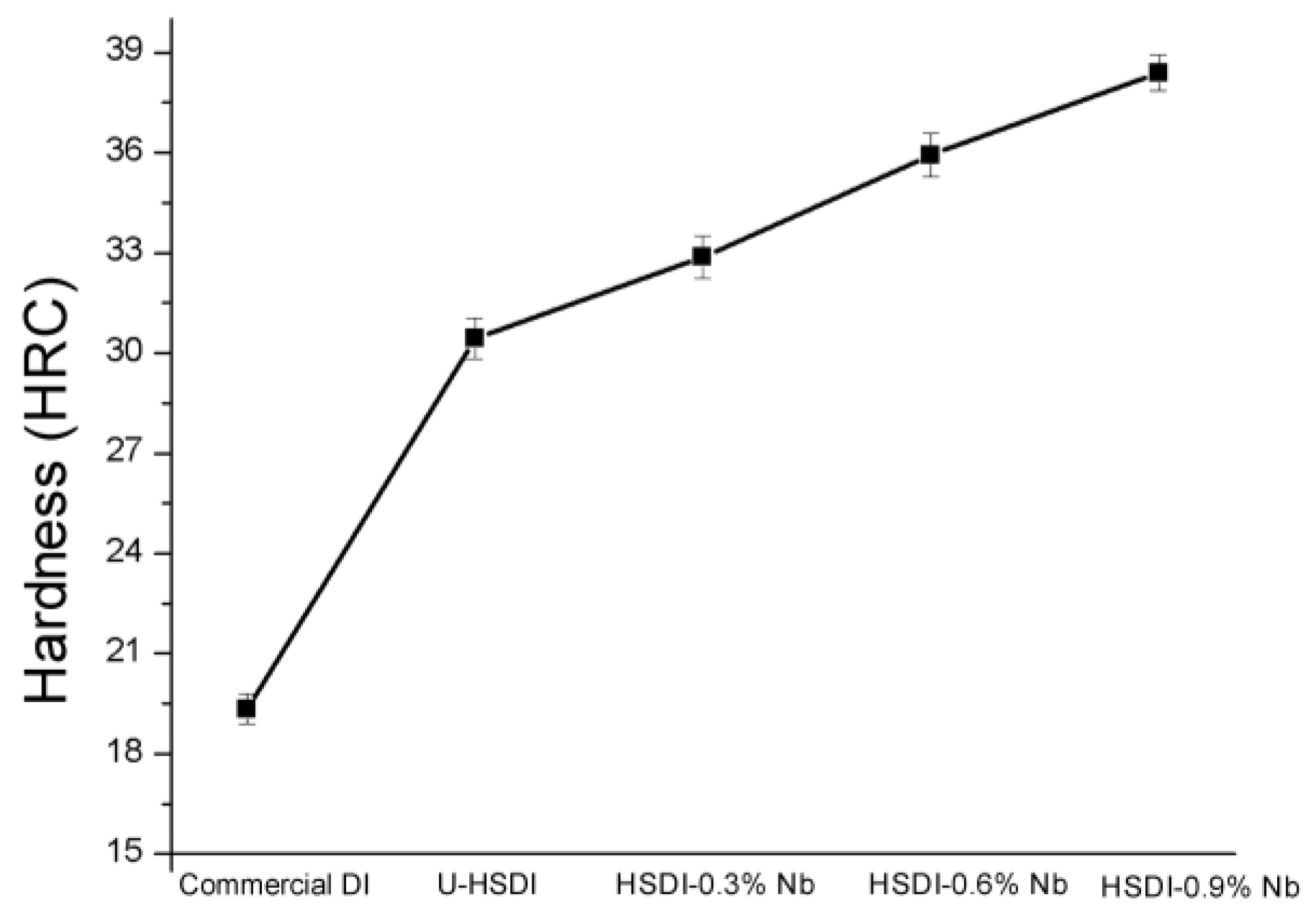
3.3. Hardness
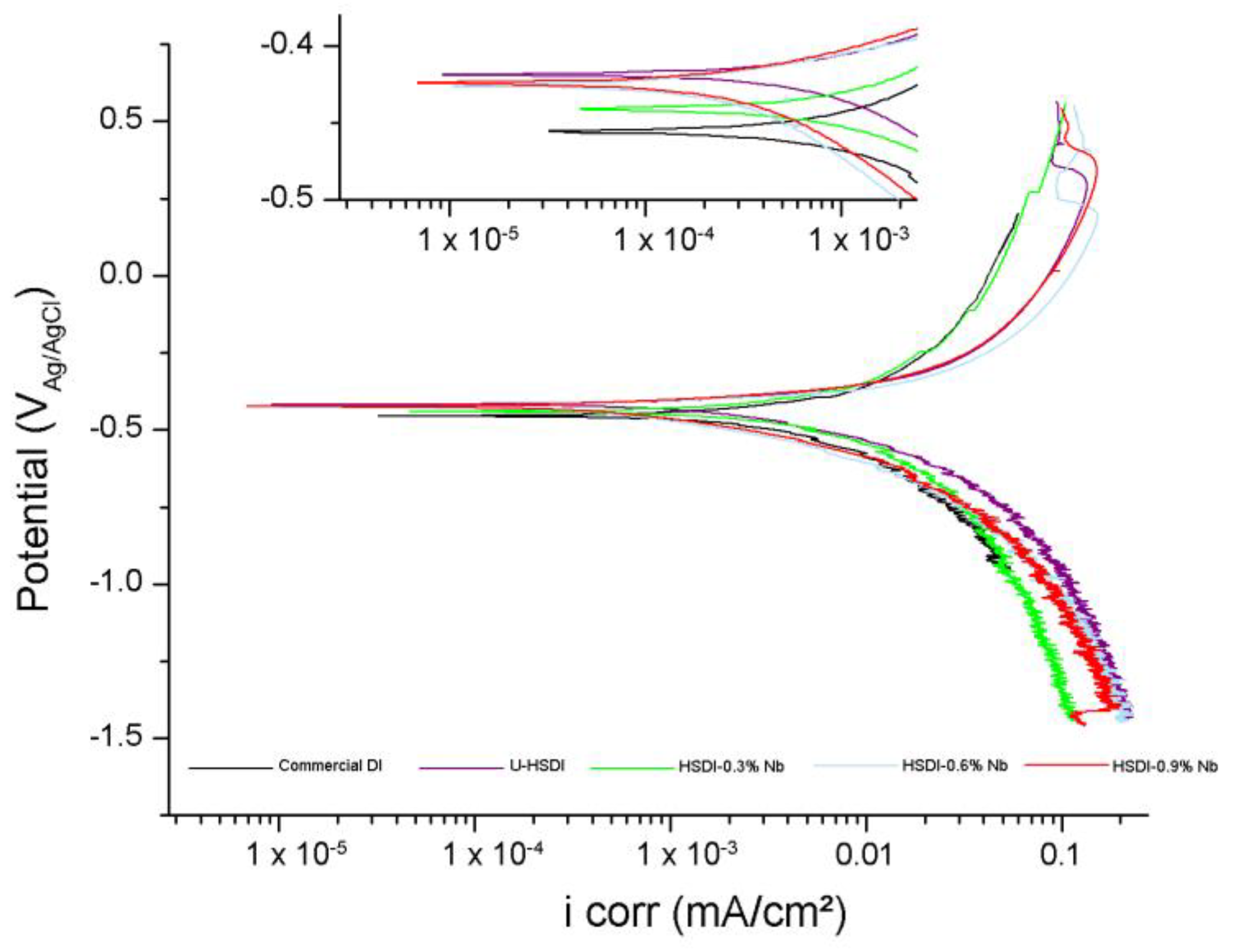
3.4. Corrosion
4. Conclusions
- The formation of polygonal NbC carbides was observed in HSDI upon the Nb addition in a range of 0.3–0.9 wt.%. These carbides have a size between 0.5 and 4.5 µm, which decreases with increasing Nb content in HSDI.
