High-Entropy Alloy Al0.2Co1.5CrFeNi1.5Ti0.5 Prepared from High-Entropy Oxide (Al0.2Co1.5CrFeNi1.5Ti0.5)3O4 by a Deoxidation Process via a CaH2-Assisted Molten Salt Method
Abstract
:1. Introduction
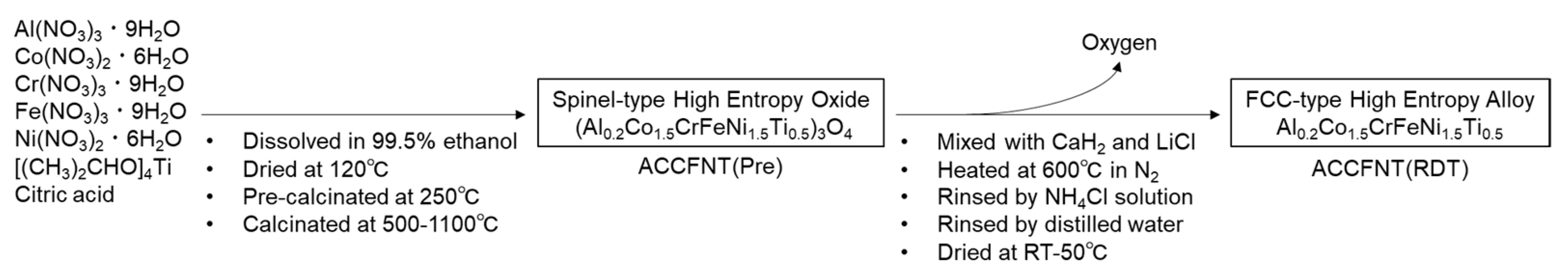
2. Materials and Methods
3. Results and Discussion
3.1. Sample Preparation and Catalytic Tests
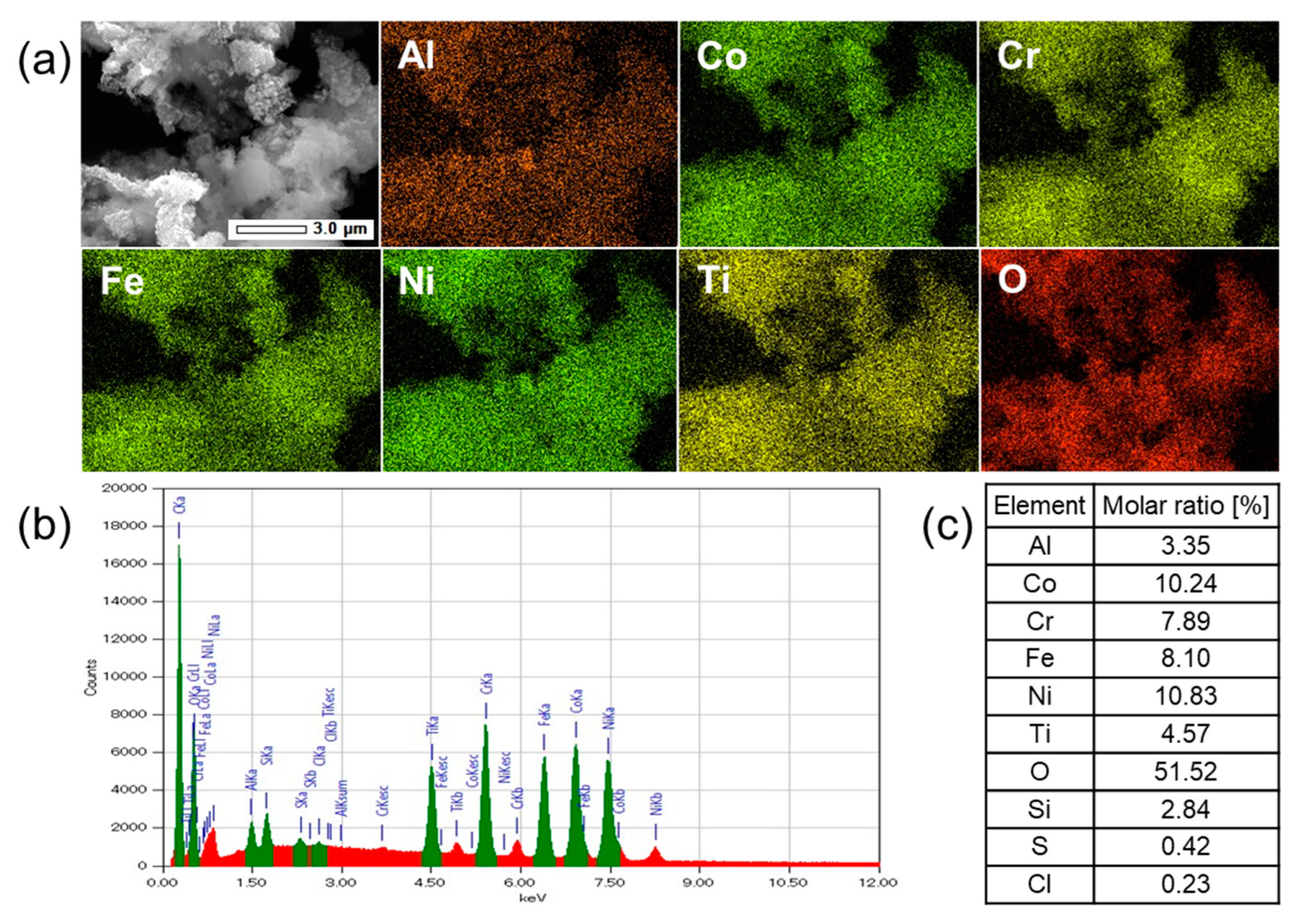
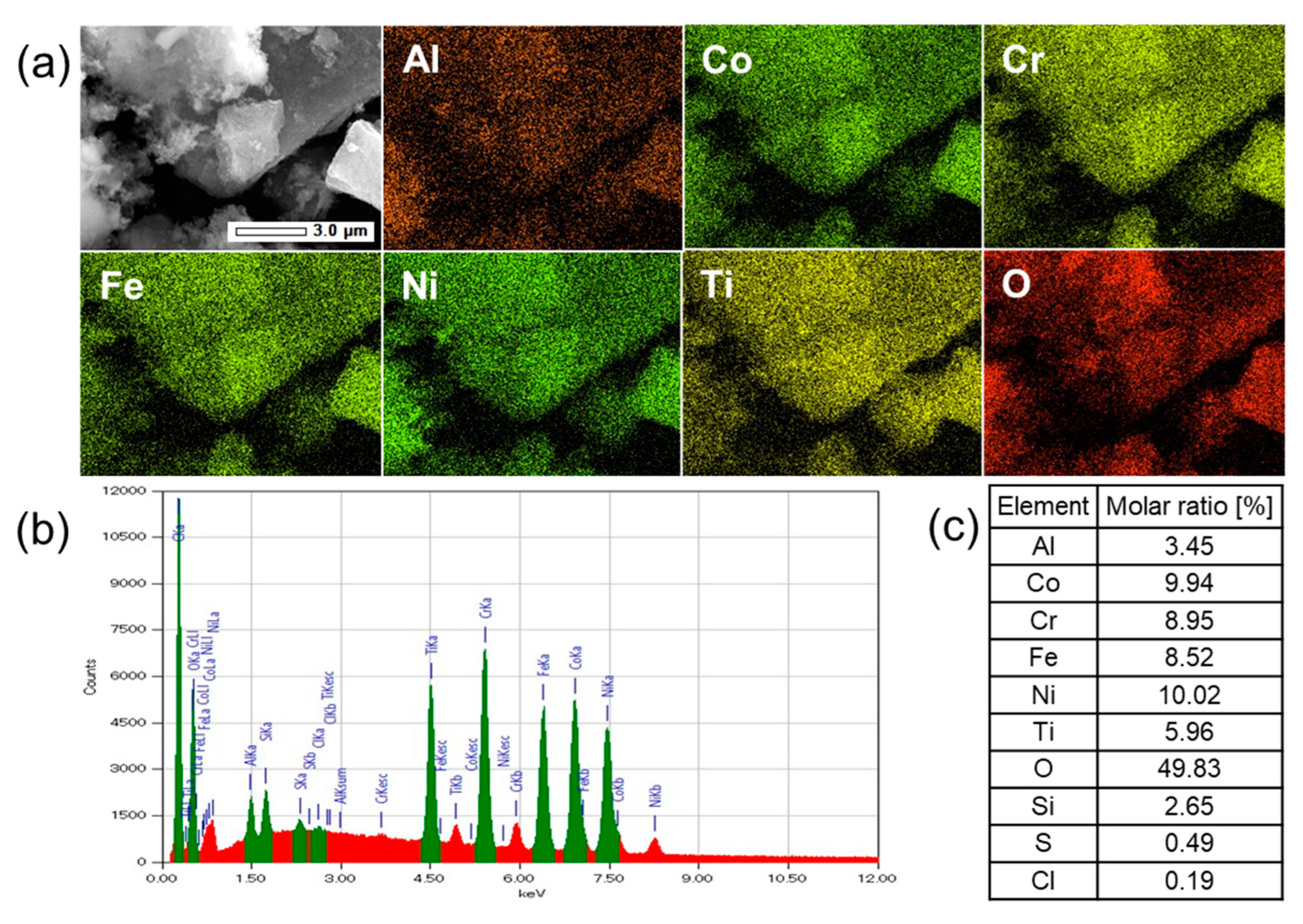
3.2. Elemental Analysis by SEM-EDX
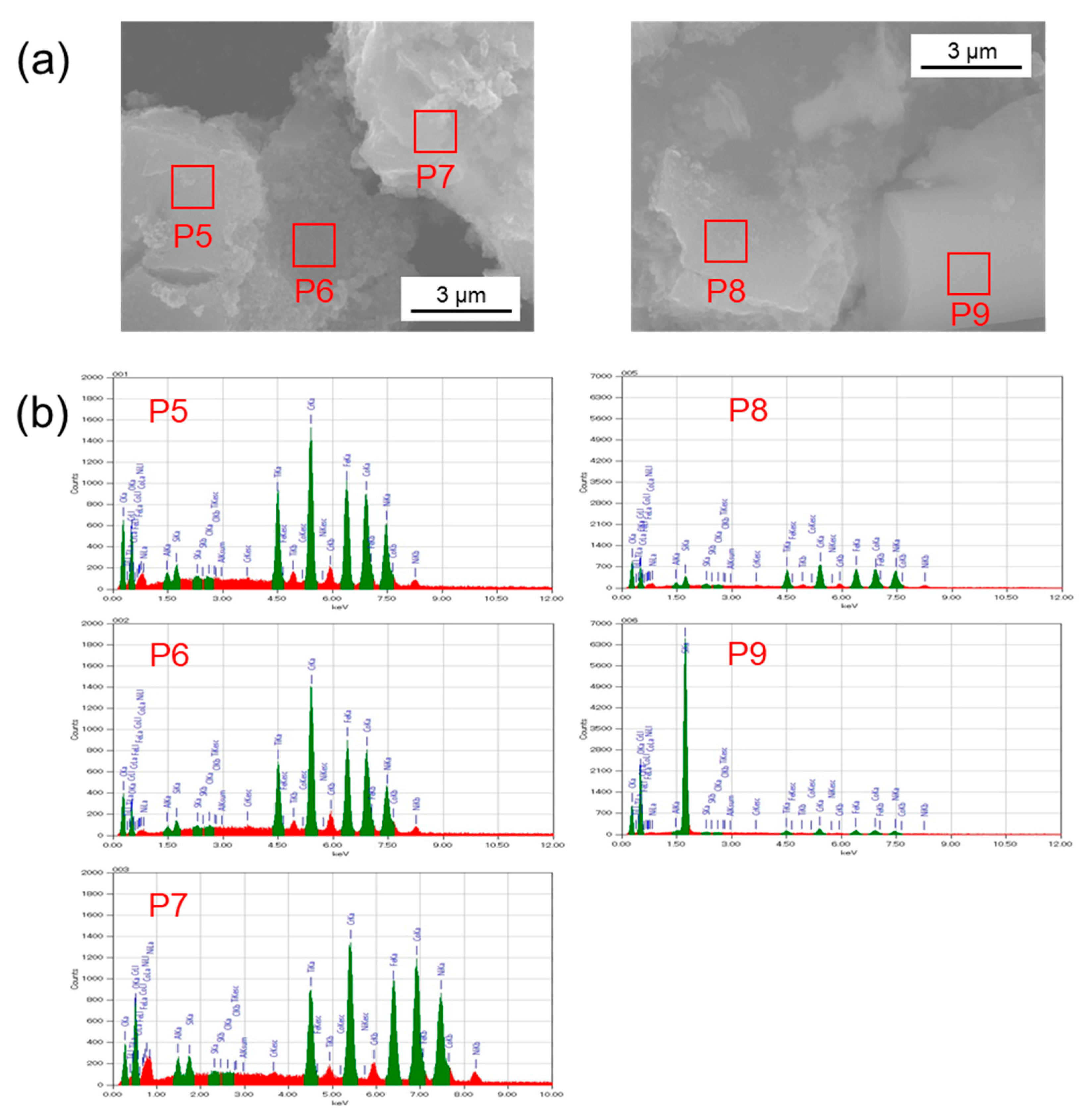
3.3. Elemental Analysis by TEM-EDX
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Yeh, J.-W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Torralba, J.M.; Alvaredo, P.; García-Junceda, A. High-entropy alloys fabricated via powder metallurgy. A critical review. Powder Metall. 2019, 62, 84–114. [Google Scholar] [CrossRef]
- Ke, B.; Sun, Y.; Zhang, Y.; Wang, W.; Wang, W.; Ma, P.; Ji, W.; Fu, Z. Powder metallurgy of high-entropy alloys and related composites: A short review. Int. J. Miner. Metall. Mater. 2021, 28, 931–943. [Google Scholar] [CrossRef]
- Yao, Y.; Huang, Z.; Xie, P.; Lacey, S.D.; Jacob, R.J.; Xie, H.; Chen, F.; Nie, A.; Pu, T.; Rehwoldt, M.; et al. Carbothermal shock synthesis of high-entropy-alloy nanoparticles. Science 2018, 359, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Yao, Y.; Huang, Z.; Liu, Z.; Zhang, J.; Li, T.; Wang, G.; Shahbazian-Yassar, R.; Hu, L.; Wang, C. Highly efficient decomposition of ammonia using high-entropy alloy catalysts. Nat. Commun. 2019, 10, 4011. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Du, Y.; Wang, C.; Guo, Y.; Zou, J.; Zhou, K.; Zeng, Z.; Liu, Y.; Li, L. High-entropy alloy nanoparticles on aligned electronspun carbon nanofibers for supercapacitors. J. Alloys Compd. 2020, 822, 153642. [Google Scholar] [CrossRef]
- Singh, M.P.; Srivastava, C. Synthesis and electron microscopy of high entropy alloy nanoparticles. Mater. Lett. 2015, 160, 419–422. [Google Scholar] [CrossRef]
