Study on Recovery of Lithium from Lithium-Containing Aluminum Electrolyte
Abstract
:1. Introduction
2. Experimental
2.1. Experimental Raw Materials
2.1.1. Mineralogical Analysis of Lithium-Containing Aluminum Electrolyte
2.1.2. Lithium-Containing Aluminum Electrolyte Mineral Phase Analysis
2.2. Additive Analysis
2.3. Evaluating Indicators
2.3.1. Lithium Leaching Rate
2.3.2. Aluminum Direct Yield
3. Results and Discussion
3.1. Thermodynamic Analysis
3.2. Experimental Programs
3.3. Effect of Reaction Conditions on Li Leaching Rate
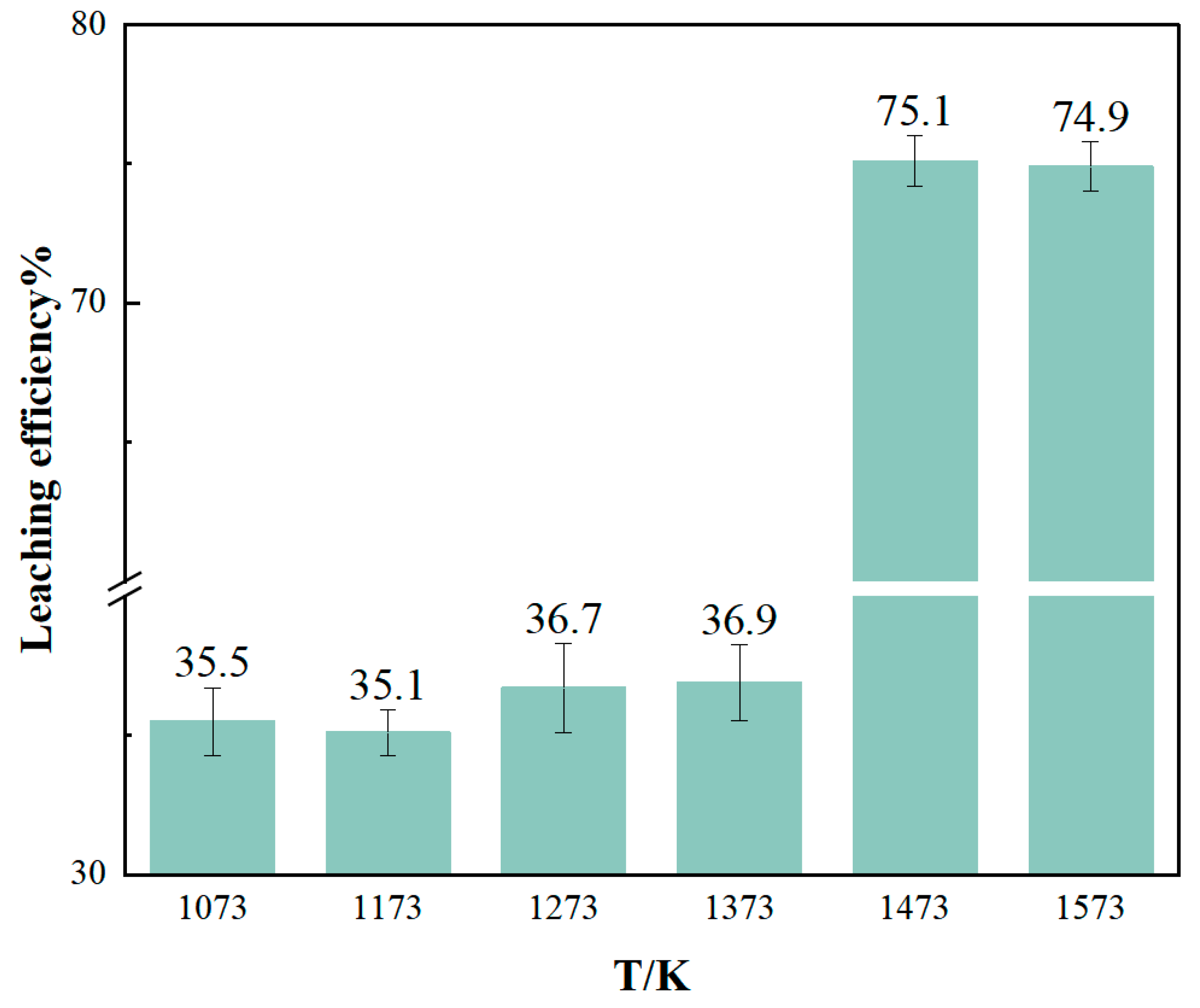
3.3.1. Effect of Chlorination Roasting Temperature on Li Leaching Rate
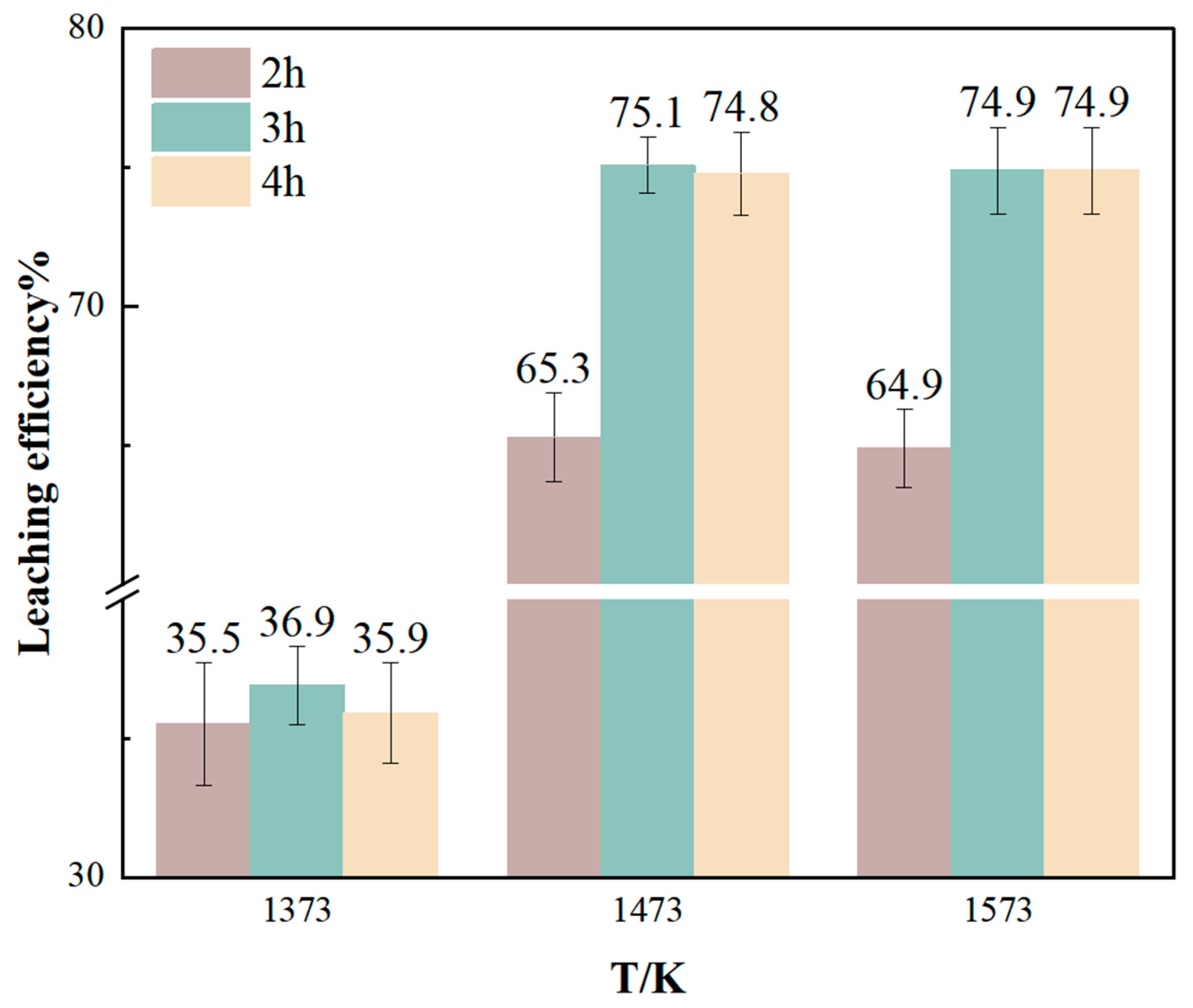
3.3.2. Effects of Chlorination Roasting Temperature and Holding Time on Li Leaching Rate
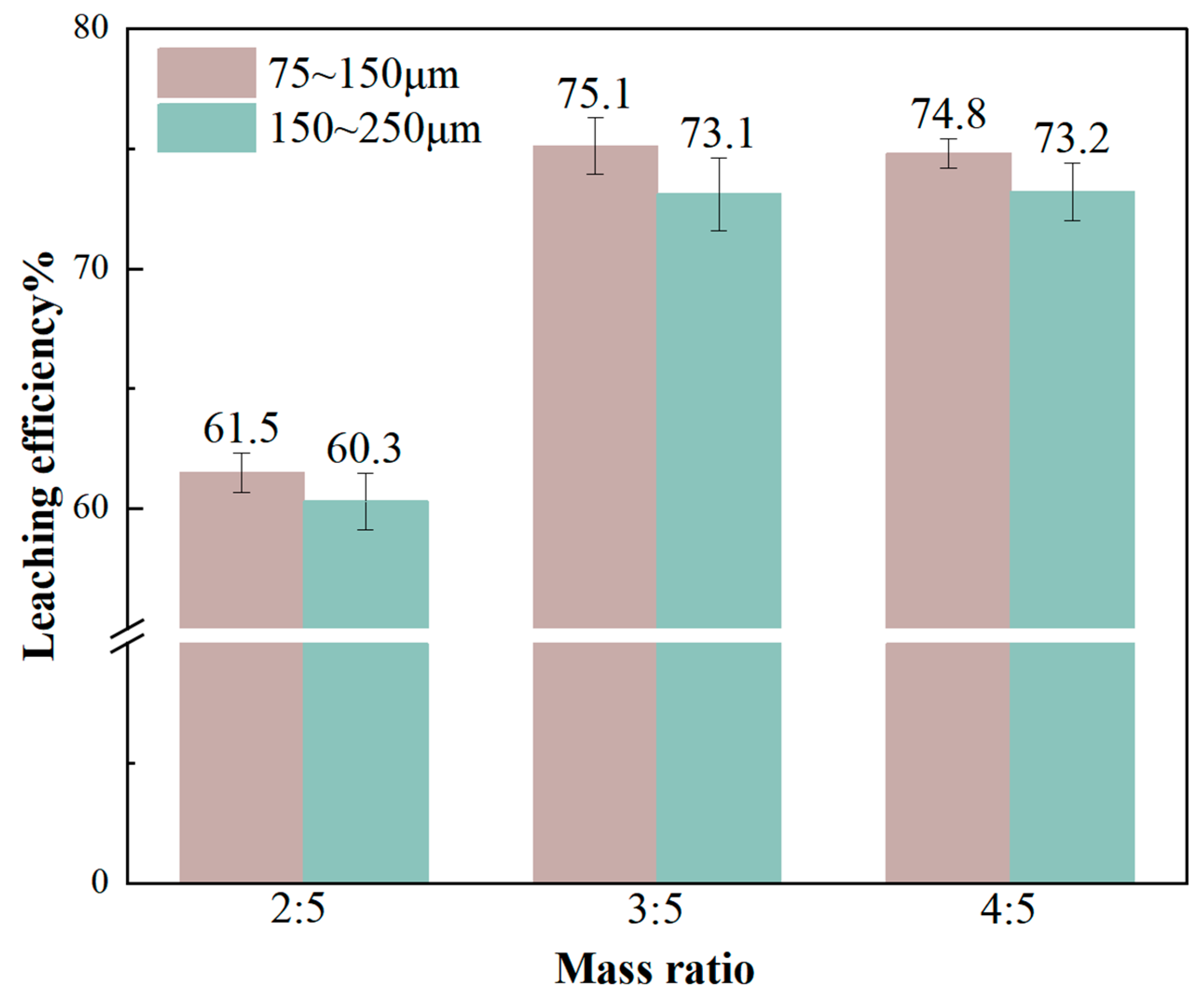
3.3.3. Effects of Raw Material Particle Size and CaCl2 Ratio on Li Leaching Rate
3.3.4. Effect of Leaching Temperature and Leaching Time on the Leaching Rate of Li
3.3.5. Analysis of Leach Residue
4. Conclusions
- (1)
- Thermodynamic calculations are carried out using HSC Chemistry 6.0 thermodynamic software, and the results show that chlorination roasting using CaCl2 with Na2LiAlF6 reacts preferentially at 1630 K to produce LiCl, NaCl, and