Study of Galena Ore Powder Sintering and Its Microstructure
Abstract
:1. Introduction
2. Some Powder Metallurgy Concepts
2.1. Green Density
- Increasing compaction pressing to the extent of endurance.
- Using a wide range of particle-size powder.
- Compacting of a ductile powder, such as Cu, Zn, Pb, Sn, and Al, or their mixture. This depends on prior knowledge of the mechanical properties of compacted powders.
- Mixing lubricant materials with the powder before compaction facilitates the particles slipping to obtain a high green density, as the additive lubricant then volatilizes during the sintering process.
2.2. Sintering
2.3. Solid State Sintering
2.4. Liquid Phase Sintering
- The liquid phase of the lower melting point particles of the mixed powders should wet the surface of the other solid particles, i.e., the reaction between mixed materials is sufficient to ensure a high degree of wetting. This means that diffusion can occur during the sintering process at the liquid state of one constituent of the mixed powders.
- Sufficient difference in the melting temperatures of the mixed powder components, such as mixing iron and copper powders to form an alloy of Fe-Cu, where the melting of Fe and Cu are around 1538 °C and 1085 °C, respectively.
- The amount of liquid should be enough to surround most or all of the solid particles to ensure high sintering density.
- The fine particle size of the mixed powders will facilitate fast infiltration and, subsequently, rapid densification. That is due to the high surface area, which normally leads to a high rate of reaction. Therefore, generally, the particle size of the compacted powder plays an essential role in producing high-sintered density products using powder metallurgy technique if required.
2.5. Sintering Density
2.6. Length to Diameter Ratio (L/D) of Compacted Part
3. Material and Instruments
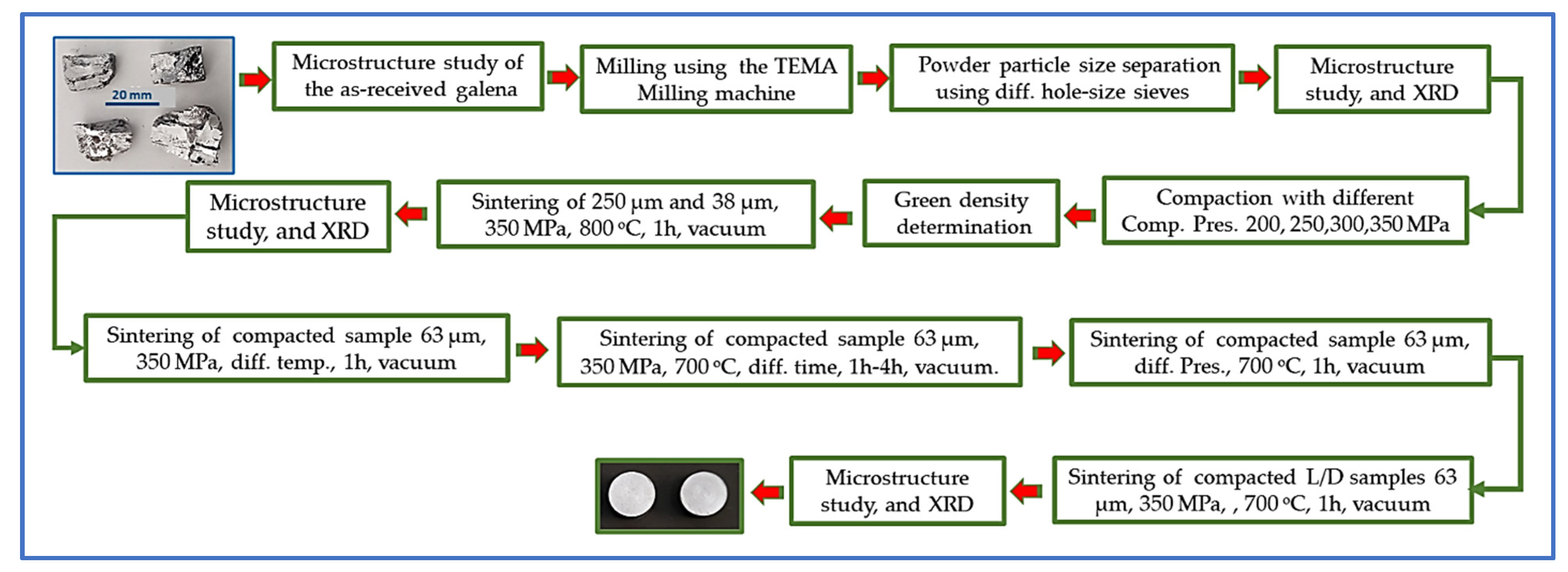
4. Experimental Procedure
4.1. SEMs Investigation of the Galena Ore
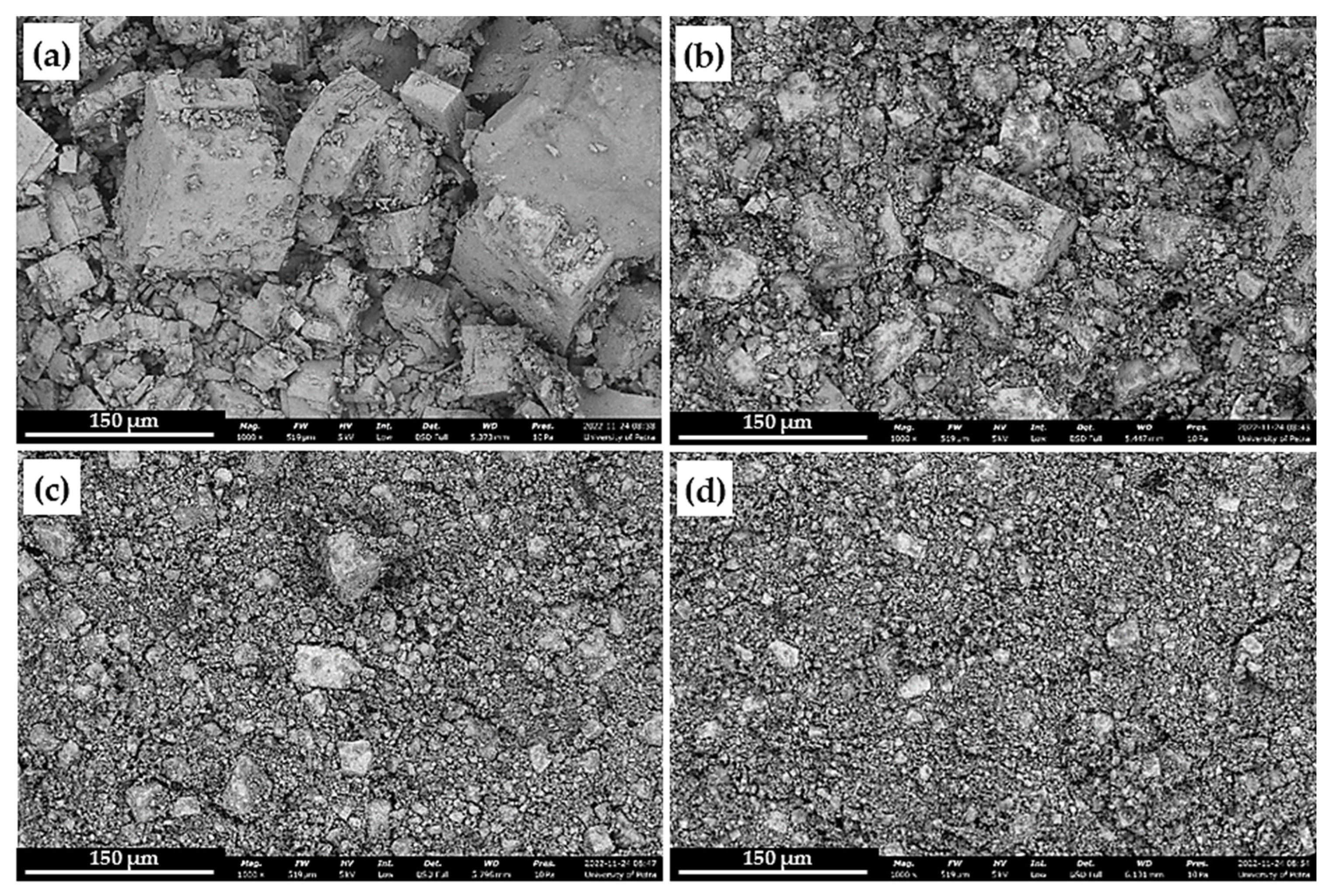