- Niu, B.; Zhang, F.; Ping, H.; Li, N.; Zhou, J.; Lei, L.; Xie, J.; Zhang, J.; Wang, W.; Fu, Z. Sol-gel autocombustion synthesis of nanocrystalline high-entropy alloys. Sci. Rep. 2017, 7, 3421. [Google Scholar] [CrossRef] [PubMed]
- Glasscott, M.W.; Pendergast, A.D.; Goines, S.; Bishop, A.R.; Hoang, A.T.; Renault, C.; Dick, J.E. Electrosynthesis of high-entropy metallic glass nanoparticles for designer, multi-functional electrocatalysis. Nat. Commun. 2019, 10, 2650. [Google Scholar] [CrossRef] [PubMed]
- Bondesgaard, M.; Broge, N.L.N.; Mamakhel, A.; Bremholm, M.; Iversen, B.B. General solvothermal synthesis method for complete solubility range bimetallic and high-entropy alloy nanocatalysts. Adv. Funct. Mater. 2019, 29, 1905933. [Google Scholar] [CrossRef]
- Wu, D.; Kusada, K.; Yamamoto, T.; Toriyama, T.; Matsumura, S.; Kawaguchi, S.; Kubota, Y.; Kitagawa, H. Platinum-group-metal high-entropy-alloy nanoparticles. J. Am. Chem. Soc. 2020, 142, 13833–13838. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, B.; Ke, X.; Xu, F.; Bozhilov, K.N.; Hu, L.; Shahbazian-Yassar, R.; Zachariah, M.R. Aerosol synthesis of high entropy alloy nanoparticles. Langmuir 2020, 36, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dong, Q.; Qiao, H.; Huang, Z.; Saray, M.T.; Zhong, G.; Lin, Z.; Cui, M.; Brozena, A.; Hong, M.; et al. Continuous synthesis of hollow high-entropy nanoparticles for energy and catalysis applications. Adv. Mater. 2020, 32, 2002853. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Kusada, K.; Yamamoto, T.; Toriyama, T.; Matsumura, S.; Gueye, I.; Seo, O.; Kim, J.; Hiroi, S.; Sakata, O.; et al. On the electronic structure and hydrogen evolution reaction activity of platinum group metal-based high-entropy-alloy nanoparticles. Chem. Sci. 2020, 11, 12731–12736. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Hao, S.; Huang, Z.; Yuan, Y.; Han, S.; Lei, L.; Zhang, X.; Shahbazian-Yassar, R.; Lu, J. Synthesis of high-entropy alloy nanoparticles on supports by the fast moving bed pyrolysis. Nat. Commun. 2020, 11, 2016. [Google Scholar] [CrossRef] [PubMed]
