High-Throughput Synthesis and Characterization Screening of Mg-Cu-Y Metallic Glass
Abstract
:1. Introduction
High-Throughput Methods for MG Synthesis
2. Materials and Methods
2.1. Materials
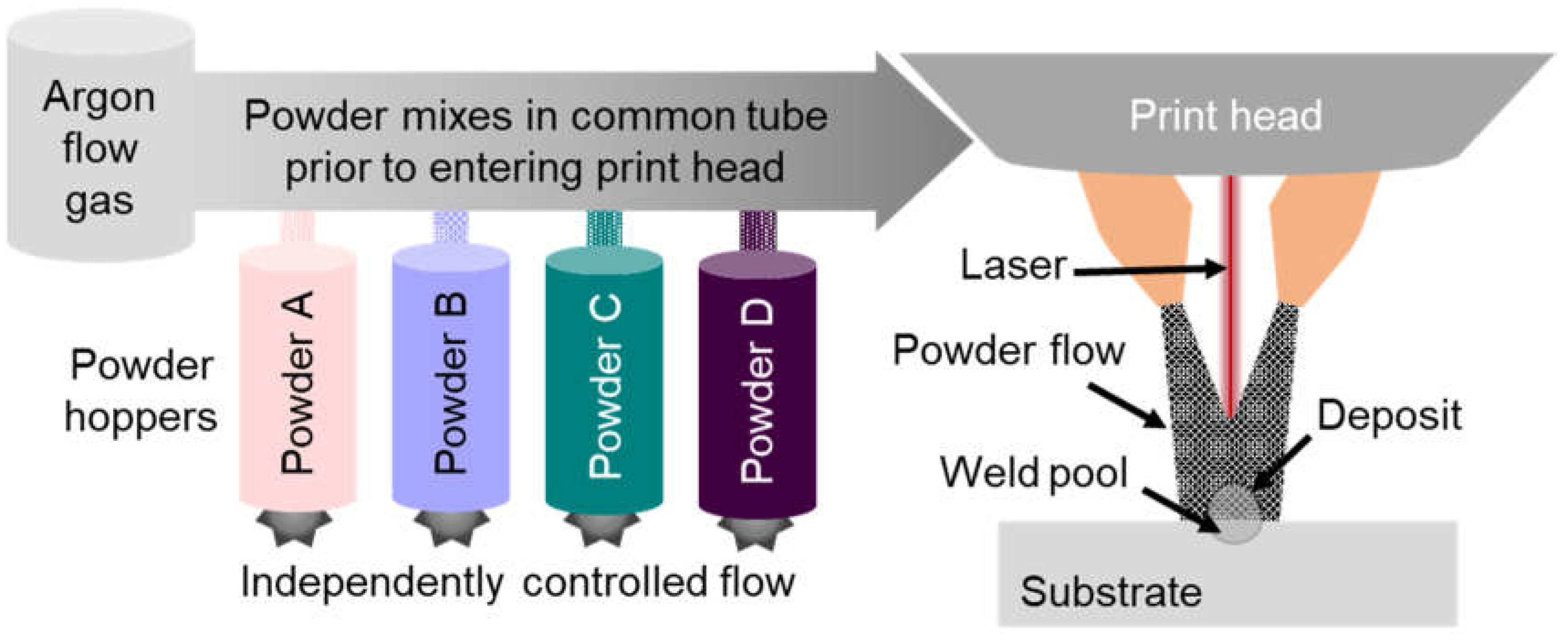
2.2. High-Throughput Processing Methodology
2.3. Characterization
