Application of a Simple Pretreatment in the Process of Acid Leaching of Electric Arc Furnace Dust
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials
2.2. Sample Preparation
2.3. Methods of Characterization of the EAF Dust Representative Sample
2.3.1. Physical and Chemical Characterization
2.3.2. Particle Size Distribution
2.3.3. Mineralogical Characterization
2.4. Pretreatment of the EAF Dust
2.4.1. Influence of the Leaching Time on the Leaching Efficiency of the EAF Dust
2.4.2. Influence of the Solid and Liquid Phase Ratio on the Leaching Efficiency of the EAF Dust
2.4.3. Influence of the Temperature on the Leaching Efficiency of the EAF Dust
2.5. Leaching of the Solid Residue Obtained after the Pretreatment
3. Results and Discussion
3.1. Characterization of a Representative EAF Dust Sample
3.1.1. Physical and Chemical Characterization
3.1.2. Particle Size Distribution
3.2. Mineralogical Characterization
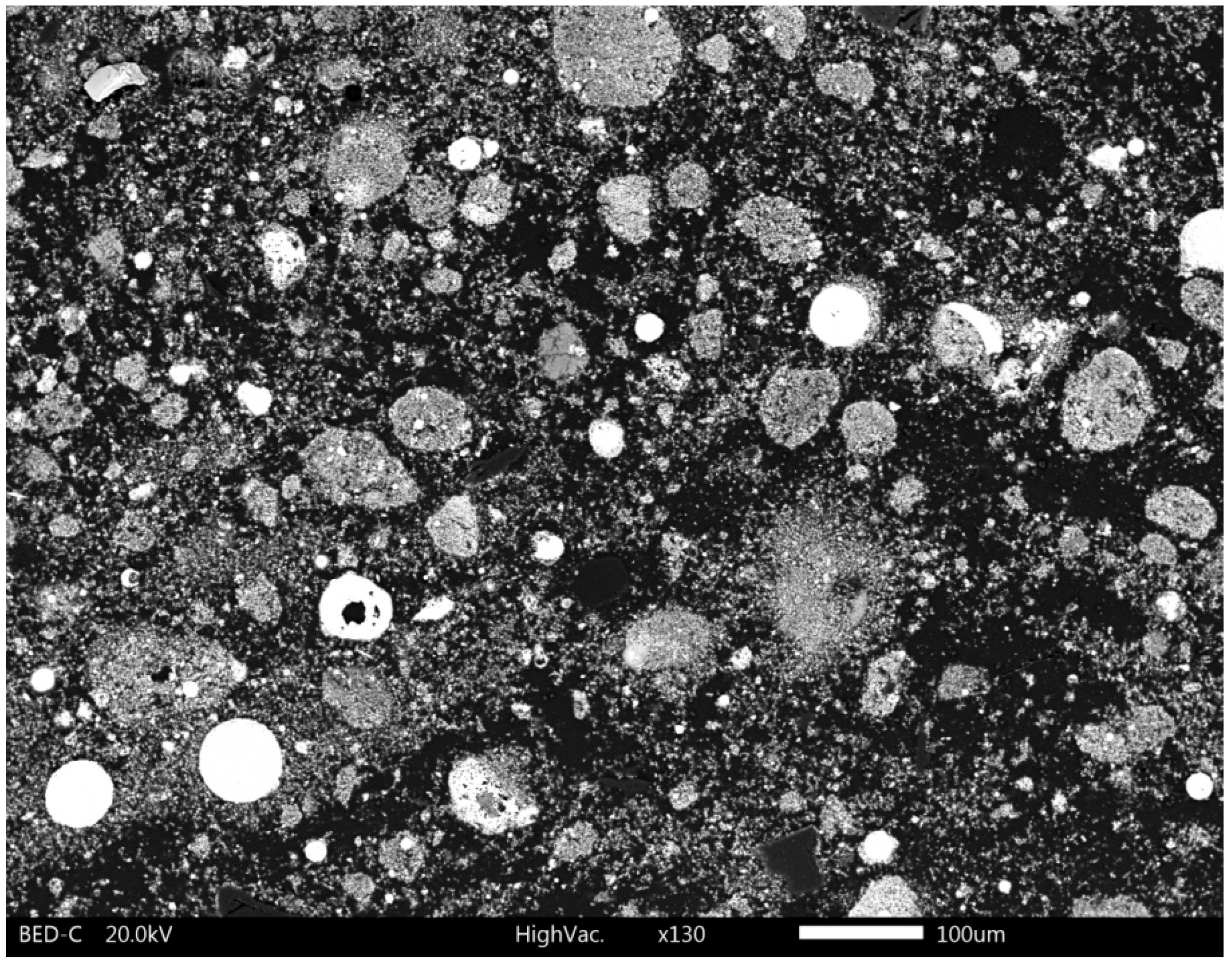
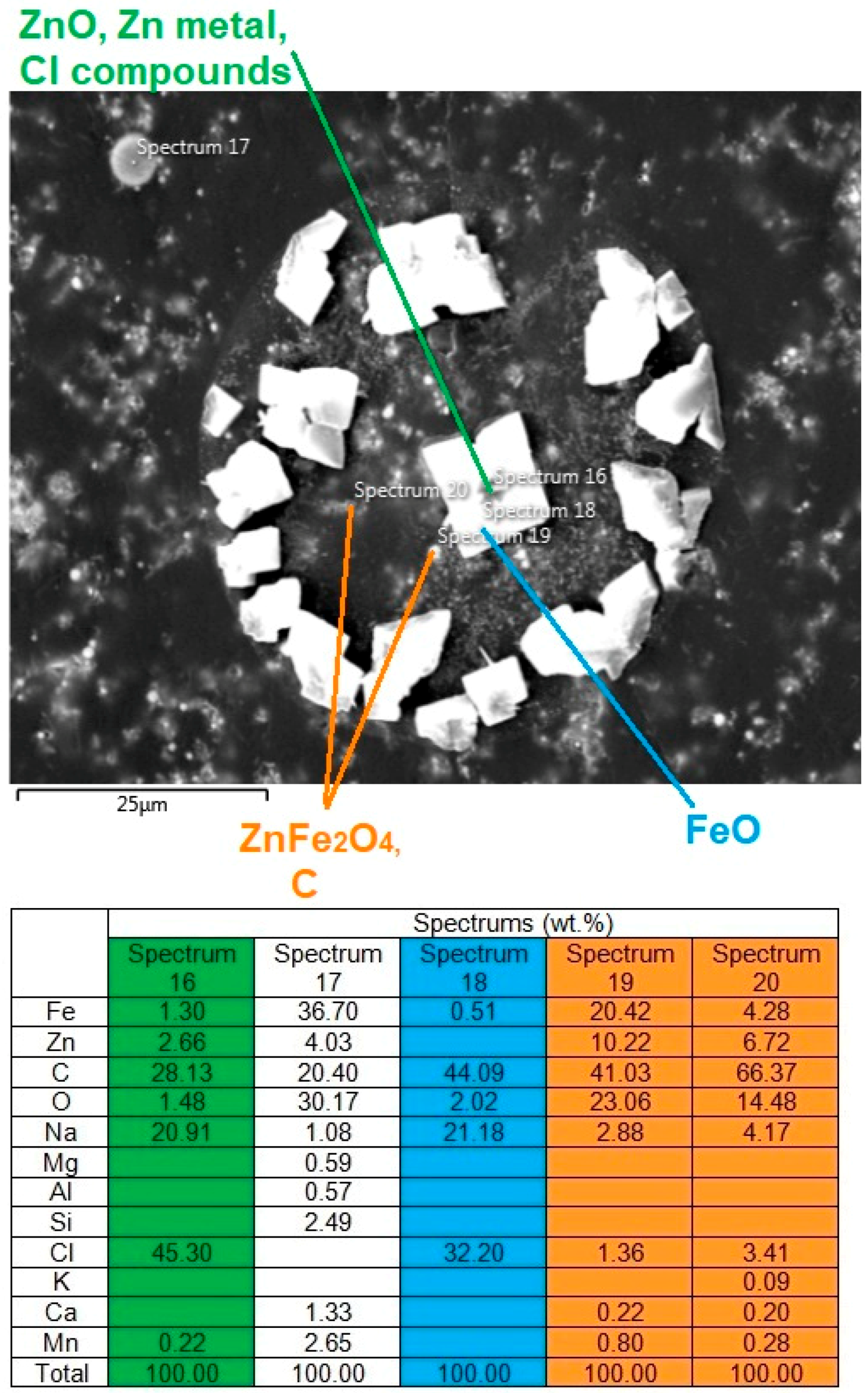
3.2.1. SEM-EDS Analysis
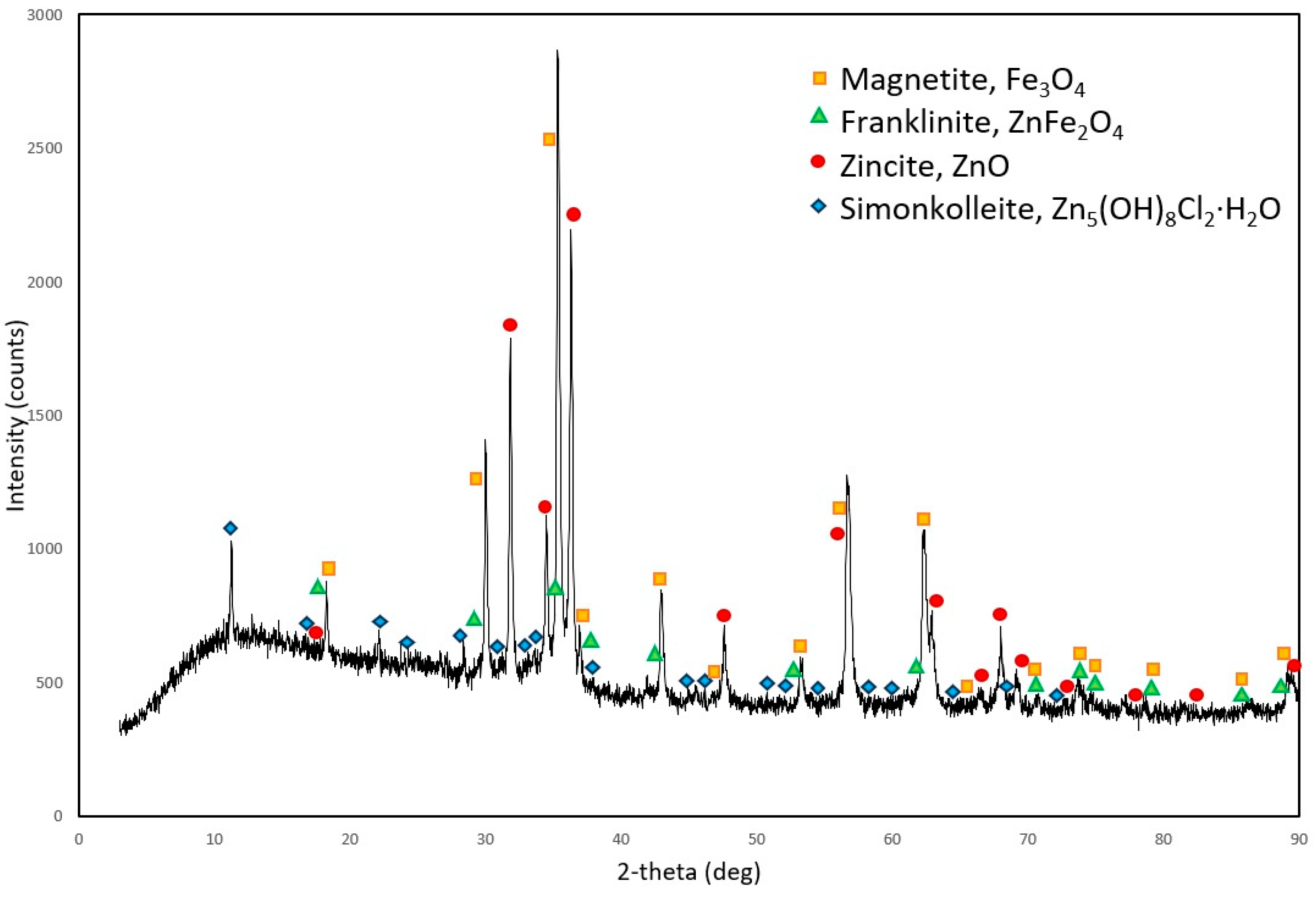
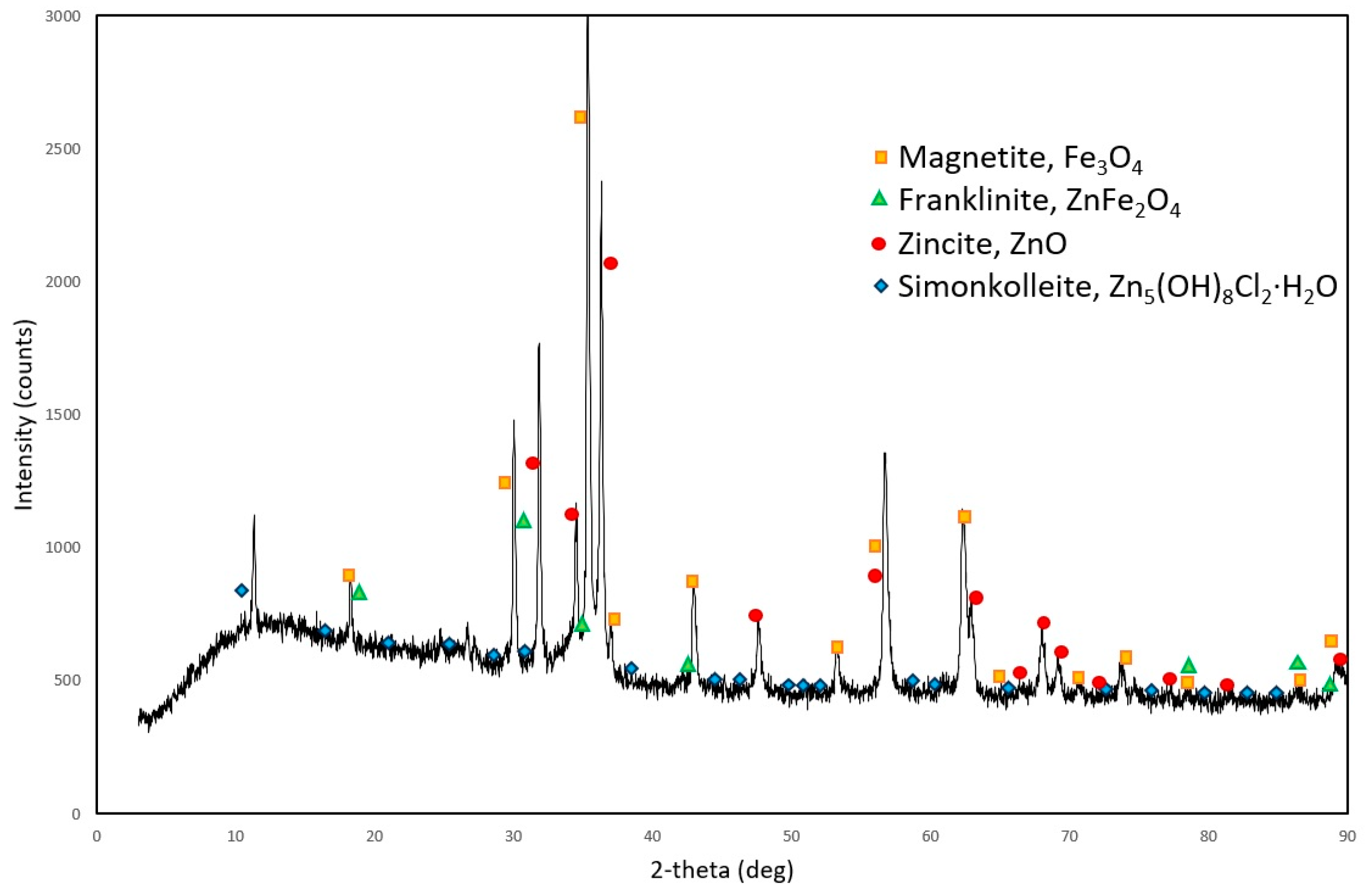
3.2.2. XRD Analysis
3.3. Pretreatment of the EAF Dust
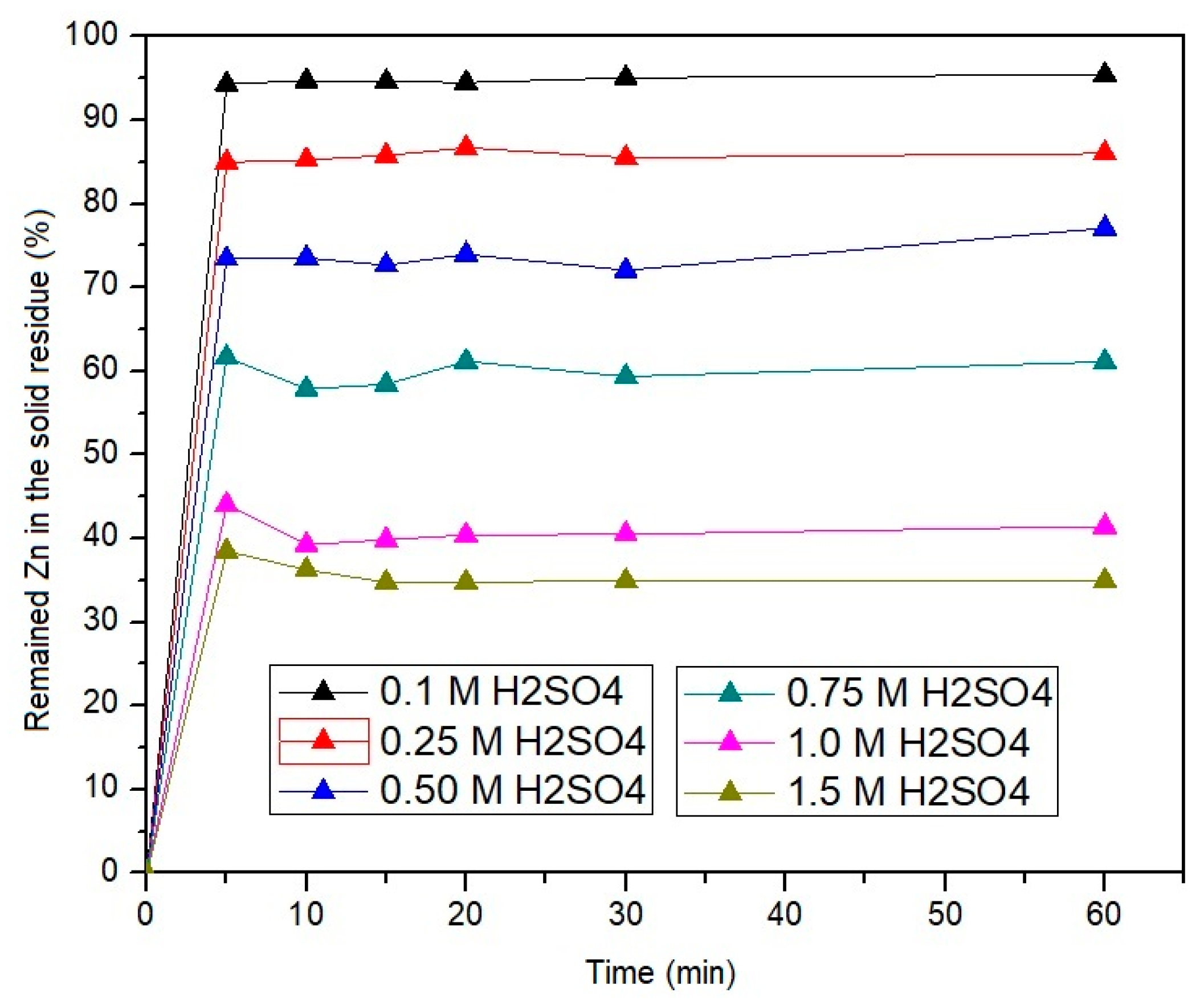
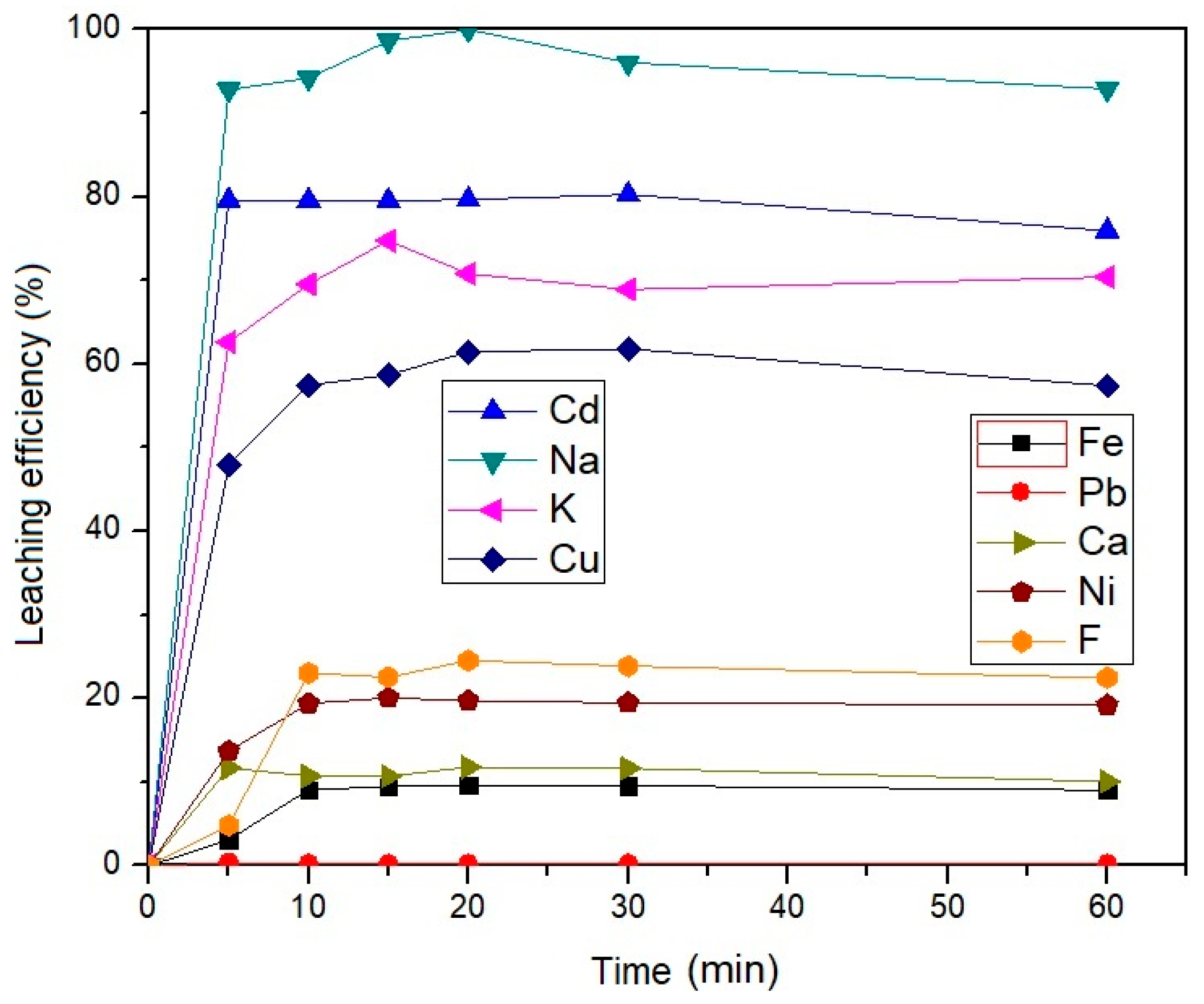
3.3.1. Influence of Leaching Time on the Leaching Efficiency of the EAF Dust
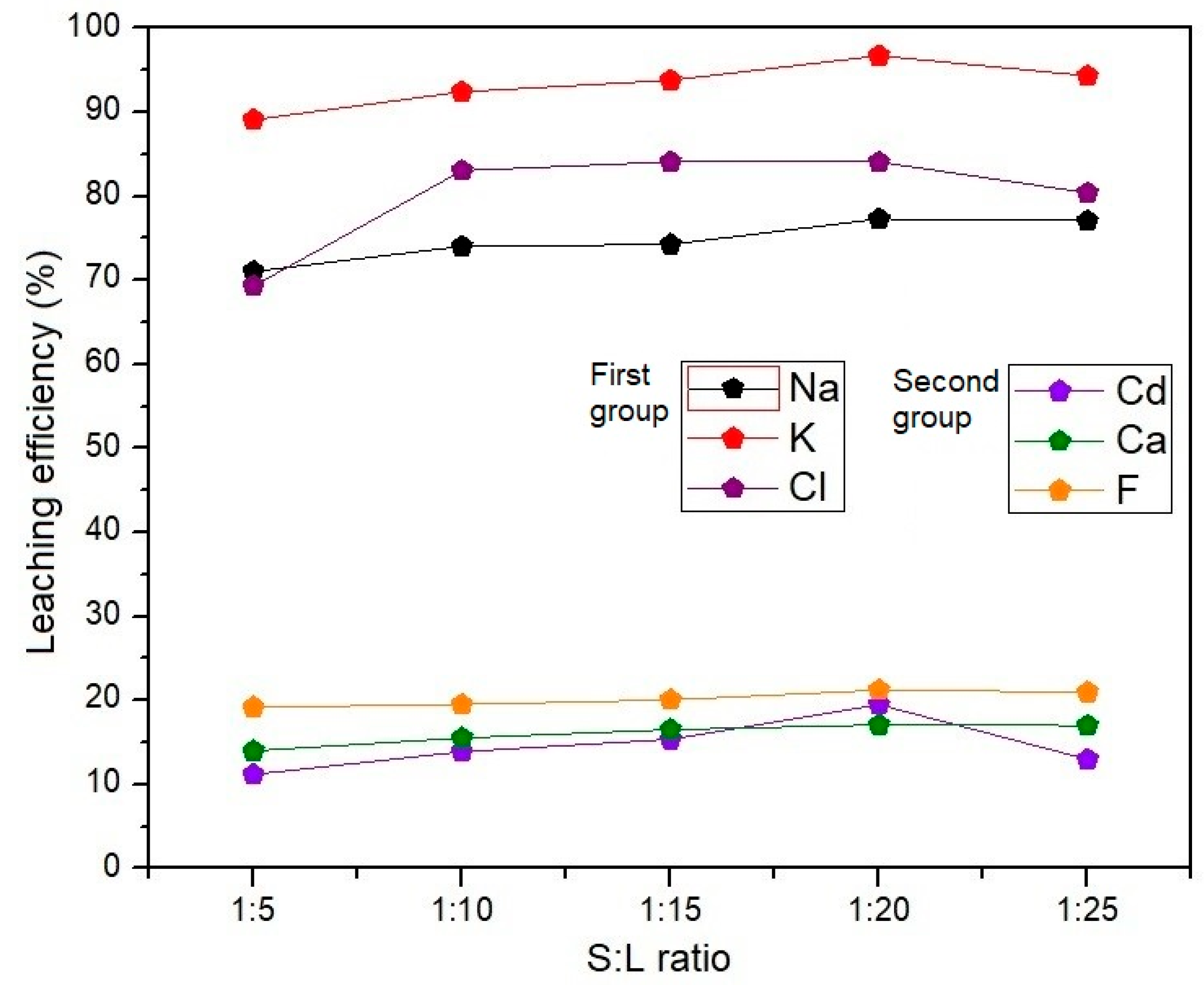
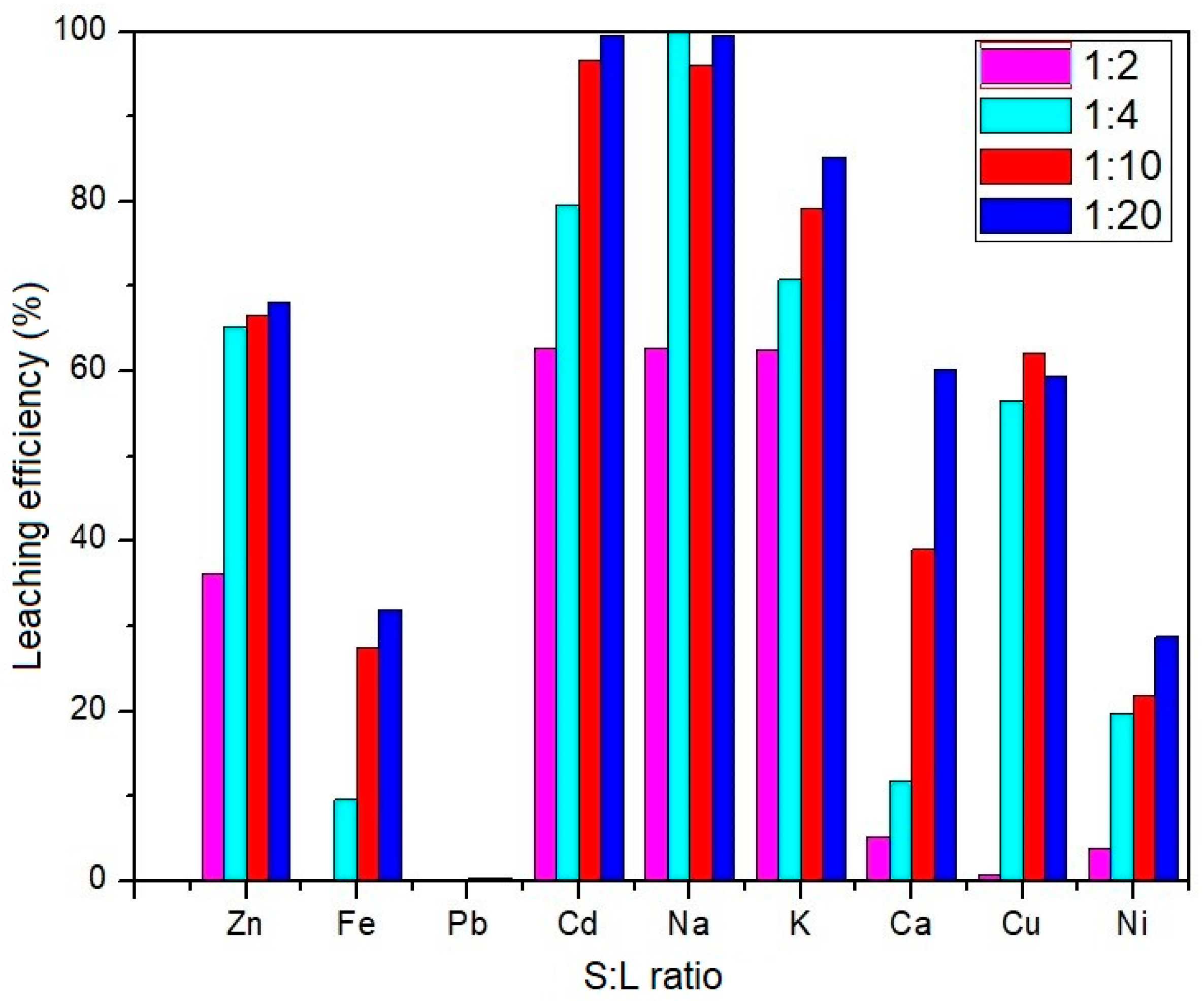
3.3.2. Influence of the Solid and Liquid Phase Ratio on the Leaching Efficiency of the EAF Dust
3.3.3. Influence of Temperature on the Leaching Efficiency of the EAF Dust
3.3.4. XRD Analysis of the Solid Residue Obtained after the Pretreatment
3.4. Leaching of the Solid Residue Obtained after the Pretreatment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pickles, C.A. Thermodynamic modeling of the multiphase pyrometallurgical processing of electric arc furnace dust. Miner. Eng. 2009, 22, 977–985. [Google Scholar] [CrossRef]
