Numerical Simulation of Macro-Segregation Phenomena in Transition Blooms with Various Carbon Contents
Abstract
:1. Introduction
2. Simulation Model
2.1. Model Description and Assumptions
- The new and old grades are considered transient, turbulent, and incompressible Newtonian fluids. The density of the molten steel is calculated using the volume-weighted mixing law within the tundish. After mixing in the tundish, it is assumed that the density of the new and old grades does not undergo a sudden change. The density of the molten steel entering the mold follows the Boussinesq assumption. Assuming that the density of the new and old grades entering the mold is constant, both are 7020 kg/m3.
- The simulation only considers the Fe elements in the new and old grades and some solute elements with higher mass fractions.
- The mushy zone is treated as a porous medium, and the flow within the mushy zone follows Darcy’s law.
- The effects of the solidification shrinkage of the molten steel and the curvature of the continuous caster on the solidification process of the bloom are neglected.
- The solid-state phase change latent heat, which is far less than the latent heat of solidification, is also ignored.
- Induction magnetic fields and the induced heat in the molten steel, as well as the impact of the molten steel flow on the magnetic field, are disregarded. Average electromagnetic forces are used instead of transient values.
2.2. Governing Equations
2.2.1. Fluid Flow
2.2.2. Solidification and Heat Transfer
2.2.3. Species Transfer
2.3. Boundary Conditions
- 1.
- The SEN is defined as a velocity inlet with a velocity magnitude of 0.72 m/s. The turbulence intensity is calculated using Equation (20), with a value of 0.043.
