Stress Corrosion Cracking of Ultrafine-Grained Ti-2Fe-0.1B Alloying after Equal Channel Angular Pressing
Abstract
1. Introduction
2. Materials and Methods
3. Results
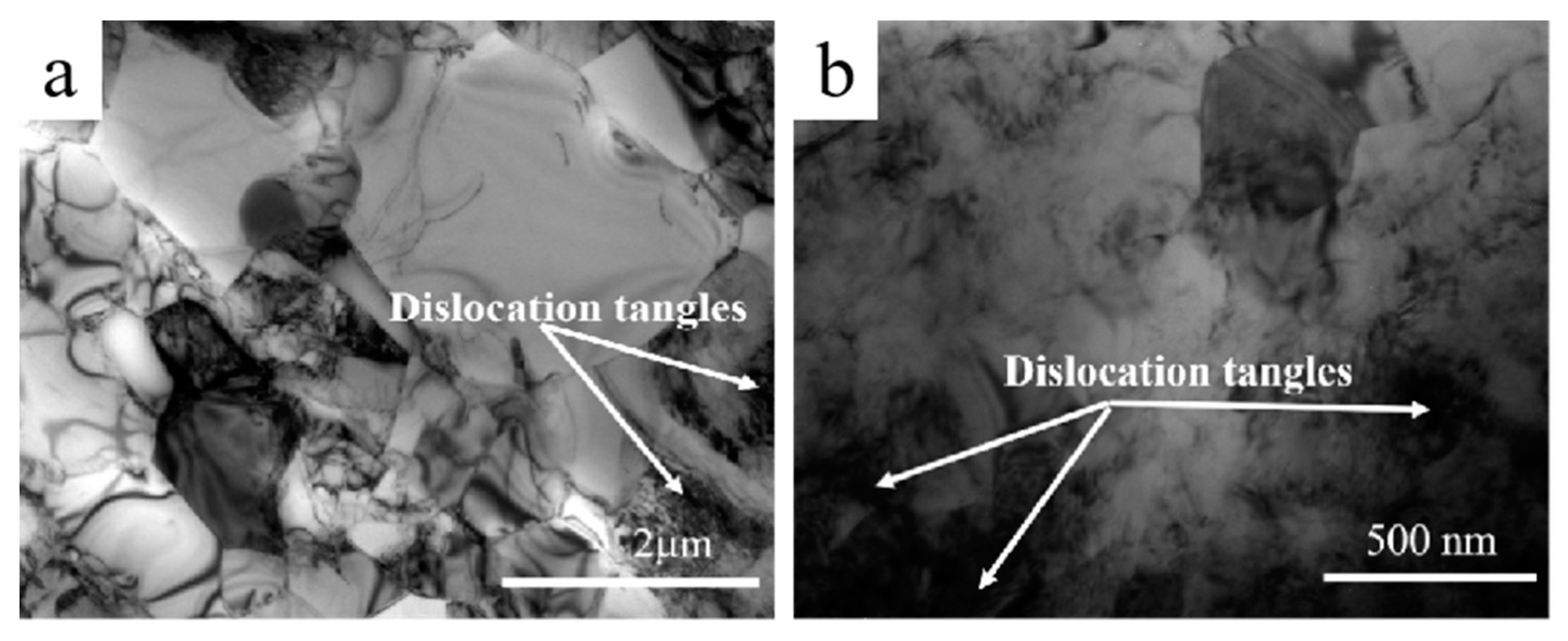
3.1. TEM Microstructural Characterization
3.2. Mechanical Testing
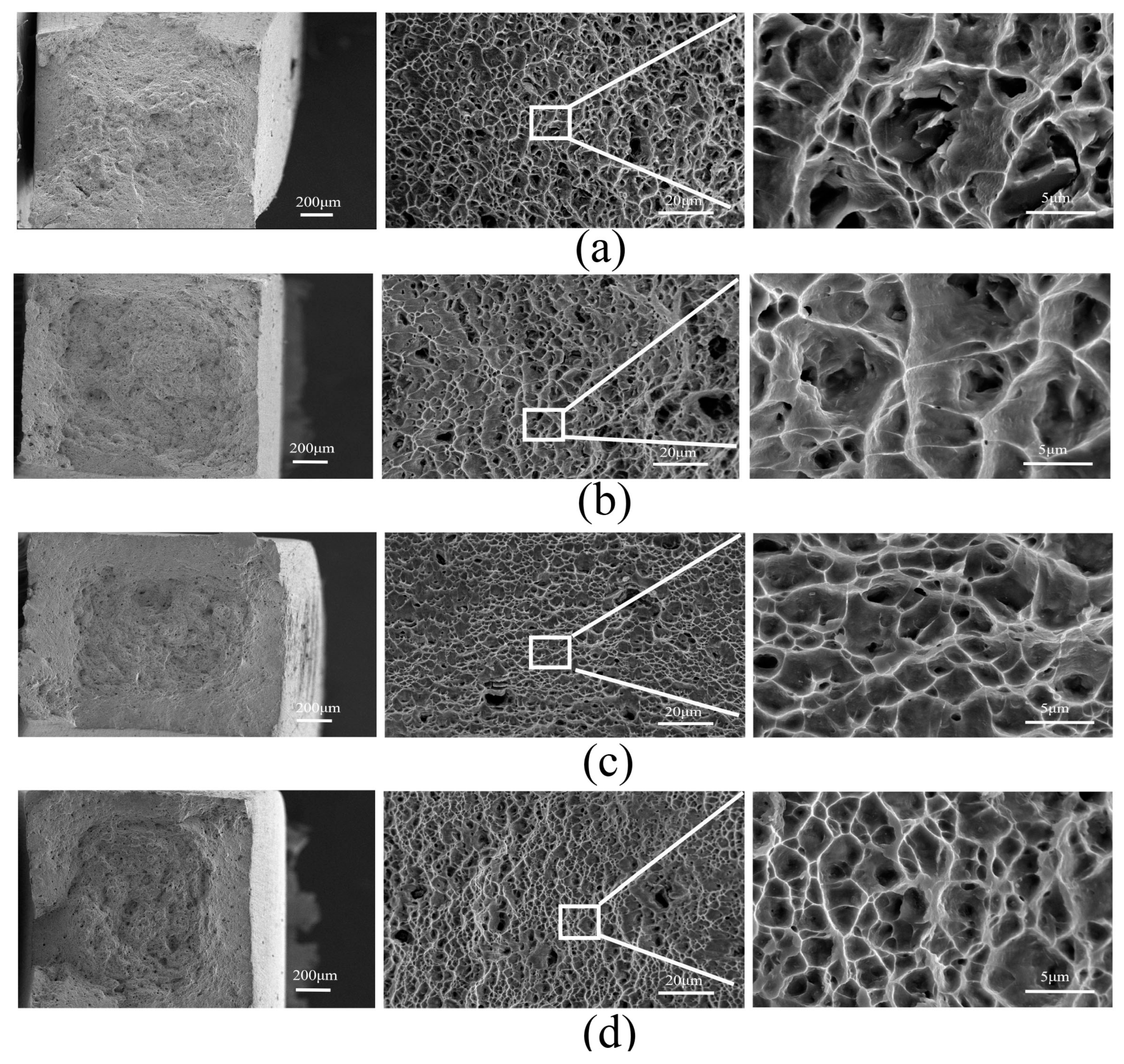
3.3. Morphology of SCC
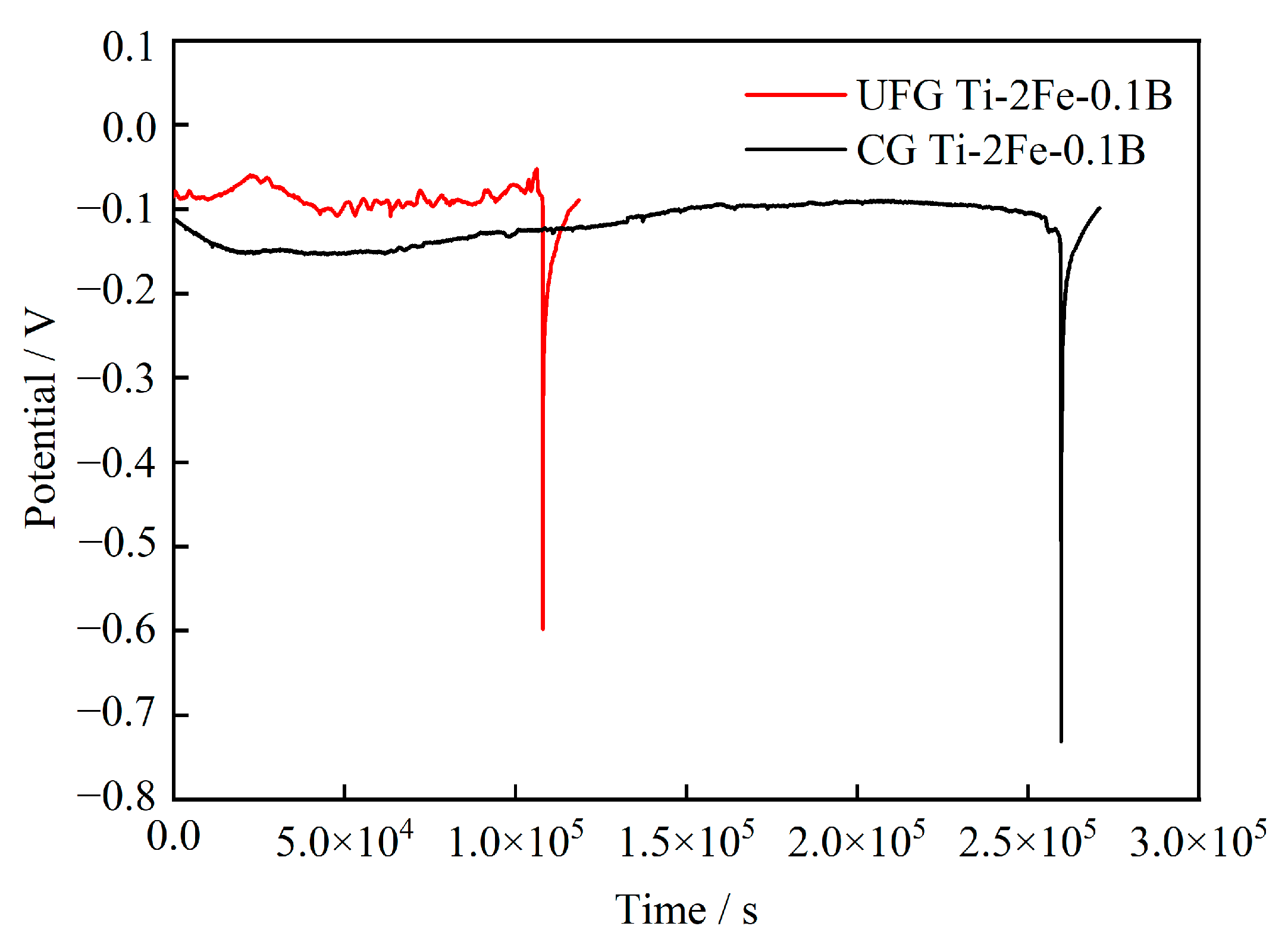
3.4. In-Situ Open Circuit Potential Result
3.5. Passivation Film Characterization
4. Discussion
5. Conclusions
- (1)
- Both UFG and CG Ti-2Fe-0.1B alloys show certain stress-corrosion sensitivity. However, the stress corrosion sensitivity of UFG Ti-2Fe-0.1B processed by ECAP is much lower than that of CG Ti-2Fe-0.1B; the ISSRT value decreased from 0.0555 to 0.0337;
- (2)
- Refinement of grain size and increase in dislocation density in UFG Ti-2Fe-0.1B can provide more sites for the nucleation of the passivation film, which enhances the passivation kinetics and increases the TiO2 content and re-passivation ability of the passivation film;
- (3)
- The UFG sample possesses high strength but low ductility; it took a relative short time and low plastic deformation from crack initiation until failure, which weakened the effect of corrosion during SSRT.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qarni, M.J.; Sivaswamy, G.; Rosochowski, A.; Boczkal, S. Effect of incremental equal channel angular pressing (I-ECAP) on the microstructural characteristics and mechanical behaviour of commercially pure titanium. Mater. Des. 2017, 122, 385–402. [Google Scholar] [CrossRef]
