Study of Electroless Nickel Plating on Super Duplex Stainless Steel for Lithium-Ion Battery Cases: Electrochemical Behaviour and Effects of Plating Time
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
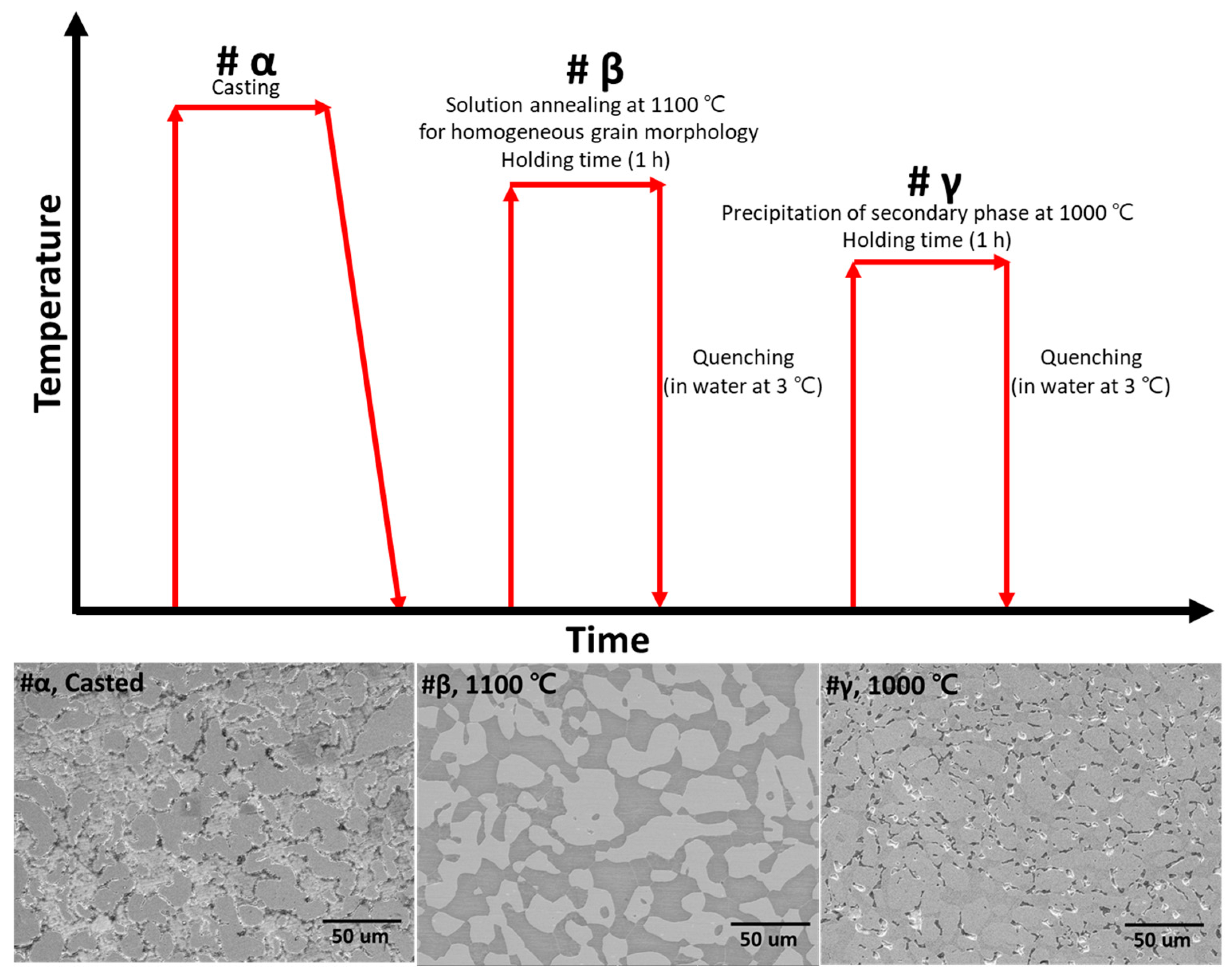
2.2. Heat Treatment Conditions
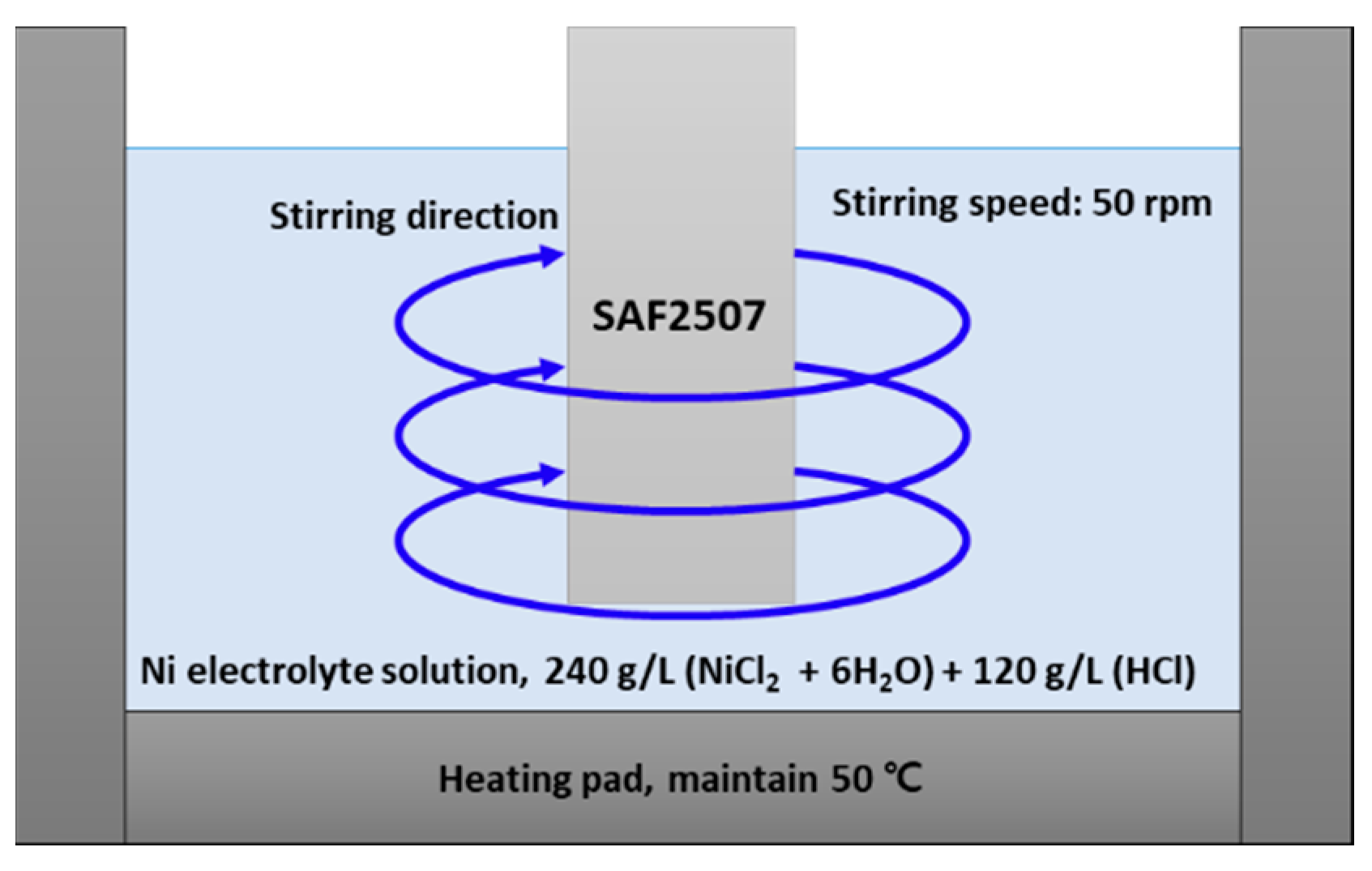
2.3. Electroless Ni Plating
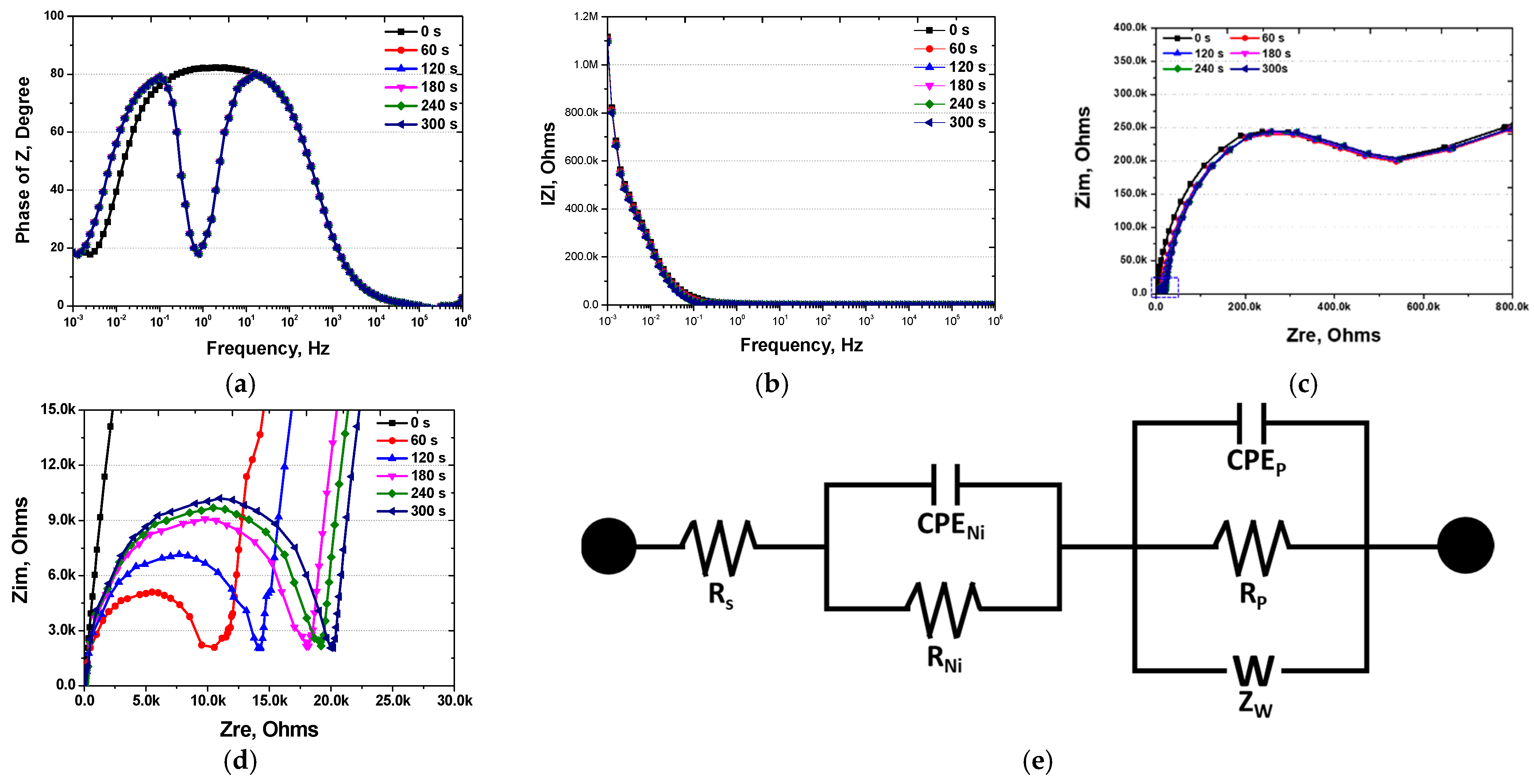
2.4. Electrochemical Properties
3. Results
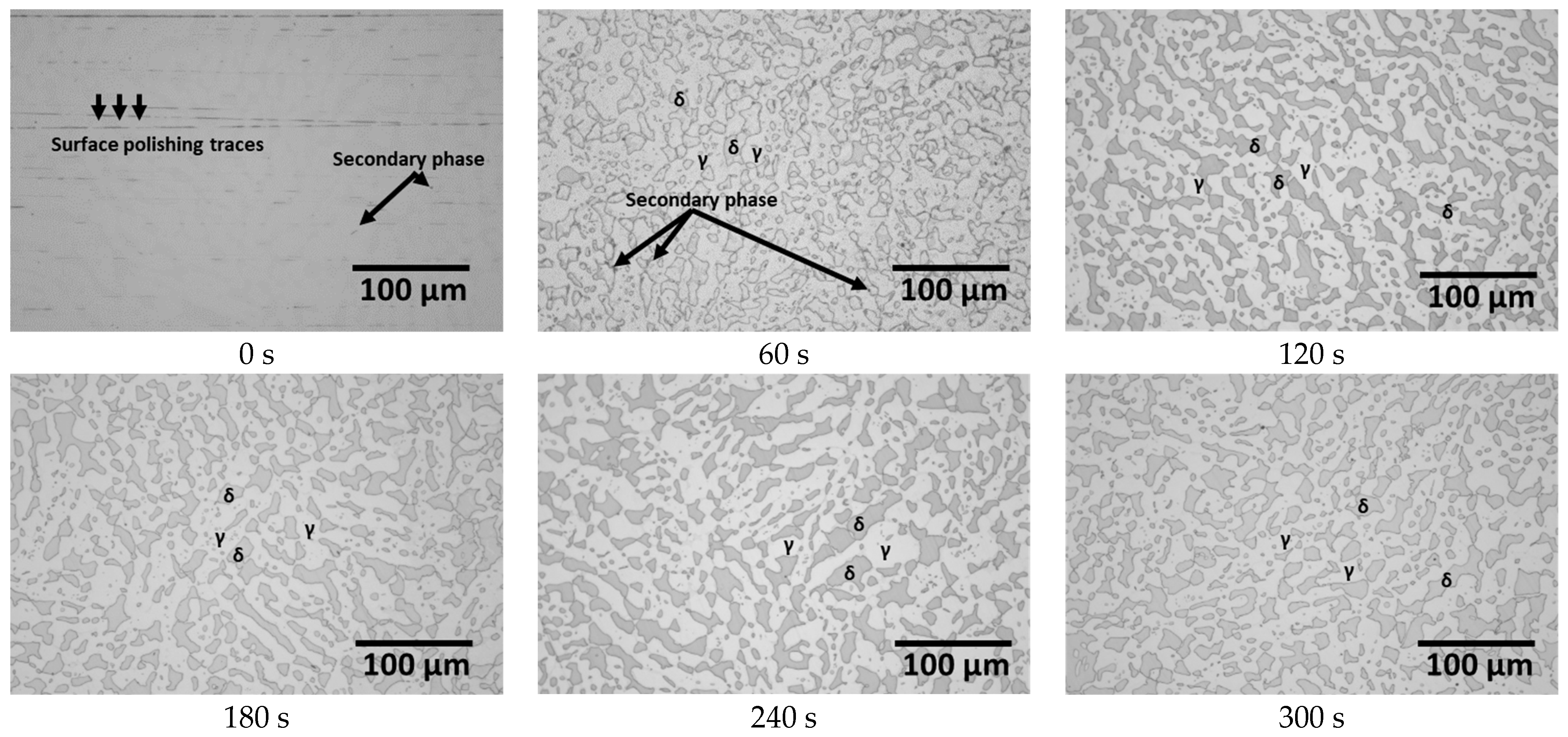
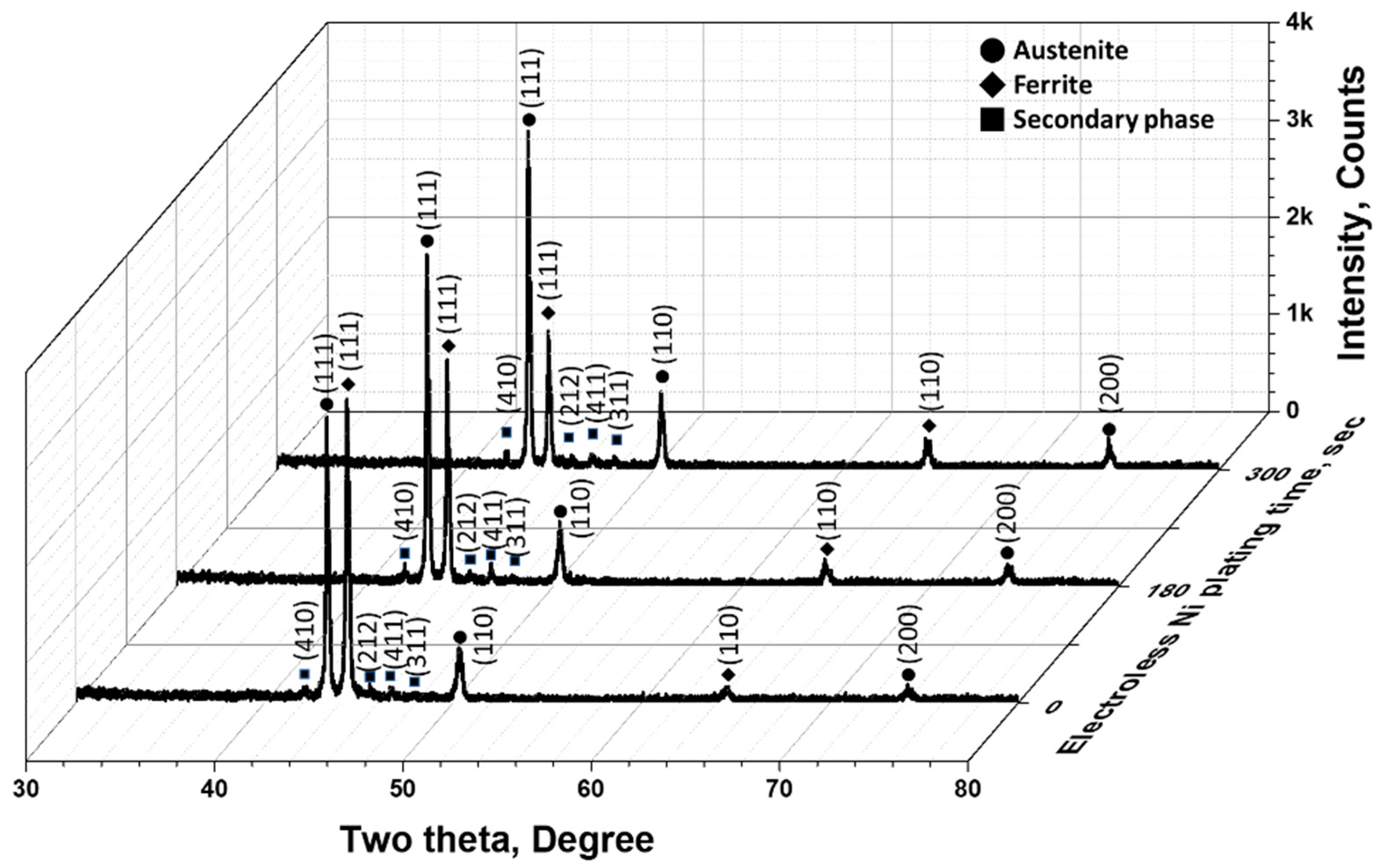
3.1. Electroless Ni Plating Behaviour with Time