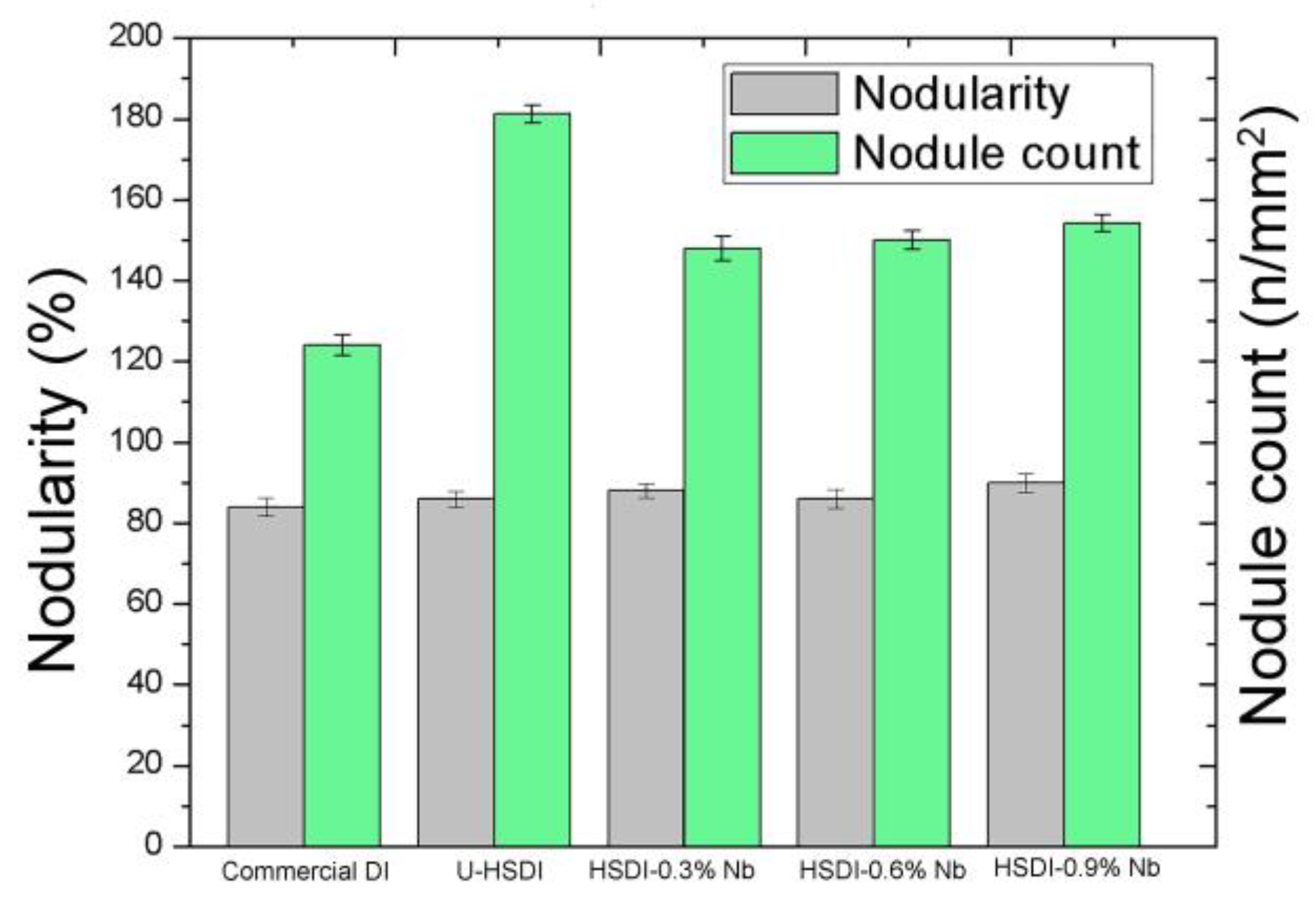
- The presence of NbC carbides increases with Nb content, resulting in heterogeneous graphite nodule nucleation.