4.2. Milling of Galena Lumps
4.3. Compaction and Sintering of Galena Powders and the SEM Characterization of Sintered Products
- All sintering processes of compacted samples were carried out under a vacuum of approximately 10−3 torr. To start the sintering with as clean an atmosphere as possible, the procedure to prepare the used furnace was evacuated to the vacuum above, and the argon gas was pumped several times while the evacuation continued. Then, argon was flushed out, and the vacuum was set to reach 10−3 torr again prior to the sintering step according to the research plan. The rate of heating was around 10 °C/min. for all sintered samples, and the samples were furnace-cooled to room temperature at a rate of cooling of approximately 2.5 °C/min as a mean value. All sintered samples were doubled to take the average of green and sintered densities.
- The first attempt at compaction was at a pressure of 300 MPa to produce four samples using the homemade single-die compaction unit, two of each 38 µm and 250 µm sieved galena milled powder. The samples were then sintered at 800 °C for 1 h under vacuum to find out the nearest suitable sintering temperature for galena powder. The result showed the formation of a yellow powdery layer, which was then analyzed using the SEM. Thus, the subsequent sintering temperatures were less than 800 °C. The two sintered samples of 38 µm and 250 µm were photographed for comparison purposes to show the difference in their features after sintering.
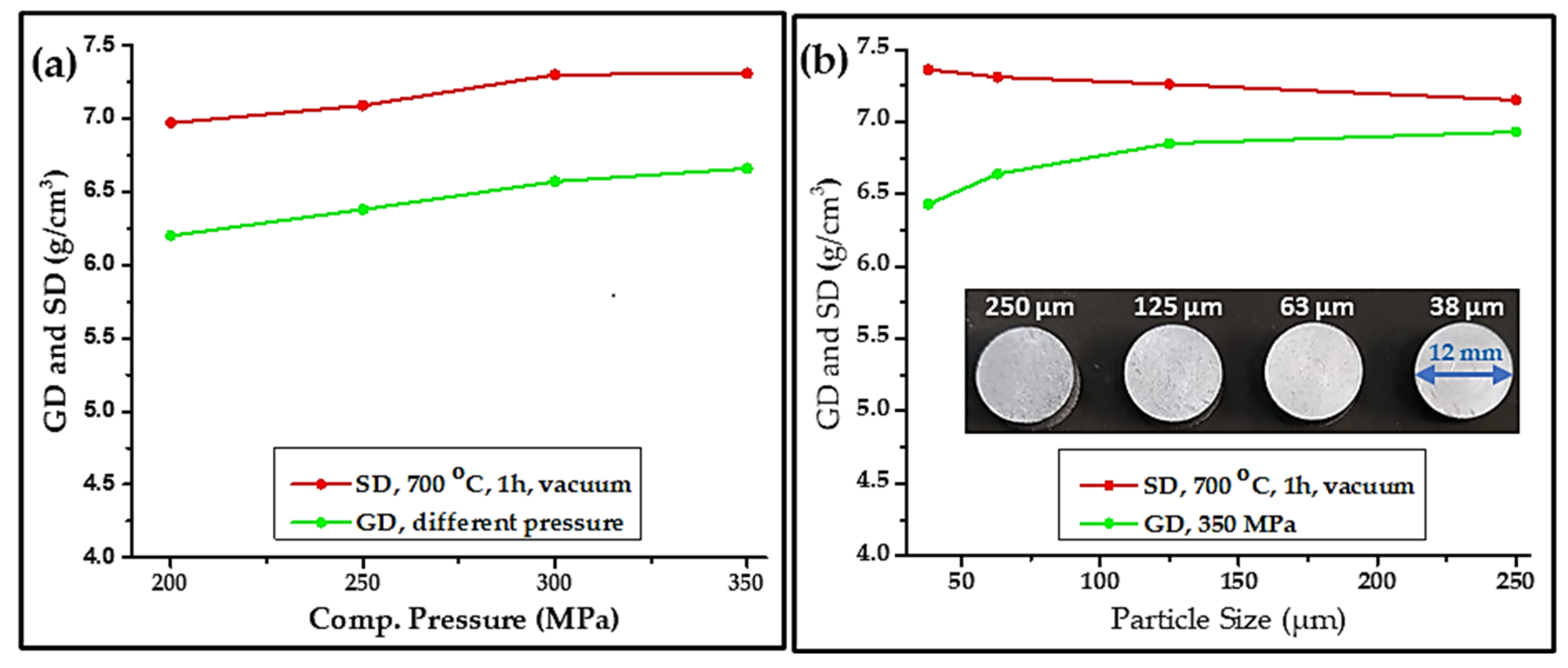
- Compaction of sieved powders was continued as required. Four compaction pressures 200, 250, 300, and 350 MPa were utilized on the 63 µm galena powder to realize the effect of compaction pressure on GD and consequently on SD products as a result. The produced green bulks were sintered at 700 °C for 1 h to find out the highest SD with regard to compaction pressure, to be adopted in the subsequent sintering stage. The highest SD was at a pressure of 350 MPa, where the result was graphed.