3.2. Effect of Electroless Ni Plating Time on Electrochemical Properties
4. Discussion
5. Conclusions
- (1)
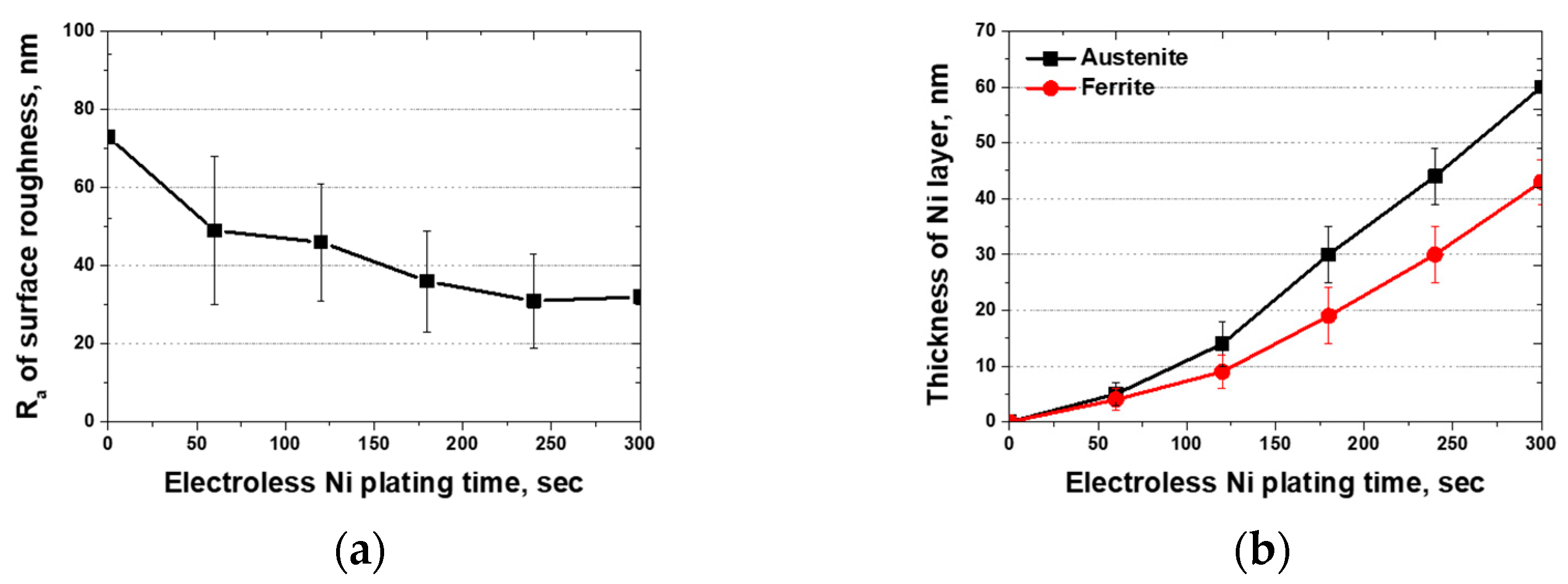
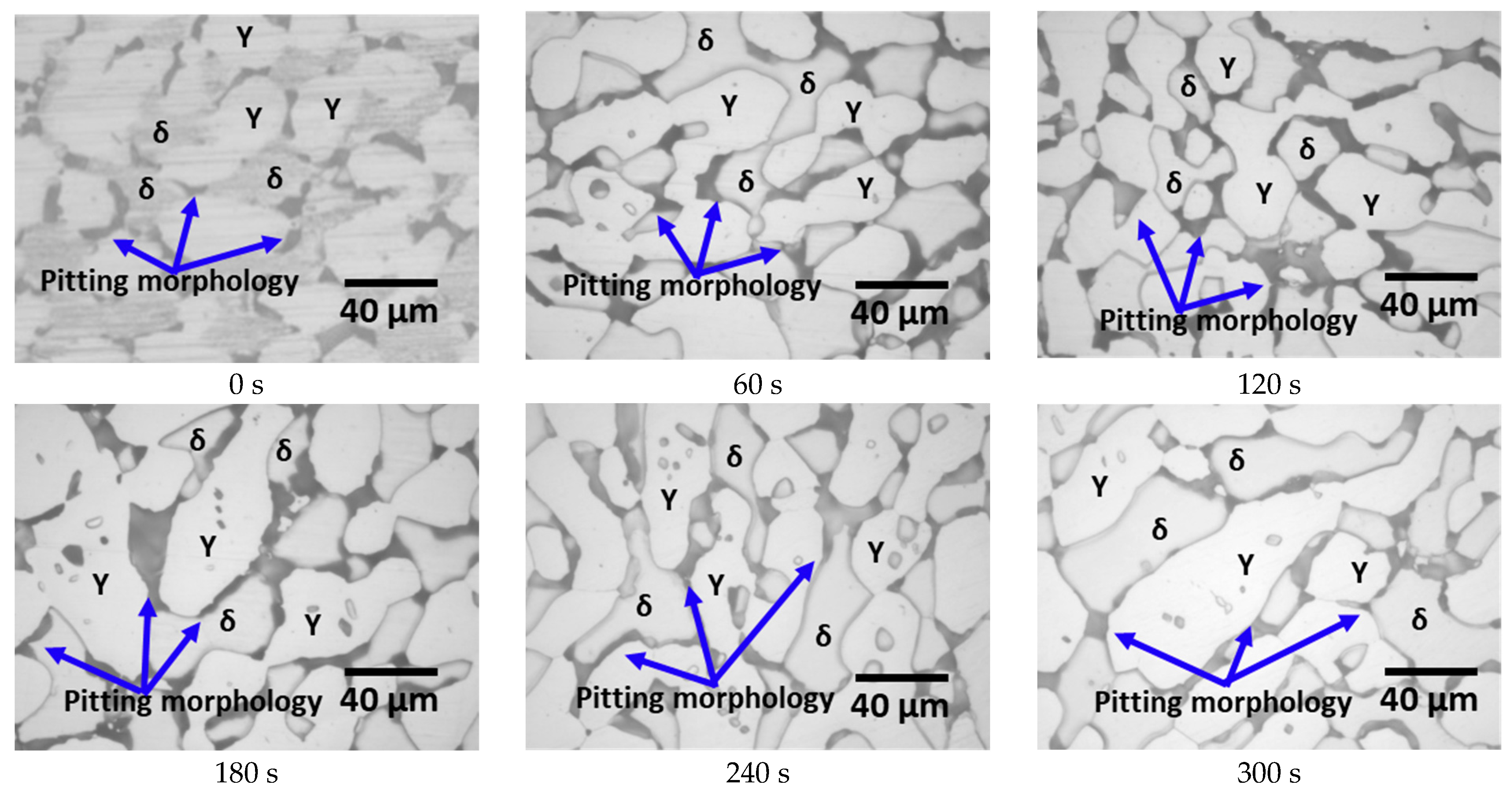
- SAF2507, heat-treated at 1000 °C, precipitated a 13% secondary phase primarily at the ferrite–austenite boundaries. The secondary phase was formed through the precipitation of Cr- and Mo-rich clusters, with the chemical composition of Cr and Mo exceeding 30 and 10 wt.%, respectively. The differences in the chemical composition and lattice structure of the secondary phase influenced the Ni plating. The secondary-phase plating rate was equivalent to that of ferrite and slower than austenite. However, uniform plating layers were formed after 180 s. Examination of the plating thickness via AFM revealed that the plating rate improved from an increase of less than 10 μm to over 15 μm after 180 s. Therefore, uniform plating enhances the plating rate and improves electroless Ni plating ability.
- (2)
- The secondary phase was homogeneously plated within 180 s of the electroless plating. The plating reaction showed a preference for the (111) plane at 42° of austenite, making the Ni plating layer distinguishable between the austenite and ferrite regions. Considering the phase proportions, the secondary phase exhibited a reaction rate equivalent to that of ferrite, suggesting that it grew together with the ferrite. Therefore, the phases have a greater effect than the material composition on Ni plating on SAF2507. The phase composition affects the plating rate, and since SDSS consists of both austenite and ferrite, an electroless Ni plating time of over 180 s is required to form a stable plating layer over 20 nm.