- Gianelle, M.; Kundu, A.; Anderson, K.P.; Roy, A.; Balasubramanian, G.; Chan, H.M. A novel ceramic derived processing route for Multi-Principal Element Alloys. Mater. Sci. Eng. A 2020, 793, 139892. [Google Scholar] [CrossRef]
- Guo, X.; Liu, P.; Xia, Y.; Dong, Z.; Liu, H.; Chen, Y. Two-step hydrogen reduction of oxides for making FeCoNiCu high entropy alloy: Part I—Process and mechanical properties. Mater. Charact. 2020, 193, 112271. [Google Scholar] [CrossRef]
- Gianelle, M.A.; Clapp, C.; Kundu, A.; Chan, H.M. Solid state processing of the cantor derived alloy CoCrFeMnNi by oxide reduction. Results Mater. 2022, 14, 100286. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Suzuki, D.; Yokoyama, S.; Shoji, R. Molten salt synthesis of high-entropy alloy AlCoCrFeNiV nanoparticles for the catalytic hydrogenation of p-nitrophenol by NaBH4. Int. J. Hydrogen Energy 2022, 47, 3722–3732. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Teah, H.Y.; Yokoyama, S.; Shoji, R. Nobuko Hanada, Environmentally friendly molten salt synthesis of high-entropy AlCoCrFeNi alloy powder with high catalytic hydrogenation activity. Int. J. Hydrogen Energy 2023, 48, 30963–30973. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Teah, H.Y.; Yokoyama, S.; Shoji, R.; Hanada, N. A Molten Salt Synthesis Method of the High-Entropy Alloy CrMnFeCoNi for High Catalytic Performance and Low Life Cycle GHG Emissions. ACS Sustain. Chem. Eng. 2022, 10, 15046–15057. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Yokoyama, S.; Shoji, R. Core–Shell Multicomponent Alloys with High Specific Surface Areas Prepared by Molten Salt Synthesis for Catalytic Hydrogenation of p-Nitrophenol by NaBH4. ACS Appl. Eng. Mater. 2023, 1, 152–164. [Google Scholar] [CrossRef]