- A compaction set of the four-milled galena powder of 38, 63, 125, and 250 µm with constant pressure of 350 MPa was carried out for particle-size effect on GD and SD of finished pieces. The green compacted samples were sintered at 700 °C for 1 h in the same sintering condition above, and the result was plotted.
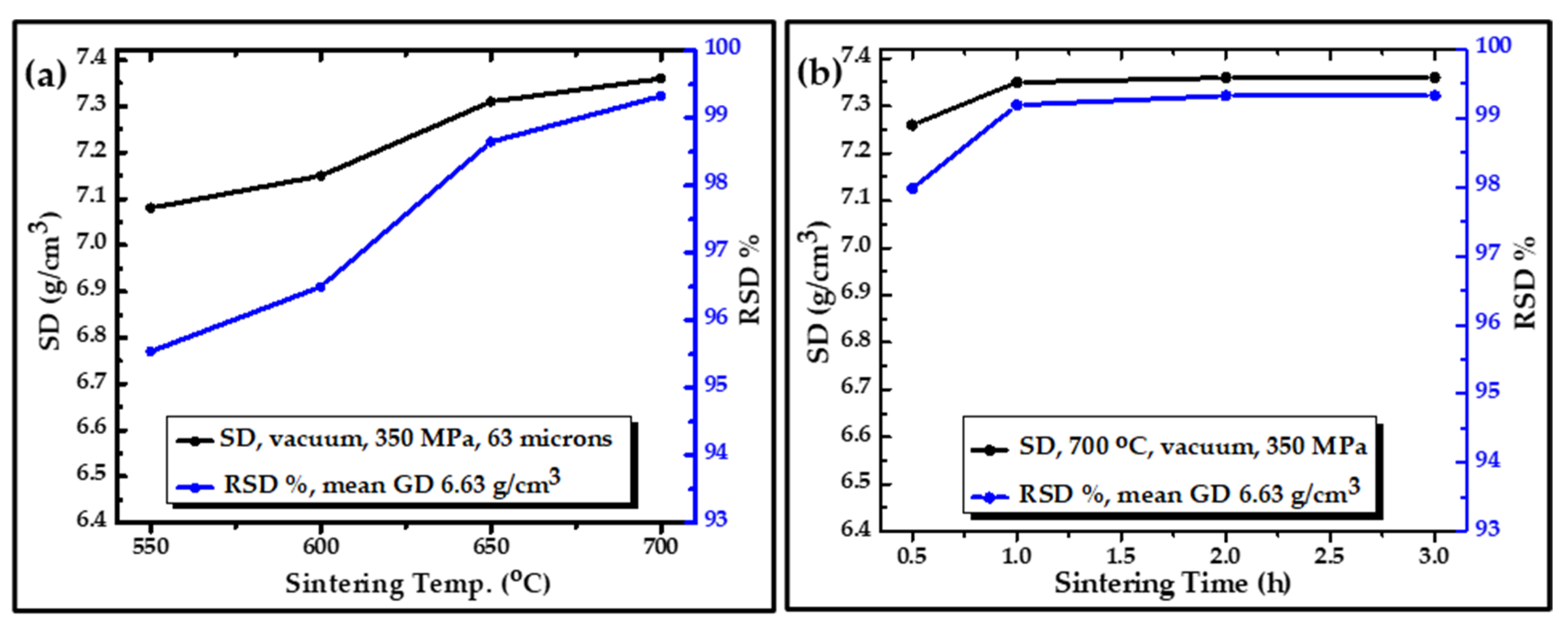
- A group of compacted samples of 63 µm powder at a constant pressure of 350 MPa was sintered with different temperatures of 550, 600, 650, and 700 °C for 1 h to realize the change in the SD with sintering temperatures. The SD of all cases was graphed as a function of sintering temperature and relative sintered density (RSD%), where the obtained SD values were treated according to the following equation:
- Also, the SD with respect to sintering time of 0.5, 1, 2, and 3 h at a constant sintering temperature of 700 °C and constant pressure of 350 MPa was investigated, and the result was plotted with the RSD%.