- (3)
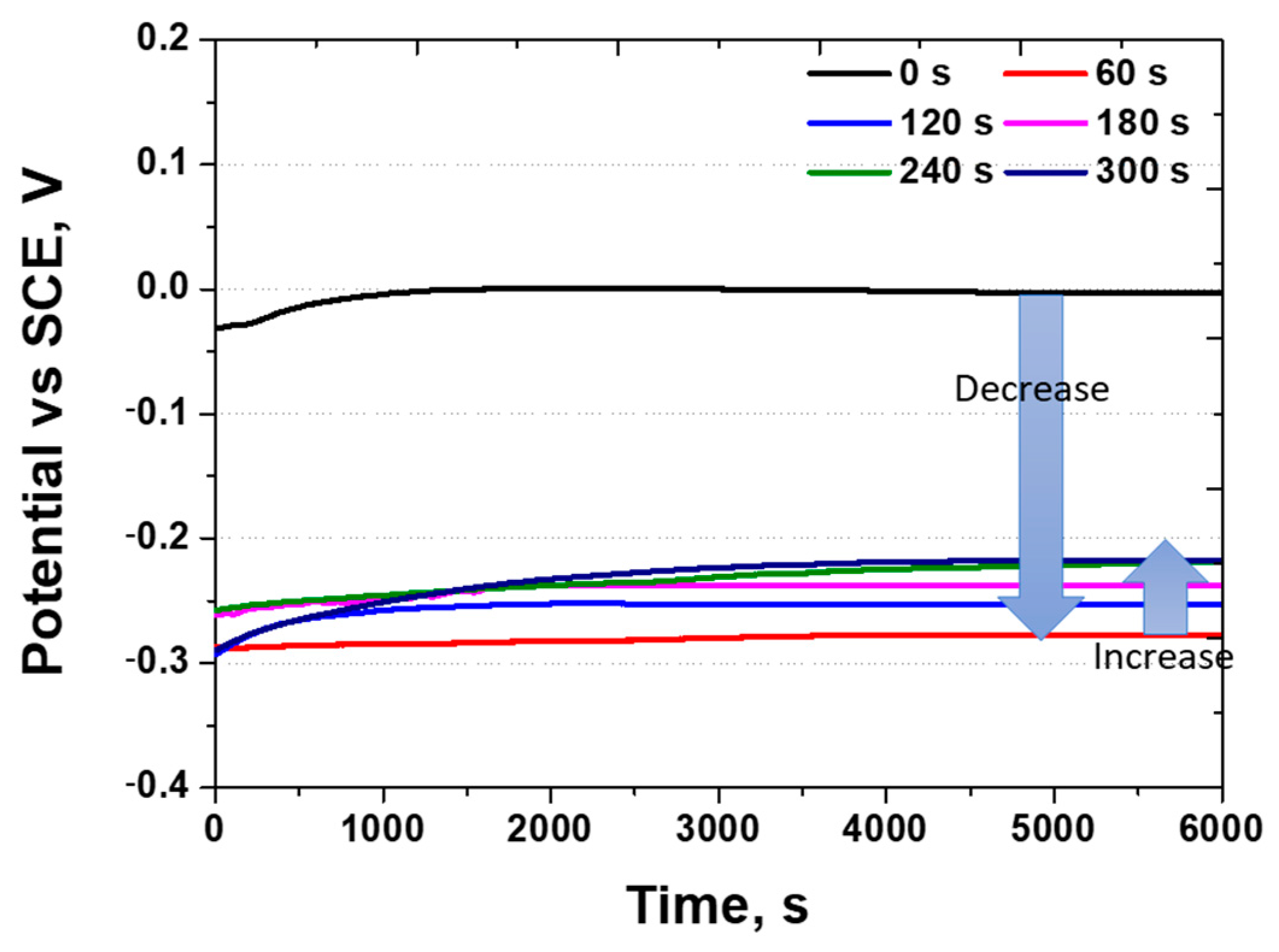
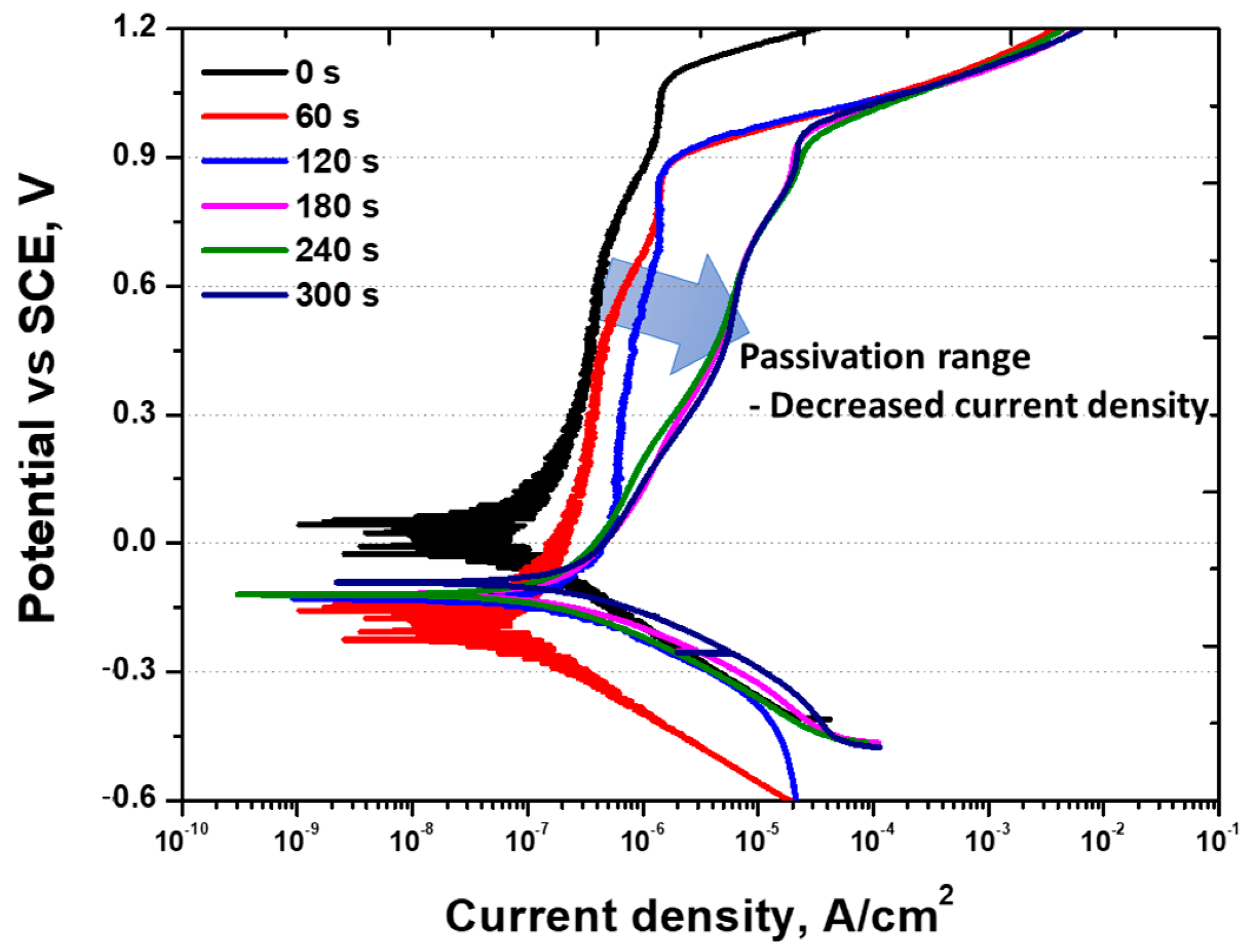
- The electrochemical characteristics were uniform after 120 s of electroless Ni plating. In contrast, the uneven Ni plating layer formed after 60 s caused galvanic corrosion with the secondary phase, resulting in a decreased OCP potential from 0.0 V to −0.28 V and activation polarisation. In addition, a dual loop was observed in the potentiodynamic polarisation curves at 60 s. After 120 s, uniform electrochemical properties were evident, showing an increased potential, current density, and reduced pitting potential. The increase in potential approached that of pure Ni after galvanic corrosion, and the increase in current density indicated an enhanced uniform corrosion rate arising from Ni plating. The pitting potential, generated in the secondary phase, decreased from 1.05 to 0.90 V due to increased corrosion after Ni plating.
