Atomic-Scale Insights into Flow-Accelerated Corrosion of Carbon Steel
Abstract
1. Introduction
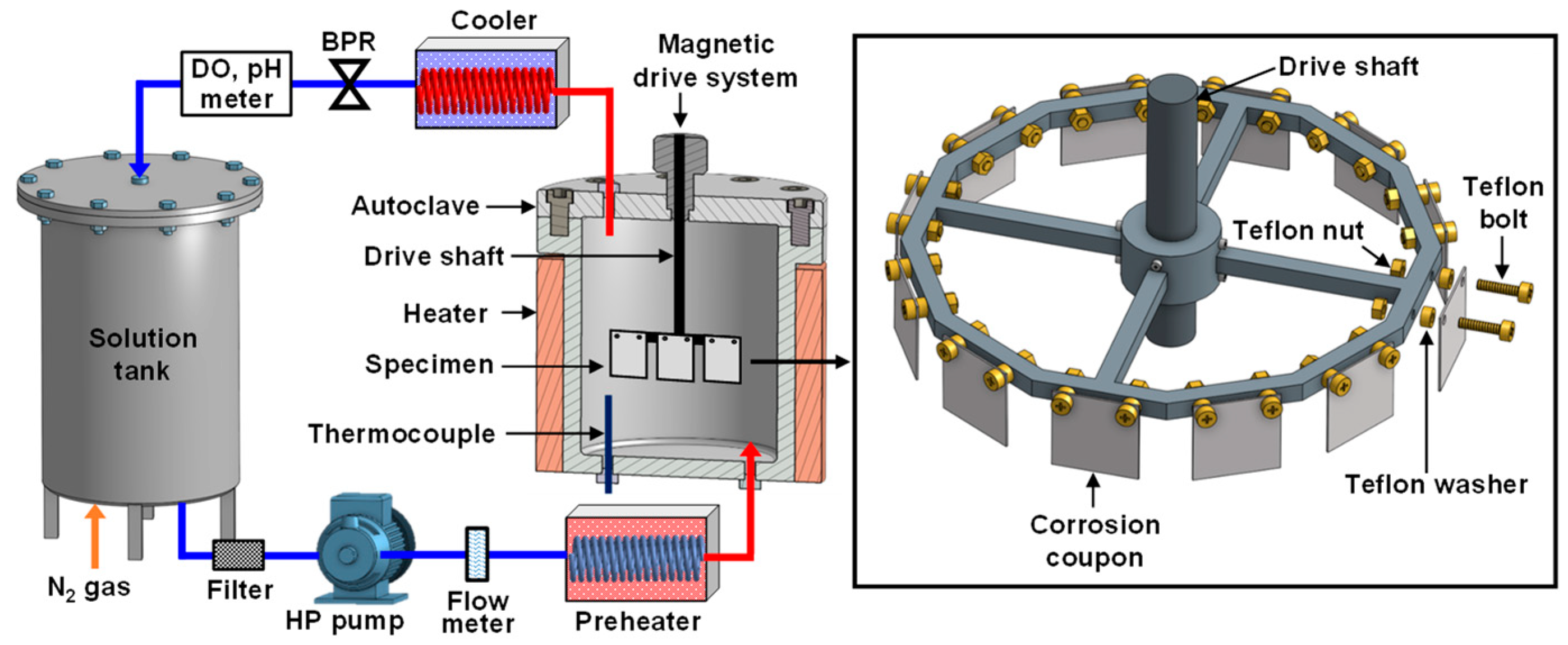
2. Materials and Methods
3. Results
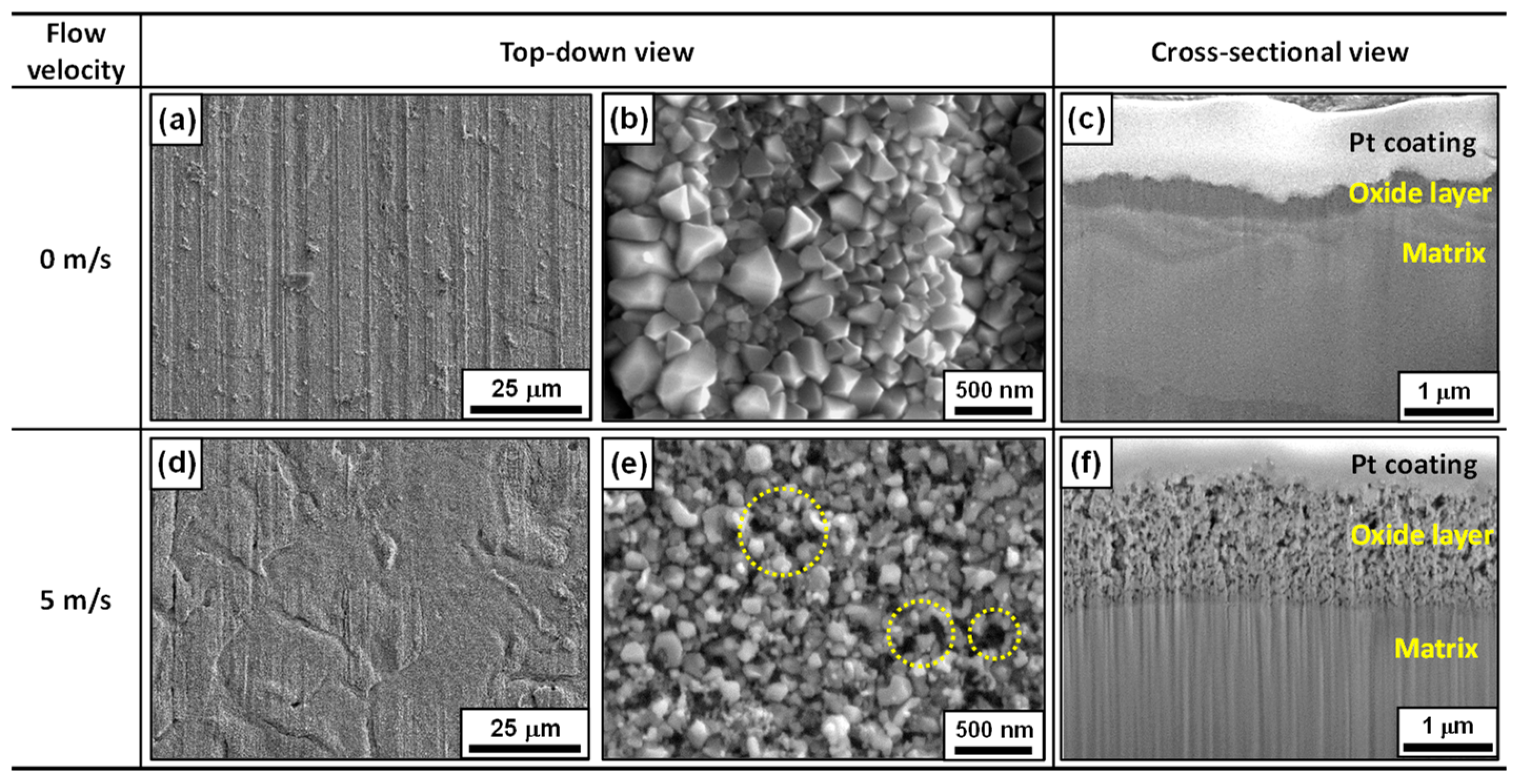
3.1. Corrosion Behavior and Oxide Layer Characteristics
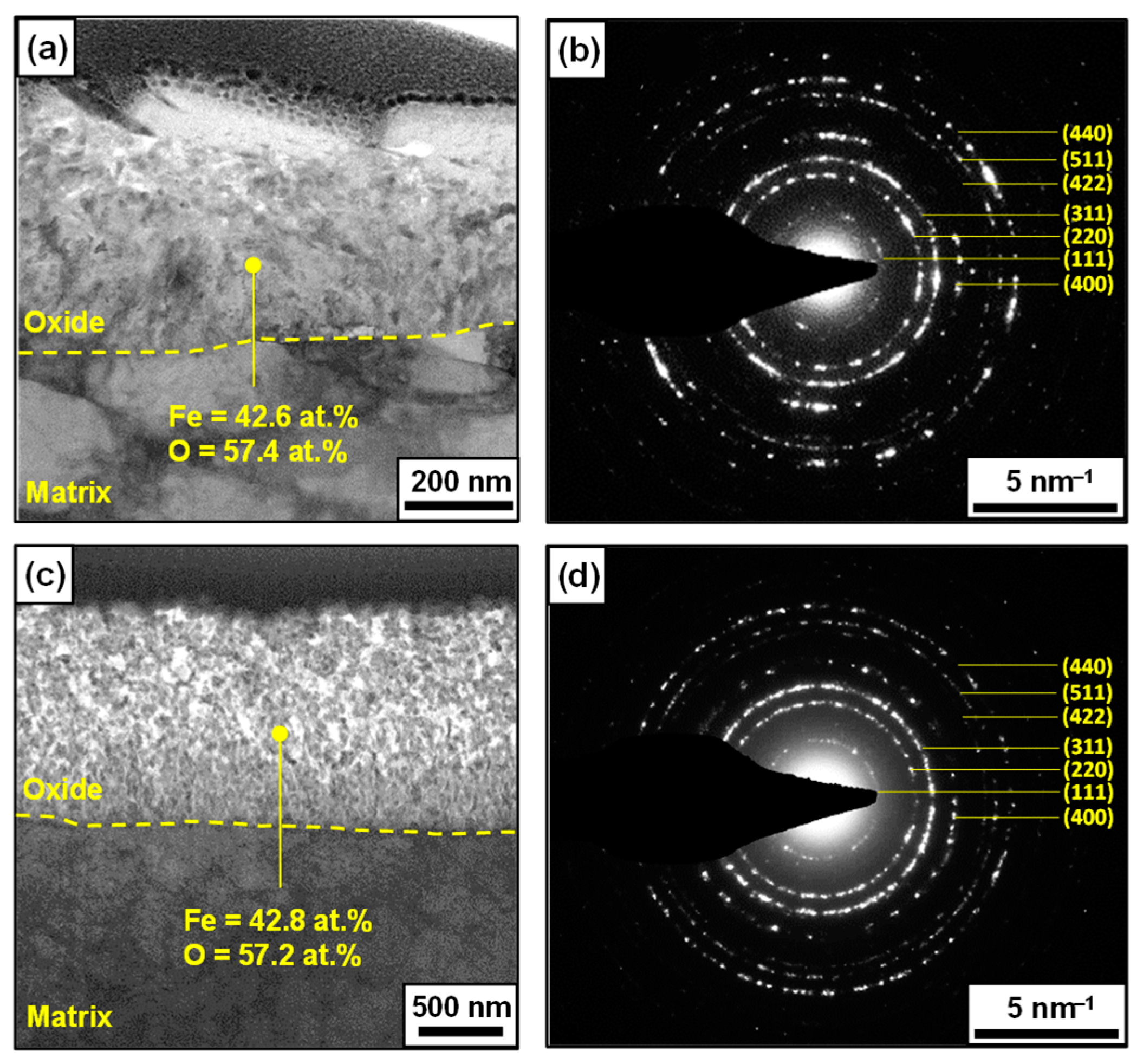
3.2. Atomic-Scale Oxide Structure
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khunphakdee, P.; Chalermsinsuwan, B. Review of flow accelerated corrosion mechanism, numerical analysis, and control measures. Chem. Eng. Res. Des. 2023, 197, 519–535. [Google Scholar] [CrossRef]
- Poulson, B. Predicting and preventing flow accelerated corrosion in nuclear power plant. Int. J. Nucl. Energy 2014, 2014, 423295. [Google Scholar] [CrossRef]
- Dooley, R.B. Flow-accelerated corrosion of fossil and combined cycle/HRSG plants. Power Plant Chem. 2008, 10, 68–89. [Google Scholar]
- Fujiwara, K.; Domae, M.; Yoneda, K.; Inada, F.; Ohira, T.; Hisamune, K. Correlation of flow accelerated corrosion rate with iron solubility. Nucl. Eng. Des. 2011, 241, 4482–4486. [Google Scholar] [CrossRef]
- Tomarov, G.V.; Shipkov, A.A. Flow-accelerated corrosion wear of power-generating equipment: Investigations, prediction, and prevention: 1. Flow-accelerated corrosion processes and regularities. Therm. Eng. 2018, 65, 493–503. [Google Scholar] [CrossRef]
- Sturla, P. Oxidation and deposition phenomena in forced circulating boilers and feedwater treatment. In Proceedings of the Fifth Nation Feedwater Conference, Prague, Czechoslovakia; 1973. [Google Scholar]
- Wang, B.; Wang, Y.; Li, Q.; Li, H.; Zhang, L.; Lu, M. Effect of chromium on the corrosion behaviour of low Cr-bearing alloy steel under an extremely high flow rate. RSC Adv. 2020, 10, 35302–35309. [Google Scholar] [CrossRef]