3. Results
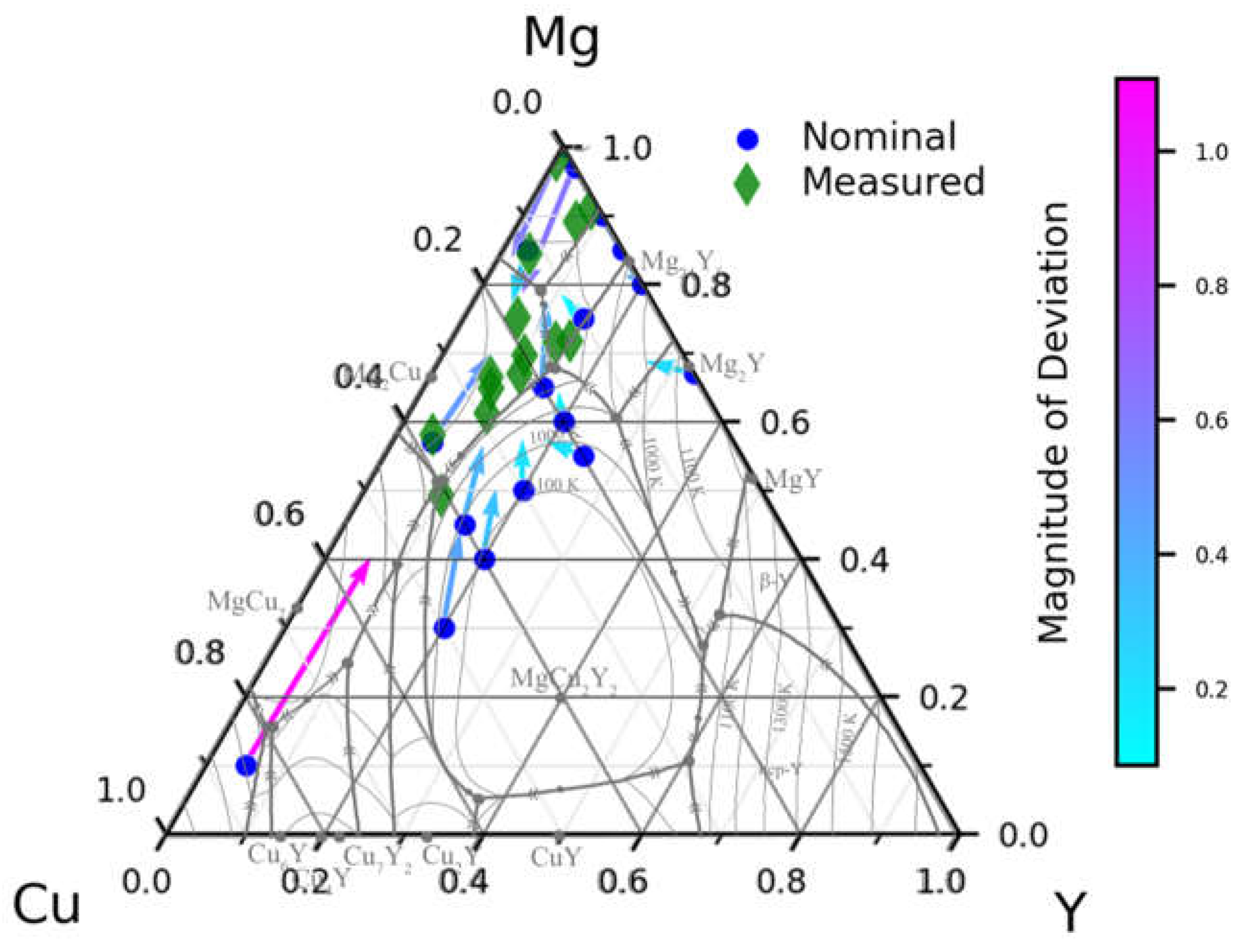
3.1. High-Throughput Synthesis of Nominal Alloy Compositions
3.2. High-Throughput Optical Investigation of Amorphous Regions
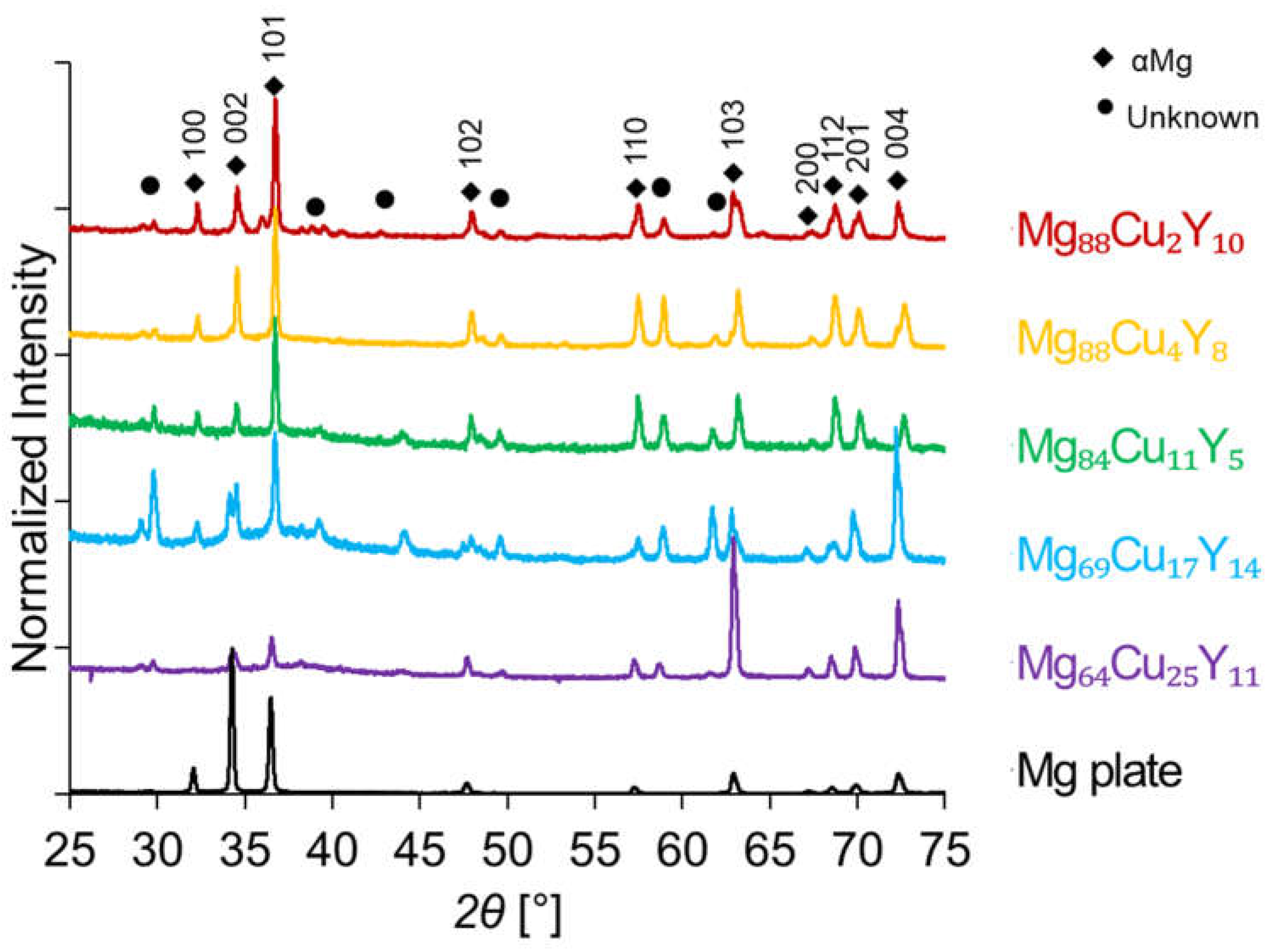
3.3. X-ray Diffraction Analysis of High Luminance Samples
