Orientation Relationship of the Intergrowth Al13Fe3 and Al13Fe4 Intermetallics Determined by Single-Crystal X-ray Diffraction
Abstract
:1. Introduction
2. Materials and Methods
3. Results
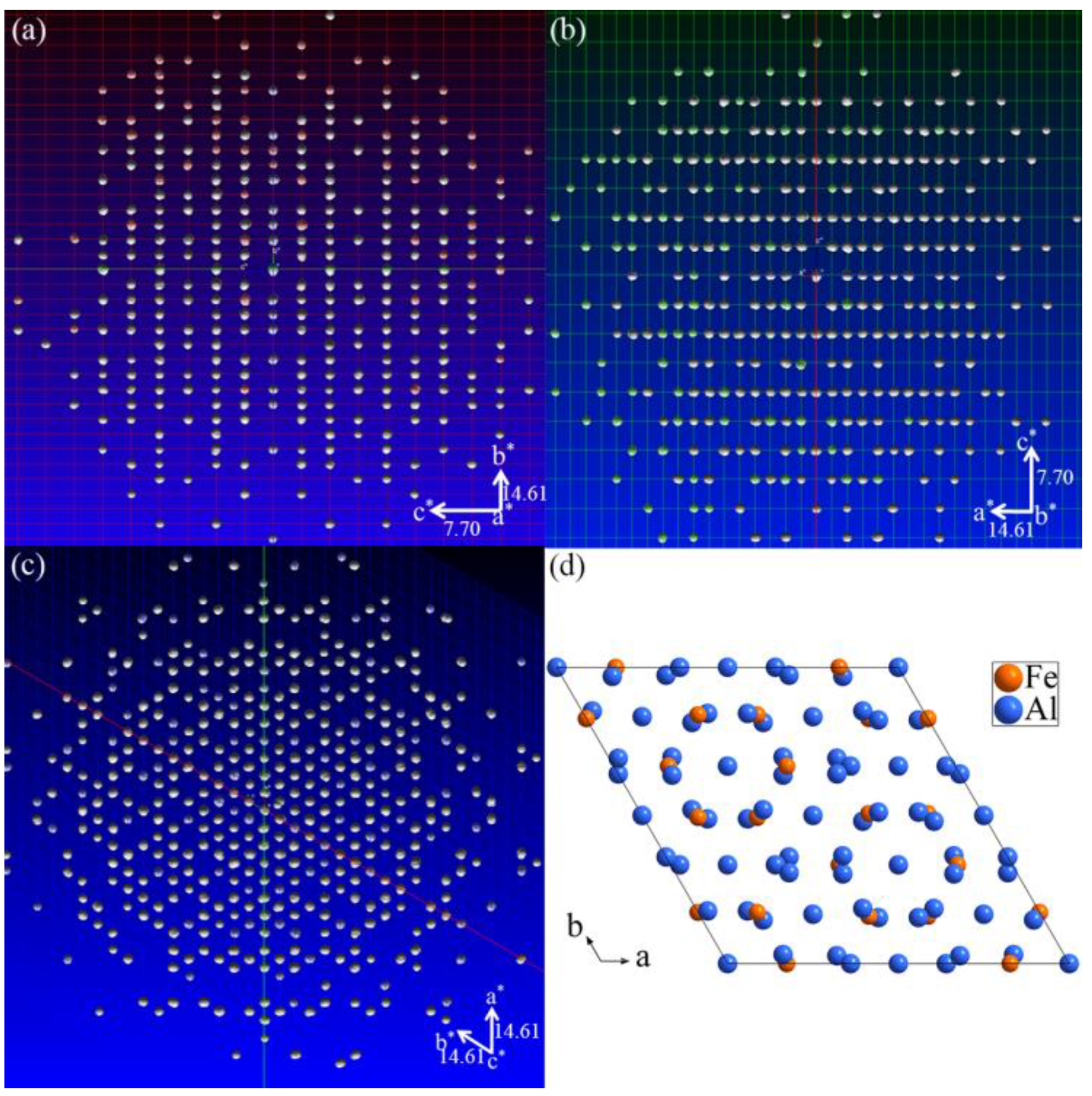
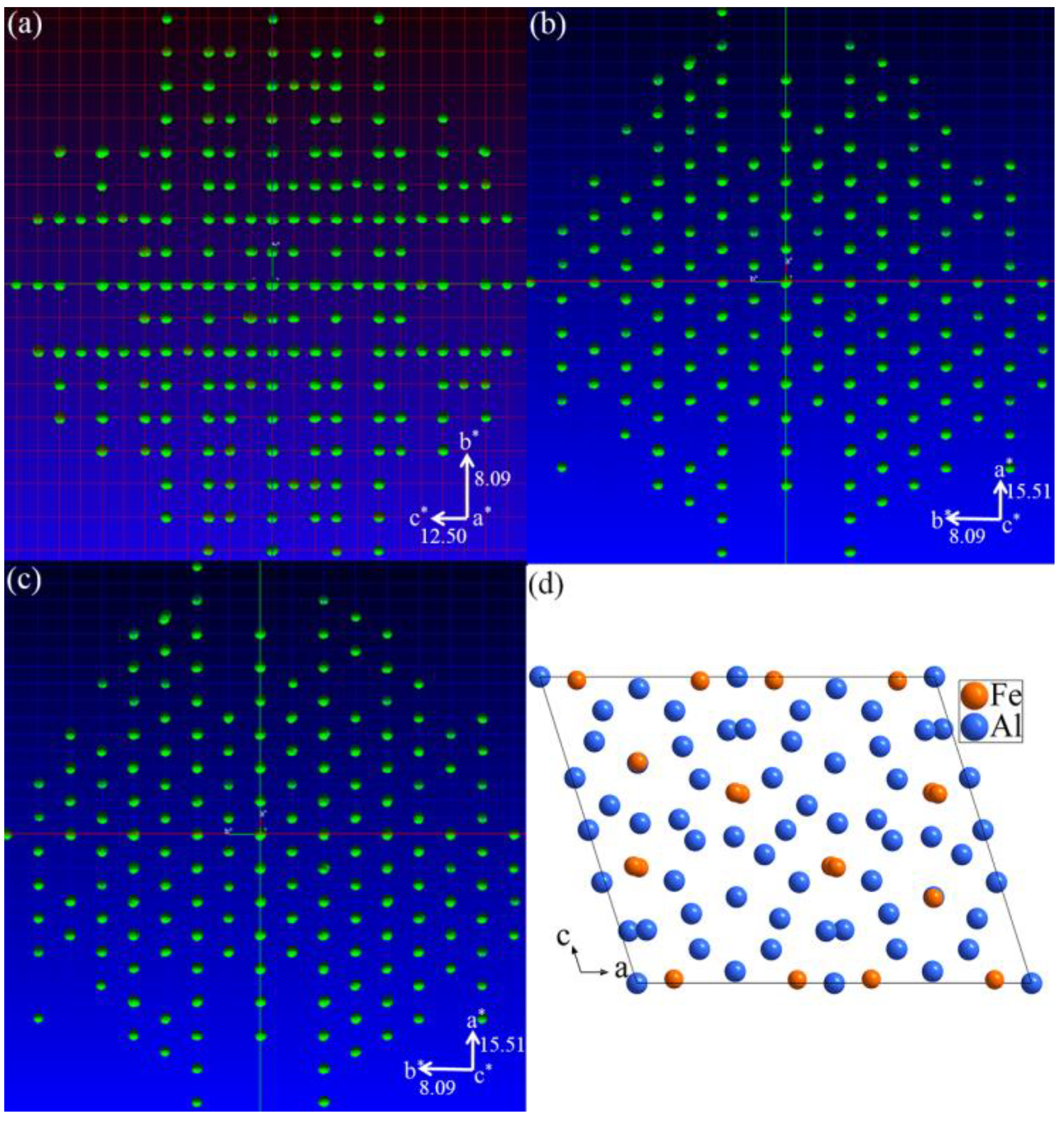
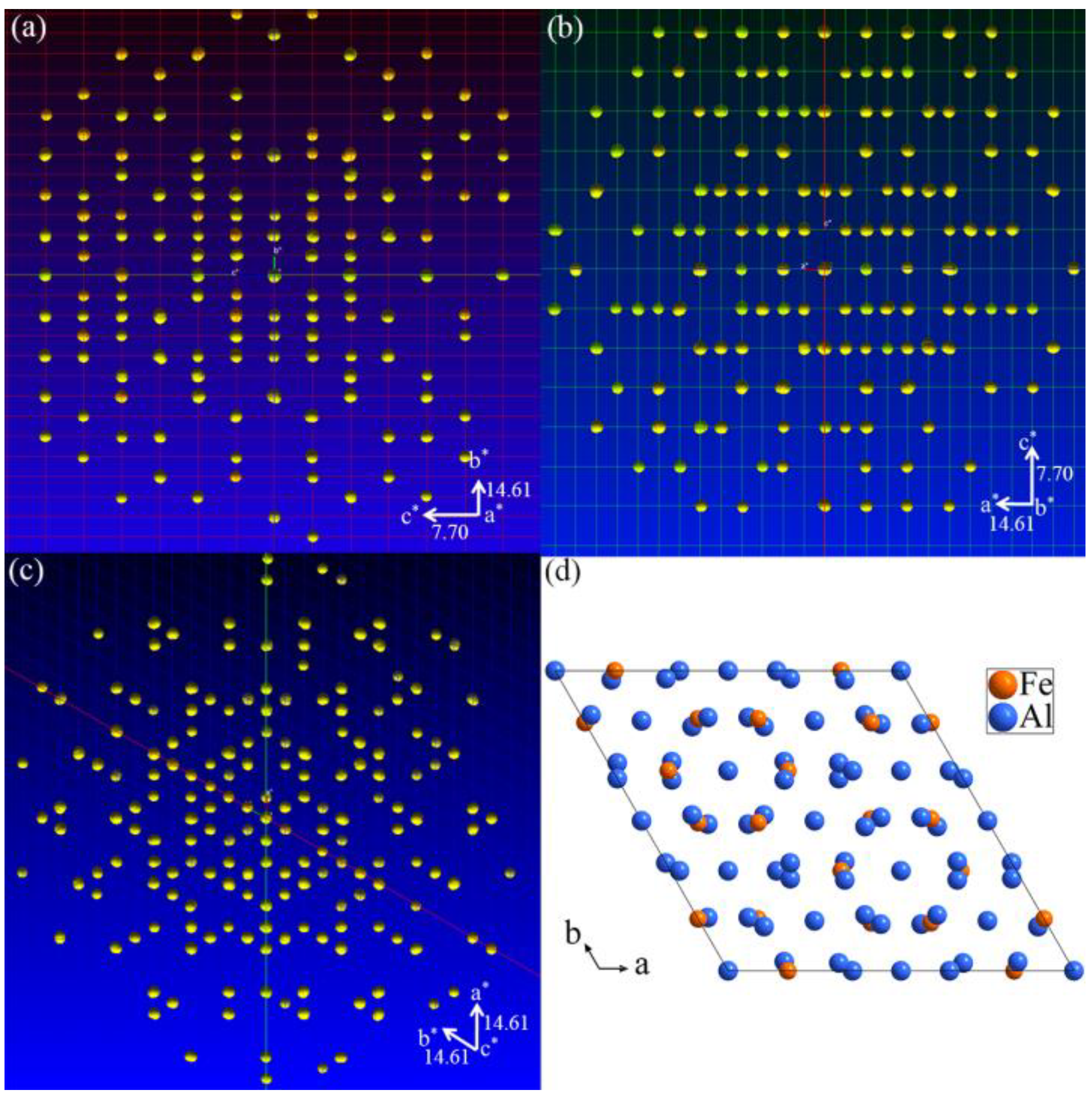
3.1. Single-Crystal XRD Patterns
3.2. Crystal Structure Refinement of Al13Fe3 Phase and Al13Fe4 Phase
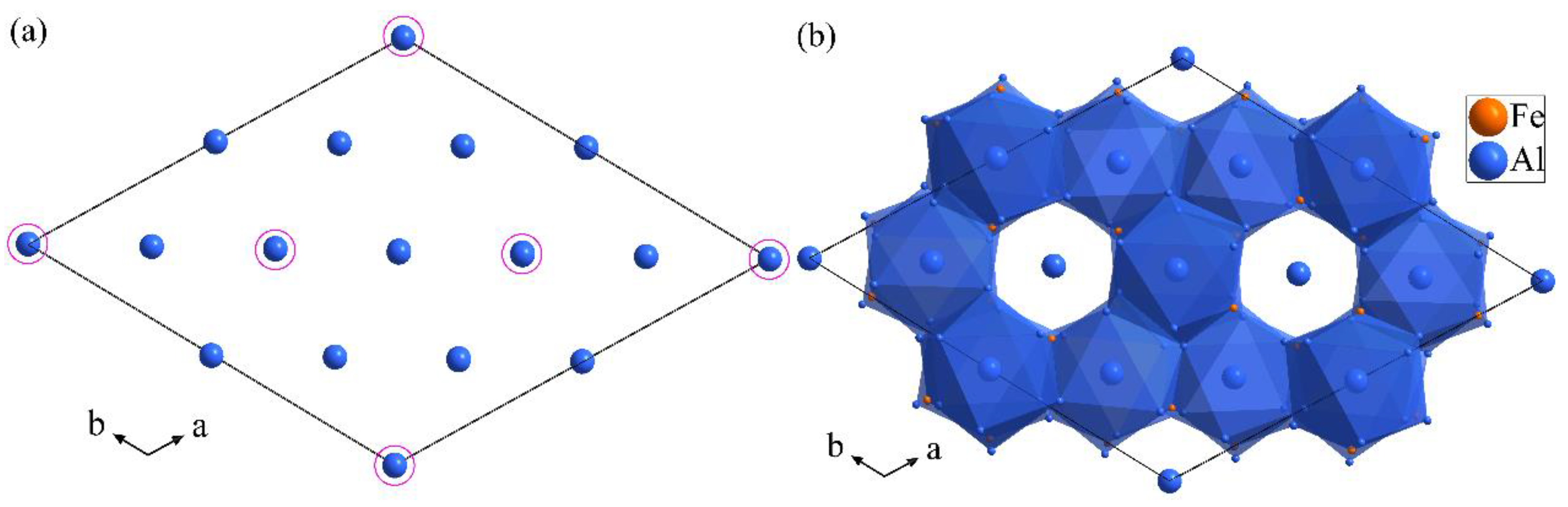
3.3. Structure Models of the Al13Fe3 and Al13Fe4 Intergrowth Phases in Real Space
3.4. Interfaces between Al13Fe3 Phase and Al13Fe4 Phases
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Tcherdyntsev, V.V.; Kaloshkin, S.D.; Gunderov, D.V.; Afonina, E.A.; Brodova, I.G.; Stolyarov, V.V.; Baldokhin, Y.V.; Shelekhov, E.V.; Tomilin, I.A. Phase composition and microhardness of rapidly quenched Al–Fe alloys after high pressure torsion deformation. Mater. Sci. Eng. A 2004, 375, 888–893. [Google Scholar] [CrossRef]
- Sasaki, T.T.; Ohkubo, T.; Hono, K. Microstructure and mechanical properties of bulk nanocrystalline Al–Fe alloy processed by mechanical alloying and spark plasma sintering. Acta Mater. 2009, 57, 3529–3538. [Google Scholar] [CrossRef]
- Goulart, P.R.; Spinelli, J.E.; Cheung, N.; Garcia, A. The effects of cell spacing and distribution of intermetallic fibers on the mechanical properties of hypoeutectic Al–Fe alloys. Mater. Chem. Phys. 2010, 119, 272–278. [Google Scholar] [CrossRef]
- Akinlade, O.; Singh, R.N.; Sommer, F. Thermodynamics of liquid Al–Fe alloys. J. Alloys Compd. 2000, 299, 163–168. [Google Scholar] [CrossRef]
