Study on Corrosion Resistance of S-Carbon Bricks for Blast Furnace Hearth in Molten Iron
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Sample Preparation
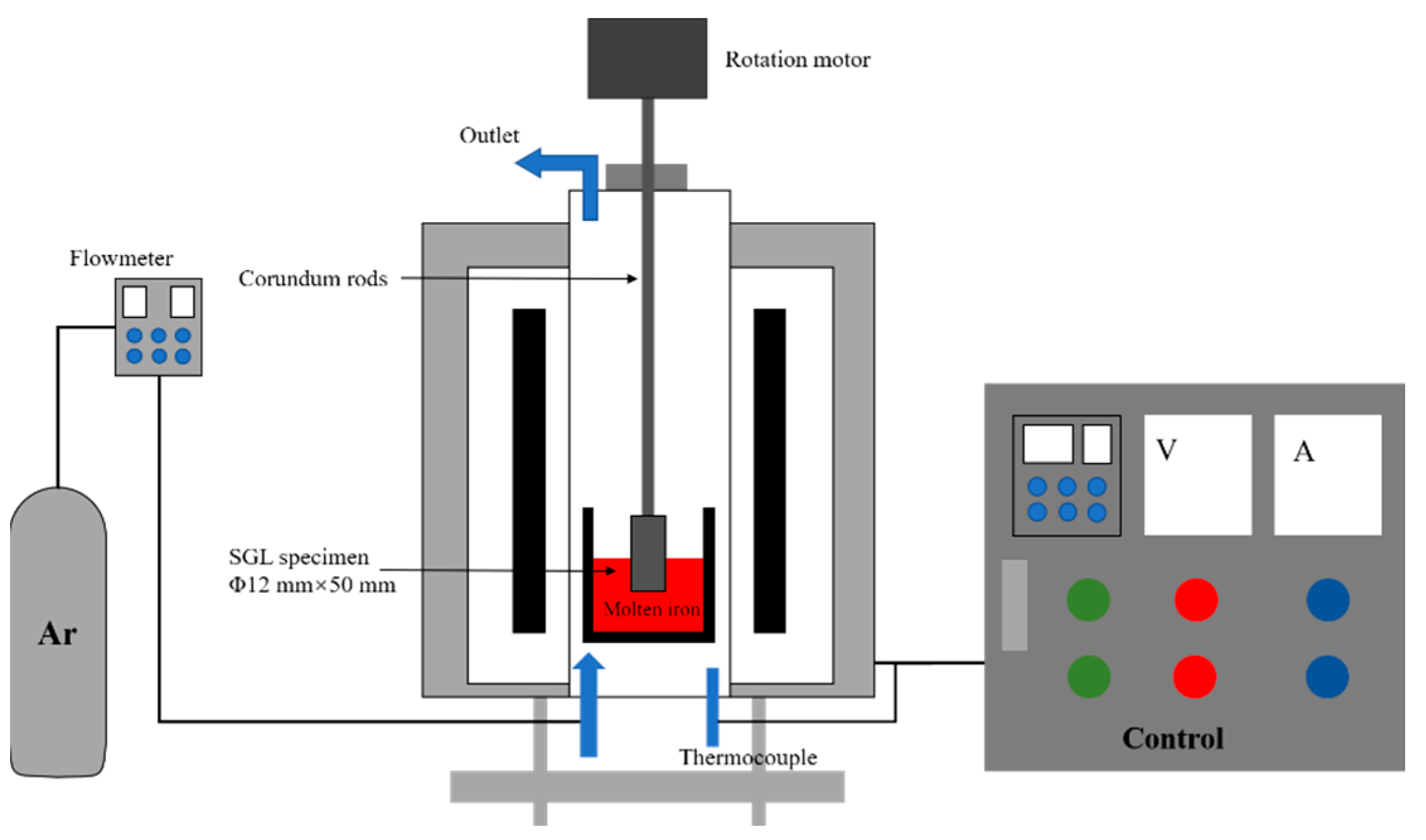
2.2. Experimental Apparatus and Procedure