3.4. EDS-SEM Analysis of High Luminance Samples and XRD Screening
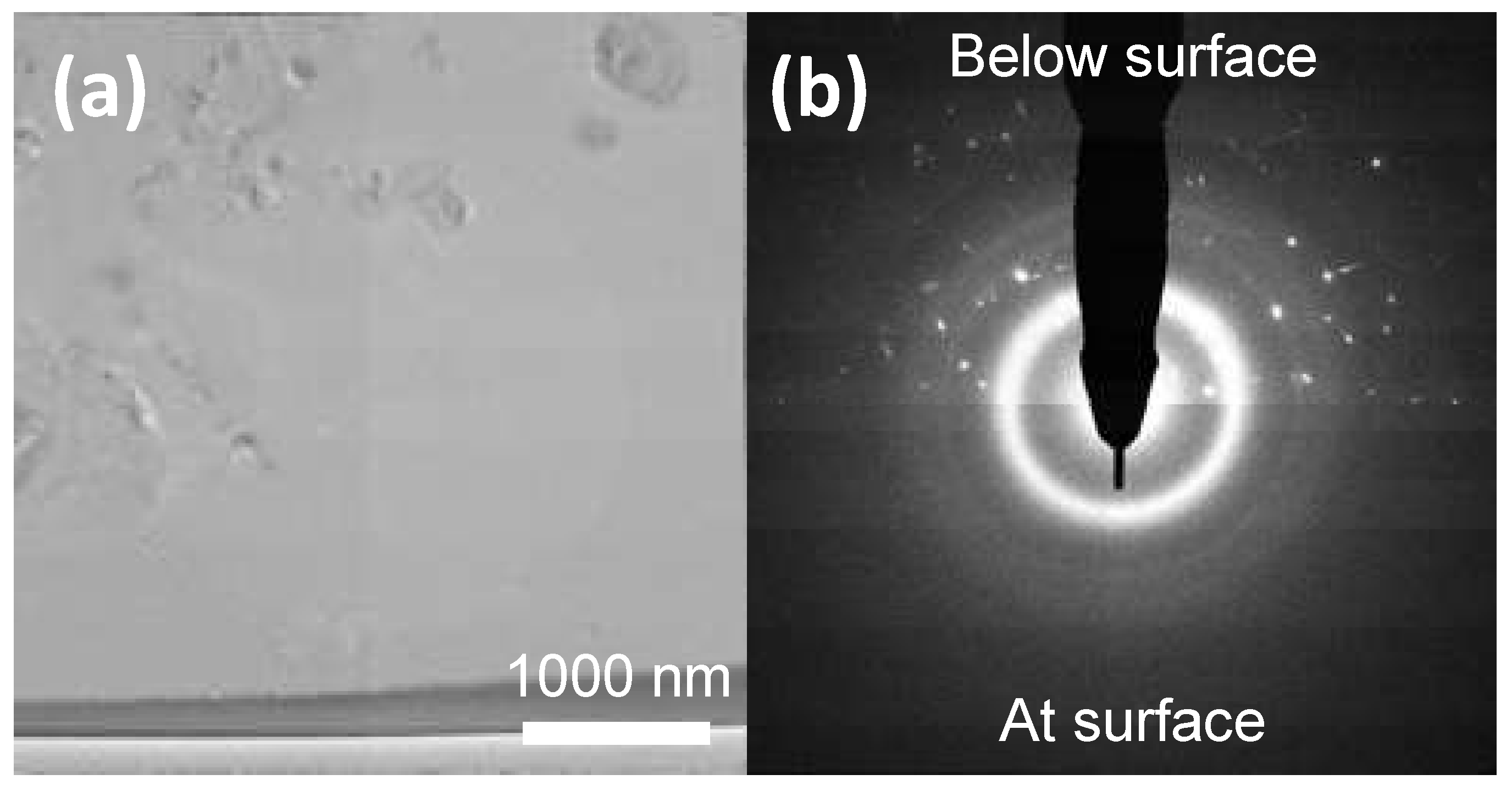
3.5. TEM Validation
4. Discussion
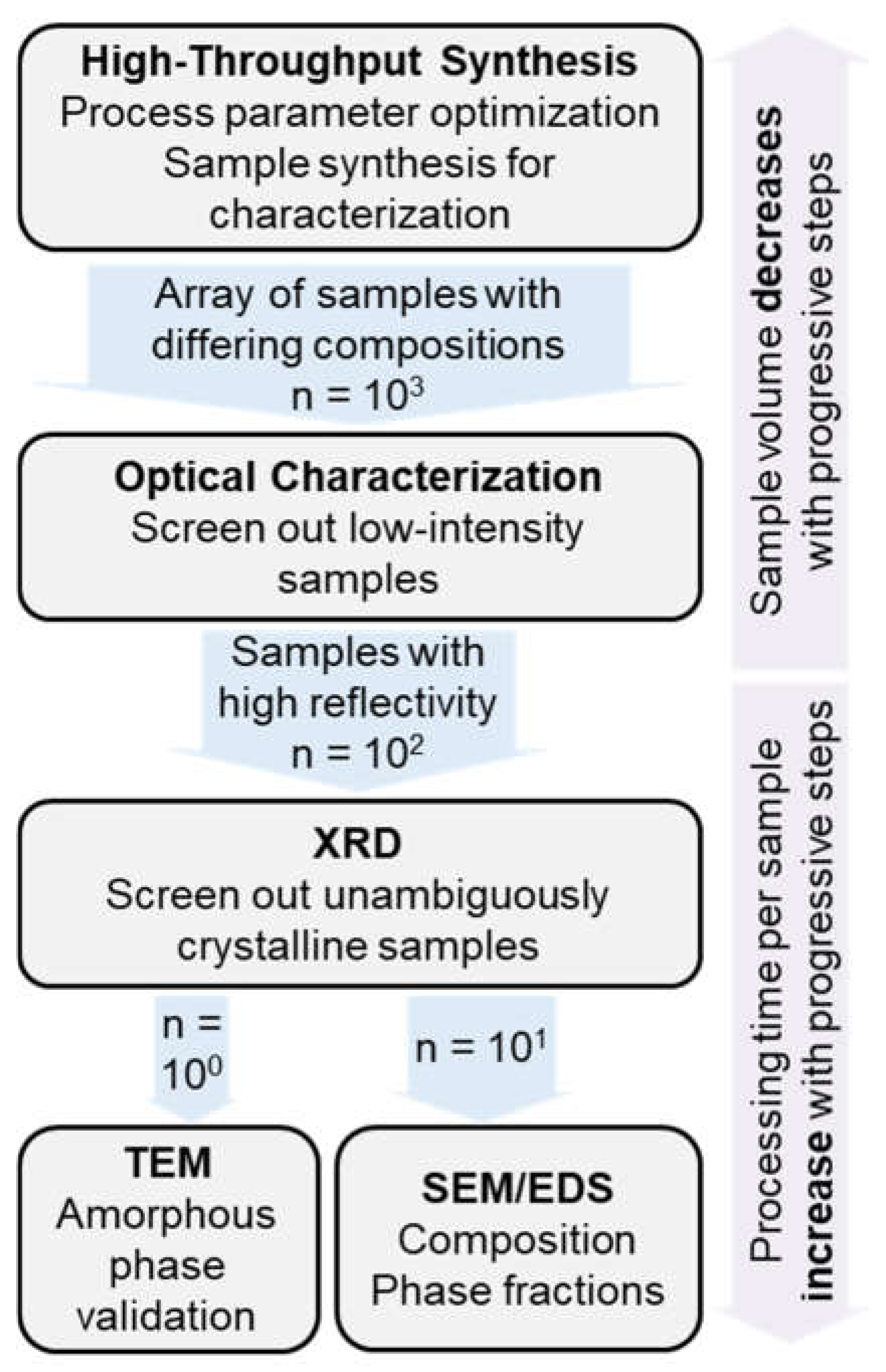
4.1. Combinatorial Experimentation: HT Screening and Down-Selection
4.2. Data Fidelity
5. Conclusions
- A total of 1000 “bulk” samples with different compositions can be synthesized in a day.
- A tiered level of HT “screening” permits a down-selection to one successful alloy within a week. The screening included optical luminance, XRD, EDS-SEM analysis, and a final validation of an amorphous composition with a TEM investigation.