- (4)
- SAF2507 precipitated with a secondary phase and showed excellent electroless Ni plating behaviour. Considering the surface conditions and electrochemical characteristics, electroless Ni plating for 180 s (Ni layer thicknesses from 20 to 40 nm and EIS resistance of 20 kΩ) or more is suitable for application as a Li-ion battery case material. Compared to conventional AISI304 stainless steel, SAF2507 offers superior strength and plating characteristics, which can enhance its safety and durability. This study confirms that SAF2507 is a potential replacement for Li-ion battery materials, anticipating the improved safety of Li-ion batteries through future material enhancements.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chang, W.-S.; Park, C.-M.; Kim, J.-H.; Kim, Y.-U.; Jeong, G.; Sohn, H.-J. Quartz (SiO2): A New Energy Storage Anode Material for Li-Ion Batteries. Energy Environ. Sci. 2012, 5, 6895–6899. [Google Scholar] [CrossRef]
- Bizeray, A.M.; Howey, D.A.; Monroe, C.W. Resolving a Discrepancy in Diffusion Potentials, with a Case Study for Li-Ion Batteries. J. Electrochem. Soc. 2016, 163, E223. [Google Scholar] [CrossRef]
- Cicconi, P.; Kumar, P.; Varshney, P. A Support Approach for the Modular Design of Li-Ion Batteries: A Test Case with PCM. J. Energy Storage 2020, 31, 101684. [Google Scholar] [CrossRef]
- Fredriksson, W.; Edström, K. XPS Study of Duplex Stainless Steel as a Possible Current Collector in a Li-Ion Battery. Electrochim. Acta 2012, 79, 82–94. [Google Scholar] [CrossRef]
- Mayyas, A.; Steward, D.; Mann, M. The Case for Recycling: Overview and Challenges in the Material Supply Chain for Automotive Li-Ion Batteries. Sustain. Mater. Technol. 2019, 19, e00087. [Google Scholar] [CrossRef]
- Kale, R.B.; More, S.S.; Khupse, N.D.; Kalubarme, R.S.; Kulkarni, M.V.; Rane, S.B.; Kale, B.B. High-Voltage Ionic Liquid-Based Flexible Solid Polymer Electrolyte for High-Performance Li-Ion Batteries. Sustain. Energy Fuels 2023, 7, 2934–2942. [Google Scholar] [CrossRef]
- Rahman, A.; Lin, X.; Wang, C. Li-Ion Battery Anode State of Charge Estimation and Degradation Monitoring Using Battery Casing via Unknown Input Observer. Energies 2022, 15, 5662. [Google Scholar] [CrossRef]
- Tudoroiu, N.; Zaheeruddin, M.; Tudoroiu, R.-E.; Radu, M.S.; Chammas, H. Investigations on Using Intelligent Learning Techniques for Anomaly Detection and Diagnosis in Sensors Signals in Li-Ion Battery—Case Study. Inventions 2023, 8, 74. [Google Scholar] [CrossRef]
- Klink, J.; Hebenbrock, A.; Grabow, J.; Orazov, N.; Nylén, U.; Benger, R.; Beck, H.-P. Comparison of Model-Based and Sensor-Based Detection of Thermal Runaway in Li-Ion Battery Modules for Automotive Application. Batteries 2022, 8, 34. [Google Scholar] [CrossRef]
- Park, J.; Fatima, S.A. A DFT Study of TiC3 as Anode Material for Li-Ion Batteries. Appl. Surf. Sci. 2023, 638, 158024. [Google Scholar] [CrossRef]
- Khan, M.M.; Alkhedher, M.; Ramadan, M.; Ghazal, M. Hybrid PCM-Based Thermal Management for Lithium-Ion Batteries: Trends and Challenges. J. Energy Storage 2023, 73, 108775. [Google Scholar] [CrossRef]
- Rahman, A.; Lin, X. Li-Ion Battery Individual Electrode State of Charge and Degradation Monitoring Using Battery Casing through Auto Curve Matching for Standard CCCV Charging Profile. Appl. Energy 2022, 321, 119367. [Google Scholar] [CrossRef]
- Tudoroiu, R.-E.; Zaheeruddin, M.; Tudoroiu, N.; Radu, S.M.; Chammas, H. Investigations of Different Approaches for Controlling the Speed of an Electric Motor with Nonlinear Dynamics Powered by a Li-Ion Battery-Case Study. In Electric Vehicles—Design, Modelling and Simulation; IntechOpen: London, UK, 2023. [Google Scholar]
- Petit, M.; Prada, E.; Sauvant-Moynot, V. Development of an Empirical Aging Model for Li-Ion Batteries and Application to Assess the Impact of Vehicle-to-Grid Strategies on Battery Lifetime. Appl. Energy 2016, 172, 398–407. [Google Scholar] [CrossRef]
- Speidel, M.O. Nitrogen Containing Austenitic Stainless Steels. Mater. Werkst. Entwickl. Fert. Prüfung Eig. Anwendungen Tech. Werkst. 2006, 37, 875–880. [Google Scholar] [CrossRef]
- Acharyya, S.G.; Khandelwal, A.; Kain, V.; Kumar, A.; Samajdar, I. Surface Working of 304L Stainless Steel: Impact on Microstructure, Electrochemical Behavior and SCC Resistance. Mater. Charact. 2012, 72, 68–76. [Google Scholar] [CrossRef]
- Yoo, Y.-R.; Choi, S.-H.; Kim, Y.-S. Effect of Laser Peening on the Corrosion Properties of 304L Stainless Steel. Materials 2023, 16, 804. [Google Scholar] [CrossRef]
- Hoosain, S.E.; Tshabalala, L.C.; Skhosana, S.; Freemantle, C.; Mndebele, N. Investigation of the Properties of Direct Energy Deposition Additive Manufactured 304 Stainless Steel. S. Afr. J. Ind. Eng. 2021, 32, 258–263. [Google Scholar] [CrossRef]
- Nilsson, J.-O. Super Duplex Stainless Steels. Mater. Sci. Technol. 1992, 8, 685–700. [Google Scholar] [CrossRef]
- Paulraj, P.; Garg, R. Effect of Intermetallic Phases on Corrosion Behavior and Mechanical Properties of Duplex Stainless Steel and Super-Duplex Stainless Steel. Adv. Sci. Technol. Res. J. 2015, 9, 87–105. [Google Scholar] [CrossRef]
- Linton, V.M.; Laycock, N.J.; Thomsen, S.J.; Klumpers, A. Failure of a Super Duplex Stainless Steel Reaction Vessel. Eng. Fail Anal. 2004, 11, 243–256. [Google Scholar] [CrossRef]
- Makhdoom, M.A.; Ahmad, A.; Kamran, M.; Abid, K.; Haider, W. Microstructural and Electrochemical Behavior of 2205 Duplex Stainless Steel Weldments. Surf. Interfaces 2017, 9, 189–195. [Google Scholar] [CrossRef]
- Tehovnik, F.; Arzensek, B.; Arh, B.; Skobir, D.; Pirnar, B.; Zuzek, B. Microstructure Evolution in SAF 2507 Super Duplex Stainless Steel. Mater. Technol. 2011, 45, 339–345. [Google Scholar]
- Elhoud, A.M.; Renton, N.C.; Deans, W.F. Hydrogen Embrittlement of Super Duplex Stainless Steel in Acid Solution. Int. J. Hydrog. Energy 2010, 35, 6455–6464. [Google Scholar] [CrossRef]