- 2.
- The outlet for the strand model is set as outflow.
- 3.
- All the other boundaries are designated as walls.
- -
- Mold zone. The heat flux density in the mold zone is calculated using Equation (21).
- -
- Secondary cooling zone. The heat flux density in the secondary cooling zone is represented by and in the foot roll zone and the other zones, respectively.
- -
- Air-cooling zone. The heat flux density in the air-cooling zone is calculated using Equation (24) [34].
2.4. Material Parameters and Simulation Schemes
3. Results and Discussion
3.1. Model Validation
3.2. Foundation before Steel Grade Transition
3.2.1. Tundish Simulation
3.2.2. F-EMS Simulation
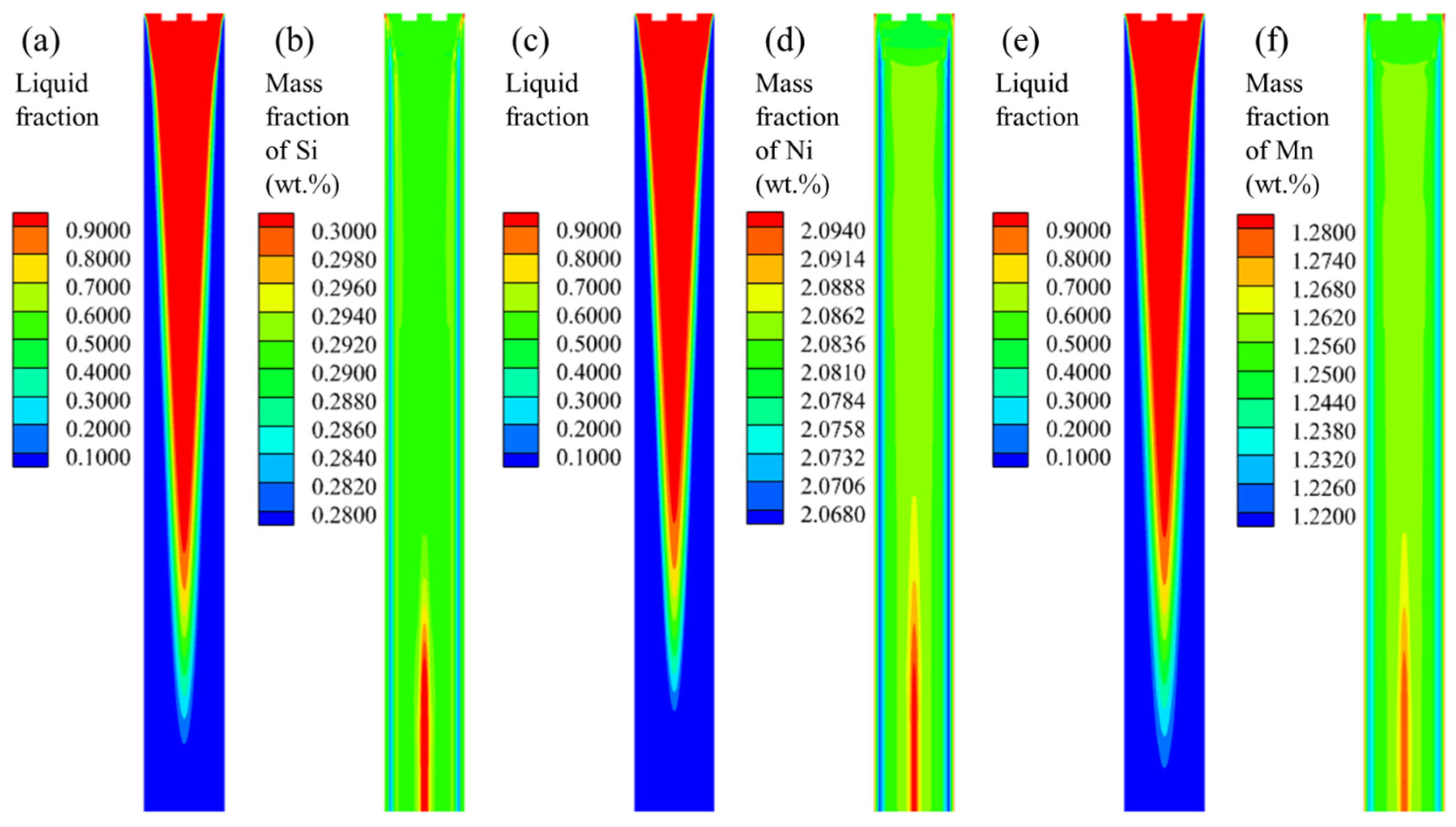
3.2.3. Old Grade Casting Simulation
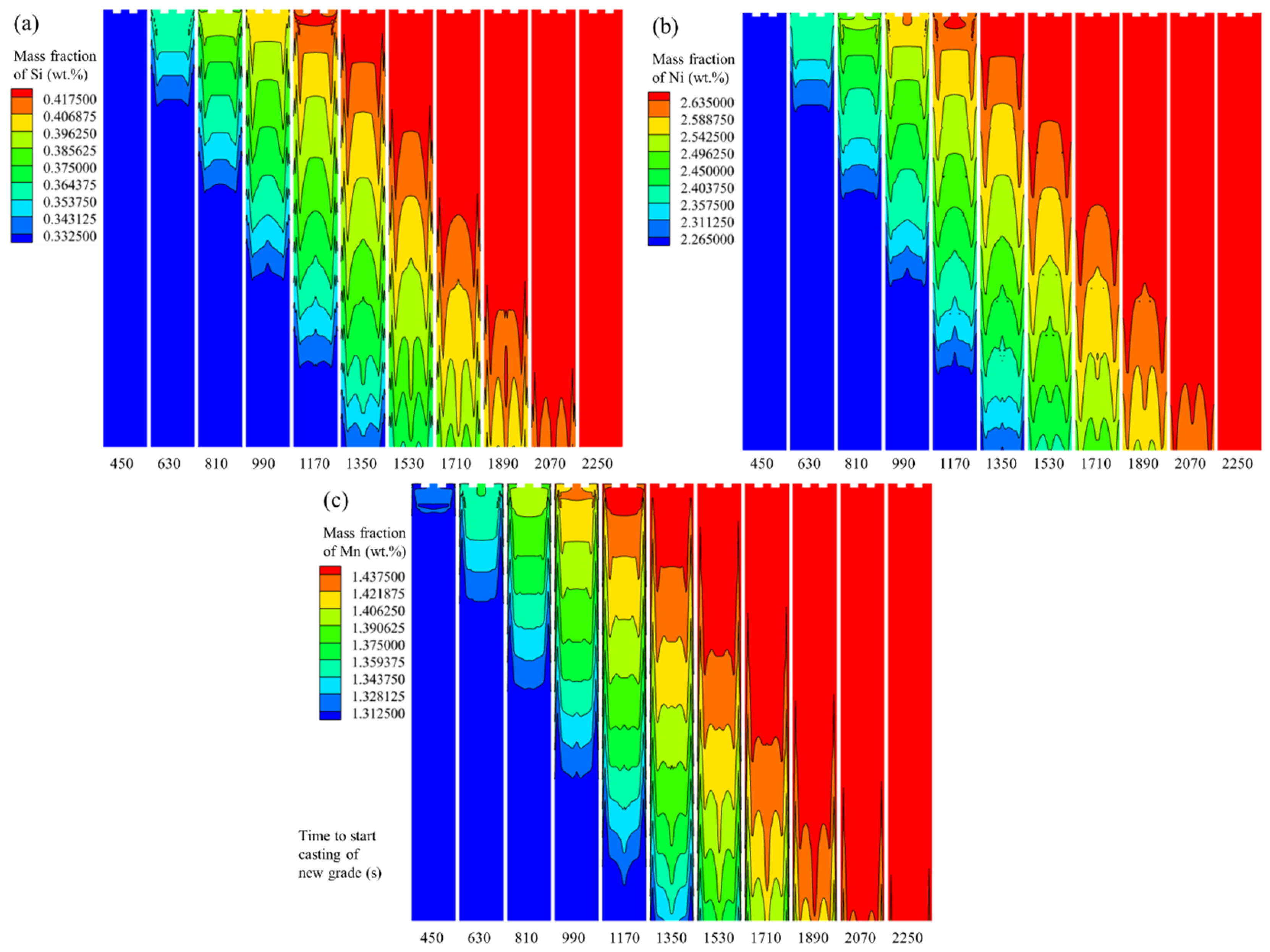
3.3. Formation Process of Transition Blooms with Different Carbon Contents
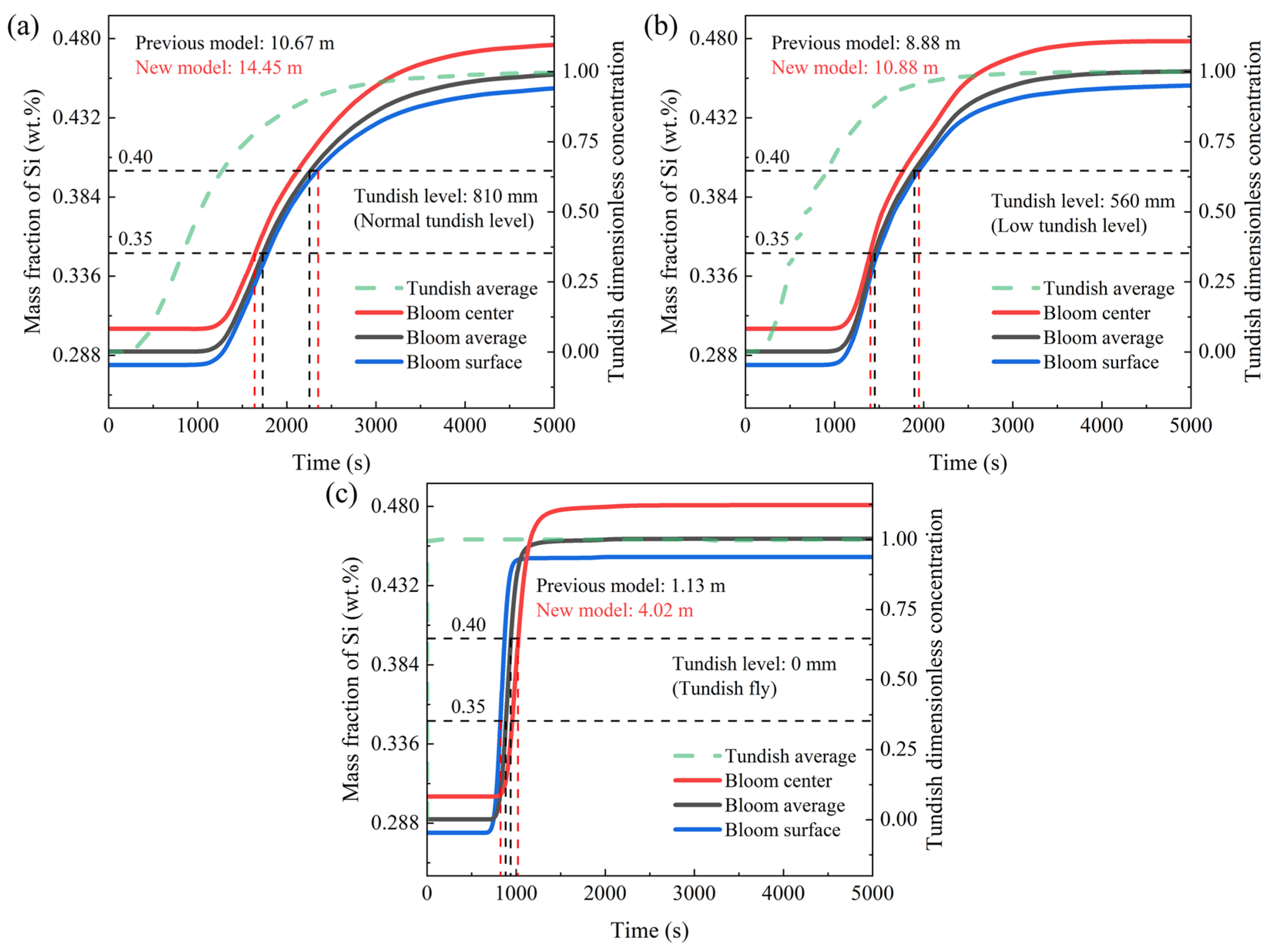
3.4. Different Tundish Levels in Steel Grade Transition
4. Conclusions
- The regions where the difference between the molten steel velocity and the casting speed is significant are mainly concentrated within 1 m below the meniscus. The influence of the molten steel flow on the mass fraction difference in the solute elements in the same cross-section of the transition bloom is relatively small. The voluminous mushy zone in the strand model leads to the continuous migration of solute elements from the solid phase through the mushy zone into the liquid phase. This results in macro-segregation, where the impact of macro-segregation on the mass fraction difference in the solute elements in the same cross-section of the transition bloom is much greater than that of the molten steel flow.