AlF3, while the decomposition produces LiF at temperatures above 2000 K, which suggests that the addition of CaCl2 reduces the reaction temperature and improves the feasibility of the reaction. Through the experiments, it is shown that CaCl2 reacts with Na2LiAlF6 to produce CaLiAlF6 when the temperature is less than 1473 K. With the increase in the reaction temperature, CaLiAlF6 is transformed into LiCl, which exists in the condensate, which proves that it is feasible to prepare soluble lithium salts using CaCl2.
- (2)
- From the experimental results, it can be seen that reducing the particle size of raw materials, increasing the leaching temperature, and increasing the leaching time are all conducive to improving the leaching rate of Li, but too high a temperature, too long a reaction time, and the refinement of the particle size of the raw materials will increase the cost of recovering lithium, so more appropriate reaction conditions should be selected. The best experimental conditions for the recovery of lithium from lithium-containing aluminum electrolyte by chloride roasting are as follows: the particle size of lithium-containing aluminum electrolyte is 75~150 μm, the mass ratio of calcium chloride to the lithium-containing aluminum electrolyte is 3:5, the reaction temperature is 1473 K, and the holding time is 3 h. The product of the reaction is crushed, and then it is leached using deionized water, the temperature of which is 368 K and the duration of which is 2 h. The solid–liquid ratio is 1/3, the stirring rate is 300 r/min, and the leaching rate of Li under the experimental conditions is as follows. Under these conditions, the leaching rate of Li can reach 75.1%, and the purity of collected AlF3 can reach 92.7%. From the lithium content in the residue of chlorination roasting, it can be seen that 98% of the lithium is converted to a gaseous state after chlorination roasting, and it should exist in the condensate. Theoretically, the leaching rate of lithium should reach more than 95%. The condensation system under the current process conditions is not perfect enough, resulting in part of the lithium chloride vapor easily escaping and being difficult to collect effectively. Therefore, the recovery of lithium can be enhanced in the future by improving the condensation collection system, and the recovery of lithium chloride can also be enhanced by simulating the variation in the thermal field in the furnace chamber to select a suitable condensation interval.