- Nodularity maintained a linear trend between 86 and 90%, and the nodule count decreased with the presence of Nb in the HSDI, from 182 n/mm2 for U-HSDI to 147–154 n/mm2 for HSDI-% Nb samples.
- An increment in hardness is due to the greater presence of NbC carbides in combination with high Si, resulting in an increase from 30.44 to 38.38 HRC for U-HSDI and HSDI-0.9 wt.% Nb, respectively.
- Electrochemical corrosion is achieved by the formation of a galvanic couple between the graphite and the metal matrix which preferentially attacks the pearlite, with the greatest corrosion occurring at HSDI-0.3 wt.% Nb.
- The combination of Nb with Si in HSDI helps to reduce the corrosion rate, since Nb promotes the development of NbC carbides and Si promotes the ferritic matrix. If the Nb content exceeds 0.3 wt.% and the Si content in the DI is more than 2.8 wt.%, protective systems against cathodic corrosion can be applied to the HSDI and it can be used in agriculture applications.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chikali, P.; Shinde, V. Analysis of machinability in ductile iron casting. Mater. Today Proc. 2020, 27, 584–588. [Google Scholar] [CrossRef]
- Scorza, D.; Ronchei, C.; Vantadori, S.; Zanichelli, A.; Carpinteri, A. A novel methodology for fatigue assessment of Ductile Cast Iron (DCI) with solidification defects. Procedia Struct. Integr. 2022, 41, 500–504. [Google Scholar] [CrossRef]
- Skaland, T.; Grong, O.; Grong, T. A model for the graphite formation in ductile cast iron: Part I. Inoculation Mechanisms. Metall. Mater. Trans. A 1993, 24, 2321–2345. [Google Scholar] [CrossRef]
- González Martínez, R.; Sertucha, J.; Lacaze, J. Effects of cobalt on mechanical properties of high silicon ductile irons. Mater. Sci. Technol. 2020, 36, 1292–1300. [Google Scholar] [CrossRef]
- Khalil-Allafi, J.; Amin-Ahmadi, B. Influence of Mold Preheating and Silicon Content on Microstructure and Casting Properties of Ductile Iron in Permanent Mold. J. Iron Steel Res. Int. 2011, 18, 34–39. [Google Scholar] [CrossRef]
- Riebisch, M.; Seiler, C.; Pustal, B.; Bührig-Polaczek, A. Microstructure of As-Cast High-Silicon Ductile Iron Produced via Permanent Mold Casting. Int. J. Met. 2018, 13, 112–120. [Google Scholar] [CrossRef]
- Almanza, A.; Dewald, D.; Licavoli, J. Effect of Cobalt Additions on the Microstructure and Mechanical Properties of As-Cast Thin-Wall Ductile Iron. Int. Met. 2021, 15, 417–432. [Google Scholar] [CrossRef]
- Franzen, D.; Weiß, P.; Pustal, B.; Bührig-Polaczek, A. Modification of silicon microsegregation in solid-solution-strengthened ductile iron by alloying with aluminum. Int. J. Met. 2020, 14, 1105–1120. [Google Scholar] [CrossRef]
- Almanza, A.; Dewald, D.; Licavoli, J.; Sanders, P.G. Influence of cobalt in the tensile properties of inch ductile iron Y-blocks. Int. Met. 2020, 15, 433–446. [Google Scholar] [CrossRef]
- Mikoleizik, P.; Geier, G. SiWind—Development of materials for offshore wind power plants of the multi megawatt range. Cast. Plant Technol. Int. (CP+T) 2014, 9, 64–69. [Google Scholar]
- Werner, H.; Lappat, I.; Aurich, B. Mischkristallverfestigte EN-GJS-Werkstoffe für Groβ-und Schwergussteile. Giesserei 2016, 103, 38–42. [Google Scholar]
- De la Torre, U.; Loizaga, A.; Lacaze, J.; Sertucha, J. As cast high silicon ductile irons with optimised mechanical properties and remarkable fatigue properties. Mater. Sci. Technol. 2014, 30, 1425–1431. [Google Scholar] [CrossRef]