- Yokoyama, S.; Kobayashi, Y.; Shoji, R. Catalytic Hydrogenation of Methyl Orange and Acid Orange 7 Using NaBH4 over Core-shell Multicomponent Alloys. J. Water Environ. Technol. 2023, 21, 249–257. [Google Scholar] [CrossRef]
- Chuang, M.-H.; Tsai, M.-H.; Wang, W.-R.; Lin, S.-J.; Yeh, J.-W. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Xin, B.; Zhang, A.; Han, J.; Su, B.; Meng, J. Tuning composition and microstructure by doping Ti and C for enhancing mechanical property and wear resistance of Al0.2Co1.5CrFeNi1.5Ti0.5 high entropy alloy matrix composites. J. Alloys Compd. 2020, 836, 155273. [Google Scholar] [CrossRef]
- Xin, B.; Zhang, A.; Han, J.; Meng, J. Improving mechanical properties and tribological performance of Al0.2Co1.5CrFeNi1.5Ti0.5 high entropy alloys via doping Si. J. Alloys Compd. 2021, 869, 159122. [Google Scholar] [CrossRef]
- Xin, B.; Zhang, A.; Han, J.; Meng, J. The tribological properties of carbon doped Al0.2Co1.5CrFeNi1.5Ti0.5 high entropy alloys. Wear 2021, 484–485, 204045. [Google Scholar] [CrossRef]
- Xin, B.; Zhang, A.; Han, J.; Zhang, J.; Meng, J. Enhancing mechanical properties of the boron doped Al0.2Co1.5CrFeNi1.5Ti0.5 high entropy alloy via tuning composition and microstructure. J. Alloys Compd. 2022, 896, 162852. [Google Scholar] [CrossRef]
- Kobayashi, Y. Synthesis of porous Ni3Al Intermetallic nano-compounds in a molten LiCl with an assistance of CaH2 as a structure-controlling agent. Chem. Lett. 2019, 48, 1496–1499. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Iwasaki, Y. Fabrication of macroporous Co3O4–MgO composite catalysts for methylene blue degradation using oxone as an oxidant. J. Chem. Eng. Jpn. 2017, 50, 821–826. [Google Scholar] [CrossRef]