- Pariona, M.M.; Teleginski, V.; Santos, K.D.; Machado, S.; Zara, A.J.; Zurba, N.K.; Riva, R. Yb-fiber laser beam effects on the surface modification of Al–Fe aerospace alloy obtaining weld filet structures, low fine porosity and corrosion resistance. Surf. Coat. Technol. 2012, 206, 2293–2301. [Google Scholar] [CrossRef]
- Staley, J.T.; Liu, J.; Hunt, W.H., Jr. Aluminum alloys for aerostructures. Adv. Mater. Process. 1997, 152, 17–21. [Google Scholar]
- Zhou, B.; Liu, B.; Zhang, S. The advancement of 7xxx series aluminum alloys for aircraft structures: A review. Metals 2021, 11, 718. [Google Scholar] [CrossRef]
- Pariona, M.M.; Teleginski, V.; dos Santos, K.; dos Santos, E.L.R.; Riva, R. AFM study of the effects of laser surface remelting on the morphology of Al–Fe aerospace alloys. Mater Charact. 2012, 74, 64–76. [Google Scholar] [CrossRef]
- Pariona, M.M.; Teleginski, V.; dos Santos, K.; de Lima, A.A.; Zara, A.J.; Micene, K.T.; Riva, R. Influence of laser surface treated on the characterization and corrosion behavior of Al–Fe aerospace alloys. Appl. Surf. Sci. 2013, 276, 76–85. [Google Scholar] [CrossRef]
- Simon, P.; Beggs, P.D. A numerical performance comparison of a dual-phase steel and aluminium alloy bumper bar system. Int. J. Crashworthiness 2010, 15, 425–442. [Google Scholar] [CrossRef]
- Hirsch, J. Recent development in aluminium for automotive applications. Trans. Nonferrous Met. Soc. China 2014, 24, 1995–2002. [Google Scholar] [CrossRef]