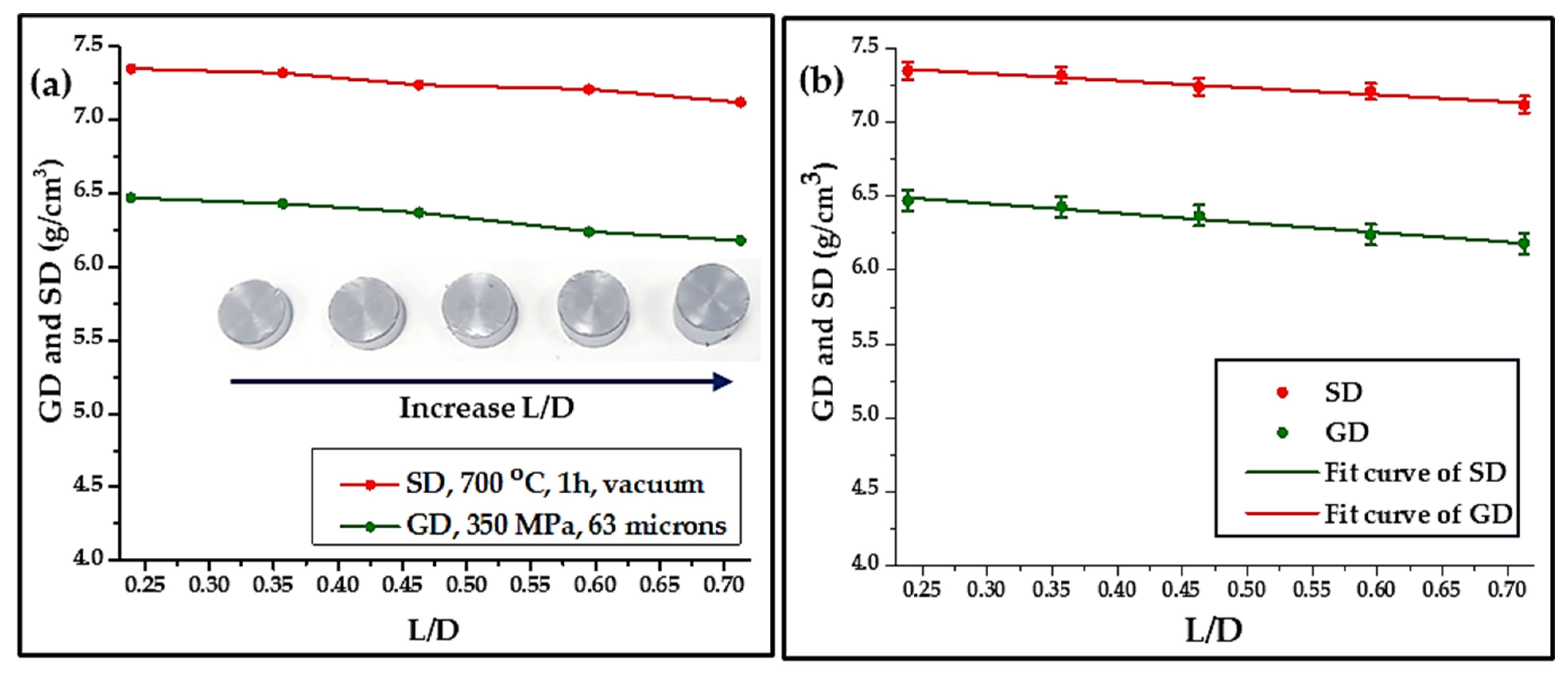
- Finally, a set of compactions at a pressure of 350 MPa using 63 µm powder was conducted to report the effect of the L/D ratio on the GD and SD.
4.4. Vickers Micro-Hardness Measurement for the As-Received Galena and Sintered Product
4.5. X-ray Diffraction (XRD) Test
5. Result and Discussion
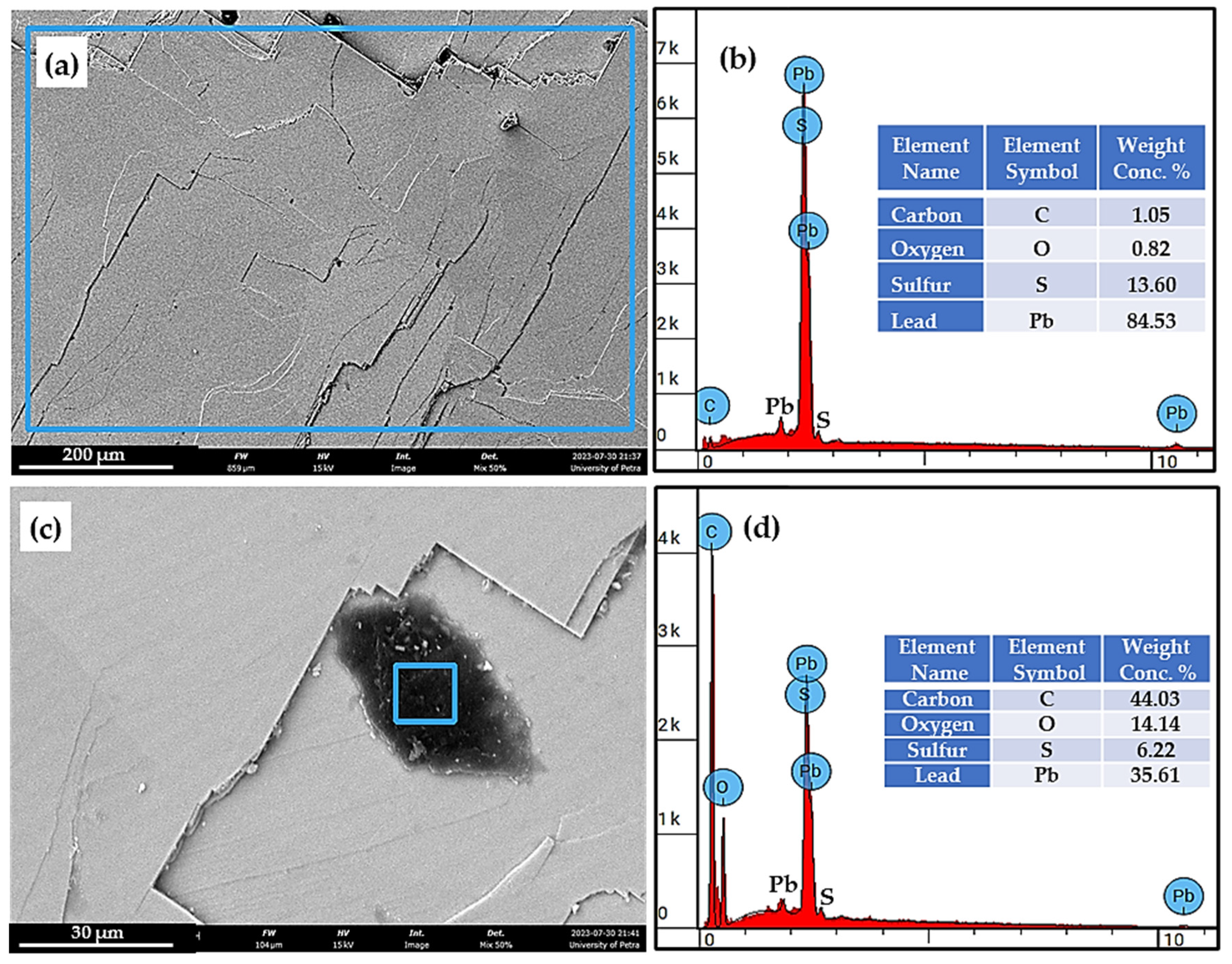
5.1. Microstructure and Chemical Composition of the As-Received Galena
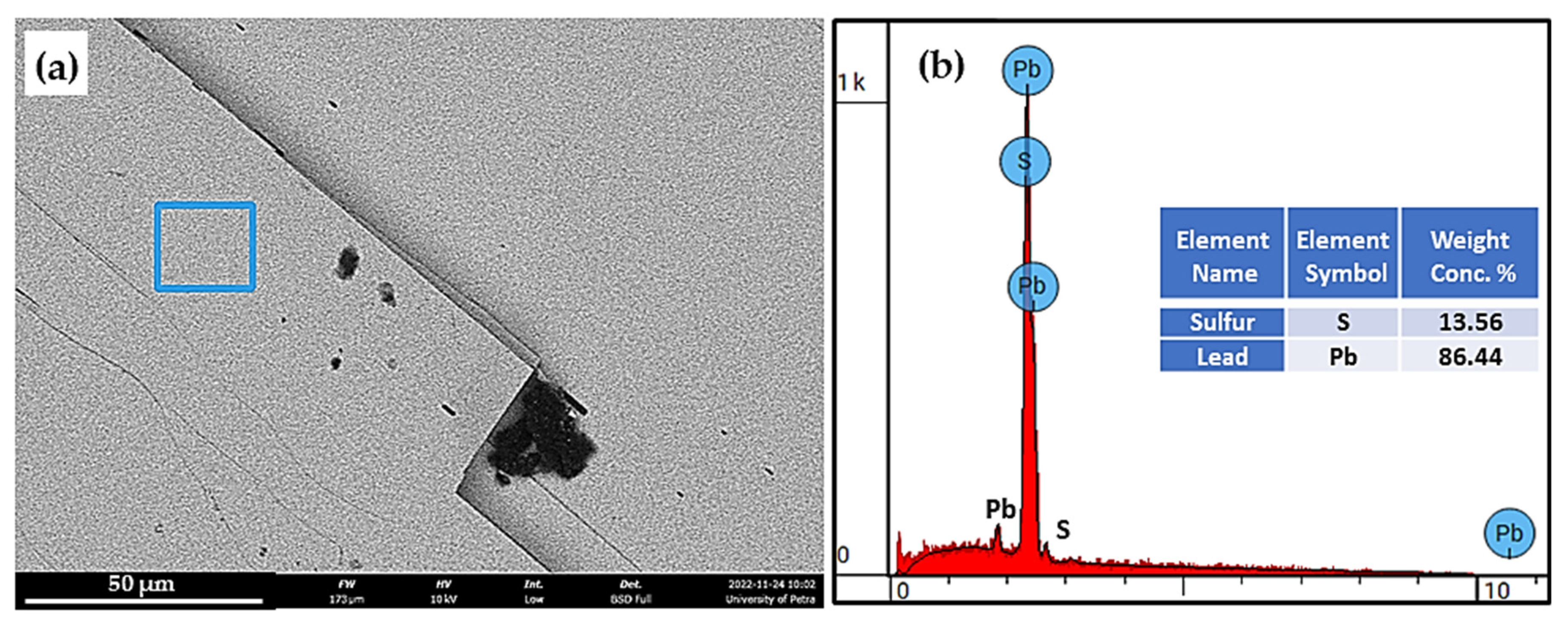
5.2. Investigation of the Milled Galena
5.3. Sintering of Galena Powder, Green and Sintered Densities, and Microstructure
5.3.1. Sintering at 800 °C under Vacuum and the SEM Investigation
5.3.2. Green and Sintered Densities Feedback and Microstructure of Sintered Products
5.4. Micro-Hardness Result of the As-Received and Sintered Product
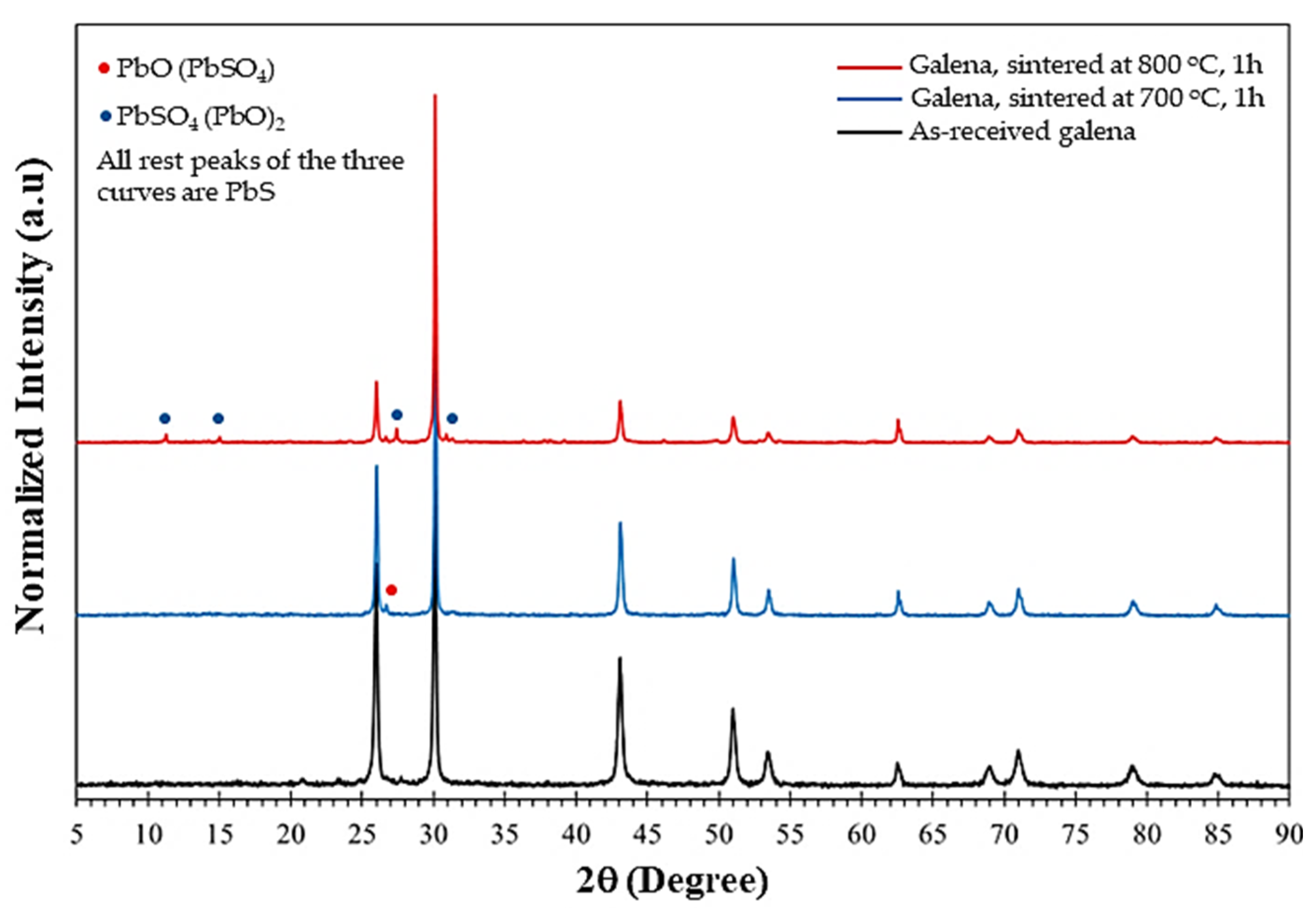
5.5. XRD Investigations
6. Conclusions
- The microfracture and chemical analysis were well investigated, and proved that the as-received galena was found to be mostly a PbS compound.
- The processes of compaction and sintering in a vacuum were very successful in producing perfect PbS pellets.