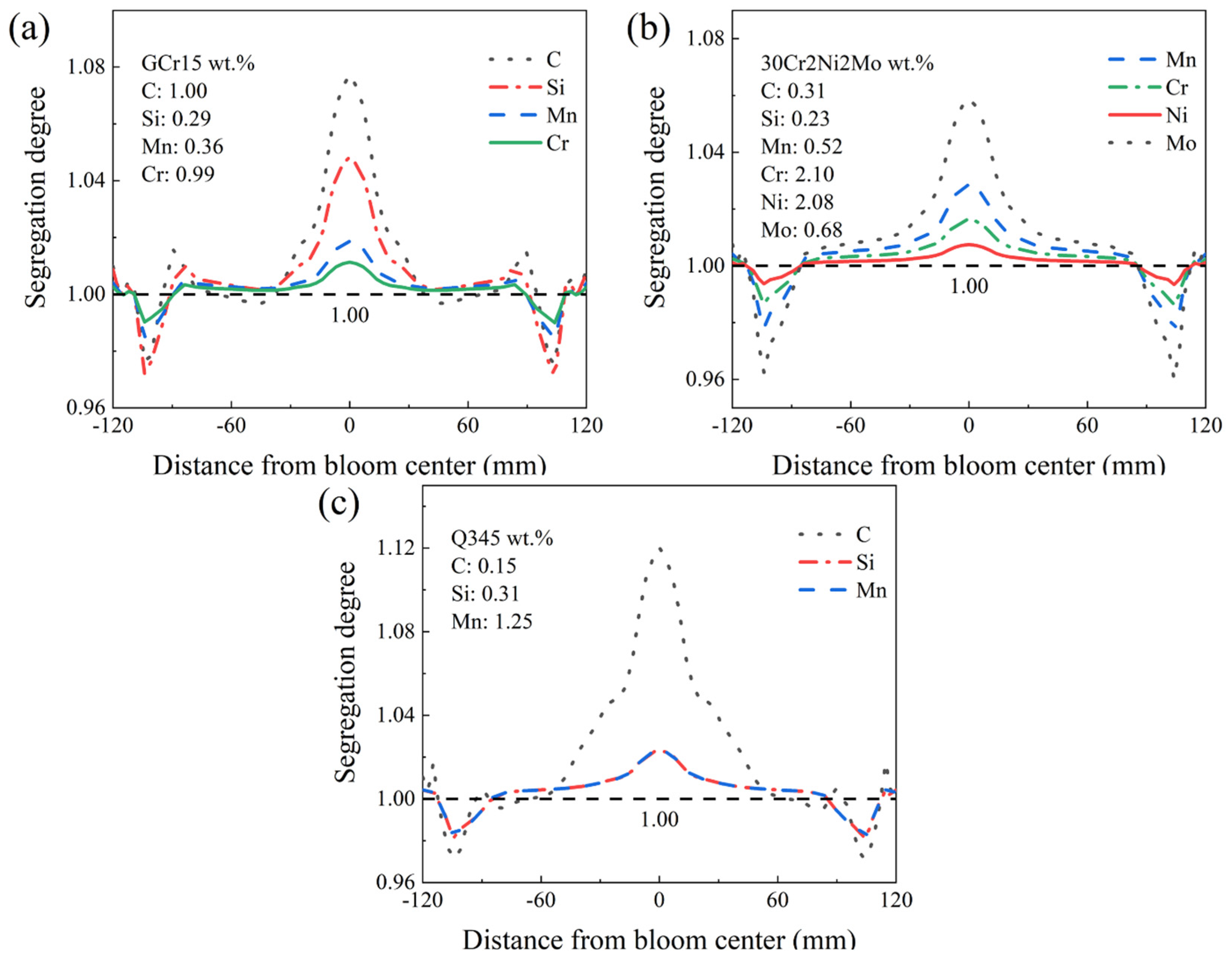
- The steel grade transition process for various solute elements in different phases exhibits similarities. The segregation degrees of the solute elements are closely related to their distribution coefficients in different phases. The segregation degree order of the solute elements in the austenite phase is as follows: C > Si > Mo > Mn > Cr > Ni. In the ferrite phase, it is C > Si = Mn.