- Karim, M.A.; Park, Y.D. A review on welding of dissimilar metals in car body manufacturing. J. Weld. Join. 2020, 38, 8–23. [Google Scholar] [CrossRef]
- Park, K.; Park, J.; Kwon, H. Fabrication and characterization of Al-SUS316L composite materials manufactured by the spark plasma sintering process. Mater. Sci. Eng. A 2017, 691, 8–15. [Google Scholar] [CrossRef]
- Mukai, T.; Suresh, S.; Kita, K.; Sasaki, H.; Kobayashi, N.; Higashi, K.; Inoue, A. Nanostructured Al–Fe alloys produced by e-beam deposition: Static and dynamic tensile properties. Acta Mater. 2003, 51, 4197–4208. [Google Scholar] [CrossRef]
- Liang, D.; Jie, W.; Jones, H. The effect of growth velocity on primary spacing of Al3Fe dendrites in hypereutectic Al-Fe alloys. J. Cryst. Growth 1994, 135, 561–564. [Google Scholar] [CrossRef]
- Senkov, O.N.; Froes, F.H.; Stolyarov, V.V.; Valiev, R.Z.; Liu, J. Microstructure of aluminum-iron alloys subjected to severe plastic deformation. Scr. Mater. 1998, 38, 1511–1516. [Google Scholar] [CrossRef]
- Medvedev, A.; Murashkin, M.; Enikeev, N.; Medvedev, E.; Sauvage, X. Influence of morphology of intermetallic particles on the microstructure and properties evolution in severely deformed Al-Fe alloys. Metals 2021, 11, 815. [Google Scholar] [CrossRef]
- Luo, S.X.; Shi, Z.M.; Li, N.Y.; Lin, Y.M.; Liang, Y.H.; Zeng, Y.D. Crystallization inhibition and microstructure refinement of Al-5Fe alloys by addition of rare earth elements. J. Alloys Compd. 2019, 789, 90–99. [Google Scholar] [CrossRef]