- The directed energy deposition synthesis process is three orders of magnitude faster than conventional bulk methods such as arc casting or melt spinning.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Klement, W.; Willens, R.H.; Duwez, P. Non-crystalline Structure in Solidified Gold–Silicon Alloys. Nature 1960, 187, 869–870. [Google Scholar] [CrossRef]
- Dambatta, M.S.; Izman, S.; Yahaya, B.; Lim, J.Y.; Kurniawan, D. Mg-based bulk metallic glasses for biodegradable implant ma-terials: A review on glass forming ability, mechanical properties, and biocompatibility. J. Non-Cryst. Solids 2015, 426, 110–115. [Google Scholar] [CrossRef]
- Li, H.F.; Zheng, Y.F. Recent advances in bulk metallic glasses for biomedical applications. Acta Biomater. 2016, 36, 1–20. [Google Scholar] [CrossRef]
- Inoue, A.; Zhang, T.; Masumoto, T. Glass-forming ability of alloys. J. Non-Cryst. Solids 1993, 156–158, 473–480. [Google Scholar] [CrossRef]
- Inoue, A.; Takeuchi, A. Recent development and application products of bulk glassy alloys. Acta Mater. 2011, 59, 2243–2267. [Google Scholar] [CrossRef]
- Kozieł, T. Estimation of cooling rates in suction casting and copper-mould casting processes. Arch. Met. Mater. 2015, 60, 767–771. [Google Scholar] [CrossRef]
- Perepezko, J.H.; Hildal, K. Analysis of solidification microstructures during wedge-casting. Philos. Mag. 2006, 86, 3681–3701. [Google Scholar] [CrossRef]
- Kozieł, T.; Matusiewicz, P.; Kopyściański, M.; Zielińska-Lipiec, A. Estimation of the cooling rate in 3 mm suction-cast rods based on the microstructural features. Met. Foundry Eng. 2013, 39, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Lu, Z.; Liu, C. Identify the best glass forming ability criterion. Intermetallics 2010, 18, 883–888. [Google Scholar] [CrossRef]
- Chattopadhyay, C.; Idury, K.S.N.S.; Bhatt, J.; Mondal, K.; Murty, B.S. Critical evaluation of glass forming ability criteria. Mater. Sci. Technol. 2016, 32, 380–400. [Google Scholar] [CrossRef]
- Liu, G.; Sohn, S.; Kube, S.A.; Raj, A.; Mertza, A.; Nawano, A.; Gilbert, A.; Shattuck, M.D.; O’Hern, C.S.; Schroers, J. Machine learning versus human learning in predicting glass-forming ability of metallic glasses. Acta Mater. 2023, 243, 1189497. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, C. A new glass-forming ability criterion for bulk metallic glasses. Acta Mater. 2002, 50, 3501–3512. [Google Scholar] [CrossRef]
- Hrubý, A. Evaluation of glass-forming tendency by means of DTA. Czechoslov. J. Phys. 1972, 22, 1187–1193. [Google Scholar] [CrossRef]
- Mondal, K.; Murty, B. On the parameters to assess the glass forming ability of liquids. J. Non-Cryst. Solids 2005, 351, 1366–1371. [Google Scholar] [CrossRef]
- Chen, Q.; Shen, J.; Zhang, D.; Fan, H.; Sun, J.; McCartney, D. A new criterion for evaluating the glass-forming ability of bulk metallic glasses. Mater. Sci. Eng. A 2006, 433, 155–160. [Google Scholar] [CrossRef]
- Xia, L.; Fang, S.S.; Wang, Q.; Dong, Y.D.; Liu, C.T. Thermodynamic modeling of glass formation in metallic glasses. Appl. Phys. Lett. 2006, 88, 171905. [Google Scholar] [CrossRef]
- Xia, M.; Zhang, S.; Ma, C.; Li, J. Evaluation of glass-forming ability for metallic glasses based on order-disorder competition. Appl. Phys. Lett. 2006, 89, 091917. [Google Scholar] [CrossRef]
- Du, X.H.; Huang, J.C.; Liu, C.T.; Lu, Z.P. New criterion of glass forming ability for bulk metallic glasses. J. Appl. Phys. 2007, 101, 086108. [Google Scholar] [CrossRef] [Green Version]
- Du, X.H.; Huang, J.C. New criterion in predicting glass forming ability of various glass-forming systems. Chin. Phys. B 2008, 17, 249–254. [Google Scholar] [CrossRef]
- Yuan, Z.-Z.; Bao, S.-L.; Lu, Y.; Zhang, D.-P.; Yao, L. A new criterion for evaluating the glass-forming ability of bulk glass forming alloys. J. Alloys Compd. 2008, 459, 251–260. [Google Scholar] [CrossRef]