- Bruckard, W.J.; Davey, K.J.; Rodopoulos, T.; Woodcock, J.T.; Italiano, J. Water leaching and magnetic separation for decreasing the chloride level and upgrading the zinc content of EAF steelmaking baghouse dust. Int. J. Miner. Process. 2005, 75, 1–20. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Cui, K.; Fu, T.; Gao, J.; Hussain, S.; AlGarni, T.S. Pyrometallurgical recovery of zinc and valuable metals from electric arc furnace dust—A review. J. Clean. Prod. 2021, 298, 126788. [Google Scholar] [CrossRef]
- Siame, M.C.; Kaoma, J.; Hlabangana, N.; Danha, G. An attainable region approach for the recovery of iron and zinc from electric arc furnace dust. S. Afr. J. Chem. Eng. 2019, 27, 35–42. [Google Scholar] [CrossRef]
- Wu, C.C.; Chang, F.C.; Chen, W.S.; Tsai, M.S.; Wang, Y.N. Reduction behavior of zinc ferrite in EAF-dust recycling with CO gas as a reducing agent. J. Environ. Manag. 2014, 143, 208–213. [Google Scholar] [CrossRef]
- Kukurugya, F.; Vindt, T.; Havlík, T. Behavior of zinc, iron and calcium from electric arc furnace (EAF) dust in hydrometallurgical processing in sulfuric acid solutions: Thermodynamic and kinetic aspects. Hydrometallurgy 2015, 154, 20–32. [Google Scholar] [CrossRef]
- Miki, T.; Chairaksa-Fujimoto, R.; Maruyama, K.; Nagasaka, T. Hydrometallurgical extraction of zinc from CaO treated EAF dust in ammonium chloride solution. J. Hazard. Mater. 2016, 302, 90–96. [Google Scholar] [CrossRef]
- Gamutan, J.; Koide, S.; Sasaki, Y.; Nagasaka, T. Selective dissolution and kinetics of leaching zinc from lime treated electric arc furnace dust by alkaline media. J. Environ. Chem. Eng. 2024, 12, 111789. [Google Scholar] [CrossRef]
- Wu, H.; Li, J.; Teng, W.; Chen, Z.; Liu, W.; Ren, S.; Yang, J.; Liu, Q. One-step extraction of zinc and separation of iron from hazardous electric arc furnace dust via sulphating roasting-water leaching. J. Environ. Chem. Eng. 2023, 11, 111155. [Google Scholar] [CrossRef]
- Murua, M.; Boto, F.; Anglada, E.; Cabero, J.M.; Fernandez, L. A slag prediction model in an electric arc furnace process for special steel production. Procedia Manuf. 2021, 54, 178–183. [Google Scholar] [CrossRef]
- Nyirenda, R.L. The processing of steelmaking flue-dust: A review. Miner. Eng. 1991, 4, 1003–1025. [Google Scholar] [CrossRef]
- Chairaksa-Fujimoto, R.; Maruyama, K.; Miki, T.; Nagasaka, T. The selective alkaline leaching of zinc oxide from Electric Arc Furnace dust pre-treated with calcium oxide. Hydrometallurgy 2016, 159, 120–125. [Google Scholar] [CrossRef]
