Study of Assimilation of Cored Wire into Liquid Steel Baths
Abstract
:1. Introduction
2. Materials and Methods
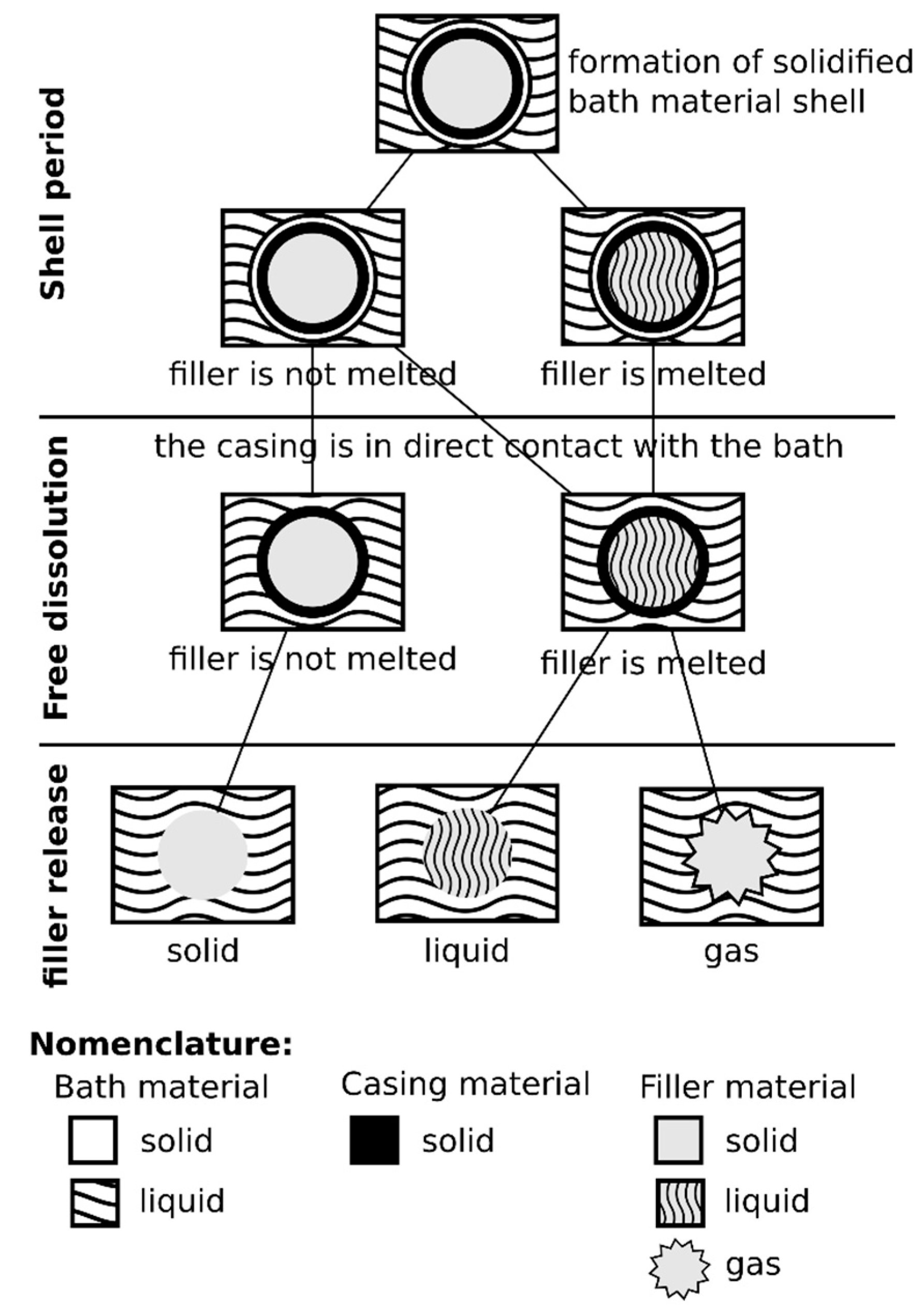
3. Results
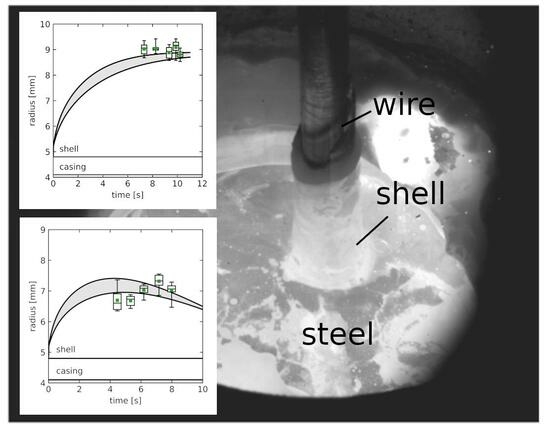
3.1. Estimations of Wire Residence Time in the Bath and Diameter of the Wire + Solidified Metal Shell Ensemble, Post Immersion
3.2. Comparison of Experimental Results and Numerical Simulations
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holappa, L. Secondary Steelmaking. In Treatise on Process Metallurgy, 1st ed.; Seetharaman, S., McLean, A., Guthrie, R., Seetharaman, S., Eds.; Elsevier: Oxford, UK, 2014; Volume 3A, pp. 301–346. [Google Scholar]
- Ren, Y.; Wang, W.; Yang, W.; Zhang, L. Modification of Non-Metallic Inclusions in Steel by Calcium Treatment: A Review. ISIJ Int. 2023, 63, 1927–1940. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, L.; Ren, Y.; Chen, W.; Liu, F. Formation and Prevention of Nozle Clogging during the Continuous Casting of Steels: A Review. ISIJ Int. 2024, 64, 1–20. [Google Scholar] [CrossRef]
- Ototani, T. Calcium Clean Steel, 1st ed.; Springer: Berlin, Germany, 1986; pp. 20–33. [Google Scholar]
- Kor, G.J.W.; Glaws, P.C. Ladle Refining and Vacuum Degassing. In The Making, Shaping and Treating of Steel, 11th ed.; Fruehan, R.J., Ed.; AISE Steel Foundation: Warrendale, PA, USA, 1998; Steelmaking and Refining Volume; pp. 661–713. [Google Scholar]
- Argyropoulos, S.A.; Li, S. Kinetics of Assimilation of Additions in Liquid Metals. In Treatise on Process Metallurgy, 1st ed.; Seetharaman, S., McLean, A., Guthrie, R., Seetharaman, S., Eds.; Elsevier: Oxford, UK, 2014; Volume 2, pp. 359–426. [Google Scholar]
- Brabie, L.C.; Kawakami, M. Kinetics of Steel Scrap Melting in Molten Fe-C Bath. High. Temp. Mater. Process 2000, 19, 241–256. [Google Scholar] [CrossRef]
- Penz, F.M.; Schenk, J. A Review of Steel Scrap Melting in Molten Iron-Carbon Melts. Steel Res. Int. 2019, 90, 1900124. [Google Scholar] [CrossRef]
- Szekely, J.; Chuang, Y.K.; Hlinka, J.W. The Melting and Dissolution of Low-Carbon Steels in Iron-Carbon Melts. Metall. Trans. 1972, 3, 2825–3328. [Google Scholar] [CrossRef]
- Kim, Y.U.; Pehlke, R.D. Mass Transfer During Dissolution of a Solid into Liquid in the Iron-Carbon System. Metall. Trans. 1974, 5, 2527–2532. [Google Scholar] [CrossRef]
- Kim, Y.U.; Pehlke, R.D. Transient Heat Transfer During Initial Stages of Steel Scrap Melting. Metall. Trans. B 1975, 6, 585–591. [Google Scholar] [CrossRef]
