Effect of Cooling Rate on Microstructure Evolution and Mechanical Properties of SCM435 Steel
Abstract
:1. Introduction
2. Materials and Methods
3. Experimental Results and Discussion
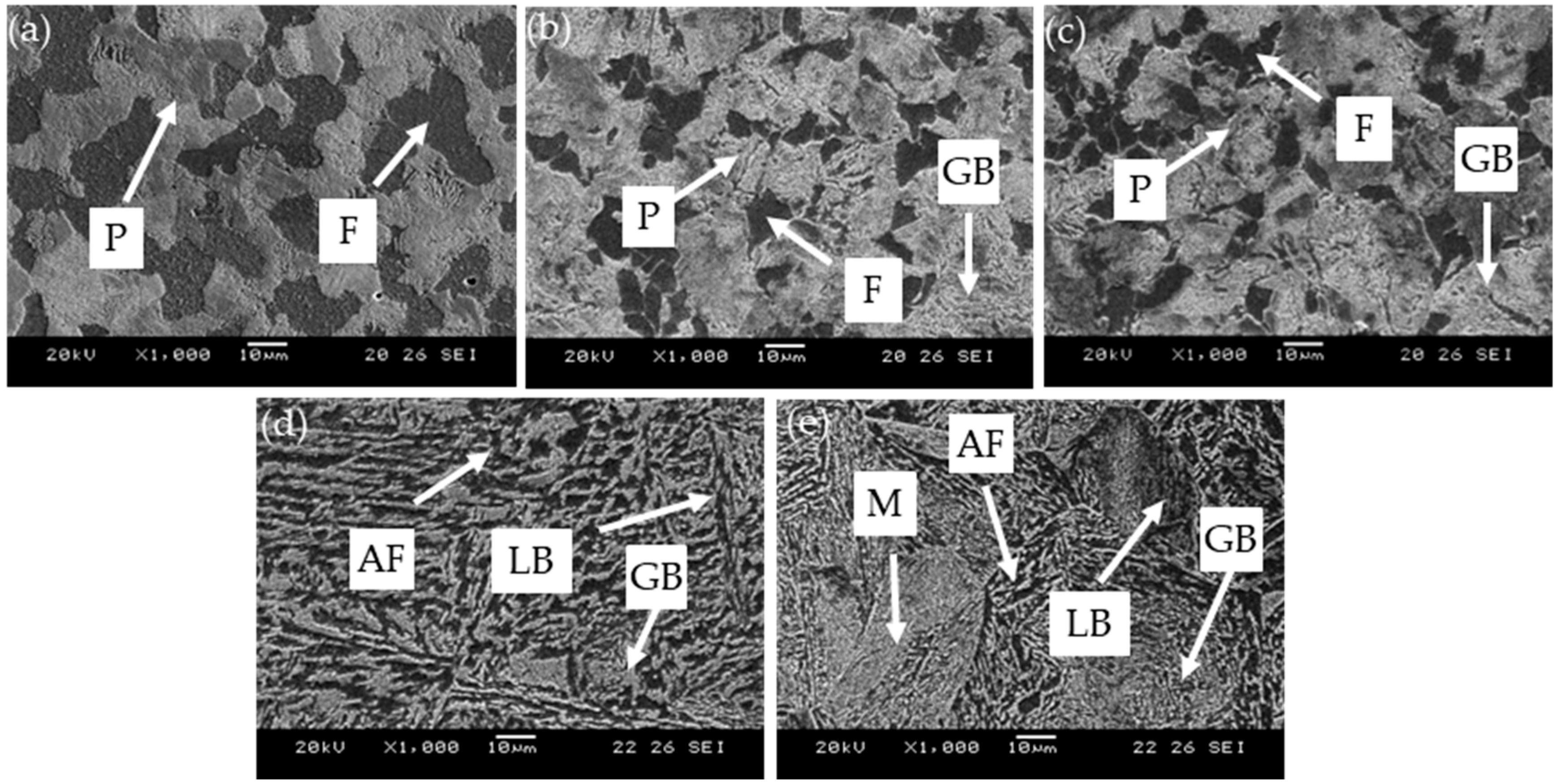
3.1. Microstructure
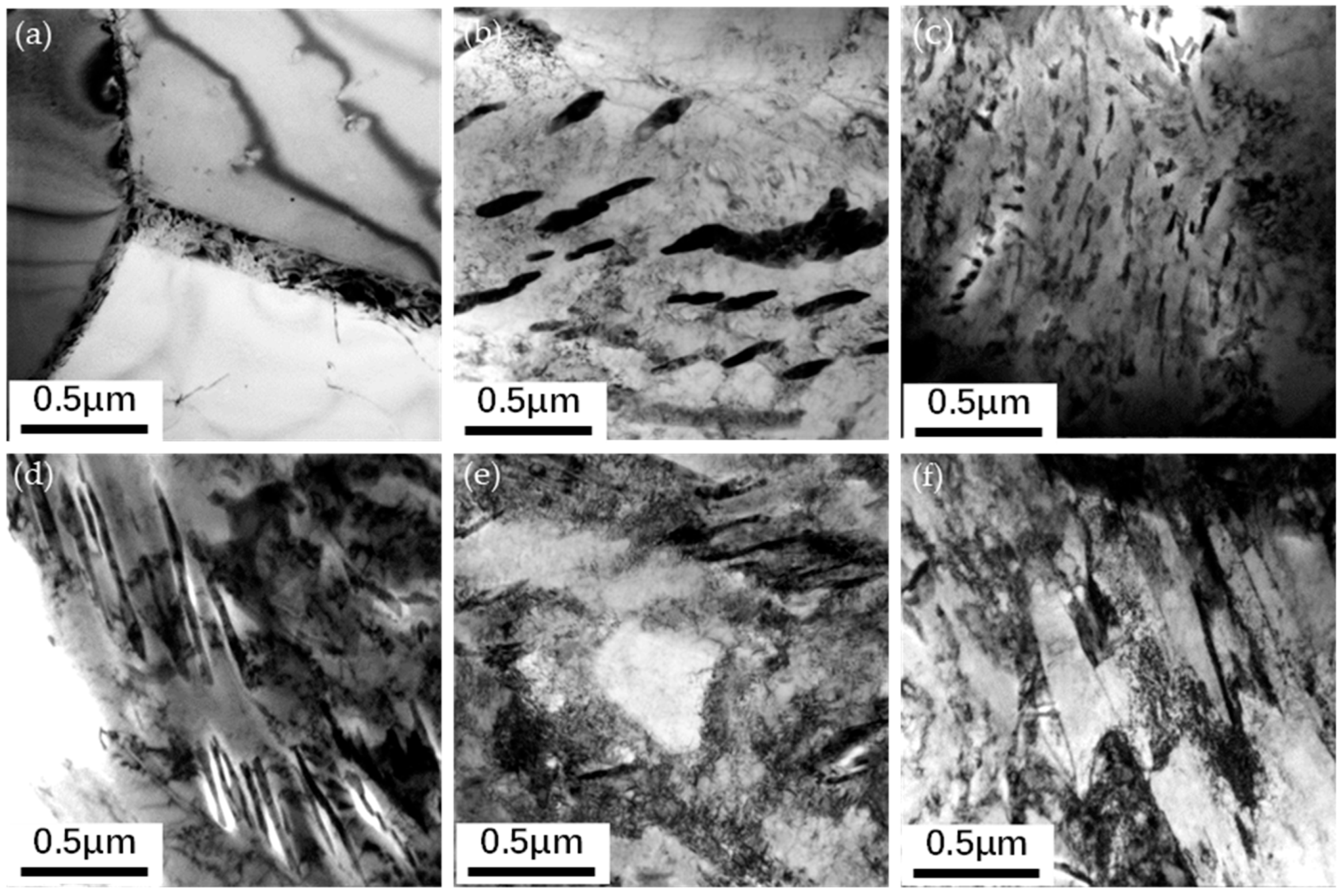
3.2. Carbide Morphology
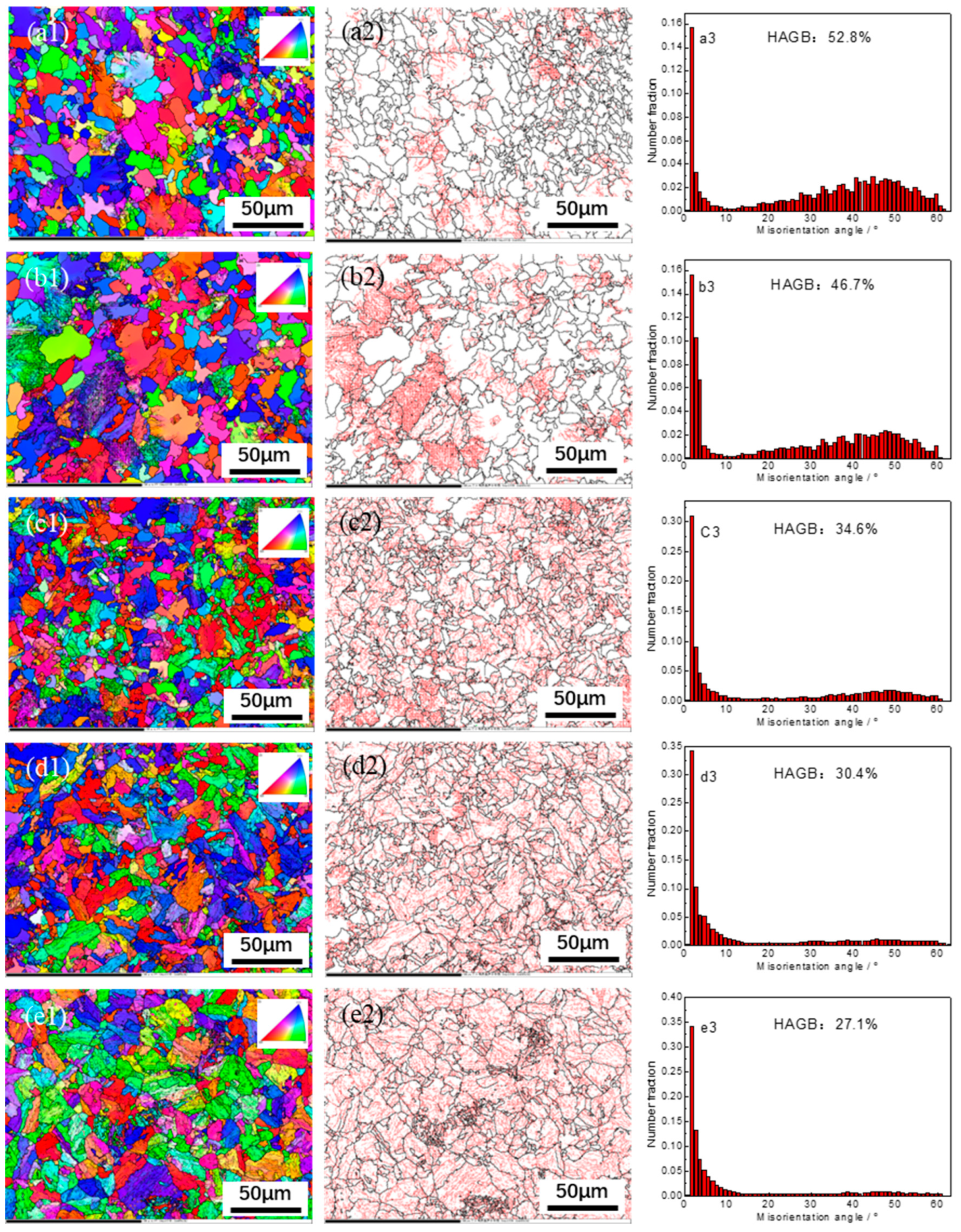
3.3. Grain Boundary Orientation
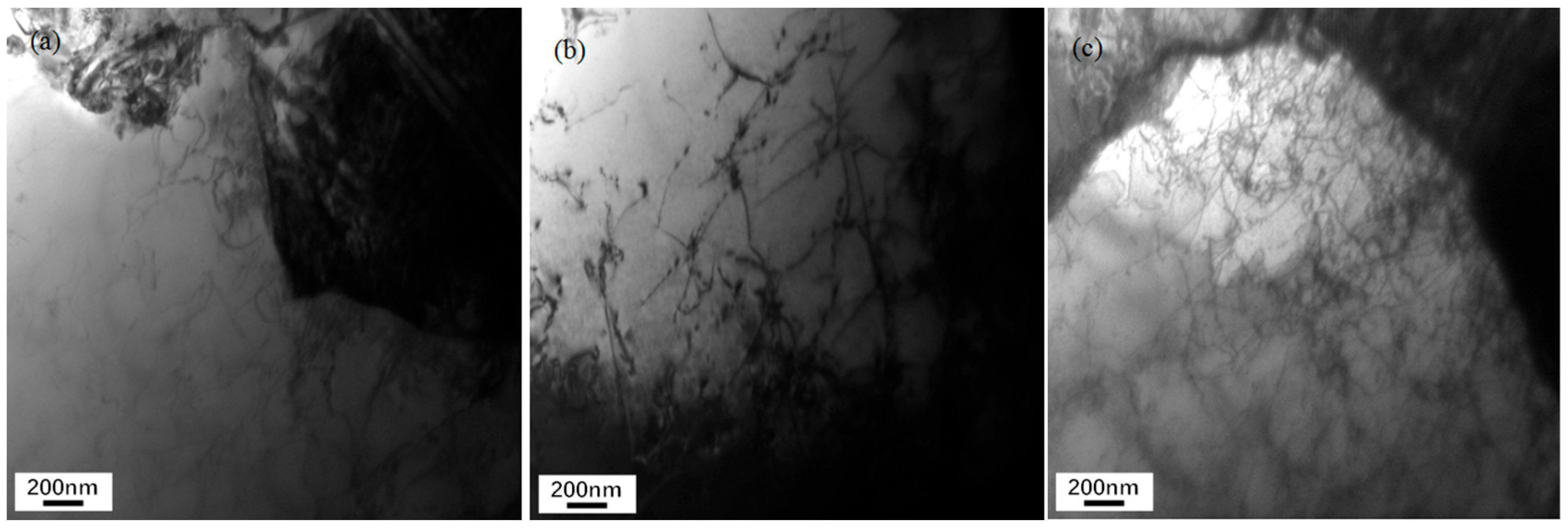
3.4. Dislocation Density
3.5. Phase Structure of Oxide
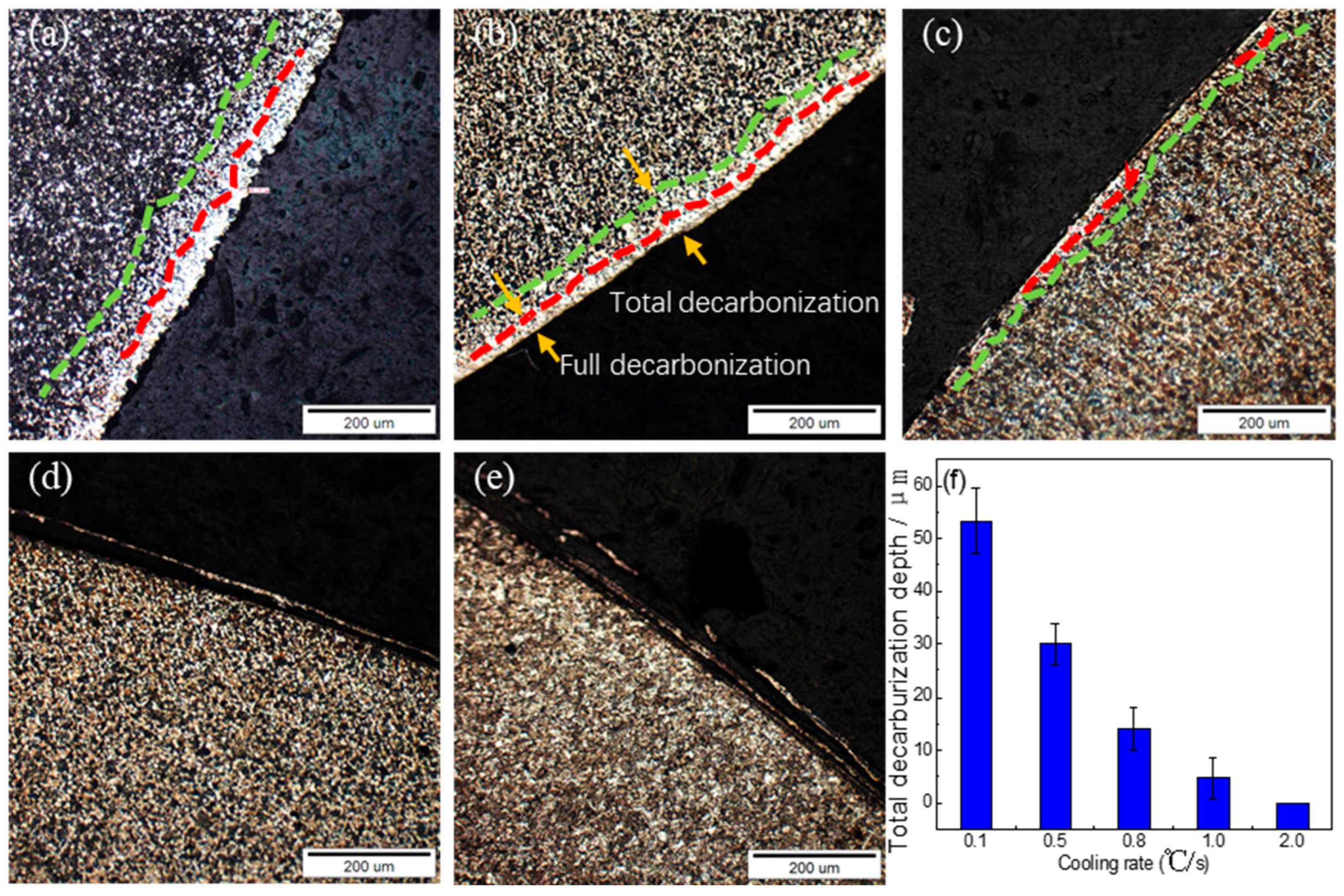
3.6. Surface Decarburization
3.7. Mechanical Property
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, H.; Lian, X.T.; Hu, C.D.; Lu, H.; Peng, W.; Zhao, H.; Xu, D. High Performance Steels: The Scenario of Theory and Technology. Acta Metall. Sin. 2020, 56, 558–582. [Google Scholar]
- Hu, C.D.; Meng, L.; Dong, H. Research and development of ultrahigh strength steels. Trans. Mater. Heat Treat. 2016, 37, 178–183. [Google Scholar]
- Hui, W.J.; Dong, H.; Weng, Y.Q. Research and development trends of high strength steel for bolts. Mater. Mech. Eng. 2002, 26, 1–4. [Google Scholar]