- Agnihotri, A.; Singh, P.K.; Singh, D.; Gupta, M. Foamy slag practice to enhance the energy efficiency of electric arc furnace: An industrial scale validation. Mater. Today Proc. 2021, 46, 1537–1542. [Google Scholar] [CrossRef]
- Havlík, T.; Vidor e Souza, B.; Bernardes, A.M.; Schneider, I.A.H.; Miškufová, A. Hydrometallurgical processing of carbon steel EAF dust. J. Hazard. Mater. 2006, B135, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Laforest, G.; Duchesne, J. Characterization and leachability of electric arc furnace dust made from remelting of stainless steel. J. Hazard. Mater. 2006, B135, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Alencastro de Araújo, J.; Schalch, V. Recycling of electric arc furnace (EAF) dust for use in steel making process. J. Mater. Res. Technol. 2014, 3, 274–279. [Google Scholar] [CrossRef]
- Nair, A.T.; Mathew, A.; Archana, A.R.; Akbar, M.A. Use of hazardous electric arc furnace dust in the construction industry: A cleaner production approach. J. Cleaner Prod. 2022, 377, 134282. [Google Scholar] [CrossRef]
- Keglevich de Buzin, P.J.W.; Heck, N.C.; Vilela, A.C.F. EAF dust: An overview on the influences of physical, chemical and mineral features in its recycling and waste incorporation routes. J. Mater. Res. Technol. 2017, 6, 194–202. [Google Scholar] [CrossRef]
- Ruiz, O.; Clemente, C.; Alonso, M.; Alguacil, F.J. Recycling of an electric arc furnace flue dust to obtain high grade ZnO. J. Hazard. Mater. 2007, 141, 33–36. [Google Scholar] [CrossRef]
- Tang, H.; Wang, L.; Sun, W.; Hu, Y.; Han, H.; Zhai, J. Electric arc furnace dust as magnetic carrier particles for removal of micro-fine particles from suspensions. Sep. Purif. Technol. 2017, 176, 220–230. [Google Scholar] [CrossRef]
- Oustadakis, P.; Tsakiridis, P.E.; Katsiapi, A.; Agatzini-Leonardou, S. Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD) Part I: Characterization and leaching by diluted sulphuric acid. J. Hazard. Mater. 2020, 179, 1–7. [Google Scholar] [CrossRef]