- Ikeda, T.; Noda, N.; Sano, Y. Conditions for notch strength to be higher than static tensile strength in high–strength ductile cast iron. Eng. Fract. Mech. 2019, 206, 75–88. [Google Scholar] [CrossRef]
- Sandikoglu, A.; Gecu, R. Microstructural, mechanical and tribological characterization of aluminum-alloyed ductile cast irons based on aluminum content. J. Alloys Compd. 2021, 879, 160428. [Google Scholar] [CrossRef]
- Kasvayee, K.A.; Ghassemali, E.; Svensson, I.L.; Olofsson, J.; Jarfors Anders, E.W. Characterization and modeling of the mechanical behavior of high silicon ductile iron. Mater. Sci. Eng. A 2017, 708, 159–170. [Google Scholar] [CrossRef]
- Bjorkgen, L.E.; Hamberg, K. Silicon alloyed ductile iron with excellent ductility and machinability. Foundryman 2001, 94, 42–51. [Google Scholar]
- Foglio, E.; Gelfi, M.; Pola, A.; Goffelli, S.; Lusuardi, D. Fatigue Characterization and Optimization of the Production Process of Heavy Section Ductile Iron Castings. Int. J. Met. 2016, 11, 33–43. [Google Scholar] [CrossRef]
- Riebisch, M.; Pustal, B.; Bührig-Polaczek, A. Influence of carbide-promoting elements on the microstructure of high-silicon ductile iron. Int. J. Met. 2020, 14, 1152–1161. [Google Scholar] [CrossRef]
- Górny, M.; Kawalec, M.; Gracz, B.; Tupaj, M. Influence of Cooling Rate on Microstructure Formation of Si–Mo Ductile Iron Castings. Metals 2021, 11, 1634. [Google Scholar] [CrossRef]
- Stets, W.; Löblich, H.; Gassner, G. Solution Strengthened Ferritic Ductile Cast Iron Properties, Production and Application. Int. Met. 2014, 8, 35–40. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Hu, H.; Mohrbacher, H.; Kang, M.; Zhang, W.; Guo, A.; Zhai, Q. Effects of niobium addition on microstructure and tensile behavior of as-cast ductile iron. Mater. Sci. Eng. A 2017, 688, 416–428. [Google Scholar] [CrossRef]
- Souza, T.N.F.; Nogueira, R.A.P.S.; Franco, F.J.S.; Aguilar, M.T.P.; Cetlin, P.R. Mechanical and microstructural characterization of Nodular Cast Iron (NCI) with Niobium Additions. Mater. Res. 2014, 17, 1167–1172. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Y.; Li, Q.; Hong, X. Mechanical properties and rolling-sliding wear performance of dual phase austempered ductile iron as potential metro wheel material. Wear 2018, 406–407, 156–165. [Google Scholar] [CrossRef]
- Bedolla-Jacuinde, A.; Solis, E.; Hernandez, B. Effect of niobium in medium alloyed ductile cast irons. Int. J. Cast Met. Res. 2003, 16, 481–486. [Google Scholar] [CrossRef]
- Sun, X.; Yin, Z.; Chang, L.; Hua, Q.; Zhai, Q. Existence of Niobium in Ductile Iron and Its Effect on the Morphology of Graphite Ball. Suppl. Proc. 2012, 2, 603–611. [Google Scholar]
- Li, H.; Yang, C.; Wei, Z.; Chen, X.; Zhai, Q. Morphology and Distribution of Nb-Rich Phase and Graphite in Nb Microalloyed Ductile Iron. Charact. Miner. Met. Mater. 2015, 2015, 579–585. [Google Scholar]
- Sckudlarek, W.; Krmasha, M.N.; Al-Rubaie, K.S.; Preti, O.; Milan, J.C.; da Costa, C.E. Effect of austempering temperature on microstructure and mechanical properties of ductile cast iron modified by niobium. J. Mater. Res. Technol. 2021, 12, 2414–2425. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, L.; Zhang, W.; Mohrbacher, H.; Wang, W.; Guo, A.; Zhai, Q. Effects of niobium alloying on microstructure, toughness, and wear resistance of austempered ductile iron. Mater. Sci. Eng. A 2019, 760, 186–194. [Google Scholar] [CrossRef]