- (3)
- The disadvantage of chlorination roasting with CaCl2 is that the current process conditions for optimal lithium extraction have a low direct yield of Al. This is mainly because the lithium-containing aluminum electrolyte feedstock also contains a large amount of stable alumina, which is not easy to react under neutral conditions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, C.X.; An, H.Y.; Bai, Y.; Liu, G.Q.; Wang, G.L.; Liu, X. Experimental Study on the Removal of Electrolytic Aluminum Slag by Flotation. Non-Met. Mines 2019, 42, 66–68. [Google Scholar] [CrossRef]
- Ishak, R.; Laroche, G.; Lamonier, J.F.; Ziegler, D.P.; Alamdari, H. Characterization of Carbon Anode Protected by Low Boron Level: An Attempt to Understand Carbon–Boron Inhibitor Mechanism. ACS Sustain. Chem. Eng. 2017, 5, 6700–6706. [Google Scholar] [CrossRef]
- Lv, X.; Shuang, Y.; Li, J.; Chen, S.; Lai, Y.; Zhang, H.; Liu, Y. Physicochemical Properties of Industrial Aluminum Electrolytes Enriching Li and K: The Liquidus Temperature. Metall. Mater. Trans. B 2017, 48, 1315–1320. [Google Scholar] [CrossRef]
- Hou, H.J. Brief Discussion of Application of Anhydrous Aluminium Fluoride Contained Lithium. Light Met. 2011, 48, 34–36. [Google Scholar] [CrossRef]
- Gheribi, A.E.; Phan, A.T.; Chartrand, P. Electrochemical Description of the Interfacial Tension between the Liquid Metal Pad and Cryolitic Melts in Industrial Electrolysis Cells. J. Mol. Liq. 2023, 369, 120843. [Google Scholar] [CrossRef]
- Cassayre, L.; Palau, P.; Chamelot, P.; Massot, L. Properties of Low-Temperature Melting Electrolytes for the Aluminum Electrolysis Process: A Review. J. Chem. Eng. Data 2010, 55, 4549–4560. [Google Scholar] [CrossRef]
- Bo, D. Study on Analytical Method of Aluminum Electrolyte. Chem. Eng. Des. Commun. 2021, 47, 59–60. [Google Scholar] [CrossRef]