2.3. Characterization
3. Results and Discussion
3.1. Experimental Results
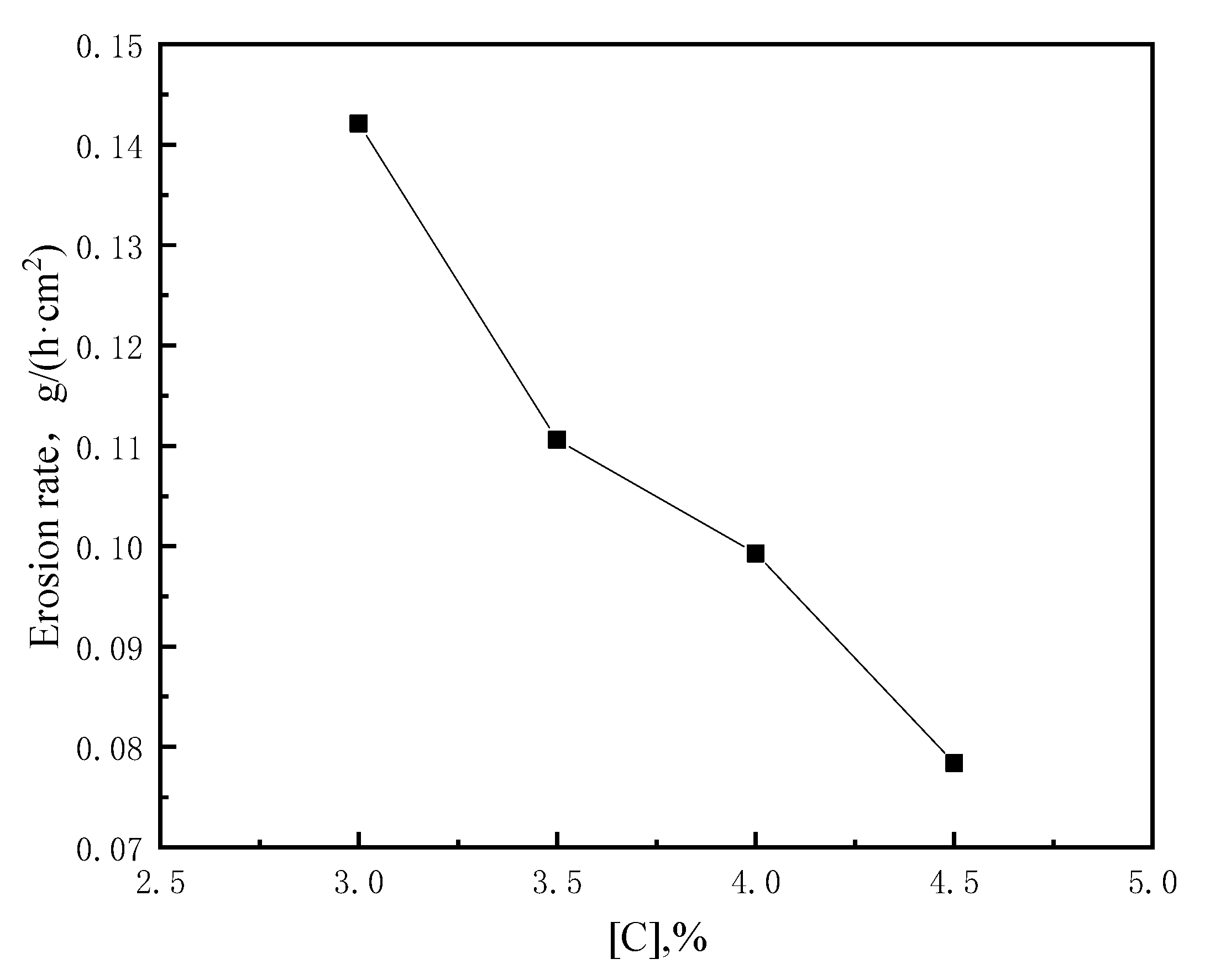
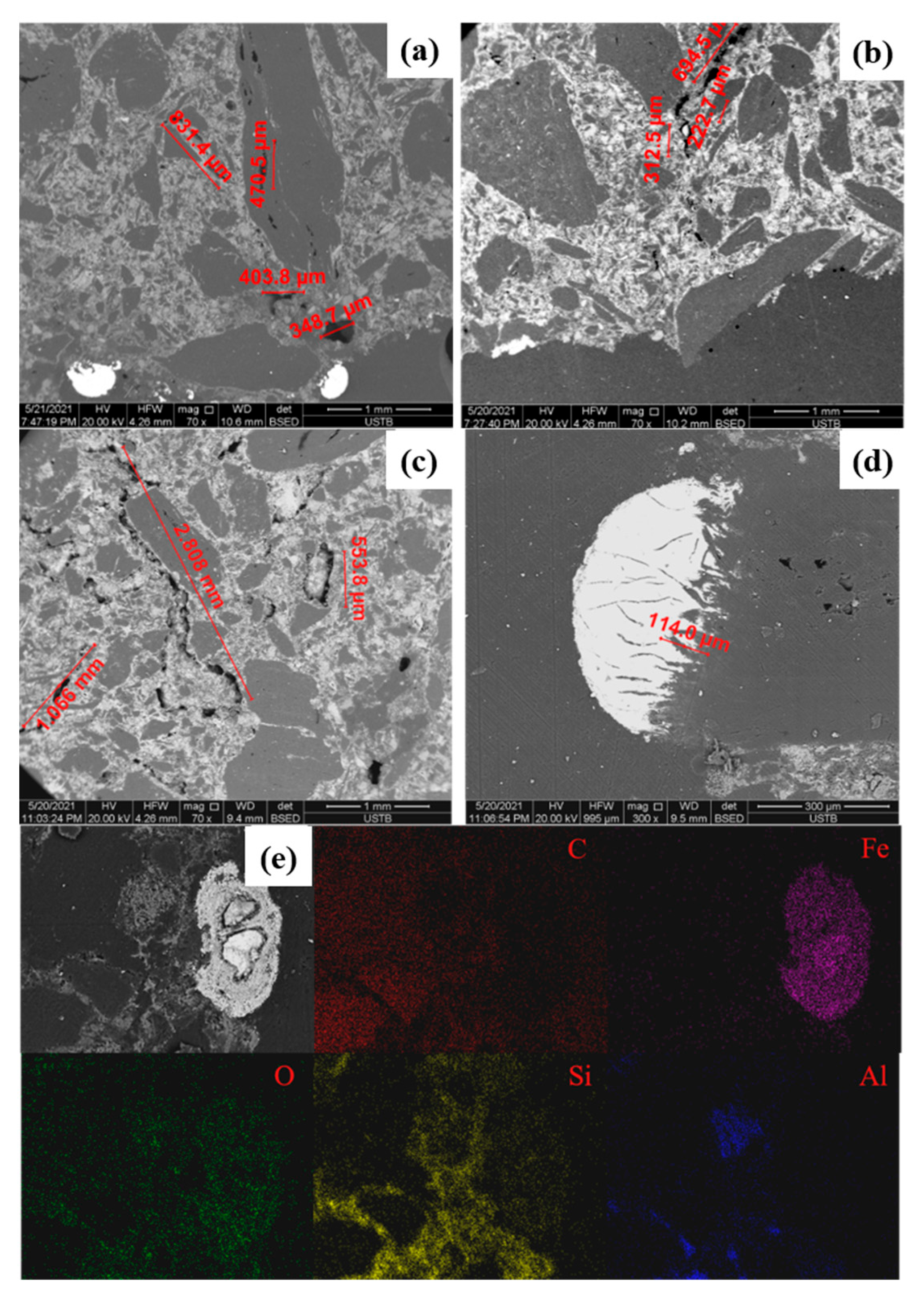
3.1.1. Influence of Carbon Content on the Corrosion of Bricks