- Sajadifar, S.V.; Yapici, G.; Demler, E.; Krooß, P.; Wegener, T.; Maier, H.; Niendorf, T. Cyclic deformation response of ultra-fine grained titanium at elevated temperatures. Int. J. Fatigue 2019, 122, 228–239. [Google Scholar] [CrossRef]
- Claudia, G.-M.; Ivan, G.; Laia, O.-M.; Emilio, J.-P.; Maria-Pau, G.; Maurizio, V.; Luís, C.J.; Marta, P. Influence of ECAP process on mechanical, corrosion and bacterial properties of Zn-2Ag alloy for wound closure devices. Mater. Des. 2023, 228, 111817. [Google Scholar] [CrossRef]
- Dalan, F.C.; de Lima Andreani, G.F.; Travessa, D.N.; Faizov, I.A.; Faizova, S.; Cardoso, K.R. Effect of ECAP processing on distribution of second phase particles, hardness and electrical conductivity of Cu-0.81 Cr-0.07 Zr alloy. Trans. Nonferrous Met. Soc. China 2022, 32, 217–232. [Google Scholar] [CrossRef]
- Duan, Y.; Tang, L.; Xu, G.; Deng, Y.; Yin, Z. Microstructure and mechanical properties of 7005 aluminum alloy processed by room temperature ECAP and subsequent annealing. J. Alloys Compd. 2016, 664, 518–529. [Google Scholar] [CrossRef]
- Banerjee, D.; Williams, J. Perspectives on titanium science and technology. Acta Mater. 2013, 61, 844–879. [Google Scholar] [CrossRef]
- Pang, J.; Blackwood, D.J. Corrosion of titanium alloys in high temperature near anaerobic seawater. Corros. Sci. 2016, 105, 17–24. [Google Scholar] [CrossRef]