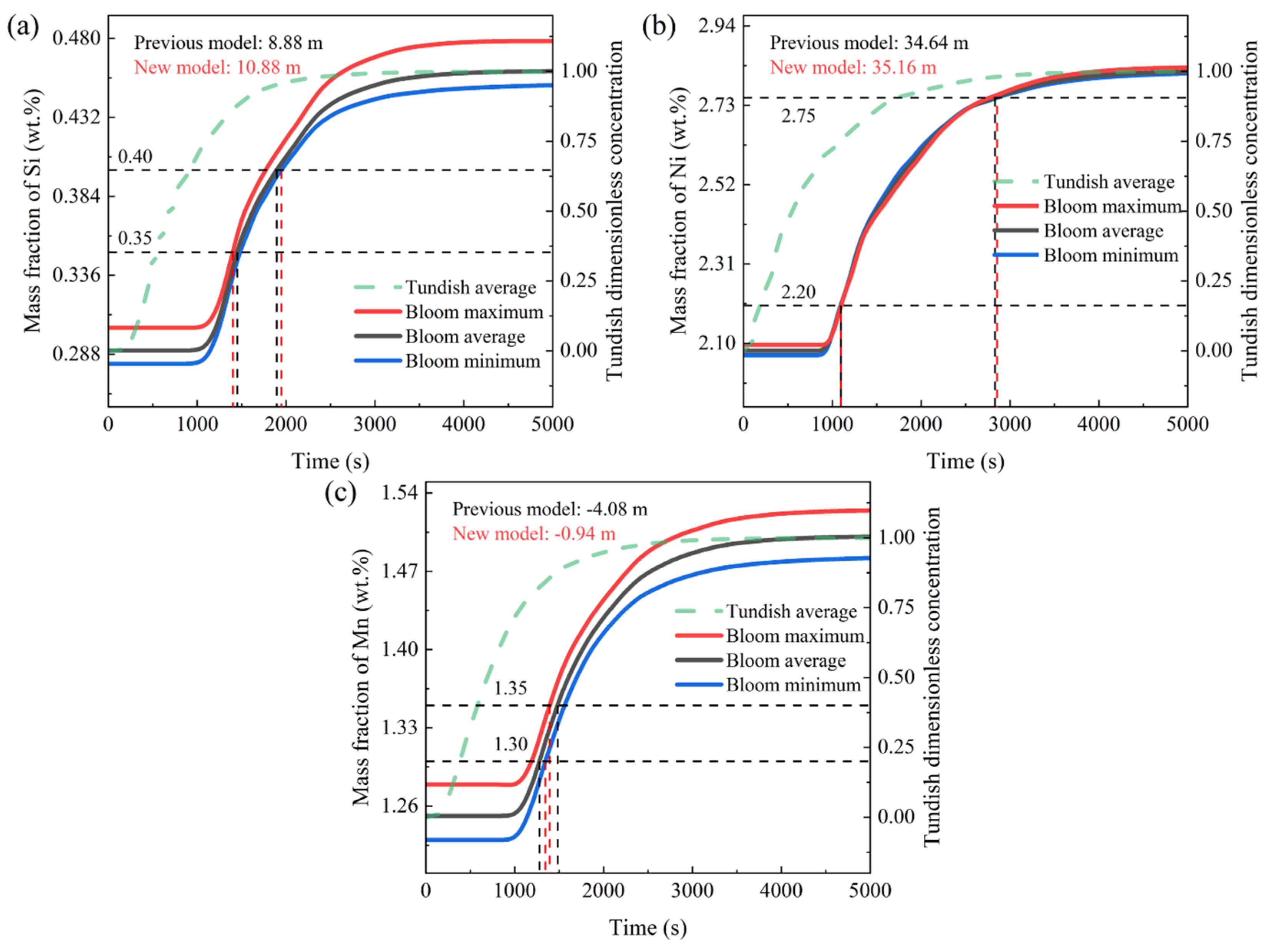
- The transition bloom division model considering macro-segregation is more rigorous than the original division method. This results in longer transition blooms when there is significant dissimilarity between the old and new grades. For example, in Scheme 1, the original division method determines a transition bloom length of 8.88 m, while the new method calculates a length of 10.88 m. In Scheme 2, the original division method gives a transition bloom length of 34.64 m, while the new method calculates a length of 35.16 m. Simultaneously, it also leads to a shorter partition interval when there is an overlap in the composition of the old and new grades. For example, in Scheme 3, the original method determines a partition interval of 4.08 m, while the new method calculates a length of 0.94 m.
- When there is an overlap in the composition of the old and new grades, it is recommended to use the normal tundish level, which can slow down the mixing of the old and new grades, allowing for a longer partition interval. When there is a significant difference in the composition of the old and new grades in the steel grade transition, it is recommended to use the minimum tundish level, which does not cause tundish powder entrapment, or to use the flying tundish method. Increasing the rate of change in the composition of the old and new grades shortens the length of the transition bloom. When using the flying tundish method, it should be combined with the use of a “grade separator” to effectively reduce the uneven distribution of the solute elements in the same cross-section of the transition bloom caused by the flow field. At the same time, attention should be paid to the actual composition of the solute elements, especially in the new steel, which differ significantly in their mass fractions, and should be kept away from the control lines of the steel composition ranges.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddiqui, M.I.H.; Kim, M.H. Optimization of flow control devices to minimize the grade mixing in steelmaking tundish. Iran. J. Sci. Technol. 2018, 32, 3213–3221. [Google Scholar] [CrossRef]
- Kumar, R.; Siddiqui, M.I.H.; Jha, P.K. Numerical investigations on the use of flow modifiers in multi-strand continuous casting tundish using RTD curves analysis. In Proceedings of the International Conference on Smart Technologies for Mechanical Engineering, Delhi, India, 25–26 October 2013; pp. 603–612. [Google Scholar]
- Siddiqui, M.I.H.; Jha, P.K. Effect of inflow rate variation on intermixing in a steelmaking tundish during ladle change-over. Steel Res. Int. 2016, 87, 733–744. [Google Scholar] [CrossRef]
- Agarwal, R.; Singh, M.K.; Kumar, R.B.; Ghosh, B.; Pathak, S. Extensive analysis of multi strand billet caster tundish using numerical technique. World J. Mech. 2019, 9, 29–51. [Google Scholar] [CrossRef]