- Yongbing, L.I.; Yunwu, M.A.; Ming, L.O.U.; Haiyang, L.; Zhongqin, L. Advances in Welding and Joining Processes of Multi-material Light weight Car Body. J. Mech. Eng. 2016, 52, 1–23. [Google Scholar]
- Sun, H.R. Review on the Fastener Steels for Automobiles. China Metall. 2011, 21, 7–9. [Google Scholar]
- Hui, W.J.; Zhang, Y.J.; Zhao, X.L.; Shao, C.; Wang, K.; Sun, W.; Yu, T. Influence of cold deformation and annealing on hydrogen embrittlement of cold hardening bainitic steel for high strength bolts. Mater. Sci. Eng. A 2016, 662, 528–536. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, J.L. Certain new techniques of wire production development in recent years. China Metall. 2014, 24, 1–4. [Google Scholar]
- Ruan, S.P.; Wang, L.J.; Chen, J.L.; Guo, M.Y.; Zhai, J.P. Effect of Control-Rolling-Cooling Process on Structure and Properties of Steel SCM435 Rod Coil. Spec. Steel 2016, 5, 45–47. [Google Scholar]
- Kitade, A.; Kawabata, T.; Kimura, S.; Takatani, H.; Kagehira, K.; Mitsuzumi, T. Clarification of micromechanism on Brittle Fracture Initiation Condition of TMCP Steel with MA as the trigger point. Procedia Struct. Integr. 2018, 13, 1845–1854. [Google Scholar] [CrossRef]