- Duchaussoy, A.; Sauvage, X.; Edalati, K.; Horita, Z.; Renou, G.; Deschamps, A.; De Geuser, F. Structure and mechanical behavior of ultrafine-grained aluminum-iron alloy stabilized by nanoscaled intermetallic particles. Acta Mater. 2019, 167, 89–102. [Google Scholar] [CrossRef]
- Guan, R.G.; Tie, D. A review on grain refinement of aluminum alloys: Progresses, challenges and prospects. Acta Metall. Sin. 2017, 30, 409–432. [Google Scholar] [CrossRef]
- Cubero-Sesin, J.M.; Horita, Z. Strengthening via Microstructure Refinement in Bulk Al–4 mass% Fe Alloy Using High-Pressure Torsion. Mater. Trans. 2012, 53, 46–55. [Google Scholar] [CrossRef]
- Fung, K.K.; Yang, C.Y.; Zhou, Y.Q.; Zhao, J.G.; Zhan, W.S.; Shen, B.G. Icosahedrally related decagonal quasicrystal in rapidly cooled Al-14-at.%-Fe alloy. Phys. Rev. Lett. 1986, 56, 2060–2063. [Google Scholar] [CrossRef] [PubMed]
- Cairney, J.M.; Munroe, P.R.; Sordelet, D.J. Microstructural analysis of a FeAl/quasicrystal-based composite prepared using a focused ion beam miller. J. Microsc. 2001, 201, 201–211. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Fan, C.; Wen, B.; Zhang, L. Crystal structure of AlFe0.95. IUCrData 2023, 8, x231065. [Google Scholar] [CrossRef]
- Stein, F.; Vogel, S.C.; Eumann, M.; Palm, M. Determination of the crystal structure of the ɛ phase in the Fe–Al system by high-temperature neutron diffraction. Intermetallics 2010, 18, 150–156. [Google Scholar] [CrossRef]