- Pettersson, N.; Pettersson, R.F.A.; Wessman, S. Precipitation of Chromium Nitrides in the Super Duplex Stainless Steel 2507. Metall. Mater. Trans. A 2015, 46, 1062–1072. [Google Scholar] [CrossRef]
- Martins, M.; Casteletti, L.C. Sigma Phase Morphologies in Cast and Aged Super Duplex Stainless Steel. Mater. Charact. 2009, 60, 792–795. [Google Scholar] [CrossRef]
- Topolska, S.; Łabanowski, J. Effect of Microstructure on Impact Toughness of Duplex and Superduplex Stainless Steels. J. Achiev. Mater. Manuf. Eng. 2009, 36, 142–149. [Google Scholar]
- Valiente Bermejo, M.A.; Thalavai Pandian, K.; Axelsson, B.; Harati, E.; Kisielewicz, A.; Karlsson, L. Microstructure of Laser Metal Deposited Duplex Stainless Steel: Influence of Shielding Gas and Heat Treatment. Weld. World 2021, 65, 525–541. [Google Scholar] [CrossRef]
- Sung, C.; Kim, K.; Chung, W.; Shin, B.-H. Electrochemical Properties of UNS S 32750 and UNS S 32760 Annealed Super Duplex Stainless Steels. Int. J. Electrochem. Sci. 2022, 17, 220526. [Google Scholar] [CrossRef]
- Kim, D.; Chung, W.; Shin, B.-H. Effects of the Volume Fraction of the Secondary Phase after Solution Annealing on Electrochemical Properties of Super Duplex Stainless Steel UNS S32750. Metals 2023, 13, 957. [Google Scholar] [CrossRef]
- Shin, B.-H.; Kim, D.; Park, S.; Hwang, M.; Park, J.; Chung, W. Precipitation Condition and Effect of Volume Fraction on Corrosion Properties of Secondary Phase on Casted Super-Duplex Stainless Steel UNS S32750. Anti-Corros. Methods Mater. 2018, 66, 61–66. [Google Scholar] [CrossRef]
- Messinese, E.; Casanova, L.; Paterlini, L.; Capelli, F.; Bolzoni, F.; Ormellese, M.; Brenna, A. A Comprehensive Investigation on the Effects of Surface Finishing on the Resistance of Stainless Steel to Localized Corrosion. Metals 2022, 12, 1751. [Google Scholar] [CrossRef]
- Nishimoto, M.; Muto, I.; Sugawara, Y. Review―Understanding and Controlling the Electrochemical Properties of Sulfide Inclusions for Improving the Pitting Corrosion Resistance of Stainless Steels. Mater. Trans. 2023, 64, 2051–2058. [Google Scholar] [CrossRef]
- Shin, B.; Park, S.; Park, J.; Kim, D.; Hwang, M.; Chung, W. Effect of Post-Weld Heat Treatment on the Corrosion Behavior of Resistance Spot Welded Super Duplex Stainless UNS S 32750. Int. J. Electrochem. Sci. 2019, 14, 2430–2441. [Google Scholar] [CrossRef]
- Sung, C.; Shin, B.-H.; Chung, W. Effect of Heat Energy Input on Electrochemical Properties of Solution-Annealed Super-Duplex Stainless Steel UNS S 32750 Laser Welding. Int. J. Electrochem. Sci. 2022, 17, 220339. [Google Scholar] [CrossRef]
- Valeriano, L.d.C.; Correa, E.O.; Mariano, N.A.; Robin, A.L.M.; Machado, M.A.G. Influence of the Solution-Treatment Temperature and Short Aging Times on the Electrochemical Corrosion Behaviour of Uns S32520 Super Duplex Stainless Steel. Mater. Res. 2019, 22, e20180774. [Google Scholar] [CrossRef]
- Maurya, A.K.; Pandey, C.; Chhibber, R. Effect of Filler Metal Composition on Microstructural and Mechanical Characterization of Dissimilar Welded Joint of Nitronic Steel and Super Duplex Stainless Steel. Arch. Civ. Mech. Eng. 2022, 22, 90. [Google Scholar] [CrossRef]
- Loto, C.A. Electroless Nickel Plating—A Review 2016. Silicon 2016, 8, 177–186. [Google Scholar] [CrossRef]
- Di Bari, G.A. Nickel Plating; ASM International: Almere, The Netherlands, 1994. [Google Scholar]
- Baudrand, D.W. Electroless Nickel Plating; ASM International: Almere, The Netherlands, 1994. [Google Scholar]
- Ghosh, S.K.; Grover, A.K.; Dey, G.K.; Totlani, M.K. Nanocrystalline Ni–Cu Alloy Plating by Pulse Electrolysis. Surf. Coat. Technol. 2000, 126, 48–63. [Google Scholar] [CrossRef]
- Rehman, A.U.; Lee, S.H. Review of the Potential of the Ni/Cu Plating Technique for Crystalline Silicon Solar Cells. Materials 2014, 7, 1318–1341. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, K.; Chung, W.; Shin, B.-H. Effect of the Plating Time on Nickel Electroless Coating Properties Deposited on the Super Duplex Stainless Steel UNS S 32750. Int. J. Electrochem. Sci. 2022, 17, 220630. [Google Scholar] [CrossRef]
- Lee, H.-B.; Sheu, H.-H.; Jian, J.-S.; Chang, S.-Y.; Yen, C.-H.; Lin, H.-E. Supercritical-CO2 Electroless Nickel Plating Enhanced Anti-Corrosion Properties of Micro-Arc Oxidized AZ31 Magnesium Alloy. Mater. Today Commun. 2022, 33, 104475. [Google Scholar] [CrossRef]
- Nilsson, J.O.; Wilson, A. Influence of Isothermal Phase Transformations on Toughness and Pitting Corrosion of Super Duplex Stainless Steel SAF 2507. Mater. Sci. Technol. 1993, 9, 545–554. [Google Scholar] [CrossRef]
- Köse, C. Effect of Heat Input and Post Weld Heat Treatment on the Texture, Microstructure and Mechanical Properties of Laser Beam Welded AISI 317L Austenitic Stainless Steel. Mater. Sci. Eng. A 2022, 855, 143966. [Google Scholar] [CrossRef]
- Shin, B.-H.; Park, J.; Jeon, J.; Heo, S.; Chung, W. Effect of Cooling Rate after Heat Treatment on Pitting Corrosion of Super Duplex Stainless Steel UNS S 32750. Anti-Corros. Methods Mater. 2018, 65, 492–498. [Google Scholar] [CrossRef]
- Beziou, O.; Hamdi, I.; Boumerzoug, Z.; Brisset, F.; Baudin, T. Effect of Heat Treatment on the Welded Joint of X70 Steel Joined to Duplex Stainless Steel by Gas Tungsten Arc Welding. Int. J. Adv. Manuf. Technol. 2023, 127, 2799–2814. [Google Scholar] [CrossRef]
- Moniruzzaman, F.N.U.M.; Shakil, S.I.; Shaha, S.K.; Kacher, J.; Nasiri, A.; Haghshenas, M.; Hadadzadeh, A. Study of Direct Aging Heat Treatment of Additively Manufactured PH13–8Mo Stainless Steel: Role of the Manufacturing Process, Phase Transformation Kinetics, and Microstructure Evolution. J. Mater. Res. Technol. 2023, 24, 3772–3787. [Google Scholar] [CrossRef]