- Elhigazi, F.; Artemev, A. The influence of carbide formation in ferrite on the bainitic type transformation. Comp. Mater. Sci. 2021, 186, 109961. [Google Scholar] [CrossRef]
- Elhigazi, F.; Artemev, A. The interaction between the displacive transformation and the diffusion process in the bainitic type transformation. Comp. Mater. Sci. 2019, 169, 109079. [Google Scholar] [CrossRef]
- Ranjan, R.; Singh, S.B. Isothermal bainite transformation in low-alloy steels: Mechanism of transformation. Acta Mater. 2021, 202, 302–316. [Google Scholar] [CrossRef]
- Chen, G.H.; Xu, Y.W.; Liu, M. Effect of high-temperature deformation and undercooling on bainite transformation of a medium-carbon bainitic steel. J. Iron Steel Res. Int. 2020, 32, 984–989. [Google Scholar]
- Ravi, A.M.; Kumar, A.; Herbig, M.; Sietsma, J.; Santofimia, M.J. Impact of austenite grain boundaries and ferrite nucleation on bainite formation in steels. Acta Mater. 2020, 188, 424–434. [Google Scholar] [CrossRef]
- Yong, Q.L.; Ma, M.T.; Wu, B.R. Microalloyed Steel-Physical and Mechanical Metallurgy; China Machine Press: Beijing, China, 1989; p. 57. [Google Scholar]
- Zheng, C.S.; Li, L.F.; Yang, W.Y.; Sun, Z. Microstructure evolution and mechanical properties of eutectoid steel with ultrafine or fine (ferrite+cementite) structure. Mater. Sci. Eng. A 2014, 599, 16–24. [Google Scholar] [CrossRef]
- Liang, Z.Y.; Wang, Y.G.; Gui, Y.; Zhao, Q. Micro-structural evolution of oxide scales formed on a Nb-Stabilizing heat-resistant steel at the initial stage in high-temperature water vapor. Mater. Chem. Phys. 2020, 242, 122443. [Google Scholar] [CrossRef]
- Liu, Y.B.; Zhang, W.; Tong, Q.; Wang, L. Effects of temperature and oxygen concentration on the characteristics of decarburization of 55SiCr spring steel. ISIJ Int. 2014, 54, 1920–1926. [Google Scholar] [CrossRef]
- Noh, W.; Lee, J.M.; Kim, D.J.; Song, J.H.; Lee, M.G. Effects of the residual stress, interfacial roughness and scale thickness on the spallation of oxide scale grown on hot rolled steel sheet. Mater. Sci. Eng. A 2019, 739, 301–316. [Google Scholar] [CrossRef]