- Ahmed, M.; Soliman, M.; Youssef, M.; Bähr, R.; Nofal, A. Effect of niobium on the microstructure and mechanical properties of alloyed ductile irons and austempered ductile irons. Metals 2021, 11, 703. [Google Scholar] [CrossRef]
- Alias, S.K.; Abdullah, B.; Jaffar, A.; Abdullah, A.H.; Jenal, N. Development of high strength ductile iron with niobium addition. Adv. Mater. Res. 2012, 576, 366–369. [Google Scholar] [CrossRef]
- Souza Oliveira, P.A.; Luiz, G.W.; Rodrigues Custódio da Silva, W.J.; Dolabella Portella, P.; Woydt, M.; Burbank, J. Abrasive wear behavior of austempered ductile iron with niobium additions. Wear 2019, 440–441, 203065. [Google Scholar] [CrossRef]
- Zhou, W.; Zhu, H.; Zheng, D.; Zheng, H.; Hua, Q.; Zhai, Q. Niobium alloying effect in high carbon equivalent grey cast iron. China Foundry 2011, 08, 36–40. [Google Scholar]
- Mohrbacher, H.; Zhai, Q. Niobium alloying in grey cast iron for vehicle brake discs. In Proceedings of the Materials Science & Technology Conference, Columbus, OH, USA, 16–20 October 2011; ASM International: Detroit, MI, USA, 2011; pp. 434–445. [Google Scholar]
- Sun, Y.; Hu, S.; Xiao, Z.; You, S.; Zhao, J.; Lv, Y. Effects of nickel on low-temperature impact toughness and corrosion resistance of high-ductility ductile iron. Mater. Des. 2012, 41, 37–42. [Google Scholar] [CrossRef]
- Gutiérrez Pérez, V.H.; Cruz Ramírez, A.; Olvera Vázquez, S.L.; Colin García, E.; Sánchez Alvarado, R.G.; Delgado Pámanes, M.F.; Rivera Salinas, J.E. Study of the effects of vanadium and molybdenum on the microstructure of ductile iron (DI) and austempered ductile iron (ADI) and their corrosion resistance. Acta Univ. 2022, 32, 1–20. [Google Scholar] [CrossRef]
- Elliott, R. Cast Iron Technology; Butterworth-Heinemann: Oxford, UK, 1988. [Google Scholar]
- Celik Çelik, G.A.; Tzini, M.-I.T.; Polat, S.; Hakan Atapek, Ş.H.; Haidemenopoulos, G.N. Thermal and microstructural characterization of a novel ductile cast iron modified by aluminum addition. Int. J. Miner. Met. Mater. 2020, 27, 190–199. [Google Scholar] [CrossRef]
- Adebayo, A.O.; Alaneme, K.K.; Oyetunji, A. Corrosion evaluation of austempered aluminium-alloyed ductile irons in well water and 0.5M NaCl solution. J. Chem. Technol. Metall. 2021, 56, 180–193. [Google Scholar]
- Günen, A.; Kalkandelen, M.; Gök, M.S.; Kanca, E.; Kurt, B.; Karakaş, M.S.; Karahan, I.H.; Çetin, M. Characteristics and high temperature wear behavior of chrome vanadium carbide composite coatings produced by thermo-reactive diffusion. Surf. Coat. Technol. 2020, 402, 126402. [Google Scholar] [CrossRef]
- Gúnen, A.; Soylu, B.; Karakaş, O. Titanium carbide coating to improve surface characteristic, wear and corrosion resistance of spheroidal graphite cast irons. Surf. Coat. Technol. 2022, 437, 128280. [Google Scholar] [CrossRef]
- Mariani, F.E.; Takeya, G.S.; Lombardi, A.N.; Picone, C.A.; Casteletti, L.C. Wear and corrosion resistance of Nb-V carbide layers produced in vermicular cast iron using TRD treatments. Surf. Coat. Technol. 2020, 397, 126050. [Google Scholar] [CrossRef]
- ASTM A536-84; Standard Specification for Ductile Iron Castings. ASTM International: West Conshohocken, PA, USA, 2019.