- Lin, Y.C.; Liu, Y.; Yin, G.; Dang, C.G.; Xiong, R.Y. Research on Fluoride Whole-Process Prevention and Control in the Electrolytic Aluminum Enterprise Based on Material Flow Analysis. J. Environ. Eng. Technol. 2023, 13, 800–807. [Google Scholar] [CrossRef]
- Hao, J.T.; Wen, F.Y.; Li, X. Study on Extracting Lithium from Electrolytic Aluminum Waste Residue. Inorg. Chem. Ind. 2019, 51, 69–71. [Google Scholar] [CrossRef]
- Xu, H.; Fu, C.C.; Xu, R. Extraction Toxicity Identification of Anode Slime and Anode Scrap Produced by Electrolytic Aluminum Enterprises and Recommendations on Their Management. Environ. Monit. China 2015, 31, 22–25. [Google Scholar] [CrossRef]
- Li, Q.; Cui, X.D.; Gao, W.Y. Countermeasures and Origins of Carbon Residue in Aluminum Reduction Production. Light Met. 2015, 52, 36–38. [Google Scholar] [CrossRef]
- Huang, L.H.; Shi, H.L.; Hou, A.Y. Influence of Lithium-Rich Alumina on Production of Aluminum Electrolysis. Nonferrous Met. Des. 2018, 45, 98–100, 110. [Google Scholar] [CrossRef]
- Cao, A.L.; Yao, S.H. Analysis on Enrichment Mechanism of Li in the Aluminum Electrolyte and Its Measures. Light Met. 2017, 54, 27–31. [Google Scholar] [CrossRef]
- Arahman, N.; Mulyati, S.; Lubis, M.R.; Takagi, R.; Matsuyama, H. The Removal of Fluoride from Water Based on Applied Current and Membrane Types in Electrodialyis. J. Fluorine Chem. 2016, 191, 97–102. [Google Scholar] [CrossRef]
- Corral-Capulin, N.G.; Vilchis-Nestor, A.R.; Gutiérrez-Segura, E.; Solache-Ríos, M. The Influence of Chemical and Thermal Treatments on the Fluoride Removal from Water by Three Mineral Structures and Their Characterization. J. Fluor. Chem. 2018, 213, 42–50. [Google Scholar] [CrossRef]
- Yelatontsev, D.; Mukhachev, A. Processing of Lithium Ores: Industrial Technologies and Case Studies—A Review. Hydrometallurgy 2021, 201, 105578. [Google Scholar] [CrossRef]