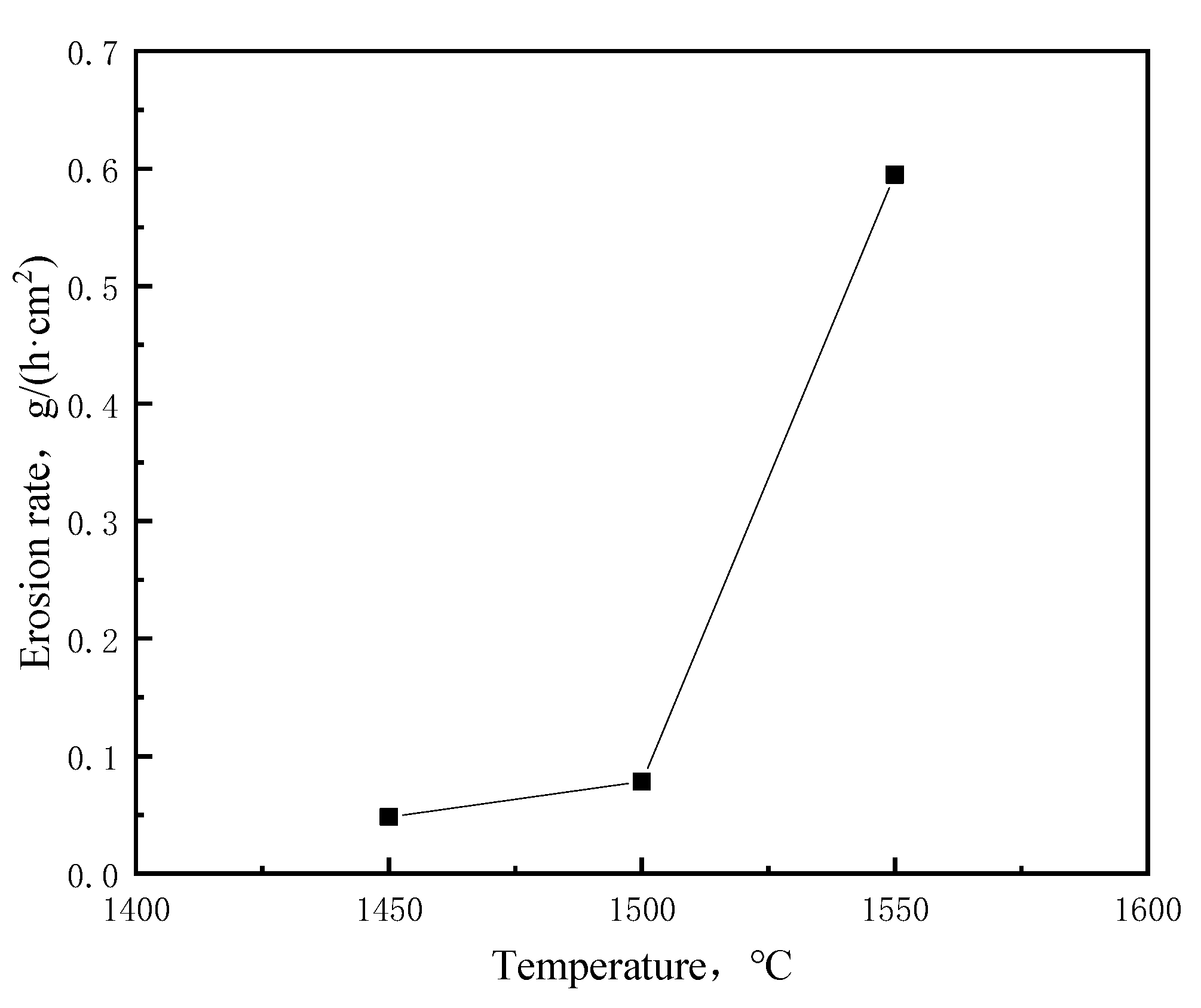
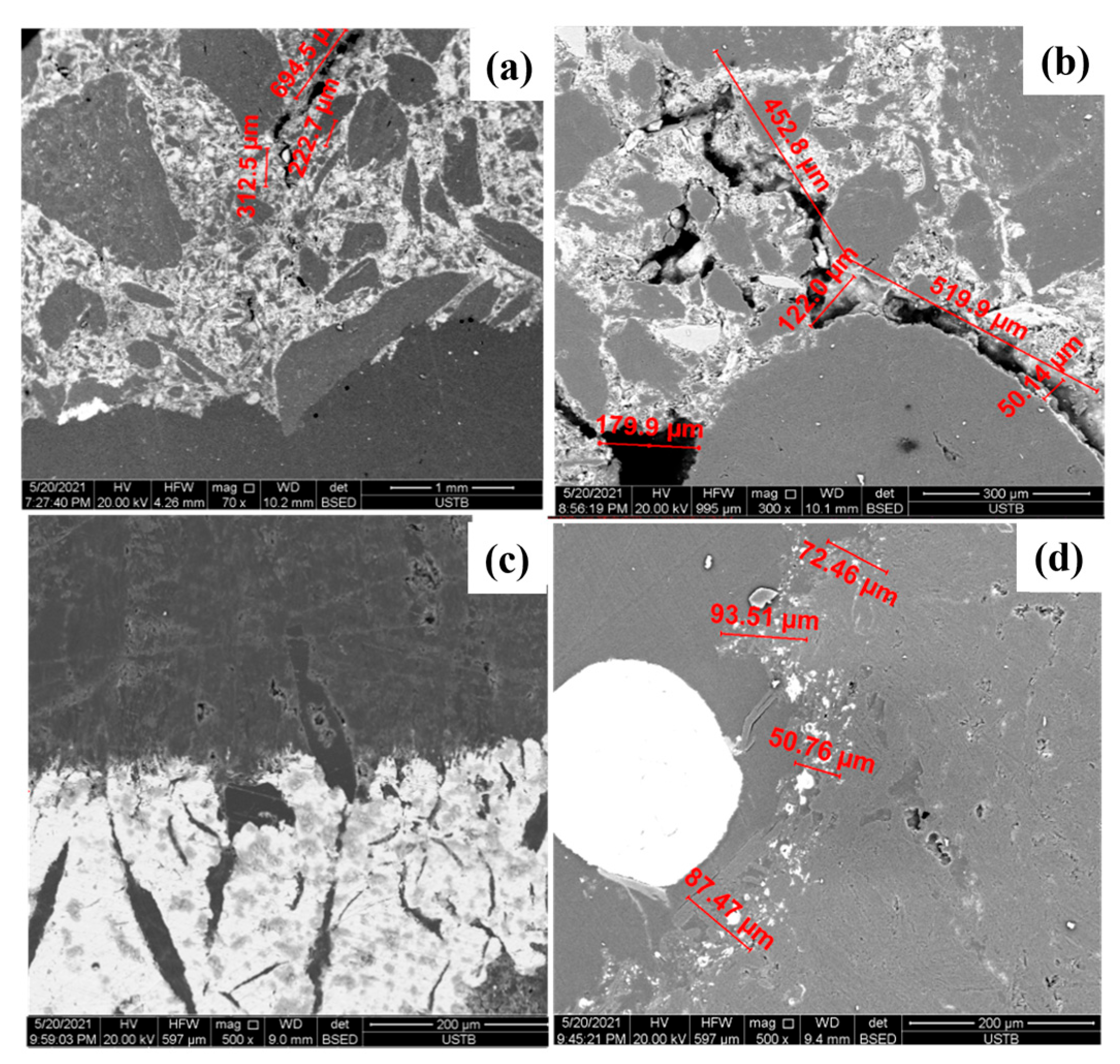
3.1.2. Influence of Temperature on the Corrosion of Bricks
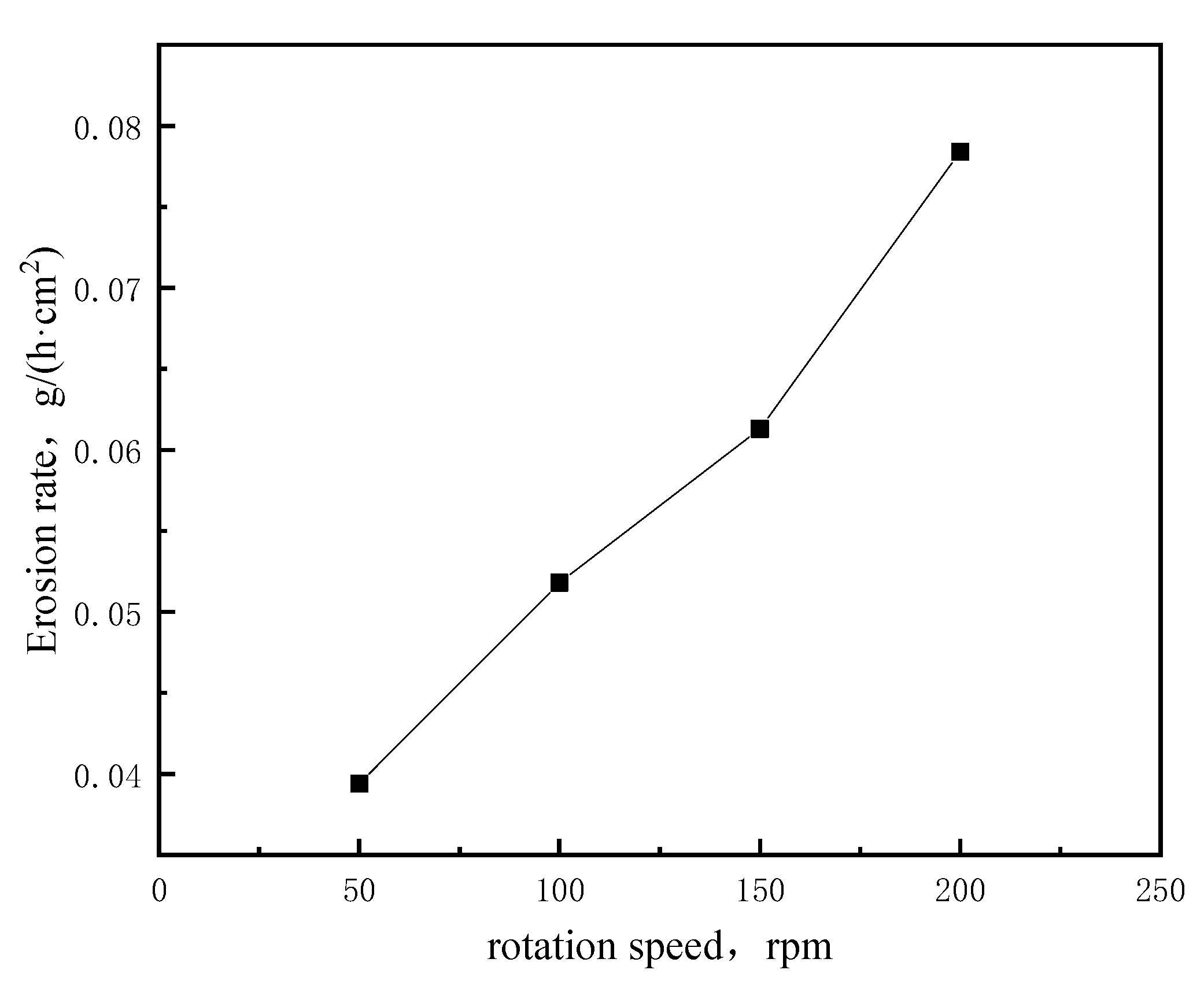
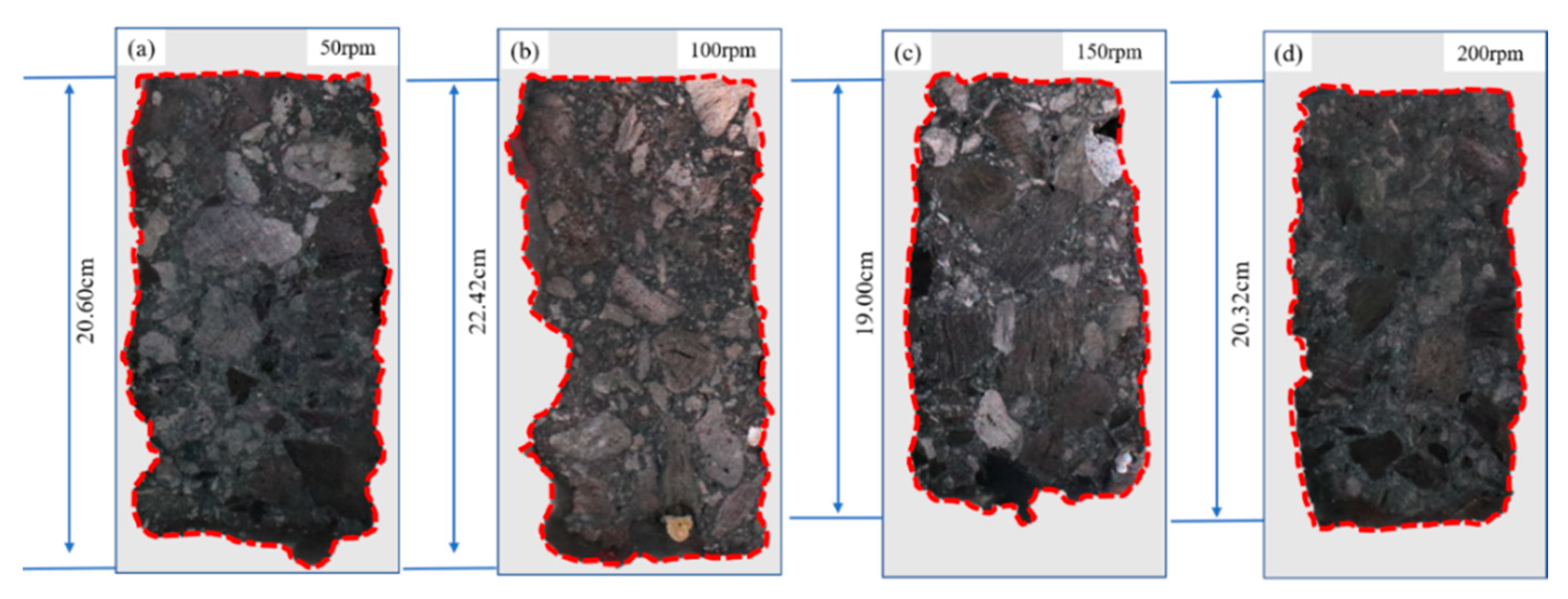