- Wang, B.; Huang, J.; Fan, J.; Dou, Y.; Zhu, H.; Wang, D. Preparation of FeCoNiCrMn high entropy alloy by electrochemical reduction of solid oxides in molten salt and its corrosion behavior in aqueous solution. J. Electrochem. Soc. 2017, 164, E575–E579. [Google Scholar] [CrossRef]
- Mao, A.; Xiang, H.-Z.; Zhang, Z.-G.; Koji, K.; Zhang, H.; Jia, Y. A new class of spinel high-entropy oxides with controllable magnetic properties. J. Magn. Magn. Mater. 2020, 497, 165884. [Google Scholar] [CrossRef]
- Akrami, S.; Edalati, P.; Fuji, M.; Edalati, K. High-entropy ceramics: Review of principles, production and applications. Mater. Sci. Eng. R Rep. 2021, 146, 100644. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Yamamoto, K.; Shoji, R. A CaH2-Assisted Reduction Method to Prepare Nanoscale Zero-Valent Iron (nZVI) from Fe2O3 for Water Remediation Application. Minerals 2023, 13, 1385. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Yokoyama, S.; Shoji, R. Molten salt synthesis of CrMnFeNi alloy nanopowder passivated by TiOx–ZrOy shell used as a superior catalyst support in liquid-phase hydrogenation. RSC Adv. 2023, 13, 10790. [Google Scholar] [CrossRef]
- Qiu, H.; Qiu, F.; Han, X.; Li, J.; Yang, J. Microwave-irradiated preparation of reduced graphene oxide-Ninanostructures and their enhanced performance for catalyticreduction of 4-nitrophenol. Appl. Surf. Sci. 2017, 407, 509–517. [Google Scholar] [CrossRef]
- Li, H.; Liao, J.; Du, Y.; You, T.; Liao, W.; Wen, L. Magnetic-field-induced deposition to fabricate multifunctional nanostructured Co, Ni, and CoNi alloy films as catalysts, ferromagnetic and superhydrophobic materials. Chem. Commun. 2013, 49, 1768–1770. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Z.; Shang, Y.; Zhang, Y.; Lou, Q.; Li, B.; Xu, J. Well dispersive Ni nanoparticles embedded in core-shell supports as efficient catalysts for 4-nitrophenol reduction. J. Nanopart. Res. 2019, 21, 120. [Google Scholar] [CrossRef]