- Michalek, K.; Gryc, K.; Tkadleková, M.; Morávka, J.; Huczala, T.; Bocek, D.; Horáková, D. Type of submerged entry nozzle vs. concentration profiles in the intermixed zone of round blooms with a diameter of 525 mm. Mater. Technol. 2012, 46, 581–587. [Google Scholar]
- Huang, X.; Thomas, B.G. Intermixing model of continuous casting during a grade transition. Metall. Mater. Trans. B 1996, 27, 617–632. [Google Scholar] [CrossRef]
- Thomas, B.G. Modeling study of intermixing in tundish and strand during a continuous-casting grade transition. Iron Steelmak. 1997, 24, 83–96. [Google Scholar]
- Thomas, B.G. The importance of computational models for further improvements of the continuous casting process. In Proceedings of the Voest Alpine Conference on Continuous Casting, Linz, Austria, 5–7 June 2000. [Google Scholar]
- Rappaz, M. Modelling of microstructure formation in solidification processes. Int. Mater. Rev. 1989, 34, 93–124. [Google Scholar] [CrossRef]
- Rappaz, M.; Gandin, C.A.; Desbiolles, J.L.; Thévoz, P. Prediction of grain structures in various solidification processes. Metall. Mater. Trans. A 1996, 27, 695–705. [Google Scholar] [CrossRef]
- Campanella, T.; Charbon, C.; Rappaz, M. Grain refinement induced by electromagnetic stirring: A dendrite fragmentation criterion. Metall. Mater. Trans. A 2004, 35, 3201–3210. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, G. Delta-ferrite distribution in a continuous casting slab of Fe-Cr-Mn austenitic stainless steel. Metall. Mater. Trans. B 2017, 48, 2324–2333. [Google Scholar] [CrossRef]
- Dong, Q.; Zhang, J.; Yin, Y.; Nagaumi, H. Numerical simulation of macrosegregation in billet continuous casting influenced by electromagnetic stirring. J. Iron Steel Res. Int. 2022, 29, 612–627. [Google Scholar] [CrossRef]
- Chen, H.; Long, M.; Chen, D.; Liu, T.; Duan, H. Numerical study on the characteristics of solute distribution and the formation of centerline segregation in continuous casting (CC) slab. Int. J. Heat Mass Transfer 2018, 126, 843–853. [Google Scholar] [CrossRef]
- Li, S.; Han, Z.; Zhang, J. Numerical modeling of the macrosegregation improvement in continuous casting blooms by using F-EMS. JOM 2020, 72, 4117–4126. [Google Scholar] [CrossRef]
- Hou, Z.; Cheng, G.; Wu, C.; Chen, C. Time-series analysis technologies applied to the study of carbon element distribution along casting direction in continuous-casting billet. Metall. Mater. Trans. B 2012, 43, 1517–1529. [Google Scholar] [CrossRef]
- Szmyd, J.S.; Suzuki, K. Modelling of Transport Phenomena in Crystal Growth; WIT Press: Southampton, UK, 2000. [Google Scholar]
- Huang, X.; Thomas, B.G. Modeling of steel grade transition in continuous slab casting processes. Metall. Mater. Trans. B 1993, 24, 379–393. [Google Scholar] [CrossRef]