- Wright, J.K. Steel Dissolution in Quiescent and Gas Stirred Fe/C Melts. Metall. Trans. B 1989, 20, 363–374. [Google Scholar] [CrossRef]
- Isobe, K.; Maede, H.; Ozawa, K.; Umezawa, K.; Saito, C. Analysis of the Scrap Melting in High Carbon Molten Iron. Tetsu-To-Hagane 1990, 76, 2033–2040. [Google Scholar] [CrossRef]
- Kawakami, M.; Takatani, K.; Brabie, L.C. Heat and Mass Transfer Analysis of Scrap Melting in Steel Bath. Tetsu-To-Hagane 1999, 85, 658–665. [Google Scholar] [CrossRef]
- Yamamura, H.; Mizukami, Y.; Ueshima, Y.; Miyazawa, K. Effect of Molten Steel Flow on Nonuniformity of Initially Solidified Shell. High. Temp. Mater. Process 2000, 19, 231–240. [Google Scholar] [CrossRef]
- Wei, G.; Zhu, R.; Tang, T.; Dong, K. Study on the Melting Characteristics of Steel Scrap in Molten Steel. Ironmak. Steelmak. 2019, 46, 609–617. [Google Scholar] [CrossRef]
- Xi, X.; Li, S.; Yang, S.; Zhao, M.; Li, J. Melting Characteristics of Steel Scrap with Different Carbon Contents in Liquid Steel. Ironmak. Steelmak. 2020, 47, 1087–1099. [Google Scholar] [CrossRef]
- Gao, M.; Gao, J.T.; Zhang, Y.L. Evaluation of Mass Transfer Coefficient during Scrap Melting. Metals 2021, 11, 1368. [Google Scholar] [CrossRef]
- Li, J.; Provatas, N. Kinetics of Scrap Melting in Liquid Steel: Multipiece Scrap Melting. Metall. Mater. Trans. B 2008, 39, 268–279. [Google Scholar] [CrossRef]
- Xi, X.; Chen, S.; Yang, S.; Ye, M.; Li, J. Melting Characteristics of Multipiece Steel Scrap in Liquid Steel. ISIJ Int. 2021, 61, 190–199. [Google Scholar] [CrossRef]
- Arzpeyma, N.; Windlund, O.; Ersson, M.; Jönsson, P. Mathematical Modeling of Scrap Melting in an EAF Using Electromagnetic Stirring. ISIJ Int. 2013, 53, 48–55. [Google Scholar] [CrossRef]
- Kruskopf, A. A Model for Scrap Melting in Steel Converter. Metall. Mater. Trans. B 2015, 46, 1195–1206. [Google Scholar] [CrossRef]
- Deng, S.; Xu, A.; Yang, G.; Wang, H. Analyses and Calculation of Steel Scrap Melting in a Multifunctional Hot Metal Ladle. Steel Res. Int. 2019, 90, 1800435. [Google Scholar] [CrossRef]
- Chen, Y.; Ryan, S.; Silaen, A.K.; Zhou, C.Q. Simulation of Scrap Melting Process in an AC Electric Arc Furnace: CFD Model Development and Experimental Validation. Metall. Mater. Trans. B 2022, 53, 2675–2694. [Google Scholar] [CrossRef]