- At a compaction pressure of 350 MPa and a sintering temperature of 700 °C for 1 h, the results confirmed reaching a high RSD% of approximately 99.3%, which is excellent in the case of solid-state sintering upon using the powder metallurgy route.
- After sintering, SEM and XRD investigations confirmed maintaining the PbS phase as it was in the as-received starting galena.
- After sintering at 800 °C for 1 h, yellow and white colored layers were formed on the surface of the 38 µm and the 250 µm sintered pellets, respectively.
- The micro-hardness measurement showed a sharp reduction of about 52% in the hardness of the sintered 38 µm sample in comparison with the as-received galena.
- The ratio of length to diameter (L/D) of compacted and sintered samples was studied, and the result indicated that the GD and SD decrease as the ratio increases.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nnanwube, I.A.; Onukwuli, O.D. Hydrometallurgical Processing of a Nigerian Galena Ore in Nitric Acid: Characterization and Dissolution Kinetics. J. Miner. Mater. Charact. Eng. 2018, 6, 271–293. [Google Scholar] [CrossRef]
- Raza, M.A.; Bhatti, M.A.; Nasir, S.; Bashir, F.; Mahmood, Z.; Kazmi, K.R.; Hafeez, I. Study on Low-Grade Galena-Barite Ore Beneficiation In Khuzdar, Balochistan, Pakistan. Min. Miner. Depos. 2019, 13, 1–8. [Google Scholar] [CrossRef]
- Chaerun, S.K.; Putri1, E.A.; Mubarok, M.Z. Bioleaching of Indonesian Galena Concentrate with an Iron- and Sulfur-Oxidizing Mixotrophic Bacterium at Room Temperature. Front. Microbiol. 2020, 11, 557548. [Google Scholar] [CrossRef] [PubMed]
- Al-Saqarat, B.S.; Al-Mobydeen, A.; Al-Masri, A.N.; Esaifan, M.; Hamadneh, I.; Moosa, I.S.; AlShamaileh, E. Facile Production Method of PbS Nanoparticles via Mechanical Milling of Galena Ore. Micromachines 2023, 14, 564. [Google Scholar] [CrossRef]
- Ogwata, C.M.; Onwughalu, M.K. Occurrence of Galena and its Potentials for Economic and Green Energy Revolution in Nigeria. IRE J. 2019, 3, 139–142. Available online: https://www.academia.edu/43430505/Occurrence_of_Galena_and_its_Potentials_for_Economic_and_Green_Energy_Revolution_in_Nigeria (accessed on 27 March 2024).