- Williamson, A.J.; Folens, K.; Damme, K.V.; Olaoye, O.; Atia, T.A.; Mees, B.; Nicomel, N.R.; Verbruggen, F.; Spooren, J.; Boon, N.; et al. Conjoint bioleaching and zinc recovery from an iron oxide mineral residue by a continuous electrodialysis system. Hydrometallurgy 2020, 195, 105409. [Google Scholar] [CrossRef]
- Menad, N.; Ayala, J.N.; Garcia-Carcedo, F.; Ruiz-Ayủcar, E.; Hernảndez, A. Study of the presence of fluorine in the recycled fractions during carbothermal treatment of EAF dust. Waste Manag. 2003, 23, 483–491. [Google Scholar] [CrossRef]
- Hamann, C.; Piehl, P.; Weingart, E.; Stolle, D.; Al-Sabbagh, D.; Ostermann, M.; Auer, G.; Adam, C. Selective removal of zinc and lead from electric arc furnace dust by chlorination–evaporation reactions. J. Hazard. Mater. 2024, 465, 133421. [Google Scholar] [CrossRef]
- Gamea, E.G.; Anwar, A.; Ezzat, A.A.; El-Rafey, E. Utilization of electric arc furnace dust as a filler for unsaturated polyester resin. Process Saf. Environ. Prot. 2022, 159, 1194–1202. [Google Scholar] [CrossRef]
- Xia, D.K. Recovery of Zinc from Zinc Ferrite and Electric Arc Furnace Dust. Master’s Thesis, Queen’s University, Kingston, ON, Canada, 1997. [Google Scholar]
- Simonyan, L.M.; Alpatova, A.A.; Demidova, N.V. The EAF dust chemical and phase composition research techniques. J. Mater. Res. Technol. 2019, 8, 1601–1607. [Google Scholar] [CrossRef]
- Trifunović, V.; Milić, S.; Avramović, L.; Jonović, R.; Gardić, V.; Đorđievski, S.; Dimitrijević, S. Investigation of Hazardous Waste—A Case Study of Electric Arc Furnace Dust Characterization. Chem. Ind. 2022, 76, 237–249. [Google Scholar] [CrossRef]
- Prasetyo, E.; Anderson, C.; Nurjaman, F.; Al Muttaqii, M.; Handoko, A.S.; Bahfie, F.; Mufakhir, F.R. Monosodium Glutamate as Selective Lixiviant for Alkaline Leaching of Zinc and Copper from Electric Arc Furnace Dust. Metals 2020, 10, 644. [Google Scholar] [CrossRef]
- Palimąka, P.; Pietrzyk, S.; Stępień, M.; Ciećko, K.; Nejman, I. Zinc Recovery from Steelmaking Dust by Hydrometallurgical Methods. Metals 2018, 8, 547. [Google Scholar] [CrossRef]
- Santos, E.B.C.; Muniz, D.D.; Barbosa, N.P.; Correia, E.A.S.; Alves, L.D.M.; Vieira de Melo, M.B.F. Brazilian steel waste: Electrical arc furnace dust chemical, thermal and morphological characterization. J. Mater. Res. Technol. 2021, 14, 2775–2781. [Google Scholar] [CrossRef]