- Yu, Z.; Dong, Y.; Li, X.; Niu, J.; Alexandrov, I.; Chang, H. Study on corrosion behavior of ultrafine-grained Ti-6Al-7Nb fabricated by equal channel angular pressing. Metals 2020, 10, 950. [Google Scholar] [CrossRef]
- Maleki-Ghaleh, H.; Hajizadeh, K.; Hadjizadeh, A.; Shakeri, M.; Alamdari, S.G.; Masoudfar, S.; Aghaie, E.; Javidi, M.; Zdunek, J.; Kurzydlowski, K. Electrochemical and cellular behavior of ultrafine-grained titanium in vitro. Mater. Sci. Eng. C 2014, 39, 299–304. [Google Scholar] [CrossRef]
- Kim, H.S.; Yoo, S.J.; Ahn, J.W.; Kim, D.H.; Kim, W.J. Ultrafine grained titanium sheets with high strength and high corrosion resistance. Mater. Sci. Eng. A 2011, 528, 8479–8485. [Google Scholar] [CrossRef]
- Nie, M.; Wang, C.T.; Qu, M.; Gao, N.; Wharton, J.A.; Langdon, T.G. The corrosion behaviour of commercial purity titanium processed by high-pressure torsion. J. Mater. Sci. 2014, 49, 2824–2831. [Google Scholar] [CrossRef]
- Garbacz, H.; Pisarek, M.; Kurzydłowski, K. Corrosion resistance of nanostructured titanium. Biomol. Eng. 2007, 24, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Balyanov, A.; Kutnyakova, J.; Amirkhanova, N.; Stolyarov, V.; Valiev, R.; Liao, X.; Zhao, Y.; Jiang, Y.; Xu, H.; Lowe, T. Corrosion resistance of ultra fine-grained Ti. Scr. Mater. 2004, 51, 225–229. [Google Scholar] [CrossRef]