- Burkhardt, U.; Grin, Y.; Ellner, M.; Peters, K. Structure refinement of the iron–aluminium phase with the approximate composition Fe2Al5. Acta. Crystallogr. B Struct. Sci. 1994, 50, 313–316. [Google Scholar] [CrossRef]
- Chumak, I.; Richter, K.W.; Ehrenberg, H. Redetermination of iron dialuminide, FeAl2. Acta Crystallogr. C 2010, 66, i87–i88. [Google Scholar] [CrossRef] [PubMed]
- Grin, J.; Burkhardt, U.; Ellner, M.; Peters, K. Refinement of the Fe4Al13 structure and its relationship to the quasihomological homeotypical structures. Z Krist. Cryst. Mater. 1994, 209, 479–487. [Google Scholar] [CrossRef]
- Xia, Z.; Liu, C.; Fan, C. Al13Fe3. IUCrData 2018, 3, x180593. [Google Scholar] [CrossRef]
- Black, P.J. The structure of FeAl3. II. Acta Crystallogr. 1955, 8, 175–182. [Google Scholar] [CrossRef]
- Saitoh, K.; Yokosawa, T.; Tanaka, M.; Tsai, A.P. Structural studies of monoclinic approximants of Al13Fe4 and τ2-inflated Al13Co4 by the high-angle annular dark-field method. Microscopy 1999, 48, 105–114. [Google Scholar]
- Yanson, T.I.; Manyako, N.B.; Bodak, O.I.; Cêrny, R.; Gladyshevskii, R.E.; Yvon, K. Crystal structure of Fe4Ti0.93Al12.07, a substitutional variant of the Fe4Al13 structure type. J. Alloys Compd. 1995, 219, 135–138. [Google Scholar] [CrossRef]
- Popčević, P.; Smontara, A.; Ivkov, J.; Wencka, M.; Komelj, M.; Jeglic, P.; Vrtnik, S.; Bobnar, M.; Jaglicic, Z.; Bauer, B.; et al. Anisotropic physical properties of the Al13Fe4 complex intermetallic and its ternary derivative Al13(Fe,Ni)4. Phys. Rev. B 2010, 81, 184203. [Google Scholar] [CrossRef]