- Wang, Q.; Tan, C.; Huang, A.; Yan, W.; Gu, H.; He, Z.; Li, G. Numerical simulation on refractory wear and inclusion formation in continuous casting tundish. Metall. Mater. Trans. B 2021, 52, 1344–1356. [Google Scholar] [CrossRef]
- Li, Q.; Qin, B.; Zhang, J.; Dong, H.; Li, M.; Tao, B.; Mao, X.; Liu, Q. Design Improvement of Four-Strand Continuous-Casting Tundish Using Physical and Numerical Simulation. Materials 2023, 16, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.; Zhang, G.; Jiang, Q.; Zhang, P.; Feng, Z.; Tian, N. The Removal of Inclusions with Different Diameters in Tundish by Channel Induction Heating: A Numerical Simulation Study. Materials 2023, 16, 5254–5266. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Z.; Li, F.; Lyu, B.; Chen, W.; Zhang, L. Numerical Simulation on Multiphase Flow and Slag Entrainment during Casting Start of a Slab Continuous Casting Tundish. Metall. Mater. Trans. B 2023, 54, 2048–2065. [Google Scholar] [CrossRef]
- Figueroa-Fierros, A.E.; Ramos-Banderas, J.Á.; Hernández-Bocanegra, C.A.; López-Granados, N.M.; Solorio-Díaz, G. Thermal-Fluid Dynamic Behavior on Intermixed Steel Calculation during a Grade Change in a Slab Tundish by Numerical Simulation. ISIJ Int. 2023, 63, 1998–2009. [Google Scholar] [CrossRef]
- Pieprzyca, J.; Merder, T.; Saternus, M.; Tkadlecková, M.; Cupek, J.; Walek, J. Modeling research on limitation of transition zone during continuous steel casting. Metalurgija 2024, 63, 3–5. [Google Scholar]
- Jeon, S.; Lee, S.; Ha, S.; Kim, S.; You, D. Effects of a moving weir on tundish flow during continuous-casting grade-transition. J. Mech. Sci. Technol. 2021, 35, 4001–4009. [Google Scholar] [CrossRef]
- Yin, S.; Luo, S.; Zhang, W.; Wang, W.; Zhu, M. Numerical simulation of macrosegregation in continuously cast gear steel 20CrMnTi with final electromagnetic stirring. J. Iron Steel Res. Int. 2021, 28, 424–436. [Google Scholar] [CrossRef]
- Wu, H.; Xu, C.; Lei, C.; Wang, T.; Gao, Y.; Zhang, X.; Jin, H. Numerical Simulation and Industrial Experiment of the Fluid Flow and Heat Transfer in Large Vertical Round Billets with Helical Final Electromagnetic Stirring. JOM 2023, 75, 1439–1449. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Qiu, H.; Wang, Z. Numerical simulation of coupled fluid flow and solidification in a curved round bloom continuous caster with a combined rotary electromagnetic stirring. Ironmak. Steelmak. 2022, 49, 506–521. [Google Scholar] [CrossRef]
- Guan, R.; Ji, C.; Zhu, M. Modeling the effect of combined electromagnetic stirring modes on macrosegregation in continuous casting blooms. Metall. Mater. Trans. B 2020, 51, 1137–1153. [Google Scholar] [CrossRef]
- Tan, R.; Liu, W.; Song, B.; Yang, S.; Chen, Y.; Zuo, X.; Huang, Y. Numerical simulation on solidification behavior and structure of 38CrMoAl large round bloom using CAFE model. J. Iron Steel Res. Int. 2023, 30, 1222–1233. [Google Scholar] [CrossRef]