- Silva, V.S.; Silva, J.S.; Costa, B.S.; Labes, C.; Oliveira, R.M.P.B. Preparation of glaze using electric-arc furnace dust as raw material. J. Mater. Res. Technol. 2019, 8, 5505–5514. [Google Scholar] [CrossRef]
- Yubonmhat, K.; Gunhakoon, P.; Sopapan, P.; Prasertchiewchan, N.; Katekaew, W. Ordinary-Portland-cement solidification of Cs-137 contaminated electric arc furnace dust from steel production industry in Thailand. Heliyon 2024, 10, e25792. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Hu, K.; Jiang, E.; Feng, J.; Song, X.; Ning, P.; Yu, Q.; Wang, H. A new strategy for the reuse of typical hazardous solid waste electric arc furnace dust (EAFD): Efficient desulfurization by EAFD slurry. Sep. Purif. Technol. 2023, 308, 122980. [Google Scholar] [CrossRef]
- Chen, Y.; Teng, W.; Feng, X.; Li, J.; Liu, W.; Ren, S.; Yang, J.; Liu, Q. Efficient extraction and separation of zinc and iron from electric arc furnace dust by roasting with FeSO4⋅7H2O followed by water leaching. Sep. Purif. Technol. 2022, 281, 119936. [Google Scholar] [CrossRef]
- Almeida, M.M.; Saczk, A.A.; Felix, F.S.; Penido, E.S.; Santos, T.A.R.; Teixeira, A.S.; Magalhães, F. Characterization of electric arc furnace dust and its application in photocatalytic reactions to degrade organic contaminants in synthetic and real samples. J. Photochem. Photobiol. A 2023, 438, 114585. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Altarawneh, S.; Al-Omari, M.; Altarawneh, M.; Kingman, S.; Dodds, C. Leaching behavior of zinc and lead from electric arc furnace dust—Poly (vinyl) chloride residues after oxidative thermal treatment. J. Clean. Prod. 2021, 328, 129622. [Google Scholar] [CrossRef]
- Halli, P.; Hamuyuni, J.; Revitzer, H.; Lundström, M. Selection of leaching media for metal dissolution from electric arc furnace dust. J. Clean. Prod. 2017, 164, 265–276. [Google Scholar] [CrossRef]
- Havlik, T.; Turzakova, M.; Stopic, S.; Friedrich, B. Atmospheric leaching of EAF dust with diluted sulphuric acid. Hydrometallurgy 2005, 77, 41–50. [Google Scholar] [CrossRef]