- Chen, Y.; Ryan, S.; Silaen, A.K.; Zhou, C.Q. An Investigation into EAF Burner Preheating and Melting Characteristics: CFD Model Development and Experimental Validation. Metall. Mater. Trans. B 2023, 54, 1068–1087. [Google Scholar] [CrossRef]
- Singha, P. Scrap Dissolution effect in BOF Converter Process. Ironmak. Steelmak. 2023, 50, 1434–1442. [Google Scholar] [CrossRef]
- Zhang, L.; Oeters, F. Melting and Mixing of Alloying Agents in Steel Melts: Methods of Mathematical Modeling, 2nd ed.; Verlag Stahleisen GmbH: Dusseldorf, Germany, 2006; pp. 1–59. [Google Scholar]
- Wang, Y.; Karasev, A.; Park, J.H.; Jönsson, P.G. Non-metallic Inclusions in Different Ferroalloys and Their Effect on the Steel Quality: A Review. Metall. Mater. Trans. B 2021, 52, 2892–2925. [Google Scholar] [CrossRef]
- Wang, Y.; Karasev, A.; Jönsson, P.G. Characterization of Nonmetallic Inclusions in Different Ferroalloys used in the Steelmaking Processes. Steel Res. Int. 2021, 92, 2100269. [Google Scholar] [CrossRef]
- Bannenberg, K.; Harste, K.; Bode, O. Aufschmelzverhalten von Fülldrähten Während des Einspulens. Stahl. Eisen 1992, 12, 101–107. [Google Scholar]
- Du Terrail, Y.; Fautrelle, Y.; Rebiere, M.; Quemener, O. Predictions of Melting or Dissolution Times with a 1D Heat Transfer Simulation Package. Trans. Eng. Sci. 1994, 5, 209–216. [Google Scholar]
- Sanyal, S.; Chandra, S.; Kumar, S.; Roy, G.G. An Improved Model of Cored Wire Injection in Steel Melts. ISIJ Int. 2004, 44, 1157–1166. [Google Scholar] [CrossRef]
- Sanyal, S.; Chandra, S.; Kumar, S.; Roy, G.G. Dissolution Kinetics of Cored Wire in Molten Steel. Steel Res. Int. 2006, 77, 542–549. [Google Scholar] [CrossRef]
- Castro-Cedeno, E.I.; Jardy, A.; Carré, A.; Gerardin, S.; Bellot, J.P. A Thermal Model of Cored Wire Injection. In Proceedings of the Liquid Metal Processing and Casting Conference LMPC 2017, Philadelphia, PA, USA, 10–13 September 2017. [Google Scholar]
- Castro-Cedeno, E.I.; Jardy, A.; Carré, A.; Gerardin, S.; Bellot, J.P. Thermal Modeling of the Injection of Standard and Thermally Insulated Cored Wire. Metall. Mater. Trans. B 2017, 48, 3316–3328. [Google Scholar] [CrossRef]
- Castro-Cedeno, E.I.; Carré, A.; Lujan, J. Advances in Cored Wire Injection and Calcium Treatment in Steelmaking. In Proceedings of the Iron & Steel Technology Conference, AISTech 2022, Pittsburgh, PA, USA, 18 May 2022. [Google Scholar]
- Huang, H.G.; Yan, M.; Sun, J.N.; Du, F.S. Heat transfer of Calcium Cored Wire and CFD Simulation on Flow and Mixing Efficiency in the Argon-Stirred Ladle. Ironmak. Steelmak. 2017, 45, 626–634. [Google Scholar] [CrossRef]