- Ding, S.H.; Chen, R. Exploration and Practice on Purification of Electrolyte System with High Lithium Potassium Salt by Segregation Method. Nonferrous Met. Des. 2020, 47, 14–18. [Google Scholar] [CrossRef]
- Gao, B.L.; Jiang, Q.W.; Qiu, Z.X.; Zhao, X.L. Phases of Complicated Aluminum Electrolytes. J. Northeast. Univ. (Nat. Sci.) 2002, 23, 28–31. [Google Scholar] [CrossRef]
- Li, D. Phases Analysis and Cryolite Ratio Determination of Aluminum Electrolytes. Master’s Thesis, Northeastern University, Shenyang, China, 2009. [Google Scholar] [CrossRef]
- Han, J.J. Study on Recovery Process of Lithium-Containing Electrolyte Produced by Electrolytic Aluminum Industry. Henan Chem. Ind. 2018, 35, 24–27. [Google Scholar] [CrossRef]
- Mei, X.Y.; Li, J.; Yu, Z.L. The Research on Recycling Carbon Residue by Floatation Process. Light Met. 2016, 53, 28–30. [Google Scholar] [CrossRef]
- Dong, L.M.; Jiao, F.; Liu, W.; Jiang, S.Q.; Wang, H.L. Research Progress of Electrolytic Aluminum Overhaul Slag Disposal. Multipurp. Util. Miner. Resour. 2023, 44, 159–168. [Google Scholar] [CrossRef]
- Tang, C.; Wang, J.; Yang, S.; Zhang, X.; Li, S.; Lai, Y.; Tian, Z.; Jin, S.; Chen, Y. Efficient Extraction and Recovery of Lithium from Waste Aluminum Cryolite Electrolyte. Resour. Conserv. Recycl. 2023, 197, 107070. [Google Scholar] [CrossRef]
- Tang, J.; Chen, X.Q.; Wang, B.; Chen, L.J. Research and Application of Low Temperature Fire Treatment Technology for Electrolytic Aluminum Carbon Slag. World Nonferrous Met. 2021, 36, 134–137. [Google Scholar] [CrossRef]
- Ma, J.L.; Shang, X.F.; Ma, Y.P.; Qiao, P.; Feng, L.; Zhang, W.Q. Directions for Development of Hazardous Waste Treatment Technologies in Electrolytic Aluminum Industry. Environ. Prot. Chem. Ind. 2016, 36, 11–16. [Google Scholar] [CrossRef]