- Tan, J.; Cao, Z.; Wang, S.; Zhong, H. Selective recovery of lead from galena-sphalerite by electro-oxidation. Hydrometallurgy 2019, 185, 218–225. [Google Scholar] [CrossRef]
- Ezekoye, B.A.; Emeakaroha, T.M.; Ezekoye, V.A.; Ighodalo1, K.O.; Offor, P.O. Optical and structural properties of lead sulphide (PbS) thin films synthesized by chemical method. Int. J. Phys. Sci. 2015, 10, 385–390. [Google Scholar] [CrossRef]
- Nafees, M.; Ikram, M.; Ali, S. Thermal stability of lead sulfide and lead oxide nano-crystalline materials. Appl. Nanosci. 2017, 7, 399–406. [Google Scholar] [CrossRef]
- Jameel, M.H.; Saleem, S.; Hashim, M.; Roslan, M.S.; Somaily, H.H.N.; Hessin, M.M.; El Bahy, Z.M.; Ashiq, M.G.B.; Hamzah, M.Q.; Jabbar, A.H.; et al. A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications. Nanotechnol. Rev. 2021, 10, 1484–1492. [Google Scholar] [CrossRef]
- Wu, S.; Qin, L.; Li, Q.; Wu, Z.; Nie, Z.; Jiang, Y.; Wang, J.; Wang, Z.; Zhou, Y.; Yu, K.; et al. Hot-carrier infrared detection in PbS with ultrafast and highly sensitive responses. Appl. Phys. Lett. 2022, 120, 42101. [Google Scholar] [CrossRef]
- Islam, M.A.; Sarkar, D.K.; Shahinuzzaman, M.; Wahab, Y.A.; Khandaker, M.U.; Tamam, N.; Sulieman, A.; Amin, N.; Akhtaruzzaman, M. Green Synthesis of Lead Sulphide Nanoparticles for High-Efficiency Perovskite Solar Cell Applications. Nanomaterials 2022, 12, 1933. [Google Scholar] [CrossRef]
- Mamiyev, Z.; Balayeva, N.O. PbS nanostructures: A review of recent advances. Mater. Today Sustain. 2023, 21, 100305. [Google Scholar] [CrossRef]
- Kang, J.; An, Y.; Xue, J.; Ma, X.; Li, J.; Chen, F.; Wang, S.; Wan, H.; Zhang, C.; Bu, X. Density Functional Theory Study of the Electronic structures of Galena. Processes 2023, 11, 619. [Google Scholar] [CrossRef]
- Yang, L.; Ren, X.; Ge, C.; Yan, Q. Status and development of powder metallurgy nickel-based disk superalloys. Int. J. Mater. Res. 2019, 110, 901–910. [Google Scholar] [CrossRef]
- Moosa, I.S.; Maqableh, B.B. Temperature difference with respect to exposure time for black paint and galena powder-black paint composite selective surfaces. In Proceedings of the World Renewable Energy Congress XVI, Murdoch University, Western Australia, Australia, 5–9 February 2017; Transition Towards 100% Renewable Energy, Ali Sayigh, Chapter 13, e. Book; Springer: Berlin/Heidelberg, Germany, 2018; pp. 139–147, ISBN 978-3-319-69844-1. [Google Scholar]
- Vergara-Hernández, H.J.; Olmos, L.; Solorio, V.M.; Bouvard, D.; Villalobos-Brito, J.; Chávez, J.; and Jimenez, O. Powder Metallurgy Fabrication and Characterization of Ti6Al4V/xCu Alloys for Biomedical Applications. Metals 2023, 13, 888. [Google Scholar] [CrossRef]
- Novák, P. Advanced Powder Metallurgy Technologies; MDPI: Basel, Switzerland, 2020; ISBN 978-3-03936-524-1. [Google Scholar]
- Čapek1, J.; Vojtěch, D. Powder Metallurgical Techniques for Fabrication of Biomaterials. Manuf. Technol. 2015, 15, 964–969. [Google Scholar] [CrossRef]
- Chatterjee, S.; Acharya, H.N.; Ratnam, V.V. Effects of sintering temperature and time on the electrical properties of pellets made from beneficiated galena ore. J. Mater. Sci. 1987, 22, 2793–2798. [Google Scholar] [CrossRef]
- Özkaplan, H. Studies of feasibilities of using galena for preparing thermoelements. Conmmun. Fac. Sei. Univ. Ank. Ser. A2 A3 1990, 39, 1–6. [Google Scholar] [CrossRef]
- BOSE, S.A. method of fabricating n-type thermoelements from galena concentrate. Bull. Mater. Sci. 1994, 17, 479–486. [Google Scholar] [CrossRef]
- Zha, L.; Li, H.; Wang, N. Preparation of high purity compact galena bulk by hot pressed sintering. Mater. Lett. 2019, 234, 233–236. [Google Scholar] [CrossRef]
- Kabir, H.; Munir, K.; Wen, C.; Li, Y. Recent research and progress of biodegradable zinc alloys and composites for biomedical applications: Biomechanical and biocorrosion perspectives. Bioact. Mater. 2021, 6, 836–879. [Google Scholar] [CrossRef]
- Danninger, H.; Calderon, R.D.; Gierl-Mayer, C. Powder Metallurgy and Sintered Materials; eBook; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2017; Available online: https://www.academia.edu/39247101/Powder_Metallurgy_and_Sintered_Materials (accessed on 27 March 2024).
- Hirschhorn, J.S. Introduction to Powder Metallurgy, 1st ed.; Colonial Press Inc.: Miami, FL, USA, 1969; p. 133. [Google Scholar]
- Julius, U.A.; Offiong, I.A.; Idorenyin, O.E. The Use of Galena as Weighting Material in Drilling Mud. JSRR 2015, 7, 455–465. [Google Scholar] [CrossRef]
- The Hudson Institute of Mineralogy (Mindat.org), Keswick, VA, USA. Available online: https://www.mindat.org/min-1641.html (accessed on 29 March 2024).