- Song, S.; Sun, Y.; Li, Y.; Zhuo, C. Effects of Temperature and Density on Transition Slab Length during Steel Grade Transition. In Proceedings of the TMS Annual Meeting & Exhibition, San Diego, CA, USA, 19–23 March 2023; pp. 227–240. [Google Scholar]
- Li, S. Numerical Study on the Macroscopic Transport Phenomena of Continuous Casting Process under Electromagnetic Force. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2020. [Google Scholar]
- Launder, B.E.; Spalding, D.B. Lectures in Mathematical Models of Turbulence; Academic Press: Cambridge, MA, USA, 1972. [Google Scholar]
- Zhong, H.; Wang, R.; Han, Q.; Fang, M.; Yuan, H.; Song, L.; Xie, X.; Zhai, Q. Solidification structure and central segregation of 6Cr13Mo stainless steel under simulated continuous casting conditions. J. Mater. Res. Technol. 2022, 20, 3408–3419. [Google Scholar] [CrossRef]
- Song, S.; Sun, Y.; An, H. Numerical modeling of grade mixing and inclusion entrapment in eight strand billet tundish. Metall. Res. Technol. 2023, 120, 1–15. [Google Scholar] [CrossRef]
- Meng, Y.A.; Thomas, B.G. Heat-transfer and solidification model of continuous slab casting: CON1D. Metall. Mater. Trans. B 2003, 34, 685–705. [Google Scholar] [CrossRef]
- Schneider, M.C.; Beckermann, C. Simulation of micro-/macrosegregation during the solidification of a low-alloy steel. ISIJ Int. 1995, 35, 665–672. [Google Scholar] [CrossRef]
- Wołczyński, W. Nature of segregation in the steel static and brass continuously cast ingots. Arch. Metall. Mater. 2018, 63, 1915–1922. [Google Scholar] [CrossRef]
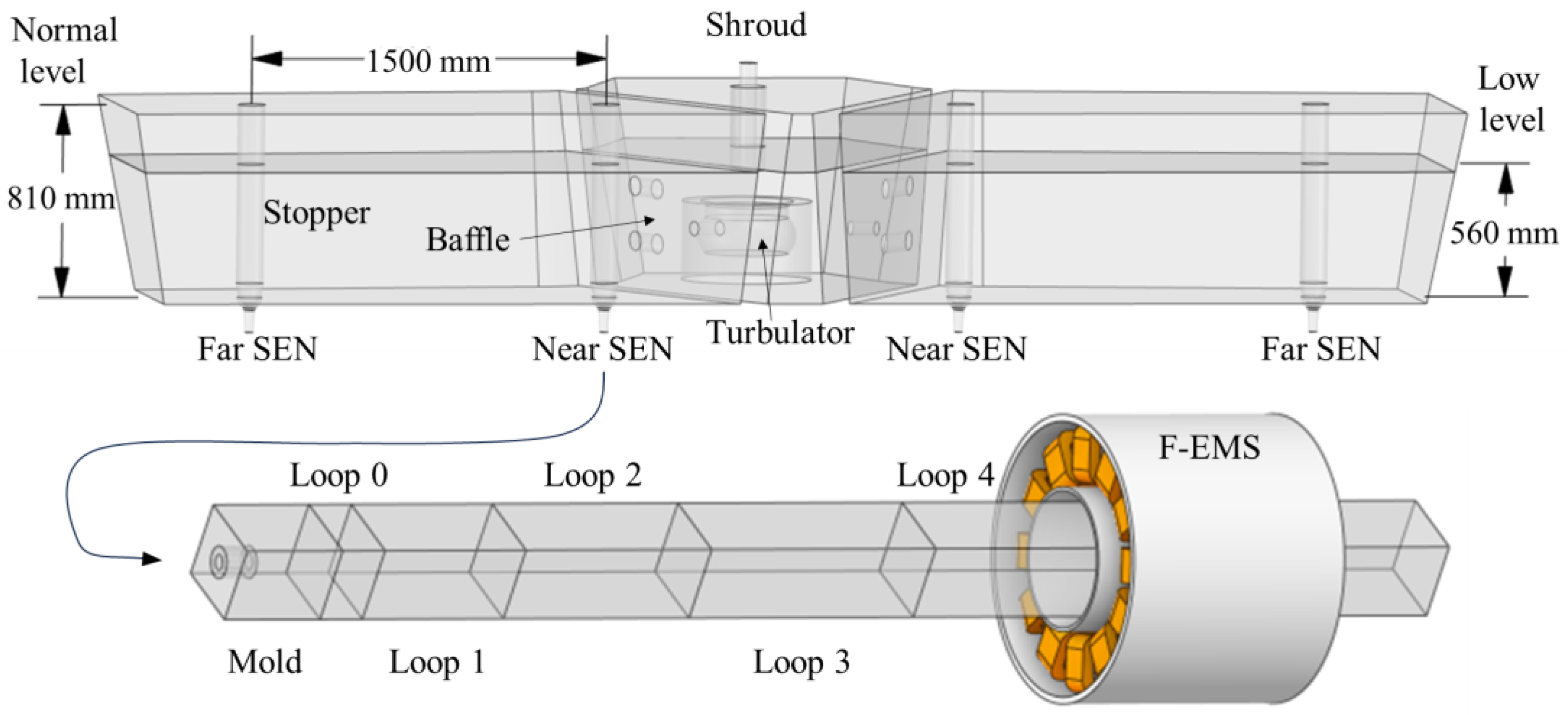

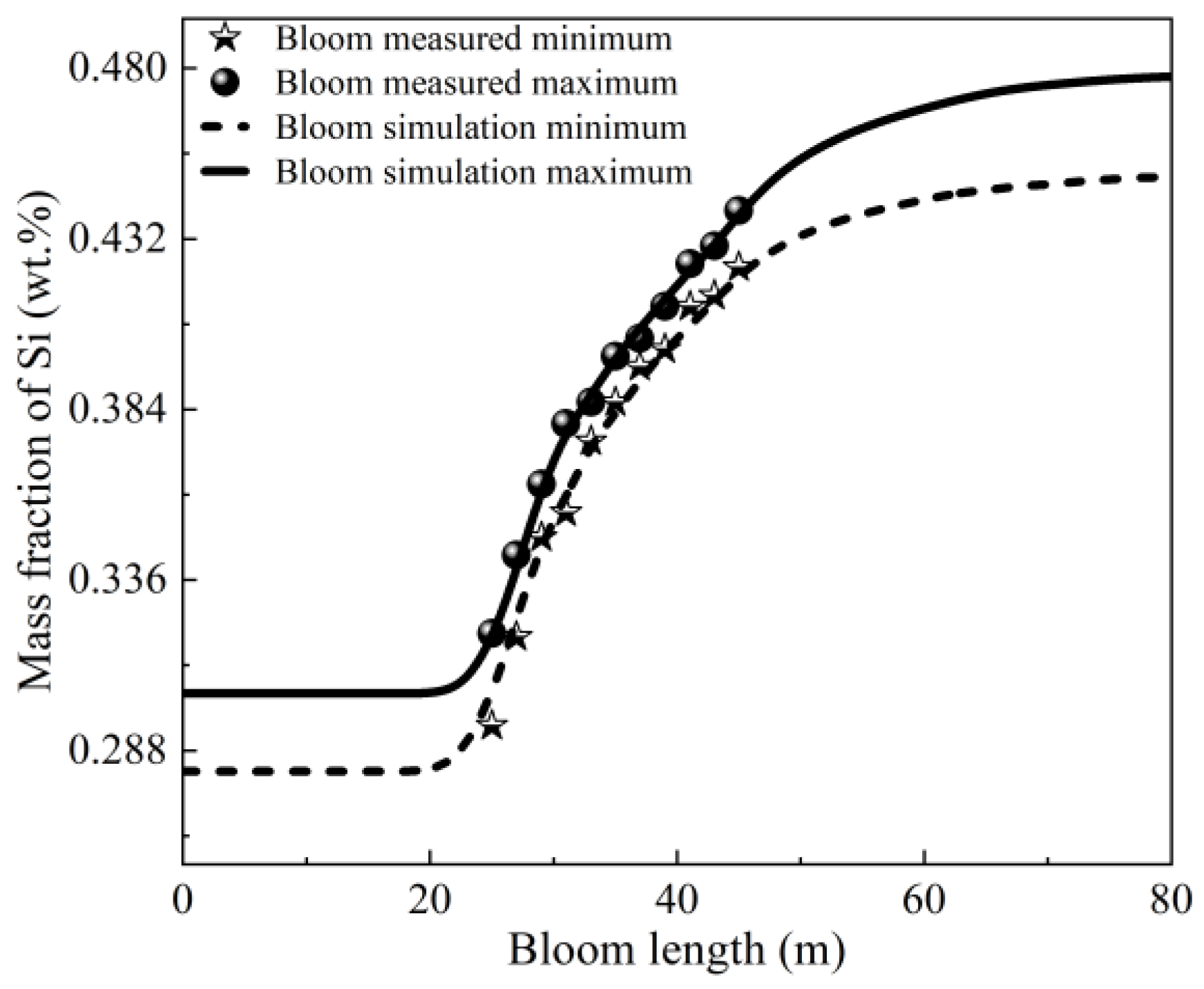
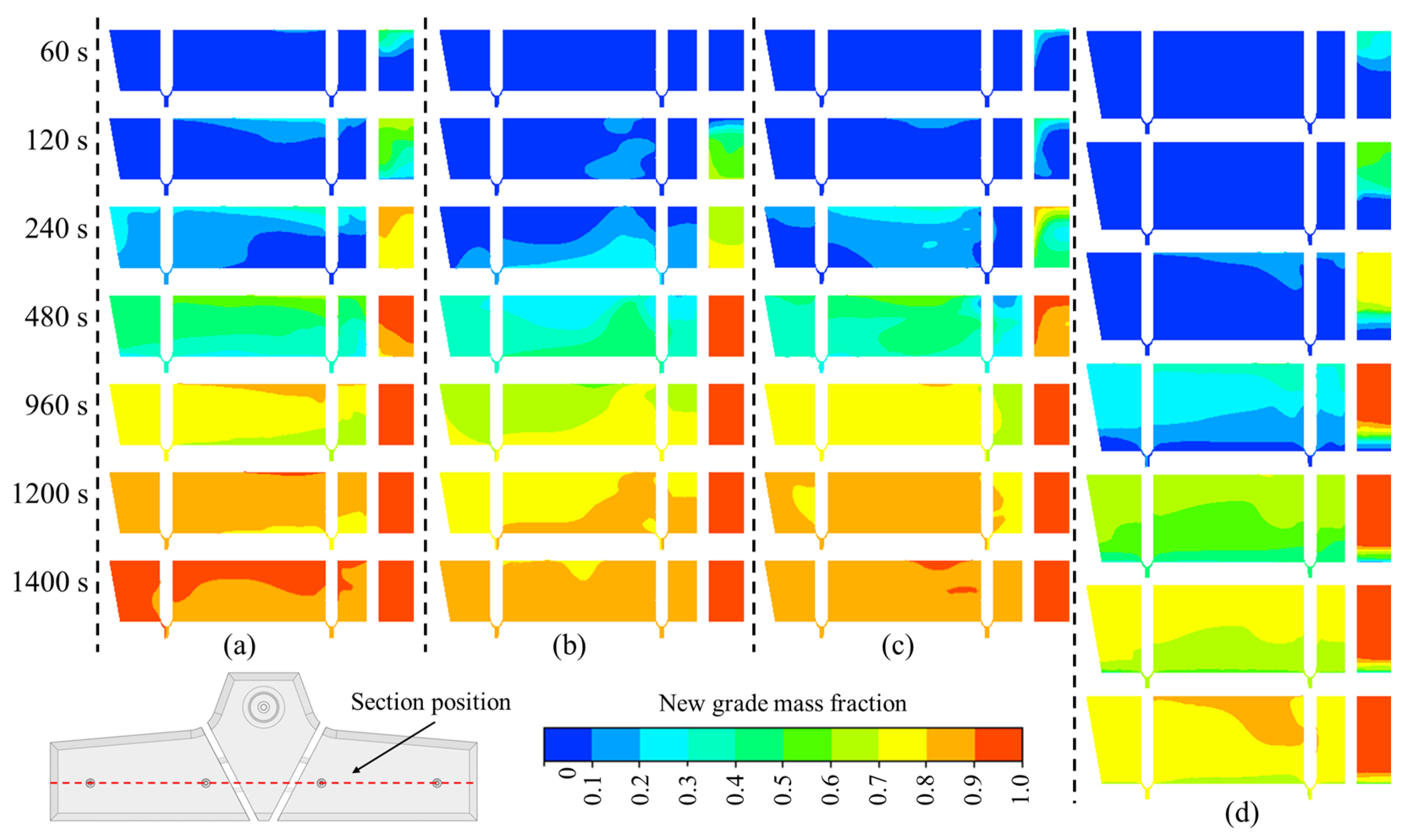


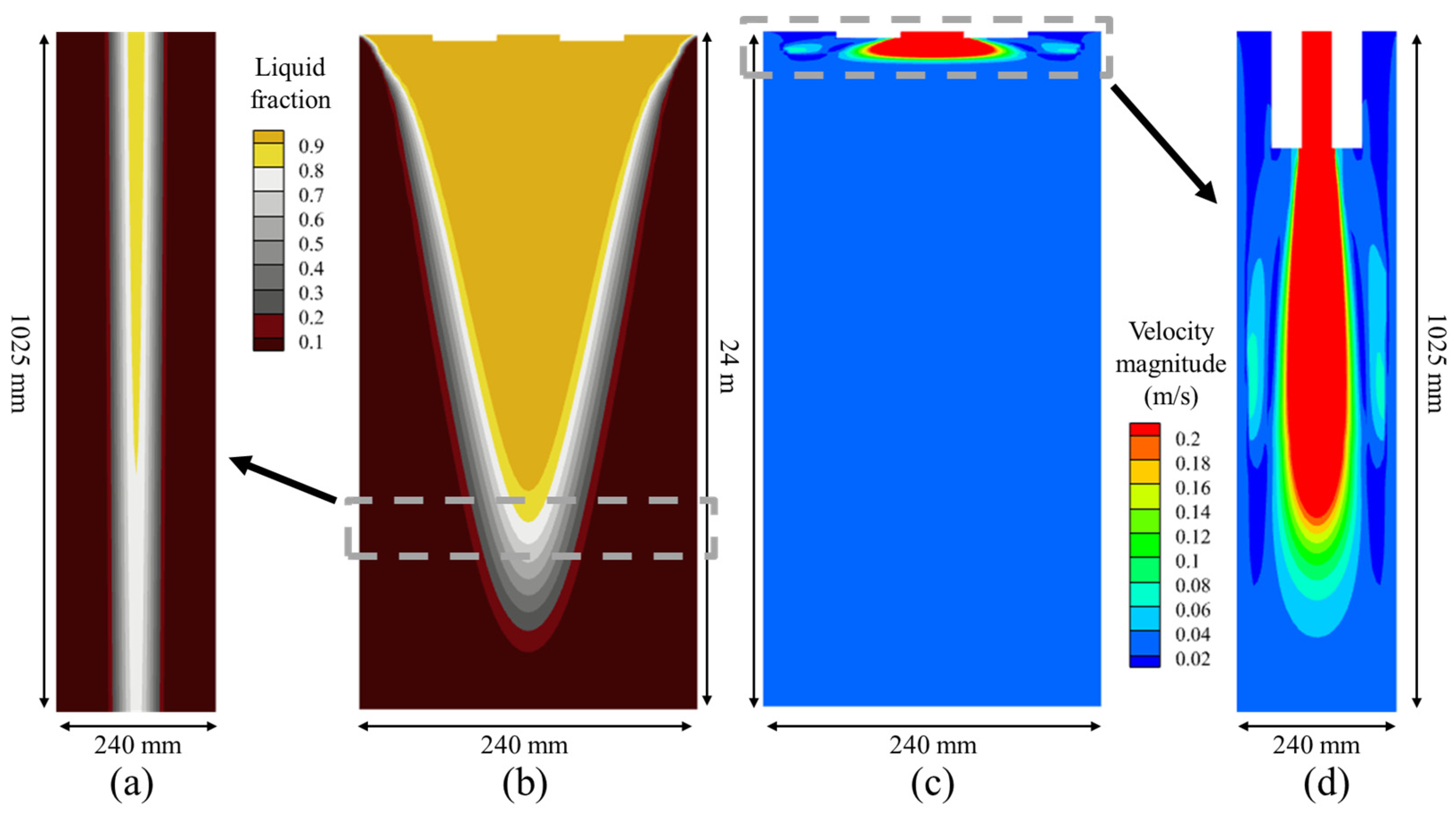

| Parameter | Value | Unit | Parameter | Value | Unit |
|---|---|---|---|---|---|
| Ladle shroud inner diameter | 75 | mm | SEN inner diameter | 45 | mm |
| Ladle shroud outer diameter | 148 | mm | SEN outer diameter | 95 | mm |
| Ladle shroud submerged depth | 350 | mm | Submerged depth of SEN | 150 | mm |
| Casting speed | 1.2 | m/min | Bloom cross-section | 240 × 240 | mm2 |
| Mold length | 700 | mm | Loop 0 length | 400 | mm |
| Loop 1 length | 2100 | mm | Loop 2 length | 2600 | mm |
| Loop 3 length | 3200 | mm | Loop 4 length | 15,000 | mm |
| F-EMS current intensity | 400 | A | F-EMS frequency | 4 | Hz |
| F-EMS core inner diameter | 660 | mm | F-EMS core outer diameter | 800 | mm |
| F-EMS coil height | 600 | mm | F-EMS inner diameter | 500 | mm |
| F-EMS core height | 500 | mm | F-EMS center position from meniscus | 17,000 | mm |
| Loop Number | Water Flow Rate (L/(m2·s)) | Convection Coefficient (W/(m2·K)) | Loop Number | Water Flow Rate (L/(m2·s)) | Convection Coefficient (W/(m2·K)) |
|---|---|---|---|---|---|
| 0 | 63.5 | 1803.1 | 1 | 105.0 | 664.0 |
| 2 | 53.6 | 425.0 | 3 | 19.4 | 246.0 |
| Parameter | Value | Unit | Parameter | Value | Unit |