- Long, Z.; Wei, H.; Ding, Y.; Zhang, P.; Xie, G.; Inoue, A. A new criterion for predicting the glass-forming ability of bulk metallic glasses. J. Alloys Compd. 2009, 475, 207–219. [Google Scholar] [CrossRef]
- Zhang, G.-H.; Chou, K.-C. A criterion for evaluating glass-forming ability of alloys. J. Appl. Phys. 2009, 106, 094902. [Google Scholar] [CrossRef]
- Egami, T.; Waseda, Y. Atomic size effect on the formability of metallic glasses. J. Non-Cryst. Solids 1984, 64, 113–134. [Google Scholar] [CrossRef]
- Fang, S.; Xiao, X.; Xia, L.; Li, W.; Dong, Y. Relationship between the widths of supercooled liquid regions and bond parameters of Mg-based bulk metallic glasses. J. Non-Cryst. Solids 2003, 321, 120–125. [Google Scholar] [CrossRef]
- Nascimento, C.; Junior, A.J. New criterion for prediction of amorphous alloy compositions: A combination of dense packing of spheres and the lambda criterion through the coordination number. Appl. Mech. Mater. 2014, 698, 411. [Google Scholar] [CrossRef]
- Krska, S.W.; DiRocco, D.A.; Dreher, S.D.; Shevlin, M. The evolution of chemical high-throughput experimentation to address challenging problems in pharmaceutical synthesis. Accounts Chem. Res. 2017, 50, 2976–2985. [Google Scholar] [CrossRef] [PubMed]
- Mennen, S.M.; Alhambra, C.; Allen, C.L.; Barberis, M.; Berritt, S.; Brandt, T.A.; Campbell, A.D.; Castañón, J.; Cherney, A.H.; Christensen, M.; et al. High-throughput experimentation in pharmaceutical development and per-spectives on the future. Org. Process Res. Dev. 2019, 23, 1213. [Google Scholar] [CrossRef]
- Wildey, M.J.; Haunso, A.; Tudor, M.; Webb, M.; Connick, J.H. Chapter Five—High-Throughput Screening. In Annual Reports in Medicinal Chemistry; Goodnow, R.A., Ed.; Academic Press: Cambridge, MA, USA, 2017; p. 149. [Google Scholar]
- Guerrero-Sanchez, C.; Paulus, R.M.; Fijten, M.W.M.; de la Mar, M.J.; Hoogenboom, R.; Schubert, U.S. High-throughput experi-mentation in synthetic polymer chemistry: From RAFT and anionic polymerizations to process development. Appl. Surf. Sci. 2006, 252, 2555–2561. [Google Scholar] [CrossRef]
- Konishi, T.; Sakamoto, T.; Kikkojin, T.; Yasumori, A.; Suehara, S.; Todoroki, S.; Inoue, S. Combinatorial approach to new glasses. Appl. Surf. Sci. 2006, 252, 2450–2455. [Google Scholar] [CrossRef]
- Moorehead, M.; Bertsch, K.; Niezgoda, M.; Parkin, C.; Elbakhshwan, M.; Sridharan, K.; Zhang, C.; Thoma, D.; Couet, A. High-throughput synthesis of Mo-Nb-Ta-W high-entropy alloys via additive manufacturing. Mater. Des. 2020, 187, 108358. [Google Scholar] [CrossRef]
- Ding, S.; Liu, Y.; Li, Y.; Liu, Z.; Sohn, S.; Walker, F.J.; Schroers, J. Combinatorial development of bulk metallic glasses. Nat. Mater. 2014, 13, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Padmanabhan, J.; Cheung, B.; Liu, J.; Chen, Z.; Scanley, B.E.; Wesolowski, D.; Pressley, M.; Broadbridge, C.C.; Altman, S.; et al. Combinatorial development of antibacterial Zr-Cu-Al-Ag thin film metallic glasses. Sci. Rep. 2016, 6, 26950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, P.; Flores, K.M. A combinatorial strategy for metallic glass design via laser deposition. Intermetallics 2014, 55, 162–166. [Google Scholar] [CrossRef]
- Polanski, M.; Kwiatkowska, M.; Kunce, I.; Bystrzycki, J. Combinatorial synthesis of alloy libraries with a progressive composition gradient using laser engineered net shaping (LENS): Hydrogen storage alloys. Int. J. Hydrogen Energy 2013, 38, 12159–12171. [Google Scholar] [CrossRef]
- Nag, S.; Banerjee, R.; Fraser, H.L. A novel combinatorial approach for understanding microstructural evolution and its rela-tionship to mechanical properties in metallic biomaterials. Acta Biomater. 2007, 3, 369–376. [Google Scholar] [CrossRef]
- Vilar, R.; Colaço, R. Laser-assisted combinatorial methods for rapid design of wear resistant iron alloys. Surf. Coat. Technol. 2009, 203, 2878–2885. [Google Scholar] [CrossRef]