- Ishida, K. Flow accelerated corrosion of carbon steel containing chromium under water chemistry conditions of boiling water reactor applying mitigation techniques of corrosive environment. J. Nucl. Sci. Technol. 2022, 59, 709–724. [Google Scholar] [CrossRef]
- Jiang, S.; Chai, F.; Su, H.; Yang, C. Influence of chromium on the flow-accelerated corrosion behavior of low alloy steels in 3.5% NaCl solution. Corros. Sci. 2017, 123, 217–227. [Google Scholar] [CrossRef]
- Ducreux, J. Theoretical and experimental investigation of the effect of chemical composition of steels on their erosion-corrosion resistance. In Proceedings of the Specialists’ Meeting on the Corrosion-Erosion of Steels in High-Temperature Water and Wet Steam, Paris, France, 11–12 May 1982. [Google Scholar]
- Nasrazadani, S.; Nakka, R.K.; Hopkins, D.; Stevens, J. Characterization of oxides on FAC susceptible small-bore carbon steel piping of a power plant. Int. J. Press. Vessel. Pip. 2009, 86, 845–852. [Google Scholar] [CrossRef]
- Gipson, E.; Trevin, S. Flow accelerated corrosion (FAC) in nuclear power plant components. In Nuclear Corrosion: Research, Progress and Challenges; Woodhead Publishing Limited: Sawston, UK, 2020; pp. 213–250. [Google Scholar]
- Ishida, K.; Wada, Y.; Tachibana, M.; Aizawa, M.; Fuse, M.; Kadoi, E.; Takiguchi, H. Hydrazine and hydrogen co-injection to mitigate stress corrosion cracking of structural materials in boiling water reactors (V): Effects of hydrazine and dissolved oxygen on flow accelerated corrosion of carbon steel. J. Nucl. Sci. Technol. 2007, 44, 222–232. [Google Scholar] [CrossRef]
- Castle, J.E.; Mann, G.M.W. The mechanism of formation of a porous oxide film on steel. Corros. Sci. 1966, 6, 253–262. [Google Scholar] [CrossRef]
- Berge, P.; Ribon, C.; Saint Paul, P. The effect of hydrogen on the corrosion of steels in high temperature water. Corrosion 1976, 32, 223–228. [Google Scholar] [CrossRef]