- Hoseini, M.; Shahryari, A.; Omanovic, S.; Szpunar, J.A. Comparative effect of grain size and texture on the corrosion behaviour of commercially pure titanium processed by equal channel angular pressing. Corros. Sci. 2009, 51, 3064–3067. [Google Scholar] [CrossRef]
- Gurao, N.; Manivasagam, G.; Govindaraj, P.; Asokamani, R.; Suwas, S. Effect of texture and grain size on bio-corrosion response of ultrafine-grained titanium. Metall. Mater. Trans. A 2013, 44, 5602–5610. [Google Scholar] [CrossRef]
- Li, X.; Dong, Y.; Dan, Z.; Chang, H.; Fang, Z.; Guo, Y. Corrosion behavior of ultrafine grained pure Ti processed by equal channel angular pressing. Acta Metall. Sin. 2019, 55, 967–975. [Google Scholar]
- Ren, S.; Du, C.; Liu, Z.; Li, X.; Xiong, J.; Li, S. Effect of fluoride ions on corrosion behaviour of commercial pure titanium in artificial seawater environment. Appl. Surf. Sci. 2020, 506, 144759. [Google Scholar] [CrossRef]
- Dong, Y.; Huang, S.; Wang, Y.; Zhang, B.; Alexandrov, I.; Chang, H.; Dan, Z.; Ma, L.; Zhou, L. Stress corrosion cracking of TC4 ELI alloy with different microstructure in 3.5% NaCl solution. Mater. Charact. 2022, 194, 112357. [Google Scholar] [CrossRef]
- Chi, G.; Jiang, B.; Yi, D.; Yang, L.; Pan, S.; Liu, H.; Feng, C.; Mao, W. Effect of lamellar α on the stress corrosion cracking of Ti-6Al-4 V alloy in simulated oilfield brine. Mater. Charact. 2021, 174, 111000. [Google Scholar] [CrossRef]