- Ma, H.; Shi, L.L.; Xu, J.; Li, Y.; Ma, E. Discovering inch-diameter metallic glasses in three-dimensional composition space. Appl. Phys. Lett. 2005, 87, 2126794. [Google Scholar] [CrossRef]
- Liu, G.; Gao, P.; Yang, S.; Xue, Z.; Zhang, M. Effects of Zn addition on the glass forming ability and mechanical properties of Mg–Cu–Gd bulk metallic glasses. J. Alloys Compd. 2014, 588, 59–63. [Google Scholar] [CrossRef]
- Su, S.Y.; He, Y.; Shiflet, G.J.; Poon, S.J. Formation and properties of Mg-based metallic glasses in Mg-TM-X alloys (TM=Cu or Ni; X=Sn, Si, Ge, Zn, Sb, Bi or In). Mater. Sci. Eng. A 1994, 185, 115–121. [Google Scholar] [CrossRef]
- Wu, X.F.; Kang, Y.; Wu, F.F.; Qiu, K.Q.; Meng, L.K. Formation of ternary Mg-Cu-Dy bulk metallic glasses. Bull. Mater. Sci. 2011, 34, 1507–1510. [Google Scholar] [CrossRef] [Green Version]
- Park, E.S.; Kang, H.G.; Kim, W.T.; Kim, D.H. The effect of Ag addition on the glass-forming ability of Mg–Cu–Y metallic glass alloys. J. Non-Cryst. Solids 2001, 279, 154–160. [Google Scholar] [CrossRef]
- Barbee, T.W.; Holmes, W.H.; Keith, D.L.; Pyzyna, M.K.; Ilonca, G. Synthesis of amorphous niobium-nickel alloys by vapor quenching. Thin Solid Films 1977, 45, 591–599. [Google Scholar] [CrossRef]
- Liu, Y.H.; Fujita, T.; Aji, D.P.B.; Matsuura, M.; Chen, M.W. Structural origins of Johari-Goldstein relaxation in a metallic glass. Nat. Commun. 2014, 5, 3238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeman, P.; Zítek, M.; Zuzjaková, Š.; Čerstvý, R. Amorphous Zr-Cu thin-film alloys with metallic glass behavior. J. Alloys Compd. 2017, 696, 1298–1306. [Google Scholar] [CrossRef]
- Wu, W.; Li, X.; Liu, Q.; Fuh, J.Y.H.; Zheng, A.; Zhou, Y.; Ren, L.; Li, G. Additive manufacturing of bulk metallic glass: Principles, materials and prospects. Mater. Today Adv. 2022, 16, 100319. [Google Scholar] [CrossRef]
- Sohrabi, N.; Jhabvala, J.; Logé, R.E. Additive manufacturing of bulk metallic glasses—process, challenges and properties: A review. Metals 2021, 11, 1279. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, M.; Chen, C. Effect of laser processing parameters on porosity, microstructure and mechanical properties of porous Mg-Ca alloys produced by laser additive manufacturing. Mater. Sci. Eng. A 2017, 703, 359–371. [Google Scholar] [CrossRef]
- Ng, C.; Savalani, M.; Lau, M.; Man, H. Microstructure and mechanical properties of selective laser melted magnesium. Appl. Surf. Sci. 2011, 257, 7447–7454. [Google Scholar] [CrossRef]
- Manvatkar, V.; De, A.; DebRoy, T. Heat transfer and material flow during laser assisted multi-layer additive manufacturing. J. Appl. Phys. 2014, 116, 124905. [Google Scholar] [CrossRef] [Green Version]
- Hofmeister, W.; Griffith, M. Solidification in direct metal deposition by LENS processing. JOM 2001, 53, 30–34. [Google Scholar] [CrossRef]
- Thoma, D.; Lewis, G.; Nemec, R. Solidification behavior during directed light fabrication. In Beam Processing of Advanced Materials; Singh, J., Ed.; ASM: Materials Park, OH, USA, 1996; pp. 247–253. [Google Scholar]
- Collins, P.C.; Banerjee, R.; Banerjee, S.; Fraser, H.L. Laser deposition of compositionally graded titanium–vanadium and tita-nium–molybdenum alloys. Mater. Sci. Eng. A 2003, 352, 118–128. [Google Scholar] [CrossRef]
- I Schwendner, K.; Banerjee, R.; Collins, P.C.; A Brice, C.; Fraser, H.L. Direct laser deposition of alloys from elemental powder blends. Scr. Mater. 2001, 45, 1123–1129. [Google Scholar] [CrossRef]
- Zhang, Y.; Meacock, C.; Vilar, R. Laser powder micro-deposition of compositional gradient Ti–Cr alloy. Mater. Des. 2010, 31, 3891–3895. [Google Scholar] [CrossRef]
- Tsai, P.; Flores, K.M. A laser deposition strategy for the efficient identification of glass-forming alloys. Met. Mater. Trans. A 2015, 46, 3876–3882. [Google Scholar] [CrossRef]
- Carvalho, P.A.; Braz, N.; Pontinha, M.M.; Ferreira, M.G.S.; Steen, W.M.; Vilar, R.; Watkins, K.G. Automated workstation for var-iable composition laser cladding—its use for rapid alloy scanning. Surf. Coat. Technol. 1995, 72, 62–70. [Google Scholar] [CrossRef]