- Fruzzetti, K. Pressurized Water Reactor Secondary Water Chemistry Guidelines—Revision 8; Electric Power Research Institute: Palo Alto, CA, USA, 2017; p. 3002010645. [Google Scholar]
- Zhao, B.; Du, Y.; Yan, Z.; Rao, L.; Chen, G.; Yuan, M.; Yang, L.; Zhang, J.; Che, R. Structural defects in phase-regulated high-entropy oxides toward superior microwave absorption properties. Adv. Funct. Mater. 2023, 33, 2209924. [Google Scholar] [CrossRef]
- Ke, X.; Zhang, M.; Zhao, K.; Su, D. Moire fringe method via scanning transmission electron microscopy. Small Methods 2022, 6, 2101040. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Zhang, L.; Zhao, X.; Wu, H. Defect induced polarization loss in multi-shelled spinel hollow spheres for electromagnetic wave absorption application. Adv. Sci. 2021, 8, 2004640. [Google Scholar] [CrossRef] [PubMed]
- Kattan, N.; Hou, B.; Fermin, D.J.; Cherns, D. Crystal structure and defects visualization of Cu2ZnSnS4 nanoparticles employing transmission electron microscopy and electron diffraction. Appl. Mater. Today 2015, 1, 52–59. [Google Scholar] [CrossRef]
- Suzki, T.; Nishi, U.; Fujimoto, M. Defect structure in homoeptitaxial non-stoichiometric strontium titanate thin films. Philos. Mag. A 2000, 80, 621–637. [Google Scholar] [CrossRef]
- Shai, W.; Wang, Q.; Zhang, D. Defect engineering of P doped Fe7S8 porous nanoparticles for high performance asymmetric supercapacitor and oxygen evolution electrocatalyst. J. Colloid Interface Sci. 2022, 617, 84–93. [Google Scholar] [CrossRef]
- Kiani, M.T.; Wang, Y.; Bertin, N.; Cai, W.; Gu, X.W. Strengthening mechanism of a single precipitate in a metallic nanocube. Nano Lett. 2019, 19, 255–260. [Google Scholar] [CrossRef]
- Kogawa, M.; Watson, E.B.; Ewing, R.C.; Utsunomiya, S. Lead in zircon at the atomic scale. Am. Miner. 2012, 97, 1094–1102. [Google Scholar] [CrossRef]