- Li, Z.; Wu, A.H.; Wang, J.C.; Lu, W.Y.; Li, Q.Q.; Zhang, L.S. Research Status and Prospects of Resource Recycling and Harmless Treatment Technology for Aluminum Electrolysis Waste Residue. Met. Mine 2023, 12, 252–262. [Google Scholar] [CrossRef]
- Zhang, N.N.; Wu, M.Q.; Han, R.; Shi, Z.Y.; Li, Z.; Yu, Y.X. Research Progress on Harmless Treatment and Resource Utilization of Electrode Waste Residue from Electrolytic Aluminum Industry. Nonferrous Met. (Extr. Metall.) 2023, 75, 102–110. [Google Scholar] [CrossRef]
- Gao, Y. Current Situation and Development Trend of Hazardous Waste Disposal Technology in Electrolytic Aluminum Industry. Nonferrous Met. Eng. Res. 2019, 40, 33–35. [Google Scholar] [CrossRef]
- Lin, Y.Q. Safety Production Management of Hydrofluoric Acid Using Process. Mod. Chem. Res. 2020, 20, 45–47. [Google Scholar] [CrossRef]
- Hou, J.; Shi, D.; Wang, Z.; Gao, B.; Shi, Z.; Hu, X. Influence of Additives on Bath Analysis in Aluminum Electrolysis. JOM 2017, 69, 2057–2064. [Google Scholar] [CrossRef]
- Ross, K.C.; Mitchell, R.H.; Chakhmouradian, A.R. The Crystal Structure of Synthetic Simmonsite, Na2LiAlF6. J. Solid State Chem. 2003, 172, 95–101. [Google Scholar] [CrossRef]
- Liu, F.Q.; Li, A.J.; Li, R.B.; Yang, X.; Wu, Z.G. Study on Recovery and Utilization of Multiple Elements in Aluminum Electrolyte by Calcified Roasting. Nonferrous Met. (Extr. Metall.) 2021, 73, 67–71. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Cheng, J.Y.; Wang, T.; Han, Q.C. Study on Impurity Removal by Secondary Cryolite Roasting. Gansu Metall. 2023, 45, 78–81. [Google Scholar] [CrossRef]
- Li, J.; Pu, G.; Chen, J.S.; Liu, Q.W. High-Temperature Volatility Characteristics and Pyrolysis Mechanism of Common Sodium Salts. CIESC J. 2020, 71, 3452–3459. [Google Scholar] [CrossRef]