- Jingang, L.; Shuomeng, S.; Weihua, W.; Zhanjun, L.; Rensheng, C.; Ning, H. Study on Heat Transfer Mechanisms of Pure Calcium Cored Wire in Molten Steel by Feeding Rate. Metal. Ital. 2019, 111, 13–19. [Google Scholar]
- Guo, Q.; Chen, M.; Xu, L. Heat Transfer and Melting Characteristics of Calcium-Cored Wire in Molten Steel. Steel Res. Int. 2023, 95, 2300249. [Google Scholar] [CrossRef]
- Wang, H.; Shi, Y.; Su, J. An Improved Simulation Modelling on the Heat Transfer Process of Wire-Feeding Spheroidization in Iron Melt. Ironmak. Steelmak. 2023, 50, 392–401. [Google Scholar] [CrossRef]
- Mucciardi, F.A. Heat Flow to Cylinders Submerged in Liquid Metal Baths. Master’s Thesis, McGill University, Montreal, QC, Canada, 1977. [Google Scholar]
- Argyropoulos, S.A. The Kinetics of Ferro-Alloy Solution in Liquid Steel. Master’s Thesis, McGill University, Montreal, QC, Canada, 1977. [Google Scholar]
- Argyropoulos, S.A. Dissolution of High Melting Point Additions in Liquid Steel. Ph.D. Thesis, McGill University, Montreal, QC, Canada, 1981. [Google Scholar]
- Geng, R.; Li, J.; Shi, C. Evolution of Inclusions with Ce Addition and Ca Treatment in Al-killed Steel during RH Refining Process. ISIJ Int. 2021, 61, 1506–1513. [Google Scholar] [CrossRef]
- Liu, C.; Kacar, Y.; Webler, B.; Pistorius, P.C. Chemical Composition Modification of Inclusions in Steels by Controlled Ca Treatment. Metall. Mater. Trans. B 2021, 52, 2837–2841. [Google Scholar] [CrossRef]
- Sun, H.; Yang, J. Evolution Behaviour and Modification Mechanism of Inclusions in NM500 Wear-Resistant Steel with Calcium Treatment. Ironmak. Steelmak. 2022, 49, 795–812. [Google Scholar] [CrossRef]
- Castro-Cedeno, E.I.; Carré, A.; Bustos-Canairca, A.A. Calcium addition cleanliness and its potential effects on steel quality. In Proceedings of the 52 Seminário de Acieria, Fundicao e Metalurgia de Nao-Ferrosos, ABM Week 2023, Sao Paolo, Brasil, 3 August 2023. [Google Scholar]
- Zhang, T.; Cheng, G.; Yang, X.; Chen, X.; Pan, J.; Li, J.; Huang, Y.; Li, S. Evolution Mechanism of Ca-Containing Inclusions in 20Mn23AlV High-Manganese Non-Magnetic Steel During the Refining Process. Ironmak. Steelmak. 2023, 50, 266–272. [Google Scholar] [CrossRef]