- Liu, R.; Xie, Y.; Jin, Y.; Cui, Y.; Liu, L.; Wang, F. Stress corrosion cracking of the titanium alloys under hydrostatic pressure resulting from the degradation of passive films. Acta Mater. 2023, 252, 118946. [Google Scholar] [CrossRef]
- Joseph, S.; Lindley, T.C.; Dye, D.; Saunders, E.A. The mechanisms of hot salt stress corrosion cracking in titanium alloy Ti-6Al-2Sn-4Zr-6Mo. Corros. Sci. 2018, 134, 169–178. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Alexandrov, I.; Ma, L.; Dong, Y.; Valiev, R.; Chang, H.; Zhang, B.; Wang, Y.; Zhou, L. Impact of equal channel angular pressing on mechanical behavior and corrosion resistance of hot-rolled Ti-2Fe-0.1 B alloy. Materials 2020, 13, 5117. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Dai, G.; Guo, Y.; Sun, Z.; Dan, Z.; Dong, Y.; Chang, H.; Alexandrov, I.V.; Zhou, L. Microstructure and mechanical properties of B modified Ti–Fe alloy manufactured by casting, forging and laser melting deposition. Compos. Part B Eng. 2021, 216, 108854. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Chang, H.; Alexandrov, I.; Sun, Z.; Dong, Y.; Valiev, R.; Wang, Y.; Zhou, L. Effect of hydrogen on microstructure evolution and deformation behaviors of Ti-2Fe-0.1 B alloy. J. Alloys Compd. 2022, 900, 163473. [Google Scholar] [CrossRef]
- HB7235-1995; Standard Practice for Slow Strain Rate Testing to Evaluate Stress Corrosion Cracking Susceptibility. Aviation Industry Corporation of China: Beijing, China, 1995.