- Yang, J.; Huang, R.; He, X.; Lv, X.; Zhu, R.; Jin, H.; Deng, X. Research for the recovery of Zn and Pb from electric arc furnace dust through vacuum carbothermal reduction. Process Saf. Environ. Prot. 2023, 170, 960–970. [Google Scholar] [CrossRef]
- Yang, C.; She, X.; Wang, R.; Wang, J.; Xue, Q. Melting and separation behaviors of electric arc furnace dust pellets under iron bath conditions. Trans. Nonferrous Met. Soc. China 2024, 34, 347–360. [Google Scholar] [CrossRef]
- Khattab, R.M.; El-Sayed Seleman, M.M.; Zawrah, M.F. Assessment of electric arc furnace dust: Powder characterization and its sinterability as ceramic product. Ceram. Int. 2017, 43, 12939–12947. [Google Scholar] [CrossRef]
- Lin, X.; Peng, Z.; Yan, J.; Li, Z.; Hwang, J.Y.; Zhang, Y.; Li, G.; Jiang, T. Pyrometallurgical recycling of electric arc furnace dust. J. Cleaner Prod. 2017, 149, 1079–1100. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Oustadakis, P.; Katsiapi, A.; Agatzini-Leonardou, S. Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD). Part II: Downstream processing and zinc recovery by electrowinning. J. Hazard. Mater. 2010, 179, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Rudnik, E. Recovery of zinc from zinc ash by leaching in sulphuric acid and Electrowinning. Hydrometallurgy 2019, 188, 256–263. [Google Scholar] [CrossRef]
- Li, Y.C.; Zhuo, S.N.; Peng, B.; Min, X.B.; Liu, H.; Ke, Y. Comprehensive recycling of zinc and iron from smelting waste containing zinc ferrite by oriented transformation with SO2. J. Clean. Prod. 2020, 263, 121468. [Google Scholar] [CrossRef]
- Rudnik, E. Investigation of industrial waste materials for hydrometallurgical recovery of zinc. Miner. Eng. 2019, 139, 105871. [Google Scholar] [CrossRef]
- Antuñano, N.; Herrero, D.; Arias, P.L.; Cambra, J.F. Electrowinning studies for metallic zinc production from double leached Waelz oxide. Process Saf. Environ. Prot. 2013, 91, 495–502. [Google Scholar] [CrossRef]
- Terrones-Saeta, J.M.; Suárez-Macías, J.; Moreno-López, E.R.; Corpas-Iglesias, F.A. Leaching of Zinc for Subsequent Recovery by Hydrometallurgical Techniques from Electric Arc Furnace Dusts and Utilisation of the Leaching Process Residues for Ceramic Materials for Construction Purposes. Metals 2021, 11, 1603. [Google Scholar] [CrossRef]