- Bae, S.; Gim, S.; Kim, H.; Hanna, K. Effect of NaBH4 on properties of nanoscale zero-valent iron and its catalytic activity for reduction of p-nitrophenol. Appl. Catal. B Environ. 2016, 182, 541–549. [Google Scholar] [CrossRef]
- Sravanthi, K.; Ayodhya, D.; Swamy, P.Y. Green synthesis, characterization and catalytic activity of 4-nitrophenol reduction and formation of benzimidazoles using bentonite supported zero valent iron nanoparticles. Mater. Sci. Energy Technol. 2019, 2, 298–307. [Google Scholar] [CrossRef]
- Lu, H.; Qiao, X.; Wang, W.; Tan, F.; Xiao, Z.; Chen, J. Chitosan stabilised nanozero-valent iron for the catalytic reduction of p-nitrophenol. Micro Nano Lett. 2014, 9, 446–450. [Google Scholar] [CrossRef]
- Wu, K.; Yu, R.; Wei, X. Monodispersed FeNi2 alloy nanostructures: Solvothermal synthesis, magnetic properties and size-dependent catalytic activity. CrystEngComm 2012, 14, 7626–7632. [Google Scholar] [CrossRef]
- Malik, M.A.; Alshehri, A.A.; Patel, R. Facile one-pot green synthesis of Ag-Fe bimetallic nanoparticles and their catalytic capability for 4-nitrophenol reduction. J. Mater. Res. Technol. 2021, 12, 455–470. [Google Scholar] [CrossRef]






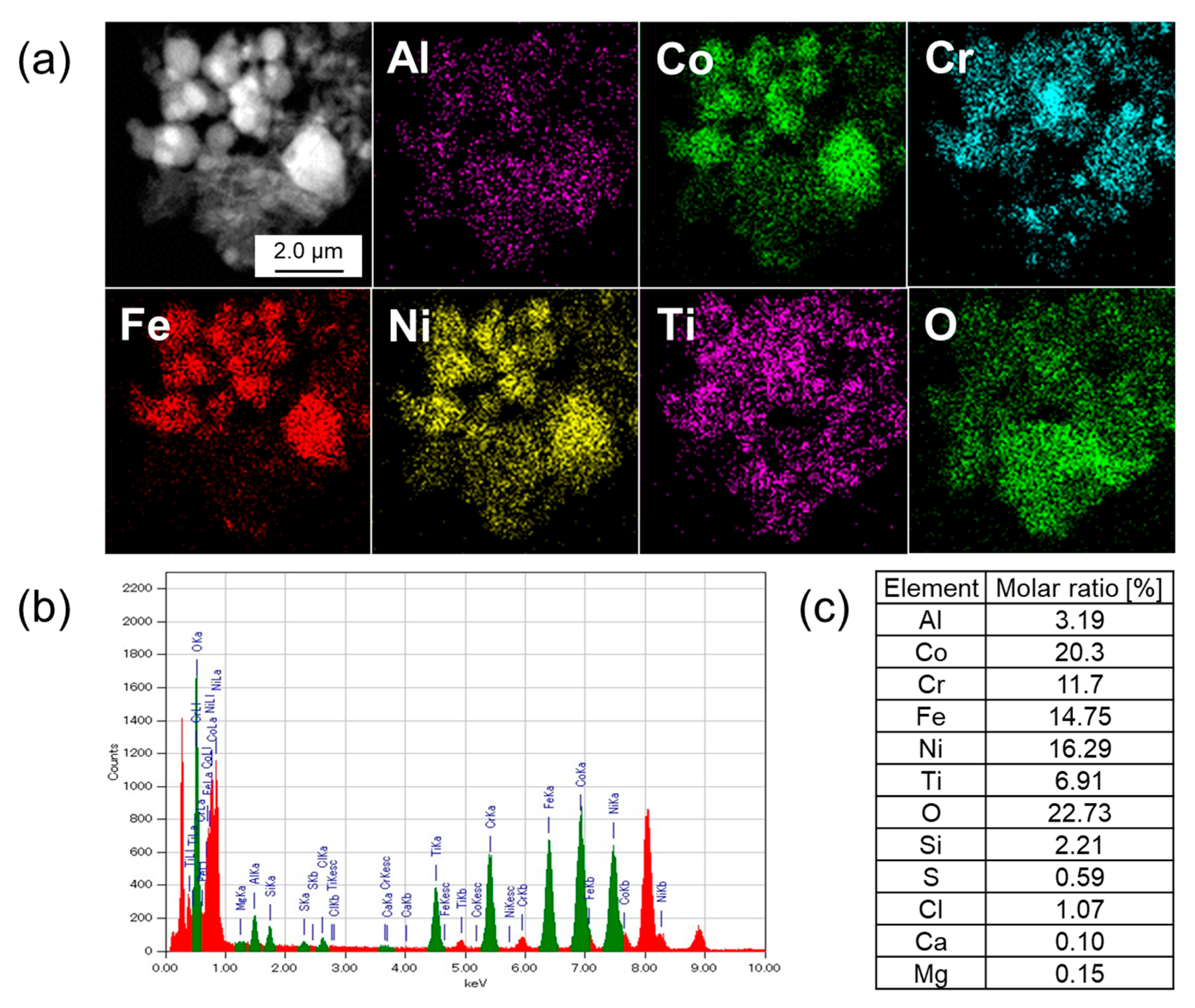


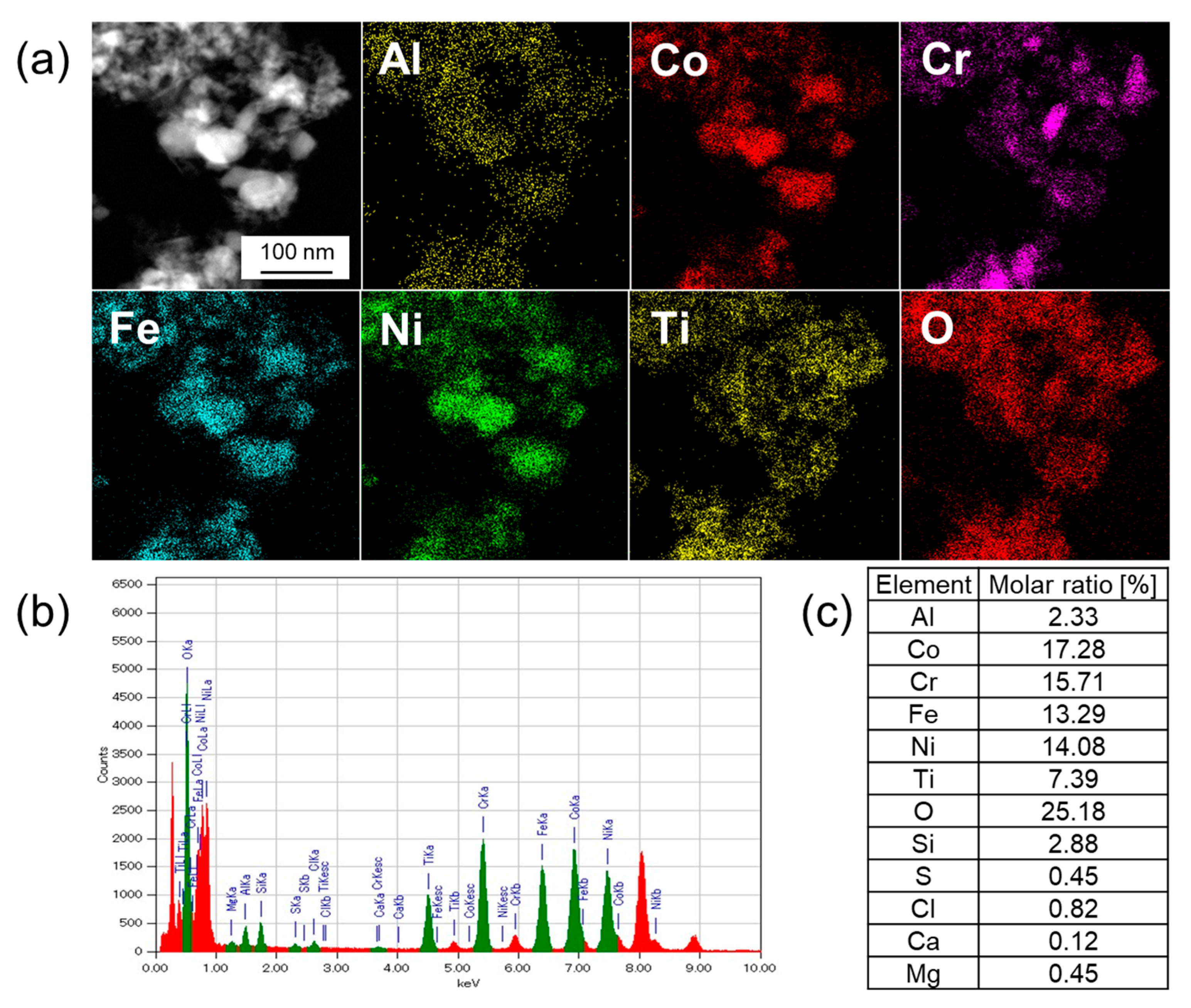
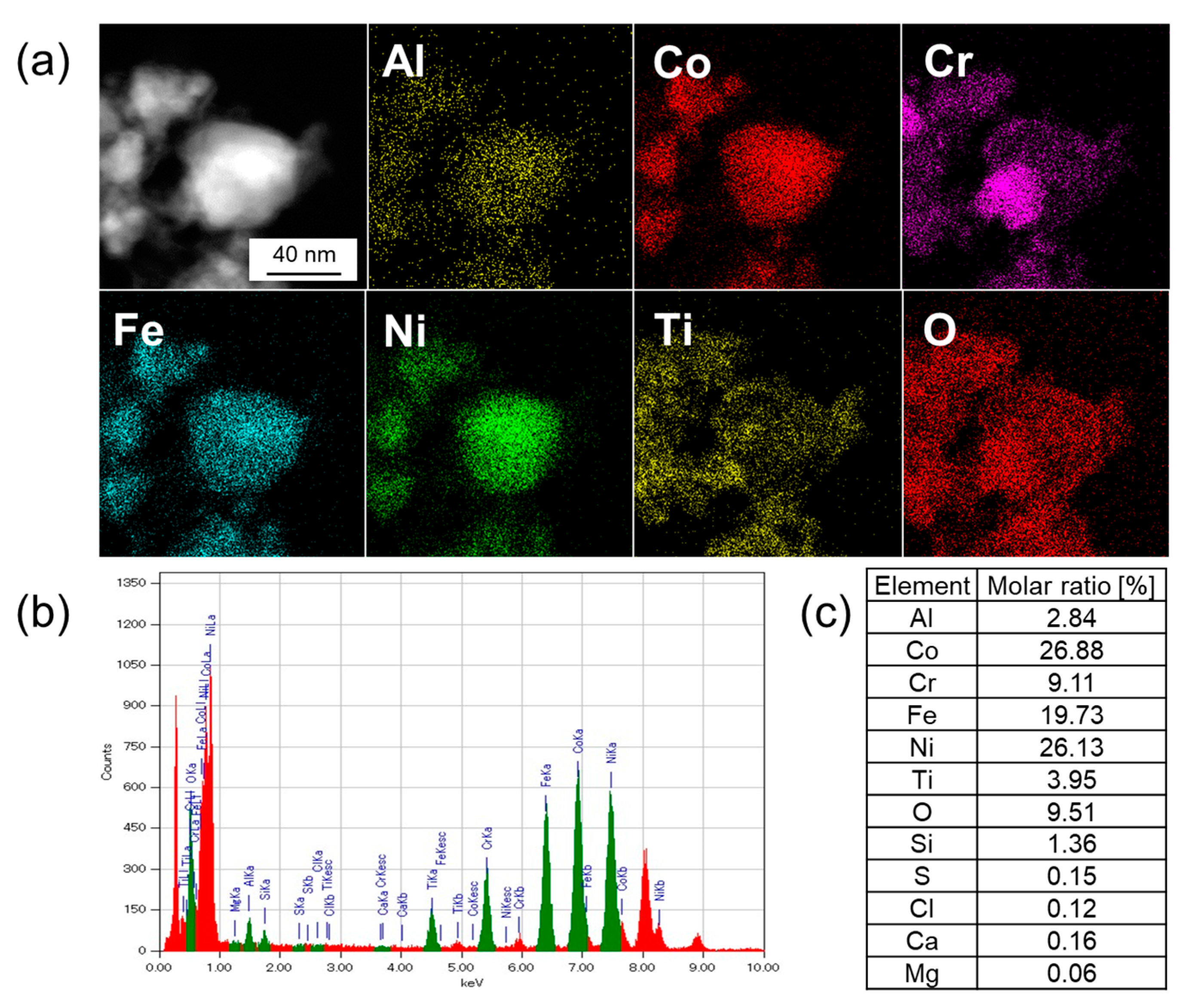
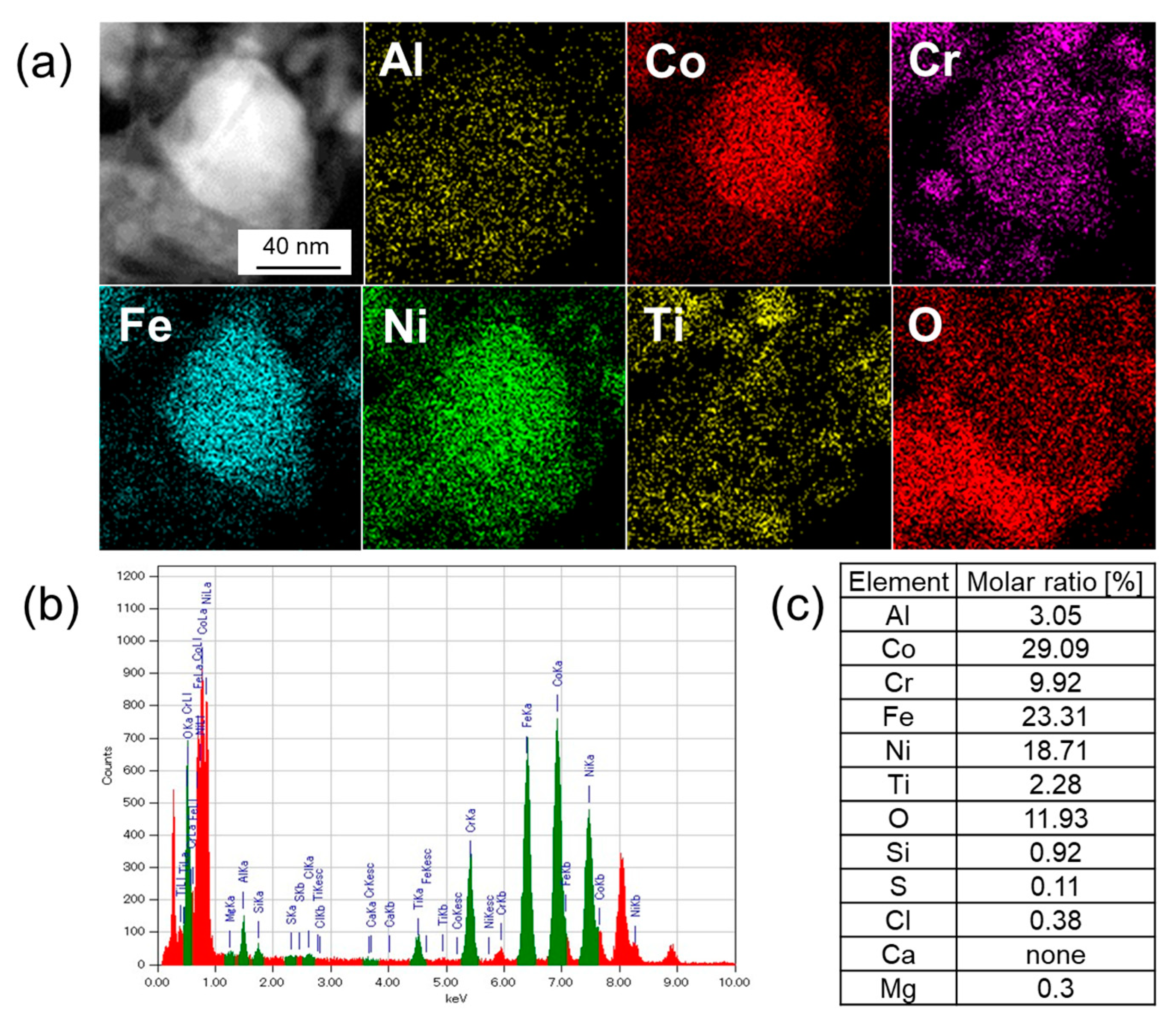
| Sample | Leached Amounts after Reactions [ppm] | |||||
|---|---|---|---|---|---|---|
| Al | Co | Cr | Fe | Ni | Ti | |
| ACCFNT(Pre500) | 0.024 | 0.017 | 1.163 | 0.013 | 0.024 | 0.002 |
| ACCFNT(RDT) | 0.021 | 0.017 | 0.015 | 0.013 | 0.026 | 0.001 |
| Catalyst | k [min−1] | Reaction Conditions | Refs. |
|---|---|---|---|
| Al0.2Co1.5CrFeNi1.5Ti0.5 | 0.2872 | 4-NP (1.6 mM) NaBH4 (47 mM) 10 mg-cat/9 mL | This study |
| CrMnFeCoNi | 0.040–0.108 | [26] | |
| AlCoCrFeNi | 0.0324 | [25] | |
| AlCoCrFeNiV | 0.0258 | [24] | |
| Nanoscale zero-valent iron | 0.040–0.048 | [39] | |