3.1.3. Influence of Rotational Speed on the Corrosion of Bricks
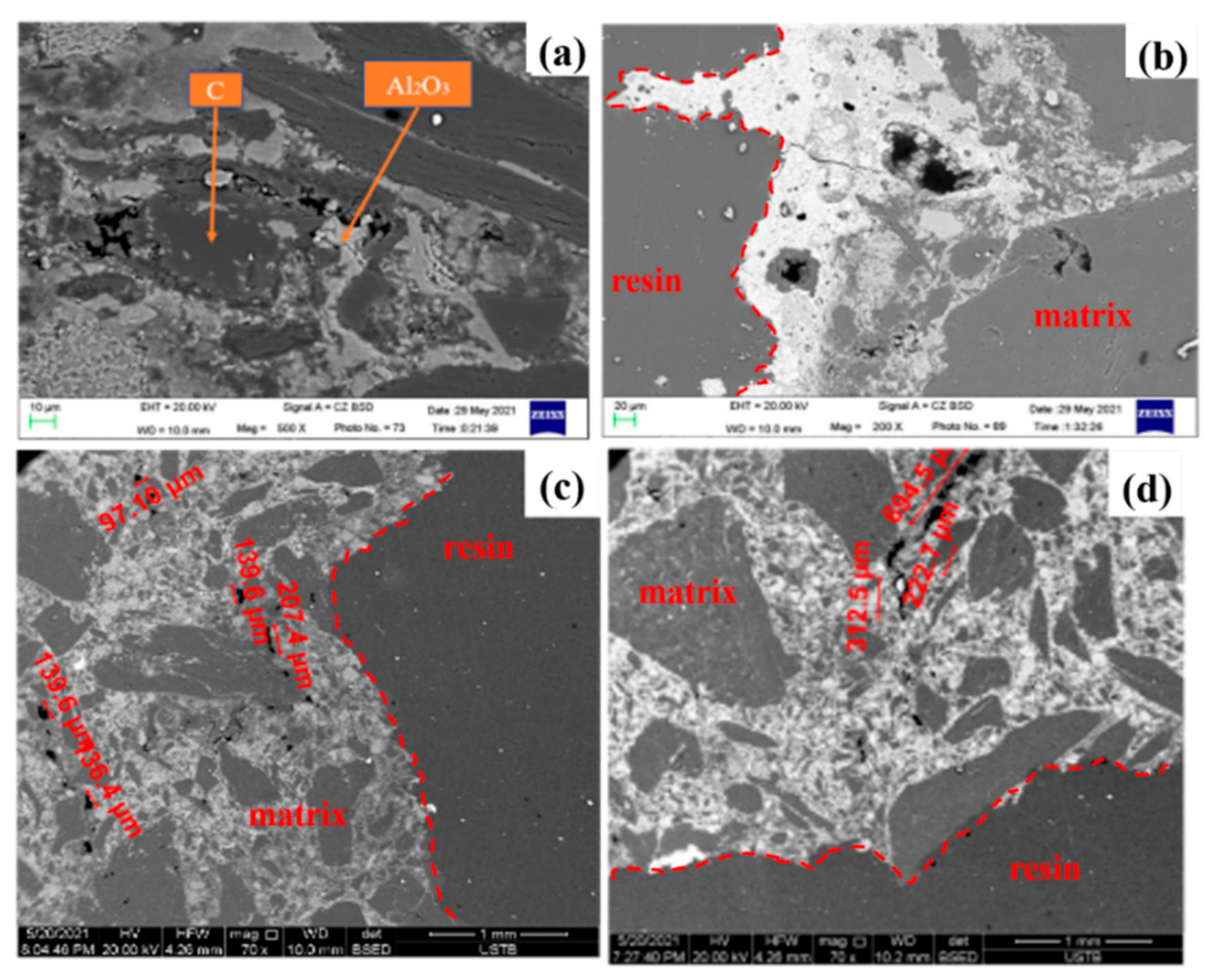
3.2. Mechanism Analysis
3.2.1. Dissolution of Carbon
3.2.2. Flow of Molten Iron
3.2.3. Erosion Mechanism
- (1)
- Molten iron is in direct contact with the carbon bricks and penetrates inwardly along the pores of the carbon bricks;
- (2)
- Molten iron will continue to permeate along the permeation channel, forming dendritic permeation;
- (3)
- Due to the infiltration of molten iron, the properties of the carbon bricks change, and the carbon bricks form an embrittlement layer and powdery particles;
- (4)
- The carbon brick dissolves into molten iron, making the iron and carbon boundaries migrate toward the carbon brick.