- Liu, C.; Fan, C. Crystal structure of the λ-Al13Fe4-type intermetallic (Al,Cu)13(Fe,Cu)4. IUCrData 2018, 3, x180363. [Google Scholar] [CrossRef]
- Barbier, J.N.; Tamura, N.; Verger-Gaugry, J.L. Monoclinic Al13Fe4 approximant phase: A link between icosahedral and decagonal phases. J. Non. Cryst. Solids. 1993, 153, 126–131. [Google Scholar] [CrossRef]
- Osawa, A.; Murata, T. On the equilibrium diagram of iron-aluminium system. Sci. Rep. Tohoku Univ. 1933, 22, 803–823. [Google Scholar]
- Phragmen, G. On the phases occurring in alloys of aluminium with copper, magnesium, manganese, iron, and silicon. J. Inst. Met. 1950, 77, 489–551. [Google Scholar]
- Black, P.J. The structure of FeAl3. I. Acta Crystallogr. 1955, 8, 43–48. [Google Scholar] [CrossRef]
- Ma, X.L.; Liebertz, H.; Köster, U. Multiple Twins of Monoclinic Al13Fe4 Showing Pseudo-Orthorhombic and Fivefold Symmetries. Phys. Status Solidi A 1996, 158, 359–367. [Google Scholar] [CrossRef]
- Griger, A.; Stefániay, V.; Turmezey, T. Crystallographic data and chemical compositions of aluminium-rich Al-Fe intermetallic phases. Int. J. Mater. Res. 1986, 77, 30–35. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Lin, Y.P.; Vincent, R.; Staniek, G. The structure and stability of some intermetallic phases in rapidly solidified AlFe. Scr. Metall. 1988, 22, 797–802. [Google Scholar] [CrossRef]
- Tsuchimori, M.; Ishimasa, T.; Fukano, Y. Crystal structures of small Al-rich Fe alloy particles formed by a gas-evaporation technique. Philos. Mag. B 1992, 66, 89–108. [Google Scholar] [CrossRef]