- Ren, L.; Xiao, W.; Chang, H.; Zhao, Y.; Ma, C.; Zhou, L. Microstructural tailoring and mechanical properties of a multi-alloyed near β titanium alloy Ti-5321 with various heat treatment. Mater. Sci. Eng. A 2018, 711, 553–561. [Google Scholar] [CrossRef]
- Bai, Y.; Gai, X.; Li, S.; Zhang, L.-C.; Liu, Y.; Hao, Y.; Zhang, X.; Yang, R.; Gao, Y. Improved corrosion behaviour of electron beam melted Ti-6Al–4V alloy in phosphate buffered saline. Corros. Sci. 2017, 123, 289–296. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Bolivar, J.; Shi, Y.; Réthoré, J.; King, A.; Fregonese, M.; Adrien, J.; Buffiere, J.Y.; Baietto, M.C. A phase field method for modeling anodic dissolution induced stress corrosion crack propagation. Corros. Sci. 2018, 132, 146–160. [Google Scholar] [CrossRef]
- Shi, Y.; Joseph, S.; Saunders, E.A.; Sandala, R.S.; Walker, A.; Lindley, T.C.; Dye, D. AgCl-induced hot salt stress corrosion cracking in a titanium alloy. Corros. Sci. 2021, 187, 109497. [Google Scholar] [CrossRef]
- Joseph, S.; Kontis, P.; Chang, Y.; Shi, Y.; Raabe, D.; Gault, B.; Dye, D. A cracking oxygen story: A new view of stress corrosion cracking in titanium alloys. Acta Mater. 2022, 227, 117687. [Google Scholar] [CrossRef]
- Cui, Y.-W.; Chen, L.-Y.; Liu, X.-X. Pitting corrosion of biomedical titanium and titanium alloys: A brief review. Curr. Nanosci. 2021, 17, 241–256. [Google Scholar] [CrossRef]
- Wang, C.M.; Zhang, L.J.; Ma, Y.J.; Zhang, S.Z.; Yang, R.; Hu, Q.M. Hydrogen-surface interaction from first-principles calculations and its implication to hydrogen embrittlement mechanisms of titanium. Appl. Surf. Sci. 2023, 621, 156871. [Google Scholar] [CrossRef]
- Gong, P.; Nutter, J.; Rivera-Diaz-Del-Castillo, P.; Rainforth, W. Hydrogen embrittlement through the formation of low-energy dislocation nanostructures in nanoprecipitation-strengthened steels. Sci. Adv. 2020, 6, eabb6152. [Google Scholar] [CrossRef]
- Danoix, F.; Hoummada, K.; Maugis, P.; Rolland, N.; Debreux, C.; Blavette, D. Grain size effect on interfacial segregation in nanomaterials. J. Phys. Chem. Solids 2022, 164, 110620. [Google Scholar] [CrossRef]
- Palumbo, G.; Erb, U. Enhancing the operating life and performance of lead-acid batteries via grain-boundary engineering. mRS Bull. 1999, 24, 27–32. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Dong, Y.; Xu, D.; Zhang, X.; Wu, J.; Gu, T.; Wang, F. Synergistic effect of chloride ion and Shewanella algae accelerates the corrosion of Ti-6Al-4V alloy. J. Mater. Sci. Technol. 2021, 71, 177–185. [Google Scholar] [CrossRef]
- Chen, L.Y.; Shen, P.; Zhang, L.; Lu, S.; Chai, L.; Yang, Z.; Zhang, L.C. Corrosion behavior of non-equilibrium Zr-Sn-Nb-Fe-Cu-O alloys in high-temperature 0.01 M LiOH aqueous solution and degradation of the surface oxide films. Corros. Sci. 2018, 136, 221–230. [Google Scholar] [CrossRef]
- Qin, T.; Lin, X.; Yu, J.; Wang, M.; Guo, P.; Li, J.; Zhang, Y.; Liu, J.; Zhang, S.; Huang, W. Performance of different microstructure on electrochemical behaviors of laser solid formed Ti-6Al-4V alloy in NaCl solution. Corros. Sci. 2021, 185, 109392. [Google Scholar] [CrossRef]