- Yetilmezsoy, K. Introduction of explicit equations for the estimation of surface tension, specific weight, and kinematic viscosity of water as a function of temperature. Fluid Mech. Res. Int. J. 2020, 4, 7–17. [Google Scholar] [CrossRef]
- Ronafoldi, A.; Roosz, A.; Veres, Z. Determination of the conditions of laminar/turbulent flow transition using pressure compensation method in the case of Ga75In25 alloy stirred by RMF. J. Cryst. Growth 2021, 654, 126078. [Google Scholar] [CrossRef]
- Ahmed, W.H. Evaluation of the proximity effect on flow-accelerated corrosion. Ann. Nucl. Energy 2010, 37, 598–605. [Google Scholar] [CrossRef]
- Kain, V. Flow accelerated corrosion: Forms, mechanisms and case studies. Procedia Eng. 2014, 86, 576–588. [Google Scholar] [CrossRef]
- Liu, G.; Yu, J.C.; Lu, G.Q.; Cheng, H.M. Crystal facet engineering of semiconductor photocatalysts: Motivations, advances and unique properties. Chem. Commun. 2011, 47, 6763–6783. [Google Scholar] [CrossRef]
- Balderas, R.I.; Ciobanu, C.V.; Richards, R.M. (111) faceted metal oxides: A review of synthetic methods. Cryst. Growth Des. 2022, 22, 6296–6322. [Google Scholar] [CrossRef]
- Lan, C.W.; Chen, C.J. Dynamic three-dimensional simulation of facet formation and segregation in Bridgman crystal growth. J. Cryst. Growth 2007, 303, 287–296. [Google Scholar] [CrossRef]
- Griffin, K.P.; Fu, L.; Moin, P. General method for determining the boundary layer thickness in nonequilibrium flows. Phys. Rev. Fluids 2021, 6, 024608. [Google Scholar] [CrossRef]
- Tanaka, Y. Mass transport in a boundary layer and in an ion exchange membrane: Mechanism of concentration polarization and water dissociation. Russ. J. Electrochem. 2012, 48, 665–681. [Google Scholar] [CrossRef]
- Geraldes, V.; Semiao, V.; Maria, N. de Pinho. The effect on mass transfer of momentum and concentration boundary layers at the entrance region of a slit with a nanofiltration membrane wall. Chem. Eng. Sci. 2002, 57, 735–748. [Google Scholar] [CrossRef]