- Dutra, A.J.B.; Paiva, P.R.P.; Tavares, L.M. Alkaline leaching of zinc from electric arc furnace steel dust. Miner. Eng. 2006, 19, 478–485. [Google Scholar] [CrossRef]
- Montenegro, V.; Agatzini-Leonardou, S.; Oustadakis, P.; Tsakiridis, P. Hydrometallurgical Treatment of EAF Dust by Direct Sulphuric Acid Leaching at Atmospheric Pressure. Waste Biomass Valorization 2016, 7, 1531–1548. [Google Scholar] [CrossRef]
- Youcai, Z.; Stanforth, R. Integrated hydrometallurgical process for production of zinc from electric arc furnace dust in alkaline medium. J. Hazard. Mater. 2000, B80, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Sun, W.; Wang, L.; Liu, R.; Han, H.; Hu, Y.; Yang, Y. Recovery of cobalt and zinc from the leaching solution of zinc smelting slag. J. Environ. Chem. Eng. 2019, 7, 102777. [Google Scholar] [CrossRef]
- Chen, W.S.; Shen, Y.H.; Tsai, M.S.; Chang, F.C. Removal of chloride from electric arc furnace dust. J. Hazard. Mater. 2011, 190, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, J.; Liu, W.; Zhang, M.; Guo, M. Recovery of metal-doped zinc ferrite from zinc-containing electric arc furnace dust: Process development and examination of elemental migration. Hydrometallurgy 2016, 166, 1–8. [Google Scholar] [CrossRef]
- Shawabkeh, R.A. Hydrometallurgical extraction of zinc from Jordanian electric arc furnace dust. Hydrometallurgy 2010, 104, 61–65. [Google Scholar] [CrossRef]
- Hazaveh, P.L.; Karimia, S.; Rashchi, F.; Sheibani, S. Purification of the leaching solution of recycling zinc from the hazardous electric arc furnace dust through an as-bearing jarosite. Ecotoxicol. Environ. Saf. 2020, 202, 110893. [Google Scholar] [CrossRef]



| Type of Treated Waste Material | Hydrometallurgical Process Phases | References | ||
|---|---|---|---|---|
| Leaching Process (Reagent Used) | Solution Purification Process | Extracting Metals/Zn Compounds Process | ||
| EAF dust | 1.5 M H2SO4 | - | - | [21] |
| EAF dust | - | Chemical precipitation of Fe, solvent extraction of Zn | Electrowinning process (EW) | [44] |
| EAF dust | 6 M NaOH | - | - | [50] |