4. Conclusions
- (1)
- The dissolution reaction of carbon bricks is affected by the carbon content, temperature and flow rate of molten iron. The erosion degree and dissolution rate of a carbon brick decrease with an increase in carbon content. The erosion degree and rate of a carbon brick increase with an increase in temperature and flow rate, while temperature has a stronger effect on erosion at temperatures above 1500 °C.
- (2)
- The iron–carbon interfacial reaction was analyzed from the perspective of thermodynamics. The main reaction for carbon brick dissolution was obtained. During the erosion process, the Fe-C reaction almost does not work, and the flow of hot iron plays a major role.
- (3)
- The mechanism of three factors influencing the erosion of carbon bricks is found. Increasing the temperature and decreasing the carbon content would lead to a decrease in carbon dissolution in molten iron. The velocity of molten iron would not only affect the shear stress caused by scouring but also increase the unsaturation of carbon in molten iron, leading to the accelerated dissolution of carbon bricks.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.J.; Zhang, J.L.; Zuo, H.B.; Yang, T.J. Recent progress on long service life design of Chinese blast furnace hearth. ISIJ Int. 2012, 52, 1713. [Google Scholar] [CrossRef] [Green Version]
- Song, M.S.; Yu, Z.J.; Zou, Z.Q. Research and development of long campaign technologies of blast furnace at WISCO. Iron Steel 2000, 7, 5–9. [Google Scholar]
- Song, M.S.; Zou, Z.Q.; Yu, Z.J. Latest development of refractory used for blast furnace lining. China Metall. 2005, 11, 6–10. [Google Scholar]
- Zhang, S.R.; Song, M.S. Necessity of applying real micro-porous carbon block for long-life blast furnace. Iron Steel 2012, 47, 9–15. [Google Scholar]
- Zuo, H.B.; Wang, C.; Zhang, J.L. Application status and important technical indexes of BF hearth refractory. Iron Steel 2015, 50, 1–6. [Google Scholar]
- Inada, T.; Kasai, A.; Nakano, K. Dissection Investigation of Blast Furnace Hearth—Kokura No. 2 Blast Furnace (2nd Campaign). ISIJ Int. 2009, 49, 470–478. [Google Scholar] [CrossRef] [Green Version]
- Jiao, K.X.; Wang, C.; Zhang, J.L. Heat Transfer Evolution Process in Hearth Based on Blast Furnace Dissection. JOM 2020, 72, 1935–1942. [Google Scholar] [CrossRef]
- Li, Y.W. Preparation of Ceramic-Bonded Carbon Block for Blast Furnace. Metall. Mater. Trans. A 2014, 45, 477–481. [Google Scholar] [CrossRef]
- Yang, D.X.; Liu, Y.G.; Fang, M.H. Study on the slag corrosion resistance of unfired Al2O3–SiC/β-Sialon/Ti(C, N)–C refractories. Ceram. Int. 2014, 40, 1593–1598. [Google Scholar] [CrossRef]
- Yong, D.; Ran, L.; Jiao, K.X. Evolution and mechanism of dissolutive wetting between hot metal and carbon brick. J. Eur. Ceram. Soc. 2022, 42, 4420–4428. [Google Scholar]
- Fan, X.Y.; Jiao, K.; Zhang, J.; Cao, R.; He, R.; Wang, K. Study on physicochemical properties of Al2O3-SiC-C castable for blast furnace. Ceram. Int. 2019, 45, 13903. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, Q.; Zhang, J.L. Erosion of Carbon Brick by Zinc in Hearth of Blast Furnace. ISIJ Int. 2019, 60, 226–232. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Jiao, K.X.; Liu, Z.J. Effects of Coke Ash on Erosion of Carbon Composite Brick. ISIJ Int. 2019, 59, 412–420. [Google Scholar] [CrossRef] [Green Version]
- Jiao, K.X.; Feng, G.X.; Zhang, J.L. Characterization and formation mechanism of graphite-rich iron protective layer in blast furnace hearth. Fuel 2021, 306, 121665. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, R.; Jiao, K.X.; Chen, Y.B. Wetting behaviour and mechanism between hot metal and carbon brick. J. Eur. Ceram. Soc. 2021, 41, 5740. [Google Scholar] [CrossRef]
- Zuo, H.B.; Wang, C.; Zhang, J.L.; Shao, J.G.; Zhao, Y.A.; Jiao, K.X. Comparison of oxidation behaviours of novel carbon composite brick with traditional carbon brick. Ceram. Int. 2015, 41, 7929. [Google Scholar] [CrossRef]