- Xie, Y.; Meng, X.; Deng, X.; Li, S. Evolution of Sulphide Inclusion in Mg-Ca Treating Gear Steel. Ironmak. Steelmak. 2023, 50, 592–598. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, W.; Zhi, J.; Su, C.; Zhang, J. Study on the Formation Mechanism of Clogging Layer of Rare Earth Microalloyed Q355 Steel’s Submerged Entry Nozzle and Process Optimization. Ironmak. Steelmak. 2023, 50, 782–793. [Google Scholar] [CrossRef]
- Chen, Z.; Pu, G.; Cai, B.; He, S.; Du, W.; Huang, H.; Chen, Y. Evolution mechanism of inclusions during refining and continuous casting process of 321 H stainless steel. Ironmak. Steelmak. 2023, 50, 837–847. [Google Scholar] [CrossRef]
- Li, X.; Wang, N.; Chen, M.; Du, Z. Effect of Molten Steel Composition on Inclusion Modification by Calcium Treatment in Al-Killed Tinplate Steel. ISIJ Int. 2023, 63, 303–312. [Google Scholar] [CrossRef]
- Zhong, H.; Jian, M.; Wang, Z.; Zhen, X.; Zhan, H.; Li, T.; Wang, X. Formation and Evolution of Inclusions in AH36 Steel During LF-RH-CC Process: The Influences of Ca-Treatment, Reoxidation, and Solidification. Metall. Mater. Trans. B 2023, 54, 593–601. [Google Scholar] [CrossRef]
- Zhou, Q.; Ba, J.; Chen, W.; Zhang, L. Evolution of Non-Metallic Inclusions in a 303-ton Calcium-Treated Heavy Ingot. Metall. Mater. Trans. B 2023, 54, 1565–1581. [Google Scholar] [CrossRef]
- Samiraj, A.R.; Haidar, S.K.; Pande, M.M.; Hazra, S.S. Optimizing Ferrotitanium Wire Injection Parameters for Improving Titanium Recovery in Ladle Furnace Steelmaking. Steel Res. Int. 2024, in press. [CrossRef]








| Material | C | Mn | Si | P | S | O | Fe |
|---|---|---|---|---|---|---|---|
| Wire filler | 0.003 | 0.114 | Bal | ||||
| Wire casing | 0.07 | 0.25 | 0.015 | 0.01 | 0.009 | Bal | |
| Steel bath | <0.002 | 0.03 | <0.001 | <0.005 | <0.01 | Bal |
| Wire | Tbath,ini | ΔTini |
|---|---|---|
| I | 1818 K (1545 °C) | 11 K |
| II | 1823 K (1550 °C) | 16 K |
| III | 1823 K (1550 °C) | 16 K |
| IV | 1853 K (1580 °C) | 46 K |
| V | 1858 K (1585 °C) | 51 K |
| VI | 1873 K (1600 °C) | 66 K |
| Wire | ttot(x) | x | Rmean | Rstdev | Rmin | RQ1 | RQ2 | RQ3 | Rmax |
|---|---|---|---|---|---|---|---|---|---|
| I (1818 K) | 7.32 | 38.5 | 9.02 | 0.23 | 8.68 | 8.83 | 9.06 | 9.18 | 9.35 |
| 8.26 | 48 | 9.04 | 0.16 | 8.85 | 8.98 | 9.02 | 9.08 | 9.42 | |
| 9.34 | 59 | 8.89 | 0.24 | 8.58 | 8.67 | 8.93 | 9.08 | 9.20 | |
| 9.88 | 64.5 | 9.09 | 0.27 | 8.58 | 8.97 | 9.17 | 9.29 | 9.42 | |
| 10.22 | 68 | 8.81 | 0.17 | 8.54 | 8.71 | 8.82 | 8.90 | 9.06 | |
| II (1823 K) | 4.8 | 33 | 7.94 | 0.24 | 7.44 | 7.84 | 7.95 | 8.01 | 8.29 |
| 5.8 | 43.5 | 8.36 | 0.23 | 7.98 | 8.24 | 8.33 | 8.48 | 8.79 | |