- Tsai, P.; Flores, K.M. High-throughput discovery and characterization of multicomponent bulk metallic glass alloys. Acta Mater. 2016, 120, 426–434. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, M.K.; Chattopadhyay, P.; Ganguly, S. Multivariate analysis and classification of bulk metallic glasses using principal component analysis. Comput. Mater. Sci. 2015, 107, 79–87. [Google Scholar] [CrossRef]
- Kakinuma, Y.; Mori, M.; Oda, Y.; Mori, T.; Kashihara, M.; Hansel, A.; Fujishima, M. Influence of metal powder characteristics on product quality with directed energy deposition of Inconel 625. CIRP Ann. 2016, 65, 209–212. [Google Scholar] [CrossRef]
- Kim, S.G.; Inoue, A.; Masumoto, T. High mechanical strengths of Mg–Ni–Y and Mg–Cu–Y amorphous alloys with significant supercooled liquid region. Mater. Trans. JIM 1990, 31, 929–934. [Google Scholar] [CrossRef] [Green Version]
- Inoue, A.; Kato, A.; Zhang, T.; Kim, S.G.; Masumoto, T. Mg–Cu–Y Amorphous Alloys with High Mechanical Strengths Produced by a Metallic Mold Casting Method. Mater. Trans. JIM 1991, 32, 609–616. [Google Scholar] [CrossRef] [Green Version]
- Inoue, A.; Nakamura, T.; Nishiyama, N.; Masumoto, T. Mg–Cu–Y bulk amorphous alloys with high tensile strength produced by a high-pressure die casting method. Mater. Trans. JIM 1992, 33, 937–945. [Google Scholar] [CrossRef] [Green Version]
- Kato, A.; Inoue, A.; Horikiri, H.; Masumoto, T. production of bulk amorphous mg85y10cu5 alloy by extrusion of atomized amorphous powder. Mater. Trans. JIM 1994, 35, 125–129. [Google Scholar] [CrossRef] [Green Version]
- Mizutani, U.; Tanaka, K.; Matsuda, T.; Suzuki, N.; Fukunaga, T.; Ozaki, Y.; Yamada, Y.; Nakayama, K. Electronic structure and electron transport properties amorphous Mg-Ni-La and Mg-Cu-Y alloys. J. Non-Cryst. Solids 1993, 156–158, 297–301. [Google Scholar] [CrossRef]
- Niikura, A.; Tsai, A.P.; Inoue, A.; Masumoto, T. Chemical structural relaxation-induced embrittlement in amorphous Mg-Cu-Y alloys. J. Non-Cryst. Solids 1993, 159, 229–234. [Google Scholar] [CrossRef]
- Kato, A.; Suganuma, T.; Horikiri, H.; Kawamura, Y.; Inoue, A.; Masumoto, T. Consolidation and mechanical properties of atom-ized Mg-based amorphous powders. Mater. Sci. Eng. A 1994, 179–180, 112–117. [Google Scholar] [CrossRef]
- Mizutani, U.; Sugiura, H.; Yamada, Y.; Sugiura, Y.; Matsuda, T. Electronic structure and electron transport properties of Al-Cu-Y and Mg-Cu-Y amorphous alloys. Mater. Sci. Eng. A 1994, 179–180, 132–136. [Google Scholar] [CrossRef]
- Mezbahul-Islam, M.; Medraj, M. Phase equilibrium in Mg-Cu-Y. Sci. Rep. 2013, 3, 3033. [Google Scholar] [CrossRef] [Green Version]
- Carow-Watamura, D.V.; Louzguine, A. Takeuchi, Physical Properties of Ternary Amorphous Alloys. Part 3: Systems from Cr-Fe-P to Si-W-Zr · Mg-Ni-Y (304). In Landolt-Bornstein—Group III Condens. Matter 37B3; Kawazoe, Y., Carow-Watamura, U., Yu, J.-Z., Eds.; Springer: Berlin/Heidelberg, Germany.
- Islam, M. Thermodynamic Modeling of the Mg-Cu-Y System; Concordia University: Montréal, QC, Canada, 2007. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thoma, D.J.; Spethson, J.T.; Francis, C.S.; Voyles, P.M.; Perepezko, J.H. High-Throughput Synthesis and Characterization Screening of Mg-Cu-Y Metallic Glass. Metals 2023, 13, 1317. https://doi.org/10.3390/met13071317
Thoma DJ, Spethson JT, Francis CS, Voyles PM, Perepezko JH. High-Throughput Synthesis and Characterization Screening of Mg-Cu-Y Metallic Glass. Metals. 2023; 13(7):1317. https://doi.org/10.3390/met13071317
Chicago/Turabian StyleThoma, Dan J., Janine T. Spethson, Carter S. Francis, Paul M. Voyles, and John H. Perepezko. 2023. "High-Throughput Synthesis and Characterization Screening of Mg-Cu-Y Metallic Glass" Metals 13, no. 7: 1317. https://doi.org/10.3390/met13071317