- SIST EN 1563:2012; Founding—Spheroidal Graphite Cast Irons. European Standard: Brussels, Belgium, 2012.
- Chen, X.R.; Zhai, Q.J.; Dong, H.; Dai, B.H.; Mohrbacher, H. Molybdenum alloying in cast iron and steel. Adv. Manuf. 2019, 8, 3–14. [Google Scholar] [CrossRef]
- Wang, B.; Barber, G.C.; Qiu, F.; Zou, Q.; Yang, H. A review: Phase transformation and wear mechanisms of single-step and dual-step austempered ductile irons. J. Mater. Res. Technol. 2020, 9, 1054–1069. [Google Scholar] [CrossRef]
- Vicente, A.D.A.; Sartori Moreno, J.R.; Santos, T.F.D.A.; Espinosa, D.C.R.; Tenório, J.A.S. Nucleation and growth of graphite particles in ductile cast iron. J. Alloys Compd. 2019, 775, 1230–1234. [Google Scholar] [CrossRef]
- Lacaze, J.; Sertucha, J.; Åberg, L.M. Microstructure of As-cast Ferritic-pearlitic Nodular Cast Irons. ISIJ Int. 2016, 56, 1606–1615. [Google Scholar] [CrossRef]
- Vdovin, K.N.; Gorlenko, D.A.; Feoktistov, N.A.; Kuryaev, D.V. Study of the effect of isothermal holding on parameters of graphite phase in indefinite chromium-nickel cast iron alloyed by nitrogen and vanadium. CIS Iron Steel Rev. 2019, 17, 30–33. [Google Scholar] [CrossRef]
- Rezvani, M.; Harding, R.A.; Campbell, J. The effect of vanadium in as-cast ductile iron. Int. J. Cast Met. Res. 1997, 10, 1–15. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, W.; Li, H.; Chen, X.; Zhai, Q. Effects of Niobium Microalloying on the Pearlite of Ductile Iron. Charact. Miner. Met. Mater. 2015, 2015, 601–606. [Google Scholar]
- Alhussein, A.; Risbet, M.; Bastien, A.; Chobaut, J.; Balloy, D.; Favergeon, J. Influence of silicon and addition elements on the mechanical behavior of ferritic ductile cast iron. Mater. Sci. Eng. A 2014, 605, 222–228. [Google Scholar] [CrossRef]
- Basso, A.; Caldera, M.; Rivera, G.; Sikora, J. High Silicon Ductile Iron: Possible Uses in the Production of Parts with Dual Phase ADI; Microstructure. ISIJ Int. 2012, 52, 1130–1134. [Google Scholar] [CrossRef]
- González, F.; Houbaert, Y. A review of ordering phenomena in iron-silicon alloys. Rev. Metal. 2013, 49, 178–199. [Google Scholar] [CrossRef]
- Weiß, P.; Tekavčič, A.; Bührig-Polaczek, A. Mechanistic approach to new design concepts for high silicon ductile iron. Mater. Sci. Eng. A 2018, 713, 67–74. [Google Scholar] [CrossRef]
- Ahmad, Z. Principles of Corrosion Engineering and Corrosion Control, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2006. [Google Scholar]
- You, Z.; Lai, Y.; Zeng, H.; Yang, Y. Influence of water and sodium chloride content on corrosion behavior of cast iron in silty clay. Constr. Build. Mater. 2020, 238, 117762. [Google Scholar] [CrossRef]