- Weinstein, O.; Miller, W. Three-dimensional calculations of facets during Czochralski crystal growth. J. Cryst. Growth 2010, 312, 989–996. [Google Scholar] [CrossRef]
- You, X.; Ye, Q.; Cheng, P. The dependence of mass transfer coefficient on the electrolyte velocity in carbon felt electrodes: Determination and validation. J. Electrochem. Soc. 2017, 164, E3386–E3394. [Google Scholar] [CrossRef]
- Li, M.; Curnan, M.T.; Gresh-Sill, M.A.; House, S.D.; Saidi, W.A.; Yang, J.C. Unusual layer-by-layer growth of epitaxial oxide islands during Cu oxidation. Nat. Commun. 2021, 12, 2781. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Y.; Fan, Q.; Qu, M.; He, N.; Deng, J.; Sun, Y.; Cheng, J.; Liao, H.G.; Sun, S.G. Atomic scale tracking of single layer oxide formation: Self-peeling and phase transition in solution. Small Methods 2021, 5, 20001234. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhu, W.; Wu, D.; Liu, Z.; Chen, X.; Yuan, L.; Wang, G.; Sharma, R.; Zhou, G. Atomic-scale mechanism of unidirectional oxide growth. Adv. Funct. Mater. 2020, 30, 1906504. [Google Scholar] [CrossRef]
- Nguyen, L.; Hashimoto, T.; Zakharov, D.N.; Stach, E.A.; Rooney, A.P.; Berkels, B.; Thompson, G.E.; Haigh, S.J.; Burnett, T.L. Atomic-scale insights into the oxidation of aluminum. ACS Appl. Mater. Interfaces 2018, 10, 2230–2235. [Google Scholar] [CrossRef]
- Hua, Y.; Barker, R.; Neville, A. Comparison of corrosion behaviour for X-65 carbon steel in supercritical CO2-saturated water and water-saturated/unsaturated supercritical CO2. J. Supercrit. Fluids 2015, 97, 224–237. [Google Scholar] [CrossRef]
- Mokaddem, M.; Volovitch, P.; Ogle, K. The anodic dissolution of zinc and zinc alloys in alkaline solution. I. Oxide formation on electrogalvanized steel. Electrochim. Acta 2010, 55, 7867–7875. [Google Scholar] [CrossRef]
- 43. In HSC Chemistry 6, version 6.12; Outotec Research Oy: Pori, Filand, 2006.