- Solimani, A.; Nguyen, T.; Zhang, J.Q.; Young, D.J.; Schütze, M.; Galetz, M.C. Morphology of oxide scales formed on chromium-silicon alloys at high temperatures. Corros. Sci. 2020, 176, 109023. [Google Scholar] [CrossRef]
- Huang, T.H.; Deng, C.M.; Song, P.; Ji, Q.; Li, C.; Chen, R.; Hua, C.; Yi, J. Investigation of oxide scale formation and internal oxidation of an Fe-based coating at 500 °C and 600 °C. Surf. Coat. Technol. 2020, 402, 126309. [Google Scholar] [CrossRef]


| Steel | C | Si | Mn | P | S | Cr | Mo |
|---|---|---|---|---|---|---|---|
| SCM435 | 0.35 | 0.18 | 0.82 | 0.001 | 0.0008 | 0.98 | 0.22 |
| Process | Heating Temperature/°C | Finishing Temperature/°C | Heat Cover | Blower | Cooling Rate/m/s |
|---|---|---|---|---|---|
| 1 | 1100 ± 10 | 900 ± 10 | Close | Close | 0.1 |
| 2 | 1100 ± 10 | 900 ± 10 | Open | Close | 0.5 |
| 3 | 1100 ± 10 | 900 ± 10 | Open | Open | 1.0 |
| 4 | 1100 ± 10 | 900 ± 10 | Open | Open | 1.5 |
| 5 | 1100 ± 10 | 900 ± 10 | Open | Open | 2.0 |
| Steel | Dislocation Density /1012·cm−2 | ||||