- Gu, Y.; Ma, A.; Jiang, J.; Li, H.; Song, D.; Wu, H.; Yuan, Y. Simultaneously improving mechanical properties and corrosion resistance of pure Ti by continuous ECAP plus short-duration annealing. Mater. Charact. 2018, 138, 38–47. [Google Scholar] [CrossRef]
- Argade, G.; Panigrahi, S.; Mishra, R. Effects of grain size on the corrosion resistance of wrought magnesium alloys containing neodymium. Corros. Sci. 2012, 58, 145–151. [Google Scholar] [CrossRef]
- Wang, J.; Song, S.; Li, D. Diffusivity of a point defect in the passive film on Ti with plastic deformation in 3.5% NaCl solution. Thin Solid Film. 2022, 754, 139304. [Google Scholar] [CrossRef]
- Macdonald, D.D. The history of the point defect model for the passive state: A brief review of film growth aspects. Electrochim. Acta 2011, 56, 1761–1772. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Lee, B.; Kim, T.; Panigrahi, B. Corrosion behaviour of ultra fine grained titanium in simulated body fluid for implant application. Trends Biomater. Artif. Organs 2008, 22, 58–64. [Google Scholar]



| Sample | σ0.2/MPa | σb/MPa | A/% | σ0.2/σb | ISSRT |
|---|---|---|---|---|---|
| CG Ti-2Fe-0.1B in air | 381 ± 5 | 629 ± 7 | 28 ± 1.2 | 0.6 | 0.0555 |
| CG Ti-2Fe-0.1B in NaCl | 360 ± 9 | 608 ± 10 | 25 ± 1.4 | 0.6 | |
| UFG Ti-2Fe-0.1B in air | 670 ± 4 | 834 ± 4 | 10 ± 0.7 | 0.8 | 0.0337 |
| UFG Ti-2Fe-0.1B in NaCl | 657 ± 6 | 807 ± 6 | 10 ± 0.9 | 0.8 |
| Composition (%) | CG Ti-2Fe-0.1B | UFG Ti-2Fe-0.1B | ||||||
|---|---|---|---|---|---|---|---|---|
| Surface | 2 nm | 4 nm | 8 nm | Surface | 2 nm | 4 nm | 8 nm | |
| TiO2 | 54.3 | 36.5 | 13.4 | 0 | 63.3 | 46.8 | 28.3 | 0 |
| Ti2O3 | 0 | 25.7 | 36.8 | 30.5 | 0 | 21.4 | 26.6 | 36.2 |
| TiO | 30.5 | 17.3 | 19.5 | 36.1 | 24.2 | 13.2 | 18.6 | 34.5 |
| Ti | 15.2 | 20.5 | 30.3 | 33.4 | 12.5 | 18.6 | 26.5 | 29.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Jin, Y.; Wang, Y.; Dong, Y.; Chang, H.; Alexandrov, I.V. Stress Corrosion Cracking of Ultrafine-Grained Ti-2Fe-0.1B Alloying after Equal Channel Angular Pressing. Metals 2023, 13, 1316. https://doi.org/10.3390/met13071316
Huang S, Jin Y, Wang Y, Dong Y, Chang H, Alexandrov IV. Stress Corrosion Cracking of Ultrafine-Grained Ti-2Fe-0.1B Alloying after Equal Channel Angular Pressing. Metals. 2023; 13(7):1316. https://doi.org/10.3390/met13071316
Chicago/Turabian StyleHuang, Shuai, Yutong Jin, Yu Wang, Yuecheng Dong, Hui Chang, and Igor V. Alexandrov. 2023. "Stress Corrosion Cracking of Ultrafine-Grained Ti-2Fe-0.1B Alloying after Equal Channel Angular Pressing" Metals 13, no. 7: 1316. https://doi.org/10.3390/met13071316
APA StyleHuang, S., Jin, Y., Wang, Y., Dong, Y., Chang, H., & Alexandrov, I. V. (2023). Stress Corrosion Cracking of Ultrafine-Grained Ti-2Fe-0.1B Alloying after Equal Channel Angular Pressing. Metals, 13(7), 1316. https://doi.org/10.3390/met13071316