| 10wt%Ni/TiZrCrMnFeNi | 0.046 | [40] | |
| 10wt%Ni/TiO2 | 0.015 | ||
| 10wt%Ni/ZrO2 | 0.0003 | ||
| Ni-RGO | 0.07 | 4-NP (0.1 mM) NaBH4 (30 mM) 10 mg-cat/104 mL | [41] |
| Ni NP | 0.02 | ||
| Ni film | 0.09 | 4-NP (0.1 mM) NaBH4 (10 mM) 15 cm2-cat/16 mL | [42] |
| Co50Ni50 film | 0.15 | ||
| Co25Ni75 film | 0.14 | ||
| SiO2@C/Ni | 2.19–3.06 | 4-NP (0.2 mM) NaBH4 (65 mM) 3 mg-cat/3.1 mL | [43] |
| Nanoscale zero-valent iron | 0.31 | 4-NP (0.1 mM) NaBH4 (50 mM) 3.5 mg-cat/L | [44] |
| Bentonite clay-supported Fe nanoparticles | 0.141 | 4-NP (0.2 mM) NaBH4 (200 mM) 10 mg-cat/L | [45] |
| Chitosan-stabilized nanozero-valent iron | 0.147 | 4-NP (0.2 mM) NaBH4 (20 mM) 1000 mg-cat/L | [46] |
| Monodispersed FeNi2 alloy nanostructures | 0.057 | 4-NP (0.03 mM) NaBH4 (20 mM) 33 mg-cat/L | [47] |
| FeAg bimetallic nanoparticles | 0.065 | 4-NP (0.07 mM) NaBH4 (3 mM) 5 mg-cat/3 mL | [48] |
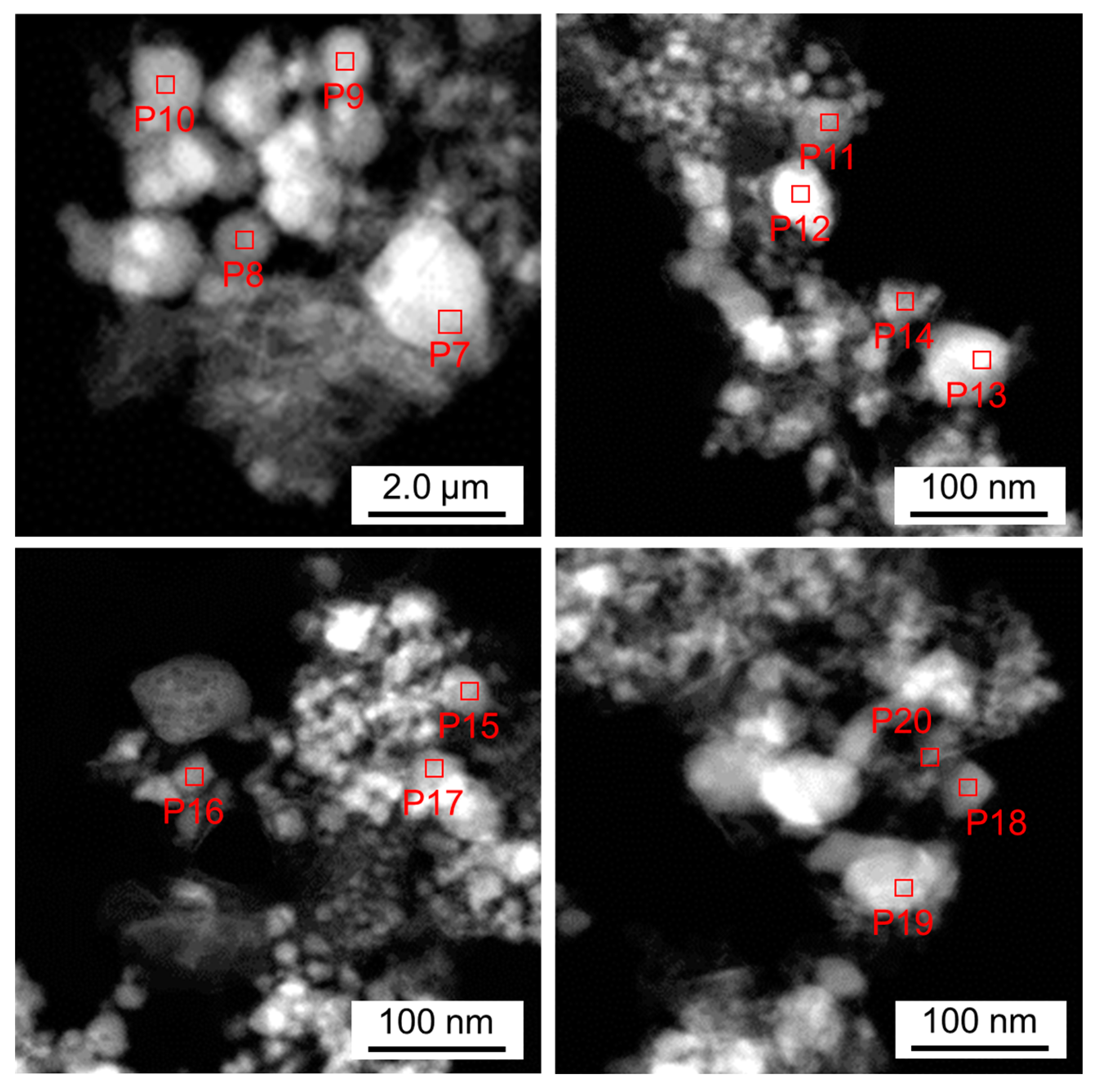
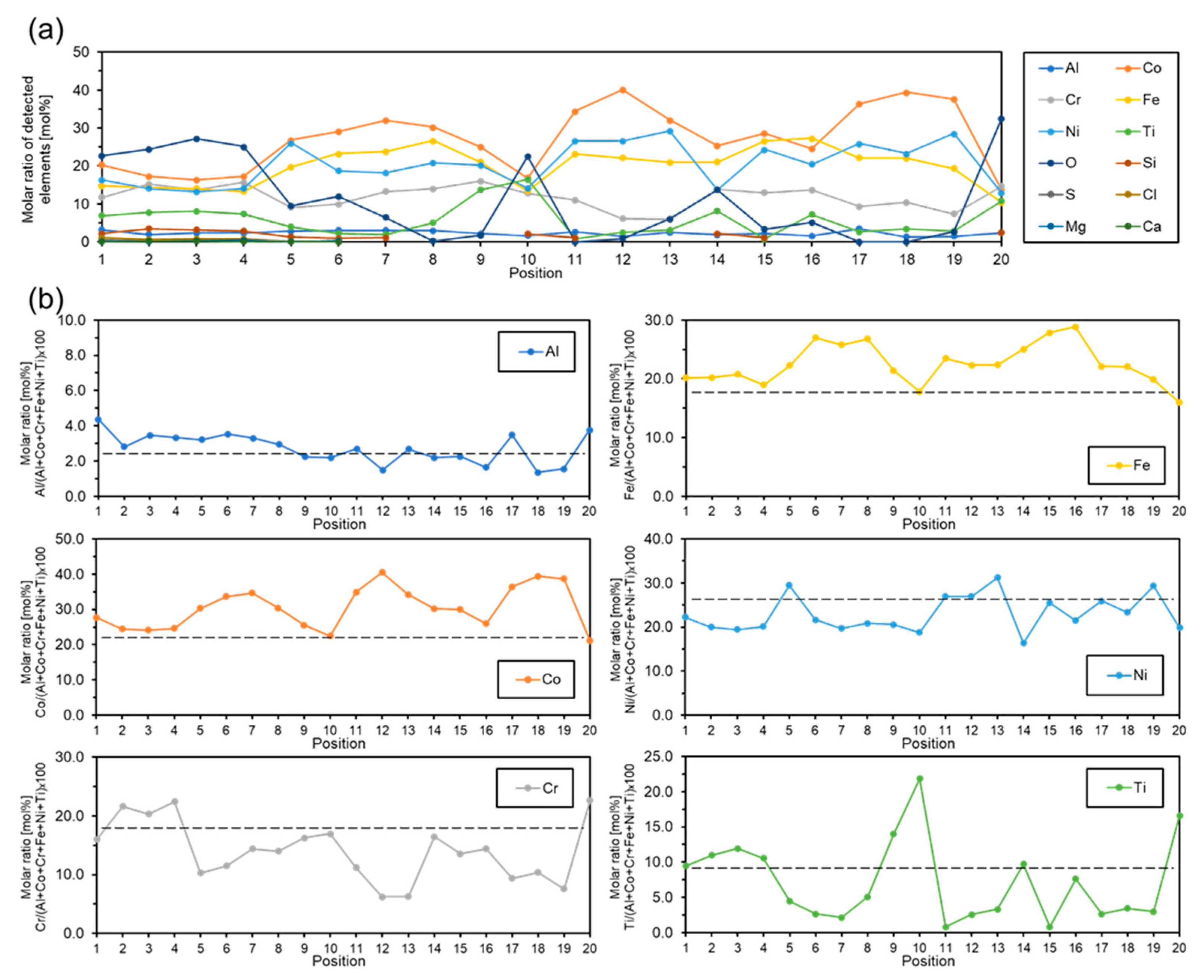
| Position | Molar Ratios of Detected Elements [mol%] | |||||
|---|---|---|---|---|---|---|
| Al | Co | Cr | Fe | Ni | Ti | |
| 1 | 7.4 | 22.8 | 17.5 | 18.0 | 24.1 | 10.2 |
| 2 | 7.4 | 21.2 | 19.1 | 18.2 | 21.4 | 12.7 |
| 3 | 5.3 | 23.7 | 18.9 | 21.8 | 19.8 | 10.6 |
| 4 | 5.4 | 21.6 | 21.0 | 22.1 | 19.5 | 10.6 |
| 5 | 3.0 | 21.8 | 24.1 | 22.3 | 16.3 | 12.4 |
| 6 | 1.5 | 22.7 | 26.6 | 22.5 | 15.9 | 10.8 |
| 7 | 5.0 | 25.1 | 19.2 | 18.6 | 21.5 | 10.6 |
| 8 | 5.8 | 22.0 | 18.5 | 19.1 | 23.4 | 11.2 |
| 9 | 5.1 | 21.5 | 21.5 | 19.5 | 21.2 | 11.0 |
| Average (SD) | 5.1 (1.8) | 22.5 (1.2) | 20.7 (2.8) | 20.2 (1.8) | 20.3 (2.6) | 11.1 (0.8) |
| Stoichiometric ratio | 3.5 | 26.3 | 17.5 | 17.5 | 26.3 | 8.8 |
| Position | Molar Ratios of Detected Elements [mol%] | |||||
|---|---|---|---|---|---|---|
| Al | Co | Cr | Fe | Ni | Ti | |
| 1 | 4.4 | 27.8 | 16.0 | 20.2 | 22.3 | 9.4 |
| 2 | 2.8 | 24.5 | 21.6 | 20.2 | 19.9 | 11.0 |