| 6.8 | 54 | 8.6 | 0.22 | 8.42 | 8.46 | 8.50 | 8.79 | 8.97 | |
| 7.94 | 66 | 7.87 | 0.16 | 7.62 | 7.74 | 7.92 | 7.98 | 8.07 | |
| 8.89 | 76 | 8.12 | 0.23 | 7.85 | 7.95 | 8.10 | 8.24 | 8.52 | |
| III (1823 K) | 10.69 | 16.5 | 7.64 | 0.14 | 7.51 | 7.51 | 7.57 | 7.77 | 7.83 |
| 11.45 | 26 | 8.1 | 0.11 | 7.94 | 8.01 | 8.12 | 8.17 | 8.28 | |
| 12.37 | 37.5 | 8.21 | 0.21 | 7.84 | 8.09 | 8.17 | 8.35 | 8.62 | |
| 13.52 | 52 | 8.59 | 0.26 | 8.22 | 8.37 | 8.57 | 8.83 | 8.92 | |
| 14.64 | 66 | 8.09 | 0.37 | 7.57 | 7.73 | 8.16 | 8.44 | 8.49 | |
| IV (1853 K) | 4.46 | 23 | 6.7 | 0.34 | 6.35 | 6.40 | 6.60 | 6.91 | 7.36 |
| 5.32 | 33 | 6.68 | 0.17 | 6.43 | 6.53 | 6.73 | 6.82 | 6.88 | |
| 6.18 | 44 | 7.03 | 0.18 | 6.70 | 6.95 | 7.09 | 7.16 | 7.23 | |
| 7.16 | 54 | 7.32 | 0.22 | 6.86 | 7.19 | 7.33 | 7.51 | 7.55 | |
| 7.98 | 69 | 7.03 | 0.24 | 6.47 | 6.97 | 7.07 | 7.17 | 7.29 | |
| V (1858 K) | 5.81 | 15 | 6.25 | 0.15 | 5.98 | 6.22 | 6.26 | 6.36 | 6.45 |
| 6.22 | 25.5 | 6.65 | 0.3 | 6.32 | 6.42 | 6.58 | 6.79 | 7.22 | |
| 6.71 | 38 | 7.02 | 0.15 | 6.79 | 6.92 | 6.98 | 7.23 | 7.23 | |
| 7.31 | 53.5 | 7 | 0.38 | 6.38 | 6.73 | 7.05 | 7.23 | 7.53 | |
| 7.71 | 63.5 | 6.75 | 0.33 | 6.34 | 6.45 | 6.78 | 6.95 | 7.38 | |
| VI (1873 K) | 8.08 | 13.5 | 5.42 | 0.36 | 5.02 | 5.13 | 5.33 | 5.75 | 5.90 |
| 9.24 | 23 | 5.24 | 0.24 | 4.90 | 5.02 | 5.30 | 5.41 | 5.57 | |
| 10.27 | 31.5 | 5.19 | 0.16 | 4.81 | 5.14 | 5.17 | 5.32 | 5.38 | |
| 11.37 | 40.5 | 4.56 | 0.27 | 4.13 | 4.36 | 4.58 | 4.82 | 4.84 | |
| 12.22 | 47.5 | 4.65 | 0.2 | 4.28 | 4.60 | 4.70 | 4.78 | 4.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Cedeno, E.-I.; Jourdan, J.; Martens, J.; Bellot, J.-P.; Jardy, A. Study of Assimilation of Cored Wire into Liquid Steel Baths. Metals 2024, 14, 462. https://doi.org/10.3390/met14040462
Castro-Cedeno E-I, Jourdan J, Martens J, Bellot J-P, Jardy A. Study of Assimilation of Cored Wire into Liquid Steel Baths. Metals. 2024; 14(4):462. https://doi.org/10.3390/met14040462
Chicago/Turabian StyleCastro-Cedeno, Edgar-Ivan, Julien Jourdan, Jonathan Martens, Jean-Pierre Bellot, and Alain Jardy. 2024. "Study of Assimilation of Cored Wire into Liquid Steel Baths" Metals 14, no. 4: 462. https://doi.org/10.3390/met14040462
APA StyleCastro-Cedeno, E.-I., Jourdan, J., Martens, J., Bellot, J.-P., & Jardy, A. (2024). Study of Assimilation of Cored Wire into Liquid Steel Baths. Metals, 14(4), 462. https://doi.org/10.3390/met14040462