- Lafaye, P.; Oishi, K.; Bourdon, M.; Harvey, J.P. Crystal chemistry and thermodynamic modelling of the Al13(Fe,TM)4 solid solutions (TM = Co, Cr, Ni, Pt). J. Alloys Compd. 2022, 920, 165779. [Google Scholar] [CrossRef]
- Lafaye, P.; Oishi, K.; Harvey, J.P. A comprehensive study on the thermodynamics of the Al13Fe4 solid solution in the Al-Fe-Mn ternary system. J. Alloys Compd. 2023, 944, 169054. [Google Scholar] [CrossRef]
- Sharma, A.; Morisada, Y.; Ushioda, K.; Fujii, H. Elucidation on the correlation between thermal stability of Al13Fe4 intermetallic phase and mechanical properties of the Al-Fe alloy fabricated via friction stir alloying. J. Alloys Compd. 2023, 967, 171732. [Google Scholar] [CrossRef]
- Cui, Y.; Gourlay, C.M. Growth twinning and morphology of Al45Cr7 and Al13Fe4. J. Alloys Compd. 2022, 893, 162318. [Google Scholar] [CrossRef]
- Pang, N.; Shi, Z.; Wang, C.; Li, N.; Lin, Y. Influence of Cr, Mn, Co and Ni Addition on Crystallization Behavior of Al13Fe4 Phase in Al-5Fe Alloys Based on ThermoDynamic Calculations. Materials 2021, 14, 768. [Google Scholar] [CrossRef]
- Song, Z.; Magdysyuk, O.V.; Tang, L.; Sparks, T.; Cai, B. Growth dynamics of faceted Al13Fe4 intermetallic revealed by high-speed synchrotron X-ray quantification. J. Alloys Compd. 2021, 861, 158604. [Google Scholar] [CrossRef]