| 3 | 3.5 | 24.1 | 20.3 | 20.8 | 19.4 | 11.9 |
| 4 | 3.3 | 24.7 | 22.4 | 19.0 | 20.1 | 10.5 |
| 5 | 3.2 | 30.3 | 10.3 | 22.3 | 29.5 | 4.5 |
| 6 | 3.5 | 33.7 | 11.5 | 27.0 | 21.7 | 2.6 |
| 7 | 3.3 | 34.7 | 14.4 | 25.8 | 19.7 | 2.1 |
| 8 | 2.9 | 30.4 | 14.0 | 26.8 | 20.8 | 5.1 |
| 9 | 2.2 | 25.5 | 16.3 | 21.4 | 20.6 | 14.0 |
| 10 | 2.2 | 22.4 | 17.0 | 17.8 | 18.8 | 21.8 |
| 11 | 2.7 | 34.9 | 11.2 | 23.5 | 26.9 | 0.8 |
| 12 | 1.5 | 40.5 | 6.2 | 22.3 | 26.9 | 2.6 |
| 13 | 2.7 | 34.2 | 6.3 | 22.4 | 31.2 | 3.3 |
| 14 | 2.2 | 30.2 | 16.4 | 25.0 | 16.4 | 9.7 |
| 15 | 2.3 | 30.0 | 13.6 | 27.9 | 25.5 | 0.8 |
| 16 | 1.6 | 26.0 | 14.4 | 28.8 | 21.5 | 7.6 |
| 17 | 3.5 | 36.5 | 9.4 | 22.1 | 25.9 | 2.7 |
| 18 | 1.4 | 39.5 | 10.4 | 22.1 | 23.3 | 3.4 |
| 19 | 1.6 | 38.7 | 7.6 | 19.9 | 29.3 | 3.0 |
| 20 | 3.8 | 21.1 | 22.6 | 16.0 | 19.9 | 16.6 |
| Average (SD) | 2.7 (0.8) | 30.5 (5.7) | 14.1 (5.0) | 22.6 (3.3) | 23.0 (4.0) | 7.2 (5.6) |
| Stoichiometric ratio | 3.5 | 26.3 | 17.5 | 17.5 | 26.3 | 8.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, Y.; Yokoyama, S.; Shoji, R. High-Entropy Alloy Al0.2Co1.5CrFeNi1.5Ti0.5 Prepared from High-Entropy Oxide (Al0.2Co1.5CrFeNi1.5Ti0.5)3O4 by a Deoxidation Process via a CaH2-Assisted Molten Salt Method. Metals 2024, 14, 443. https://doi.org/10.3390/met14040443
Kobayashi Y, Yokoyama S, Shoji R. High-Entropy Alloy Al0.2Co1.5CrFeNi1.5Ti0.5 Prepared from High-Entropy Oxide (Al0.2Co1.5CrFeNi1.5Ti0.5)3O4 by a Deoxidation Process via a CaH2-Assisted Molten Salt Method. Metals. 2024; 14(4):443. https://doi.org/10.3390/met14040443
Chicago/Turabian StyleKobayashi, Yasukazu, Shota Yokoyama, and Ryo Shoji. 2024. "High-Entropy Alloy Al0.2Co1.5CrFeNi1.5Ti0.5 Prepared from High-Entropy Oxide (Al0.2Co1.5CrFeNi1.5Ti0.5)3O4 by a Deoxidation Process via a CaH2-Assisted Molten Salt Method" Metals 14, no. 4: 443. https://doi.org/10.3390/met14040443