| EAF dust | First phase 1.0 M H2SO4 Second phase 1.5 M H2SO4 | - | - | [51] |
| EAF dust | First phase water leaching, Second phase 100 g/L (NH4)2CO3 | Cementation | Precipitation of ZnCO3, calcination ZnCO3 to ZnO | [19] |
| EAF dust | 16 different reagents (acid, base, etc.) | - | - | [38] |
| Two filter dusts and zinc ash | 25% H2SO4 | Chemical precipitation of Fe | Electrowinning process (EW) | [47] |
| EAF dust | 5 M NaOH | Chemical precipitation | Electrowinning process (EW) | [52] |
| EAF dust | 2 M NaOH, KOH, and LiOH | - | - | [8] |
| Zinc ash | 20 % H2SO4 | Chemical precipitation of Fe | Electrowinning process (EW) | [45] |
| Zn smelting slag | 2 M H2SO4 | Chemical precipitation | - | [53] |
| Element | Content, wt.% | Analytical Method 1 |
|---|---|---|
| Zn | 32.38 | V |
| Fe | 28.28 | |
| Mn | 2.29 | |
| Cu | 0.19 | |
| Pb | 1.11 | |
| Bi | <0.01 | ICP-AES |
| Co | 0.0018 | |
| Ni | 0.014 | |
| Cr | 0.40 | |
| Mo | 0.0028 | |
| S | 0.44 | CSA |
| P | 0.11 | ICP-AES |
| As | 0.045 | |
| Ba | 0.090 | |
| Li | 0.0032 | |
| Sb | 0.014 | |
| Sn | 0.035 | |
| Sr | 0.0062 | |
| Ca | 3.05 | |
| Ti | 0.083 | |
| Cd | 0.032 | |
| Al | 0.77 | |
| V | 0.0097 | |
| Na | 0.92 | |
| K | 0.74 | |
| Mg | 0.57 | |
| Cl | 2.50 | SF |
| F | 0.023 | |
| Ag | 0.0073 | AAS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifunović, V.; Milić, S.; Avramović, L.; Bugarin, M.; Đorđievski, S.; Antonijević, M.M.; Radovanović, M.B. Application of a Simple Pretreatment in the Process of Acid Leaching of Electric Arc Furnace Dust. Metals 2024, 14, 426. https://doi.org/10.3390/met14040426
Trifunović V, Milić S, Avramović L, Bugarin M, Đorđievski S, Antonijević MM, Radovanović MB. Application of a Simple Pretreatment in the Process of Acid Leaching of Electric Arc Furnace Dust. Metals. 2024; 14(4):426. https://doi.org/10.3390/met14040426
Chicago/Turabian StyleTrifunović, Vanja, Snežana Milić, Ljiljana Avramović, Mile Bugarin, Stefan Đorđievski, Milan M. Antonijević, and Milan B. Radovanović. 2024. "Application of a Simple Pretreatment in the Process of Acid Leaching of Electric Arc Furnace Dust" Metals 14, no. 4: 426. https://doi.org/10.3390/met14040426