|---|---|---|---|---|---|
| Specific heat of steel | 650 | J/(kg·K) | Viscosity of steel | 0.0062 | kg/(m·s) |
| Pure solvent melting heat | 270,000 | J/kg | Thermal conductivity | 41 | W/(m·K) |
| Liquid-phase diffusion coefficient | 2.0 × 10−9 | cm2/s |
| Grage | C | Si | Mn | Cr | Ni | Mo |
|---|---|---|---|---|---|---|
| Old grade GCr15 range | 0.95~1.05 | 0.15~0.35 | 0.25~0.45 | 0.95~1.05 | ≤0.20 | - |
| Old grade GCr15 composition | 1.00 | 0.29 | 0.36 | 0.99 | 0.01 | - |
| New grade GCr15SiMn range | 0.95~1.05 | 0.40~0.65 | 0.90~1.20 | 1.30~1.65 | ≤0.20 | - |
| New grade GCr15SiMn composition | 0.99 | 0.46 | 0.98 | 1.37 | 0.01 | - |
| Old grade 30Cr2Ni2Mo range | 0.26~0.34 | 0.17~0.37 | 0.30~0.60 | 1.80~2.20 | 1.80~2.20 | 0.50~0.80 |
| Old grade 30Cr2Ni2Mo composition | 0.31 | 0.23 | 0.52 | 2.1 | 2.08 | 0.68 |
| New grade 30CrNi3Mo range | 0.30~0.40 | 0.17~0.37 | 0.50~0.80 | 1.10~1.70 | 2.75~3.25 | 0.25~0.40 |
| New grade 30CrNi3Mo composition | 0.33 | 0.26 | 0.58 | 1.32 | 2.82 | 0.31 |
| Old grade Q345 range | 0.13~0.16 | ≤0.40 | 1.20~1.35 | ≤0.30 | ≤0.03 | ≤0.08 |
| Old grade Q345 composition | 0.15 | 0.31 | 1.25 | 0.01 | 0.01 | 0.03 |
| New grade Q355 range | 0.13~0.17 | ≤0.47 | 1.30~1.55 | ≤0.30 | ≤0.03 | ≤0.10 |
| New grade Q355 composition | 0.15 | 0.28 | 1.5 | 0.01 | 0.01 | 0.02 |
| Element | (cm2/s) | (cm2/s) | (K/wt.%) | (1/wt.%) | ||
|---|---|---|---|---|---|---|
| C | 0.19 | 0.34 | 0.0127exp(−19,450/RT) | 0.0761exp(−32,160/RT) | 78.0 | 1.10 × 10−2 |
| Si | 0.77 | 0.52 | 8.0exp(−59,500/RT) | 0.3exp(−60,100/RT) | 7.6 | 1.19 × 10−2 |
| Mn | 0.77 | 0.785 | 0.76exp(−53,640/RT) | 0.055exp(−59,600/RT) | 4.9 | 1.92 × 10−3 |
| Cr | 0.95 | 0.86 | 2.4exp(−57,310/RT) | 0.0012exp(−52,340/RT) | 1.04 | 3.97 × 10−3 |
| Ni | 0.83 | 0.95 | 1.6exp(−57,360/RT) | 0.34exp(−67,490/RT) | 4.69 | −6.85 × 10−4 |
| Mo | 0.8 | 0.585 | 3.47exp(−57,690/RT) | 0.068exp(−59,000/RT) | 2.6 | −1.92 × 10−3 |
| No. | Old Grade | New Grade | Tundish Level | No. | Old Grade | New Grade | Tundish Level |
|---|---|---|---|---|---|---|---|
| 1 | GCr15 | GCr15SiMn | 560 mm | 2 | 30Cr2Ni2Mo | 30CrNi3Mo | 560 mm |
| 3 | Q345 | Q355 | 560 mm | 4 | GCr15 | GCr15SiMn | 810 mm |
| 5 | GCr15 | GCr15SiMn | 0 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.; Sun, Y.; Chen, C. Numerical Simulation of Macro-Segregation Phenomena in Transition Blooms with Various Carbon Contents. Metals 2024, 14, 263. https://doi.org/10.3390/met14030263
Song S, Sun Y, Chen C. Numerical Simulation of Macro-Segregation Phenomena in Transition Blooms with Various Carbon Contents. Metals. 2024; 14(3):263. https://doi.org/10.3390/met14030263
Chicago/Turabian StyleSong, Sicheng, Yanhui Sun, and Chao Chen. 2024. "Numerical Simulation of Macro-Segregation Phenomena in Transition Blooms with Various Carbon Contents" Metals 14, no. 3: 263. https://doi.org/10.3390/met14030263