- GB/T4292-2017; Aluminium Fluoride. China Electricity Council (CEC): Beijing, China, 2017.
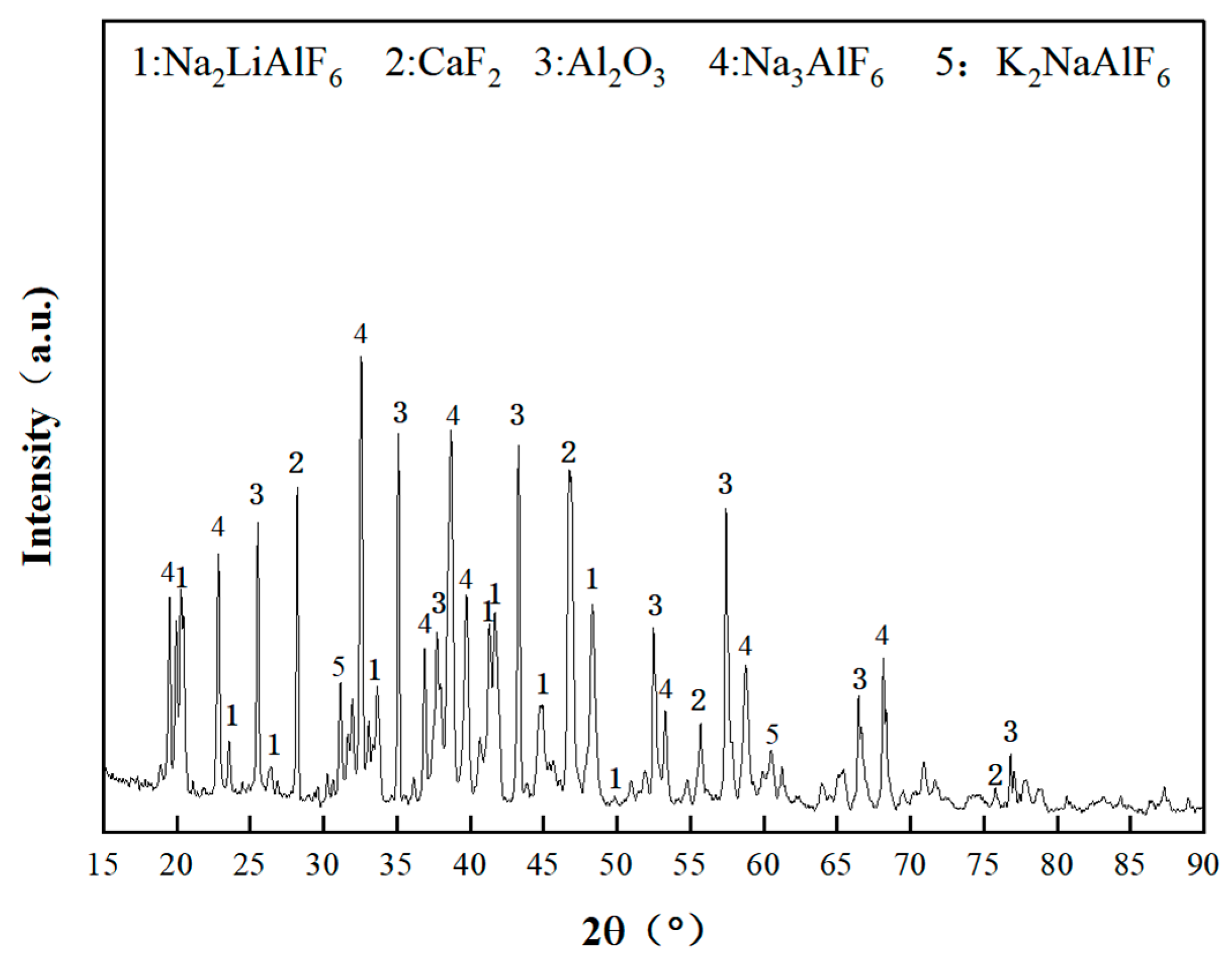


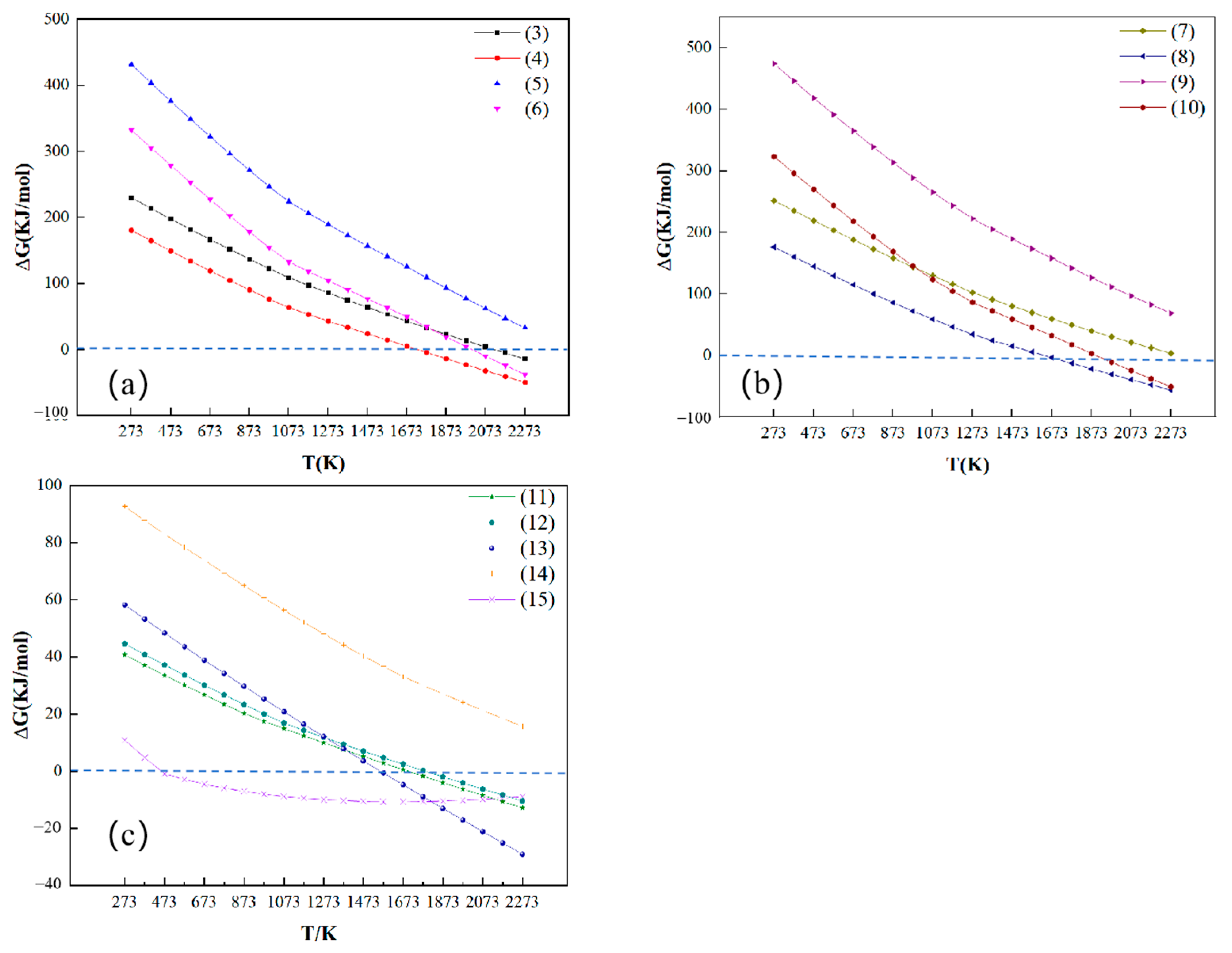

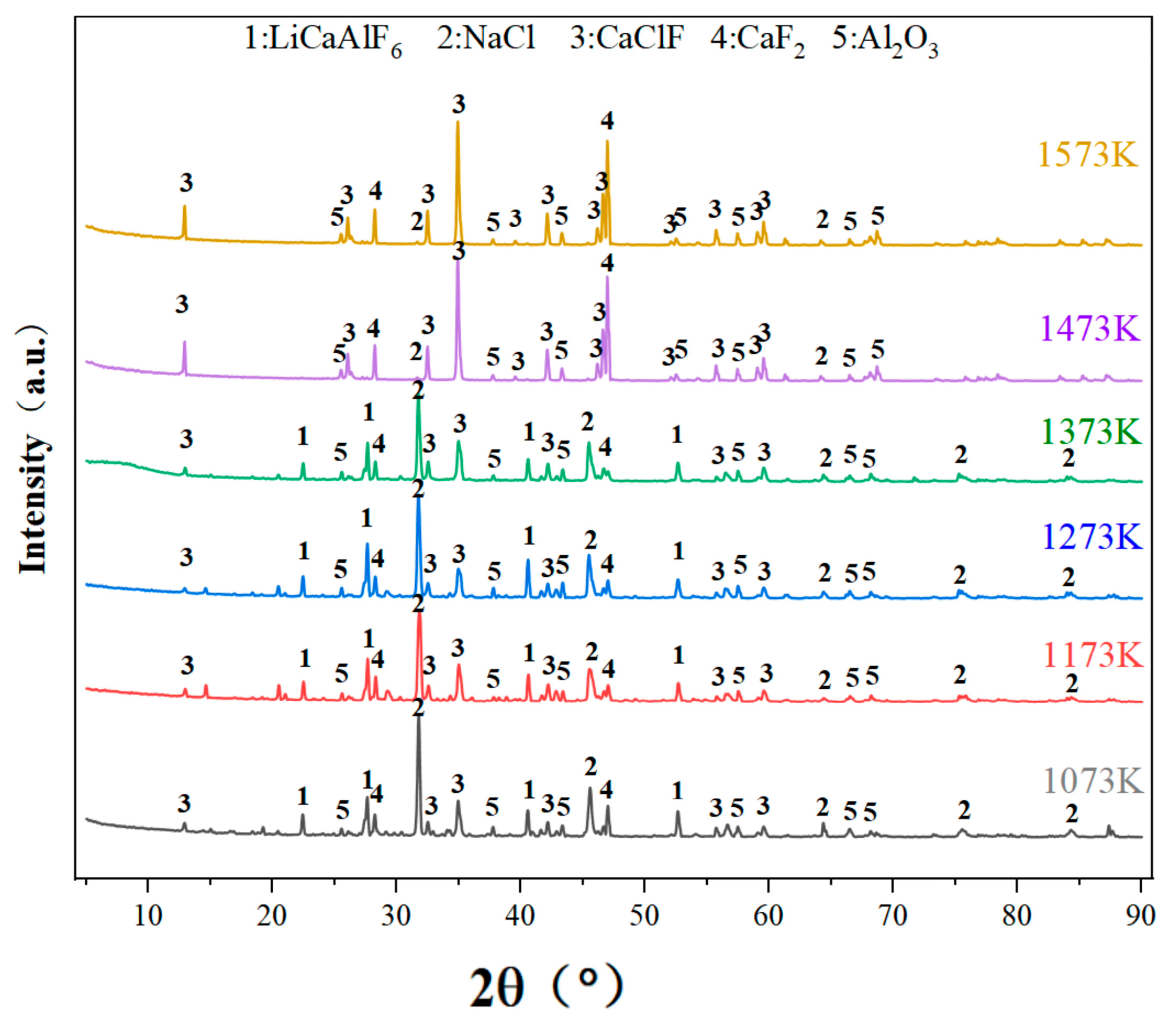
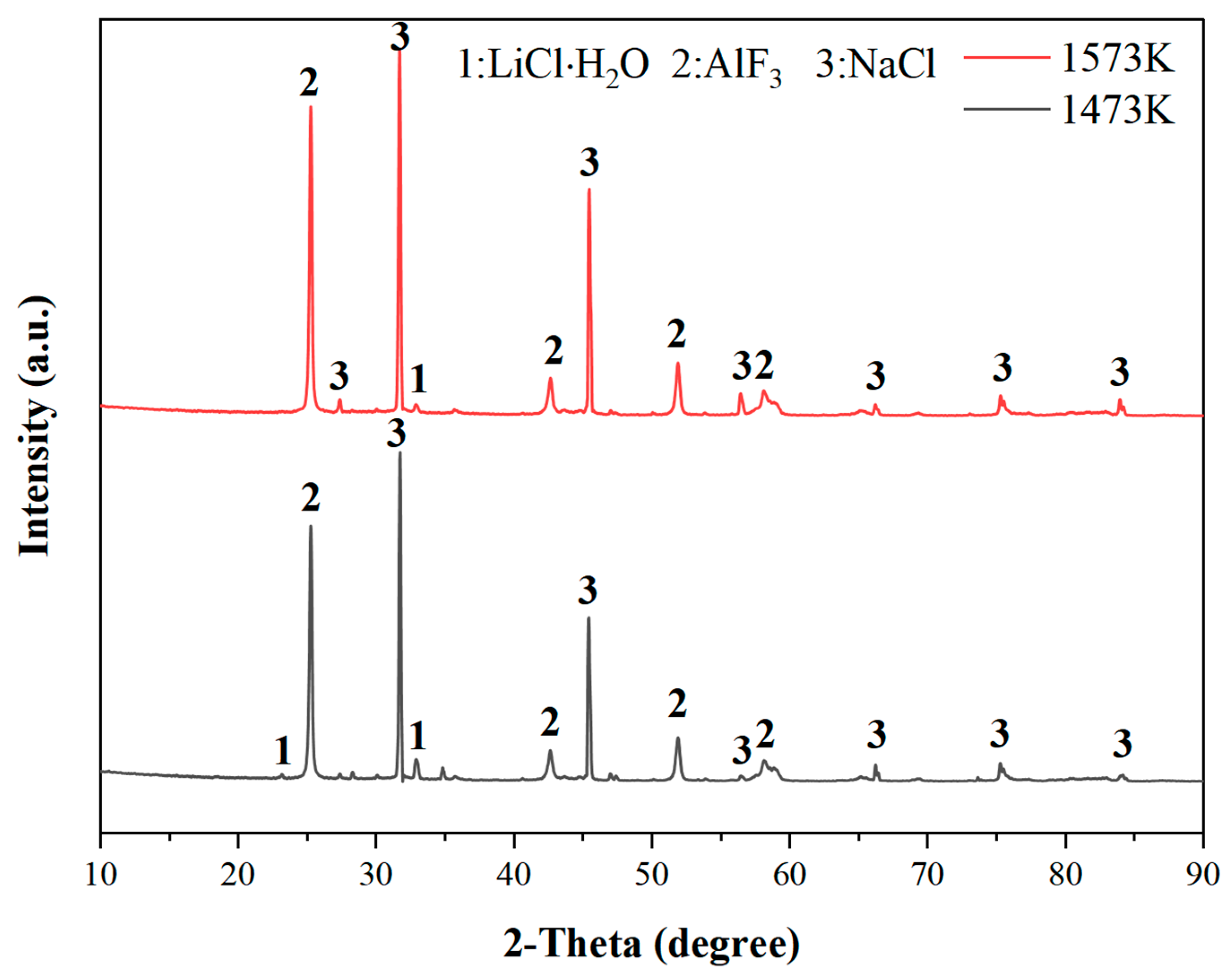


| Element | Al | Na | O | Ca | F | The Rest |
|---|---|---|---|---|---|---|
| content (wt.%) | 21.08 | 19.27 | 8.92 | 2.37 | 45.44 | 2.91 |
| Element | Li | Al | Na | K | Ca |
|---|---|---|---|---|---|
| content (wt.%) | 1.82 | 20.77 | 19.52 | 1.59 | 2.63 |
| Reaction Equation | Initial Reaction Temperature at 1 atm/K |
|---|---|
| (3) | 2117 |
| (4) | 1730 |
| (5) | 2510 |
| (6) | 2007 |
| (7) | 2318 |
| (8) | 1637 |
| (9) | 2819 |
| (10) | 1896 |
| (11) | 1700 |
| (12) | 1787 |
| (13) | 1651 |
| (14) | 2871 |
| (15) | 454 |
| Element | Al | F | Na |
|---|---|---|---|
| content(wt.%) | 31.72 | 63.25 | 0.27 |
| GB/T4292-2017(AF-0) [35] | 31.5 | 61.0 | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, R.; Cui, X.; Zhang, W.; Wang, S.; Yang, M.; Qu, T. Study on Recovery of Lithium from Lithium-Containing Aluminum Electrolyte. Metals 2024, 14, 460. https://doi.org/10.3390/met14040460
Ji R, Cui X, Zhang W, Wang S, Yang M, Qu T. Study on Recovery of Lithium from Lithium-Containing Aluminum Electrolyte. Metals. 2024; 14(4):460. https://doi.org/10.3390/met14040460
Chicago/Turabian StyleJi, Rui, Xi Cui, Wenzheng Zhang, Shichao Wang, Mingliang Yang, and Tao Qu. 2024. "Study on Recovery of Lithium from Lithium-Containing Aluminum Electrolyte" Metals 14, no. 4: 460. https://doi.org/10.3390/met14040460
APA StyleJi, R., Cui, X., Zhang, W., Wang, S., Yang, M., & Qu, T. (2024). Study on Recovery of Lithium from Lithium-Containing Aluminum Electrolyte. Metals, 14(4), 460. https://doi.org/10.3390/met14040460