- Moosa, I.S.; Johnson, G.W.; Nutting, J. The structure and magnetic properties of Nd-Fe-B magnets produced by conventional and HD routes. J. Less Common Met. 1990, 158, 51–58. [Google Scholar] [CrossRef]
- Moosa, I.S. Powder Metallurgy and its Application in the Production of Permanent Magnets. Int. J. Adv. Res. Eng. Technol. 2013, 4, 127–141. [Google Scholar]
- Ertuğ, B. Sintering Applications; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Wang, T.; Huang, Y.; Ma, Y.; Wu, L.; Yan, H.; Liu, C.; Liu, Y.; Liu, B.; Liu, W. Microstructure and mechanical properties of powder metallurgy 2024 aluminum alloy during cold rolling. J. Mater. Res. Technol. 2021, 15, 3337–3348. [Google Scholar] [CrossRef]
- Chongad, L.S.; Sharma, A.; Banerjee, M.; Jain, A. Synthesis of Lead Sulfide Nanoparticles by Chemical Precipitation Method. J. Phys. Conf. Ser. 2016, 755, 12032. [Google Scholar] [CrossRef]
- Önal, M.; Altiokka, B. Chemical Bath Deposition of PbS Thin Films. Mugla J. Sci. Technol. 2020, 6, 94–98. [Google Scholar] [CrossRef]
- Chatterjee, B.; Bandyopadhyay, A. Characterization of PbS nanoparticles synthesized using sodium lauryl sulfate at room temperature. Mater. Today Proc. 2023, 76, 114–119. [Google Scholar] [CrossRef]







| Sample | Mass before Sintering (g) | Mass after Sintering (g) | Mass Reduction (g) | Sint. Condition |
|---|---|---|---|---|
| 38 µm | 3.3611 | 3.3221 | 0.0390 | 800 °C, 1 h, vacuum, rate of heating: 10 °C/min., rate of cooling to room temp.: 2.5 °C/min. |
| 38 µm | 3.4391 | 3.3970 | 0.0421 | |
| 250 µm | 3.54281 | 3.5066 | 0.0215 | |
| 250 µm | 3.4104 | 3.3906 | 0.0198 |
| Equation: y = a + bx | |||
|---|---|---|---|
| Parameter | Value | Standard Error | |
| GD | Intercept | 6.64651 | 0.03170 |
| Slope | −0.65170 | 0.06312 | |
| R-Square | 0.95528 | - | |
| SD | Intercept | 7.47494 | 0.02590 |
| Slope | −0.47940 | 0.05156 | |
| R-Square | 0.96351 | - | |
| MHV As-Received | MHV Sintered Sample | Used Load |
|---|---|---|
| 83.23 | 43.55 | 0.98 N |
| 79.45 | 40.93 | |
| 81.67 | 42.98 | |
| 80.91 | 41.70 | |
| 77.34 | 42.09 | |
| Mean value 80.52 | Mean value: 42.25 | |
| Standard deviation: 2.00 | Standard deviation: 0.93 |
| Sample | Goodness of Fit | Phase | wt.% | Crystal Structure | Lattice Parameters |
|---|---|---|---|---|---|
| As-received galena | 1.1526 | PbS | 98.9 | Cubic | a = 5.93542 |
| PbO/PbSO4 | 1.1 | Monoclinic | a = 14.16136 b = 5.60205 c = 7.20988 | ||
| Galena-sintered 700 °C, 1 h | 1.197 | PbS | 98.5 | Cubic | a = 5.93471 |
| PbO/PbSO4 | 1.5 | Monoclinic | a = 13.75149 b = 5.69768 c = 7.07152 | ||
| Galena-sintered 800 °C, 1 h | 1.550 | PbS | 81.3 | Cubic | a = 5.93535 |
| (PbO)2/PbSO4 | 18.7 | Monoclinic | a = 7.18478 b = 5.78746 c = 8.05508 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Saqarat, B.S.; Al-Mobydeen, A.; Al-Dalahmeh, Y.; AL-Masri, A.N.; Altwaiq, A.M.; Hamadneh, I.; Abu-Afifeh, Q.; Zoubi, M.M.; Esaifan, M.; Moosa, I.S.; et al. Study of Galena Ore Powder Sintering and Its Microstructure. Metals 2024, 14, 439. https://doi.org/10.3390/met14040439
Al-Saqarat BS, Al-Mobydeen A, Al-Dalahmeh Y, AL-Masri AN, Altwaiq AM, Hamadneh I, Abu-Afifeh Q, Zoubi MM, Esaifan M, Moosa IS, et al. Study of Galena Ore Powder Sintering and Its Microstructure. Metals. 2024; 14(4):439. https://doi.org/10.3390/met14040439
Chicago/Turabian StyleAl-Saqarat, Bety S., Ahmed Al-Mobydeen, Yousef Al-Dalahmeh, Ahmed N. AL-Masri, Abdelmnim M. Altwaiq, Imad Hamadneh, Qusay Abu-Afifeh, Mutaz M. Zoubi, Muayad Esaifan, Iessa Sabbe Moosa, and et al. 2024. "Study of Galena Ore Powder Sintering and Its Microstructure" Metals 14, no. 4: 439. https://doi.org/10.3390/met14040439
APA StyleAl-Saqarat, B. S., Al-Mobydeen, A., Al-Dalahmeh, Y., AL-Masri, A. N., Altwaiq, A. M., Hamadneh, I., Abu-Afifeh, Q., Zoubi, M. M., Esaifan, M., Moosa, I. S., & AlShamaileh, E. (2024). Study of Galena Ore Powder Sintering and Its Microstructure. Metals, 14(4), 439. https://doi.org/10.3390/met14040439