|---|---|---|---|---|---|
| 0.1 °C/s | 0.5 °C/s | 1.0 °C/s | 1.5 °C/s | 2.0 °C/s | |
| 1.91 | 2.18 | 2.98 | 4.19 | 5.38 | |
| Cooling Rate/m/s | Tensile Strength/MPa | Elongation/% | Reduction in Area/% |
|---|---|---|---|
| 0.1 | 708 | 31.5 | 62 |
| 0.5 | 794 | 25.8 | 53 |
| 1.0 | 895 | 28.5 | 57 |
| 1.5 | 959 | 20.6 | 51 |
| 2.0 | 1019 | 14.5 | 47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Feng, G.; Zheng, Y.; Lin, P.; Wang, L.; Li, Y. Effect of Cooling Rate on Microstructure Evolution and Mechanical Properties of SCM435 Steel. Metals 2024, 14, 140. https://doi.org/10.3390/met14020140
Chen J, Feng G, Zheng Y, Lin P, Wang L, Li Y. Effect of Cooling Rate on Microstructure Evolution and Mechanical Properties of SCM435 Steel. Metals. 2024; 14(2):140. https://doi.org/10.3390/met14020140
Chicago/Turabian StyleChen, Jilin, Guanghong Feng, Yaxu Zheng, Peng Lin, Lijun Wang, and Yongchao Li. 2024. "Effect of Cooling Rate on Microstructure Evolution and Mechanical Properties of SCM435 Steel" Metals 14, no. 2: 140. https://doi.org/10.3390/met14020140