- Xu, R.Z.; Zhang, J.L.; Zhang, G.H.; Ma, R.Y.; Zhao, Y.A. Corrosion behaviour of carbon composite brick in high alumina slags. Ceram. Int. 2018, 44, 5242. [Google Scholar] [CrossRef]
- Jiao, K.X.; Fan, X.Y.; Zhang, J.L.; Wang, K.D.; Zhao, Y.A. Corrosion behaviour of alumina-carbon composite brick in typical blast furnace slag and iron. Ceram. Int. 2018, 44, 19981. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, J.L.; Jiao, K.X. Dissolution mechanism of carbon brick into molten iron. ISIJ Int. 2018, 58, 815. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.E.; Wang, Y.; Xie, B. Effects of oxides contents in vanadium slag on corrosion mechanism of MgO-C bricks. J. Iron Steel Res. 2012, 19, 36–42. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.L.; Chen, W. Comparative Analysis on the Corrosion Resistance to Molten Iron of Four Kinds of Carbon Bricks Used in Blast Furnace Hearth. Metals 2022, 12, 871. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, J.L.; Jiao, K.X. Effects of temperature and composition of molten iron on dissolution behaviour of carbon brick. Iron Steel 2018, 53, 25–31. [Google Scholar]
- Guo, H.J. Physical Chemistry of Metallurgical Tutorial, 2nd ed.; Metallurgical Industry Press: Beijing, China, 2006. [Google Scholar]




| Content | C | Al2O3 | SiO2 | SiC | TiO2 | Others |
|---|---|---|---|---|---|---|
| Value/% | 76.15 | 8.74 | 7.41 | 6.91 | 0.22 | 0.57 |
| Factors | No. | Temperature /°C | Speed /rpm | Composition of the Molten Iron/% | |||||
|---|---|---|---|---|---|---|---|---|---|
| (C) | (Si) | (Mn) | (P) | (S) | (Ti) | ||||
| Carbon content | C-1 | 1500 | 200 | 3.0 | 0.4 | 0.3 | 0.1 | 0.04 | 0.10 |
| C-2 | 1500 | 200 | 3.5 | 0.4 | 0.3 | 0.1 | 0.04 | 0.10 | |
| C-3 | 1500 | 200 | 4.0 | 0.4 | 0.3 | 0.1 | 0.04 | 0.10 | |
| C-4 | 1500 | 200 | 4.5 | 0.4 | 0.3 | 0.1 | 0.04 | 0.10 | |
| Temperature | T-1 | 1450 | 200 | 4.5 | 0.4 | 0.3 | 0.1 | 0.04 | 0.10 |
| T-2 | 1500 | 200 | 4.5 | 0.4 | 0.3 | 0.1 | 0.04 | 0.10 | |
| T-3 | 1550 | 200 | 4.5 | 0.4 | 0.3 | 0.1 | 0.04 | 0.10 | |
| Rotational speed | V-1 | 1500 | 50 | 4.5 | 0.4 | 0.3 | 0.1 | 0.04 | 0.10 |
| V-2 | 1500 | 100 | 4.5 | 0.4 | 0.3 | 0.1 | 0.04 | 0.10 | |
| V-3 | 1500 | 150 | 4.5 | 0.4 | 0.3 | 0.1 | 0.04 | 0.10 | |
| V-4 | 1500 | 200 | 4.5 | 0.4 | 0.3 | 0.1 | 0.04 | 0.10 | |
| Number | Pre-Reaction Diameter, mm | Post-Reaction Diameter, mm | Erosion Degree, mm/h | Erosion Rate, g/(h·cm2) |
|---|---|---|---|---|
| C-1 | 11.81 | 9.68 | 2.13 | 0.1421 |
| C-2 | 11.89 | 10.23 | 1.66 | 0.1106 |
| C-3 | 11.88 | 10.39 | 1.49 | 0.0993 |
| C-4 | 11.91 | 10.74 | 1.18 | 0.0784 |
| Number | Pre-Reaction Diameter, mm | Post-Reaction Diameter, mm | Erosion Degree, mm/h | Erosion Rate, g/(h·cm2) |
|---|---|---|---|---|
| T-1 | 11.89 | 11.17 | 0.73 | 0.0484 |
| T-2 | 11.91 | 10.74 | 1.18 | 0.0784 |
| T-3 | 11.97 | 3.05 | 8.93 | 0.5949 |
| Number | Pre-Reaction Diameter, mm | Post-Reaction Diameter, mm | Erosion Degree, mm/h | Erosion Rate, g/(h·cm2) |
|---|---|---|---|---|
| V-1 | 11.89 | 11.30 | 0.59 | 0.0394 |
| V-2 | 11.90 | 11.12 | 0.78 | 0.0518 |
| V-3 | 11.87 | 10.95 | 0.92 | 0.0613 |
| V-4 | 11.91 | 10.74 | 1.18 | 0.0784 |
| Interaction Coefficient | ||||||
|---|---|---|---|---|---|---|
| Value | 0.14 | 0.08 | −0.012 | 0.051 | 0.016 | −0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, H.; Wang, C.; Zong, Y.; Liu, Y.; Wang, Z.; Zhang, J. Study on Corrosion Resistance of S-Carbon Bricks for Blast Furnace Hearth in Molten Iron. Metals 2023, 13, 1240. https://doi.org/10.3390/met13071240
Shi H, Wang C, Zong Y, Liu Y, Wang Z, Zhang J. Study on Corrosion Resistance of S-Carbon Bricks for Blast Furnace Hearth in Molten Iron. Metals. 2023; 13(7):1240. https://doi.org/10.3390/met13071240
Chicago/Turabian StyleShi, Huangyu, Cui Wang, Yanbing Zong, Yanxiang Liu, Zhongyi Wang, and Jianliang Zhang. 2023. "Study on Corrosion Resistance of S-Carbon Bricks for Blast Furnace Hearth in Molten Iron" Metals 13, no. 7: 1240. https://doi.org/10.3390/met13071240