- Macdonald, D.D. The history of the point defect model for the passive state: A brief review of film growth aspects. Electrochim. Acta 2011, 56, 1761–1772. [Google Scholar] [CrossRef]
- Chao, C.Y.; Lin, L.F.; Macdonald, D.D. A point defect model for anodic passive films. J. Electrochem. Soc. 1981, 128, 1187–1194. [Google Scholar] [CrossRef]
- Dooley, B.; Lister, D. Flow-accelerated corrosion in steam generating plants. Power Plant Chem. 2018, 20, 194–244. [Google Scholar]
- Lou, H.R.; Tsai, D.S.; Chou, C.C. Correlation between defect density and corrosion parameter of electrochemically oxidized aluminum. Coatings 2020, 10, 20. [Google Scholar] [CrossRef]
- Lim, D.S.; Jeon, S.H.; Bae, B.J.; Choi, J.; Song, K.M.; Hur, D.H. Effect of zinc addition scenarios on general corrosion of Alloy 690 in borated and lithiated water at 330 °C. Corros. Sci. 2021, 189, 109627. [Google Scholar] [CrossRef]
- Guo, H.X.; Lu, B.T.; Luo, J.L. Study on passivation and erosion-enhanced corrosion resistance by Mott-Schottky analysis. Electrochim. Acta 2006, 52, 1108–1116. [Google Scholar] [CrossRef]
- Sun, H.; Su, G.; Zhang, Y.; Ren, J.C.; Chen, X.; Hou, H.; Ding, Z.; Zhang, T.; Liu, W. First-principles modeling of the anodic and cathodic polarization to predict the corrosion behavior of Mg and its alloys. Acta Mater. 2023, 244, 118562. [Google Scholar] [CrossRef]
- Jin, H.; Sui, Y.; Yu, X.; Feng, J.; Jiang, Y.; Wang, Q.; Sun, W. The crystallographic orientation dependent anisotropic corrosion behavior of aluminum in 3.5 wt% NaCl solution. J. Electroanal. Chem. 2023, 946, 117746. [Google Scholar] [CrossRef]
- Wang, X.; Xie, D.; Wei, L.; You, D.; Houa, M.; Leng, Y. DFT investigation of the dissolution trends of NiTi alloys with the B2 and B19’ phases during the initial oxidation stage. Phys. Chem. Chem. Phys. 2023, 25, 19804. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Chen, X.Q.; Li, R.H.; Wang, S.L.; Dong, J.H.; Ice, W. First-principles modeling of anisotropic anodic dissolution of metals and alloys in corrosive environments. Acta Mater. 2017, 130, 137–146. [Google Scholar] [CrossRef]
- Crundwell, F.K. On the mechanism of the dissolution of quartz and silica in aqueous solutions. ACS Omega 2017, 2, 1116–1127. [Google Scholar] [CrossRef]
- Duan, Y.; Lee, J.Y.; Xi, S.; Sun, Y.; Ge, J.; Ong, S.J.H.; Chen, Y.; Dou, S.; Meng, F.; Diao, C.; et al. Anodic oxidation enabled cation leaching for promoting surface reconstruction in water oxidation. Angew. Chem. Int. Ed. 2021, 60, 7418–7425. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, C.; Wang, S.; Xue, C.; Tian, G.; Su, H.; Yan, C.; Yan, Z.; Liud, X.; Wang, J. Towards quantum corrosion chemistry: Screening perfect Cr, Ni sites and stoichiometry on top of an Fe(110) surface using DFT. RSC Adv. 2023, 13, 9945. [Google Scholar] [CrossRef]