- Ding, D.; Zhang, Y.; Yu, X.; Fang, B.; Guo, J.; Li, J.; Liu, L.; Du, C. Effects of environmental factors on corrosion behavior of high-silicon cast iron in Shanxi soil medium. Anti-Corros. Methods Mater. 2018, 65, 538–546. [Google Scholar] [CrossRef]
- Krawiec, H.; Stypuła, B.; Stoch, J.; Mikołajczyk, M. Corrosion behaviour and structure of the surface layer formed on austempered ductile iron in concentrated sulphuric acid. Corros. Sci. 2006, 48, 595–607. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, J.; Zhang, Y.; Liu, W.; Pei, W.; Zhao, A.; Zhang, W.; Zeng, L. The study on corrosion behavior and corrosion resistance of ultralow carbon high silicon iron-based alloy. Mater. Res. Express 2021, 8, 026504. [Google Scholar] [CrossRef]





| C | Si | Mn | S | Cu | P | Nb | Mg | Ce | Cr | V | Mo | Fe | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Commercial DI | 3.25 | 2.60 | 0.85 | 0.006 | 0.71 | 0.018 | - | 0.053 | 0.031 | - | - | - | bal. |
| U-HSDI | 3.40 | 3.76 | 0.42 | 0.006 | 1.12 | 0.03 | 0.004 | 0.026 | 0.033 | 0.07 | 0.013 | 0.011 | bal. |
| HSDI-0.3% Nb | 3.42 | 3.76 | 0.42 | 0.005 | 1.12 | 0.03 | 0.296 | 0.024 | 0.033 | 0.08 | 0.011 | 0.012 | bal. |
| HSDI-0.6% Nb | 3.39 | 3.76 | 0.42 | 0.006 | 1.12 | 0.03 | 0.592 | 0.021 | 0.031 | 0.06 | 0.015 | 0.011 | bal. |
| HSDI-0.9% Nb | 3.40 | 3.75 | 0.42 | 0.007 | 1.12 | 0.03 | 0.921 | 0.020 | 0.031 | 0.06 | 0.012 | 0.014 | bal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñiz Valdez, C.R.; García Navarro, D.; Galindo Valdés, J.S.; Montes González, F.A.; Almanza Casas, E.; Rodríguez Rosales, N.A. Determination of Corrosion Resistance of High-Silicon Ductile Iron Alloyed with Nb. Metals 2023, 13, 917. https://doi.org/10.3390/met13050917
Muñiz Valdez CR, García Navarro D, Galindo Valdés JS, Montes González FA, Almanza Casas E, Rodríguez Rosales NA. Determination of Corrosion Resistance of High-Silicon Ductile Iron Alloyed with Nb. Metals. 2023; 13(5):917. https://doi.org/10.3390/met13050917
Chicago/Turabian StyleMuñiz Valdez, Carlos Rodrigo, Daniel García Navarro, Jesús Salvador Galindo Valdés, Félix Alan Montes González, Efrain Almanza Casas, and Nelly Abigail Rodríguez Rosales. 2023. "Determination of Corrosion Resistance of High-Silicon Ductile Iron Alloyed with Nb" Metals 13, no. 5: 917. https://doi.org/10.3390/met13050917
APA StyleMuñiz Valdez, C. R., García Navarro, D., Galindo Valdés, J. S., Montes González, F. A., Almanza Casas, E., & Rodríguez Rosales, N. A. (2023). Determination of Corrosion Resistance of High-Silicon Ductile Iron Alloyed with Nb. Metals, 13(5), 917. https://doi.org/10.3390/met13050917