- Vongsilathai, S.; Lothongkum, A.W.; Lothongkum, G. Corrosion Behavior of a New 25Cr-3Ni-7Mn-0.66 N Duplex Stainless Steel in Artificial Seawater. Mater. Test. 2021, 63, 505–511. [Google Scholar] [CrossRef]
- Kannan, A.R.; Shanmugam, N.S.; Rajkumar, V.; Vishnukumar, M. Insight into the Microstructural Features and Corrosion Properties of Wire Arc Additive Manufactured Super Duplex Stainless Steel (ER2594). Mater. Lett. 2020, 270, 127680. [Google Scholar] [CrossRef]
- Baghdadchi, A.; Hosseini, V.A.; Valiente Bermejo, M.A.; Axelsson, B.; Harati, E.; Högström, M.; Karlsson, L. Wire Laser Metal Deposition of 22% Cr Duplex Stainless Steel: As-Deposited and Heat-Treated Microstructure and Mechanical Properties. J. Mater. Sci. 2022, 57, 9556–9575. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, J.; Chen, Z.; Guo, R.; Miao, D.; Peng, L.; Wang, Y.; Shang, S. Enhanced Electro-Conductivity and Multi-Shielding Performance with Copper, Stainless Steel and Titanium Coating onto PVA Impregnated Cotton Fabric. J. Mater. Sci. Mater. Electron. 2018, 29, 5624–5633. [Google Scholar] [CrossRef]
- Gasana, E.; Westbroek, P.; Hakuzimana, J.; De Clerck, K.; Priniotakis, G.; Kiekens, P.; Tseles, D. Electroconductive Textile Structures through Electroless Deposition of Polypyrrole and Copper at Polyaramide Surfaces. Surf. Coat. Technol. 2006, 201, 3547–3551. [Google Scholar] [CrossRef]
- Rybalka, K.V.; Beketaeva, L.A.; Davydov, A.D. Electrochemical Behavior of Stainless Steel in Aerated NaCl Solutions by Electrochemical Impedance and Rotating Disk Electrode Methods. Russ. J. Electrochem. 2006, 42, 370–374. [Google Scholar] [CrossRef]
- Wang, D.; Li, F.; Liu, M.; Zhang, W.; Yu, X.; Da, W. Effect of Nanodiamond Content in the Plating Solution on the Corrosion Resistance of Nickel–Nanodiamond Composite Coatings Prepared on Annealed 45 Carbon Steel. Coatings 2022, 12, 1558. [Google Scholar] [CrossRef]
- Metikoš-Huković, M.; Babić, R.; Grubač, Z.; Petrović, Ž.; Lajçi, N. High Corrosion Resistance of Austenitic Stainless Steel Alloyed with Nitrogen in an Acid Solution. Corros. Sci. 2011, 53, 2176–2183. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, W.G.; Kim, Y.H.; Jang, H. Surface Roughness and the Corrosion Resistance of 21Cr Ferritic Stainless Steel. Corros. Sci. 2012, 63, 404–409. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Lee, T.-H.; Bae, J.-H.; Chun, D.W. Molybdenum Effects on Pitting Corrosion Resistance of FeCrMnMoNC Austenitic Stainless Steels. Metals 2018, 8, 653. [Google Scholar] [CrossRef]
- Yazdanpanah, A.; Franceschi, M.; Bergamo, G.; Bonesso, M.; Dabalà, M. On the Exceptional Stress Corrosion Cracking Susceptibility of Selective Laser Melted 316L Stainless Steel under the Individual Effect of Surface Residual Stresses. Eng. Fail Anal. 2022, 136, 106192. [Google Scholar] [CrossRef]
- Guerrini, E.; Cristiani, P.; Grattieri, M.; Santoro, C.; Li, B.; Trasatti, S. Electrochemical Behavior of Stainless Steel Anodes in Membraneless Microbial Fuel Cells. J. Electrochem. Soc. 2013, 161, H62. [Google Scholar] [CrossRef]
| Element (wt.%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | N | Mn | Ni | Cr | Mo | Cu | Fe | PRE | ||
| (a) | Total | 0.01 | 0.27 | 0.8 | 6.8 | 25.0 | 3.8 | 0.2 | Bal | 41.9 |
| (b) | Austenite | 0.01 | 0.51 | 0.9 | 7.9 | 23.3 | 3.2 | 0.2 | Bal | 42.0 |
| Ferrite | 0.01 | 0.05 | 0.8 | 5.5 | 26.6 | 4.4 | 0.2 | Bal | 41.9 | |
| Intensity | 0 s | 180 s | 300 s |
|---|---|---|---|
| Austenite (111) plane at 42° | 3000 ± 30 | 3700 ± 33 | 3800 ± 35 |
| Ferrite (111) plane at 44° | 3200 ± 15 | 2300 ± 21 | 1500 ± 25 |
| Secondary-phase (410) plane at 41° | 150 ± 29 | 170 ± 31 | 170 ± 32 |
| Secondary-phase (212) plane at 46° | 160 ± 34 | 160 ± 44 | 150 ± 40 |
| Secondary-phase (411) plane at 47° | 150 ± 25 | 180 ± 32 | 160 ± 31 |
| Secondary-phase (311) plane at 48° | 140 ± 31 | 160 ± 34 | 150 ± 32 |
| 0 s | 60 s | 120 s | 180 s | 240 s | 300 s | ||
|---|---|---|---|---|---|---|---|
| Ra of surface roughness, nm | 73 ± 21 | 49 ± 19 | 46 ± 15 | 36 ± 13 | 31 ± 12 | 32 ± 18 | |
| Thickness of Ni layer (nm) | Austenite | 0 | 5 ± 2 | 14 ± 4 | 30 ± 5 | 44 ± 5 | 60 ± 5 |
| Ferrite | 0 | 4 ± 2 | 9 ± 3 | 19 ± 5 | 30 ± 5 | 43 ± 4 | |
| 0 s | 60 s | 120 s | 180 s | 240 s | 300 s | |
|---|---|---|---|---|---|---|
| Corrosion potential at active polarisation (V) | 0.07 ± 0.02 | −0.15 ± 0.02 | −0.15 ± 0.02 | −0.14 ± 0.02 | −0.14 ± 0.02 | −0.12 ± 0.02 |
| Corrosion current density at active polarisation (A/cm2) | (1 ± 0.1) × 10−7 | (3 ± 0.5) × 10−7 | (4 ± 0.4) × 10−7 | (4 ± 0.3) × 10−7 | (4 ± 0.3) × 10−7 | (4 ± 0.3) × 10−7 |
| Pitting potential (V) | 1.05 ± 0.02 | 0.90 ± 0.04 | 0.90 ± 0.03 | 0.91 ± 0.02 | 0.90 ± 0.02 | 0.90 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, B.-H.; Park, J.; Kim, S.; Ok, J.-W.; Kim, D.-I.; Yoon, J.-H. Study of Electroless Nickel Plating on Super Duplex Stainless Steel for Lithium-Ion Battery Cases: Electrochemical Behaviour and Effects of Plating Time. Metals 2024, 14, 307. https://doi.org/10.3390/met14030307
Shin B-H, Park J, Kim S, Ok J-W, Kim D-I, Yoon J-H. Study of Electroless Nickel Plating on Super Duplex Stainless Steel for Lithium-Ion Battery Cases: Electrochemical Behaviour and Effects of Plating Time. Metals. 2024; 14(3):307. https://doi.org/10.3390/met14030307
Chicago/Turabian StyleShin, Byung-Hyun, Jinyong Park, Seongjun Kim, Jung-Woo Ok, Doo-In Kim, and Jang-Hee Yoon. 2024. "Study of Electroless Nickel Plating on Super Duplex Stainless Steel for Lithium-Ion Battery Cases: Electrochemical Behaviour and Effects of Plating Time" Metals 14, no. 3: 307. https://doi.org/10.3390/met14030307
APA StyleShin, B.-H., Park, J., Kim, S., Ok, J.-W., Kim, D.-I., & Yoon, J.-H. (2024). Study of Electroless Nickel Plating on Super Duplex Stainless Steel for Lithium-Ion Battery Cases: Electrochemical Behaviour and Effects of Plating Time. Metals, 14(3), 307. https://doi.org/10.3390/met14030307