- Liu, H.; Valentin, C.D. Band gap in magnetite above verwey temperature induced by symmetry breaking. J. Phys. Chem. C 2017, 121, 25736–25742. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.C.; Khoh, W.H.; Chin, S.F. Nanoparticulate magnetite thin films as electrode materials for the fabrication of electrochemical capacitors. J. Mater. Sci. 2010, 45, 5598–5604. [Google Scholar] [CrossRef]
- Zheng, Y.; Slade, T.J.; Hu, L.; Tan, X.Y.; Luo, Y.; Luo, Z.Z.; Xu, J.; Yan, Q.; Kanatzidis, M.G. Defect engineering in thermoelectric materials: What have we learned? Chem. Soc. Rev. 2021, 50, 9022–9054. [Google Scholar] [CrossRef]
- Li, Q.; Wang, G.T. Spatial distribution of defect luminescence in GaN nanowires. Nano Lett. 2010, 10, 1554–1558. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Mizumukai, Y.; Ohnishi, T.; Shibata, N.; Ikuhara, Y.; Yamamoto, T. High electron mobility of Nb-doped SrTiO3 films stemming from rod-type Sr vacancy clusters. ACS Nano 2015, 9, 10769–10777. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Shang, T.; Zhang, C.; Hu, J.; Zhang, Z.; Zhang, Q.; Yuan, X.; Hou, D.; Zeng, D.; Shi, T. In situ study of flow accelerated corrosion and its mitigation at different locations of a gradual contraction of N80 steel. J. Alloys Compd. 2020, 824, 153947. [Google Scholar] [CrossRef]
- Zhang, G.A.; Zeng, L.; Huang, H.L.; Guo, X.P. A study of flow accelerated corrosion at elbow of carbon steel pipeline by array electrode and computational fluid dynamics simulation. Corros. Sci. 2013, 77, 334–341. [Google Scholar] [CrossRef]
- Utanohara, Y.; Nagaya, Y.; Nakamura, A.; Murase, M.; Kamahori, K. Correlation between flow accelerated corrosion and wall shear stress downstream from an orifice. J. Power Energy Syst. 2013, 7, 138–147. [Google Scholar] [CrossRef][Green Version]
- Fingjun, L.; Lin, Y.; Li, X. Numerical simulation for carbon steel flow-induced corrosion in high-velocity flow seawater. J. Anti-Corros. Methods Mater. 2008, 55, 66–72. [Google Scholar] [CrossRef]
- Kang, D.G.; Jo, J.C. CFD application to the regulatory assessment of FAC-caused CANDU feeder pipe wall thinning issue. J. Nucl. Eng. Technol. 2008, 40, 37–48. [Google Scholar] [CrossRef]

| Ni | Cr | Mo | Cu | C | Fe |
|---|---|---|---|---|---|
| 0.02 | 0.04 | 0.01 | 0.01 | 0.19 | Balance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hur, D.-H.; Han, J.; Lee, Y.-K. Atomic-Scale Insights into Flow-Accelerated Corrosion of Carbon Steel. Metals 2024, 14, 445. https://doi.org/10.3390/met14040445
Hur D-H, Han J, Lee Y-K. Atomic-Scale Insights into Flow-Accelerated Corrosion of Carbon Steel. Metals. 2024; 14(4):445. https://doi.org/10.3390/met14040445
Chicago/Turabian StyleHur, Do-Haeng, Jeoh Han, and Young-Kook Lee. 2024. "Atomic-Scale Insights into Flow-Accelerated Corrosion of Carbon Steel" Metals 14, no. 4: 445. https://doi.org/10.3390/met14040445
APA StyleHur, D.-H., Han, J., & Lee, Y.-K. (2024). Atomic-Scale Insights into Flow-Accelerated Corrosion of Carbon Steel. Metals, 14(4), 445. https://doi.org/10.3390/met14040445