- Sahu, M.; Paul, A.; Ganguly, S. Formation of mechanical property gradient along the sheet thickness due to the patterned Fe/Al IMC layers in the interface in dissimilar FSW of HSLA steel to AA 5083. Mater. Charact. 2023, 203, 113146. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, Z.; Zhang, Y.; Su, J. Dissimilar welding of aluminium to steel: A review. J. Manuf. Process. 2024, 110, 376–397. [Google Scholar] [CrossRef]
- Chatelier, C.; Anand, K.; Gille, P.; De Weerd, M.C.; Ledieu, J.; Fournée, V.; Resta, A.; Vlad, A.; Garreau, Y.; Coati, A.; et al. Revealing the epitaxial interface between Al13Fe4 and Al5Fe2 enabling atomic Al interdiffusion. ACS Appl. Mater. Interfaces 2023, 15, 19593–19603. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fan, C.; Wen, B.; Xu, Z.; Fu, R.; Zhang, L. Orientation Relationship of Intergrowth Al2Fe and Al5Fe2 Intermetallics Determined by Single-Crystal X-ray Diffraction. Metals 2024, 14, 337. [Google Scholar] [CrossRef]
- Bruker AXS Inc. APEX3, SAINT and SADABS. In Software for Data Reduction, Absorption Correction and Structure Solution; Bruker AXS Inc.: Madison, WI, USA, 2015. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Putz, H.; Brandenburg, K. Diamond 4.2. 2–Crystal and Molecular Structure Visualization. Crystal. Impact. Kreuzherrenstr. 2017, 102, 53227. [Google Scholar]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Akhmetshina, T.G.; Blatov, V.A. A fascinating building unit: Mackay cluster in intermetallics. Struct. Chem. 2017, 28, 133–140. [Google Scholar] [CrossRef]
- Sugiyama, K.; Obata, T.; Hiraga, K. The crystalline structure of O-AlFePd. Mater. Trans. 2012, 53, 1357–1362. [Google Scholar] [CrossRef]

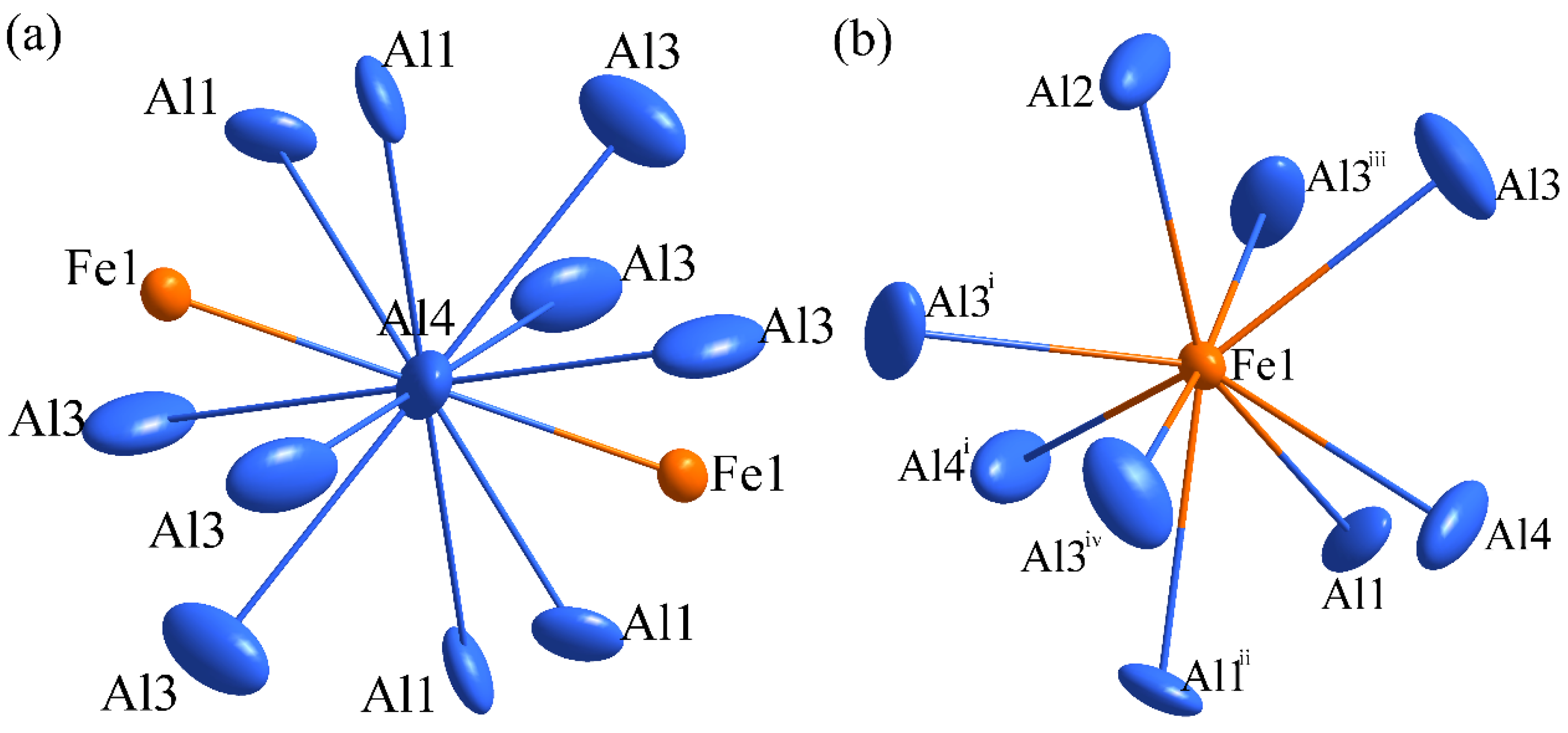
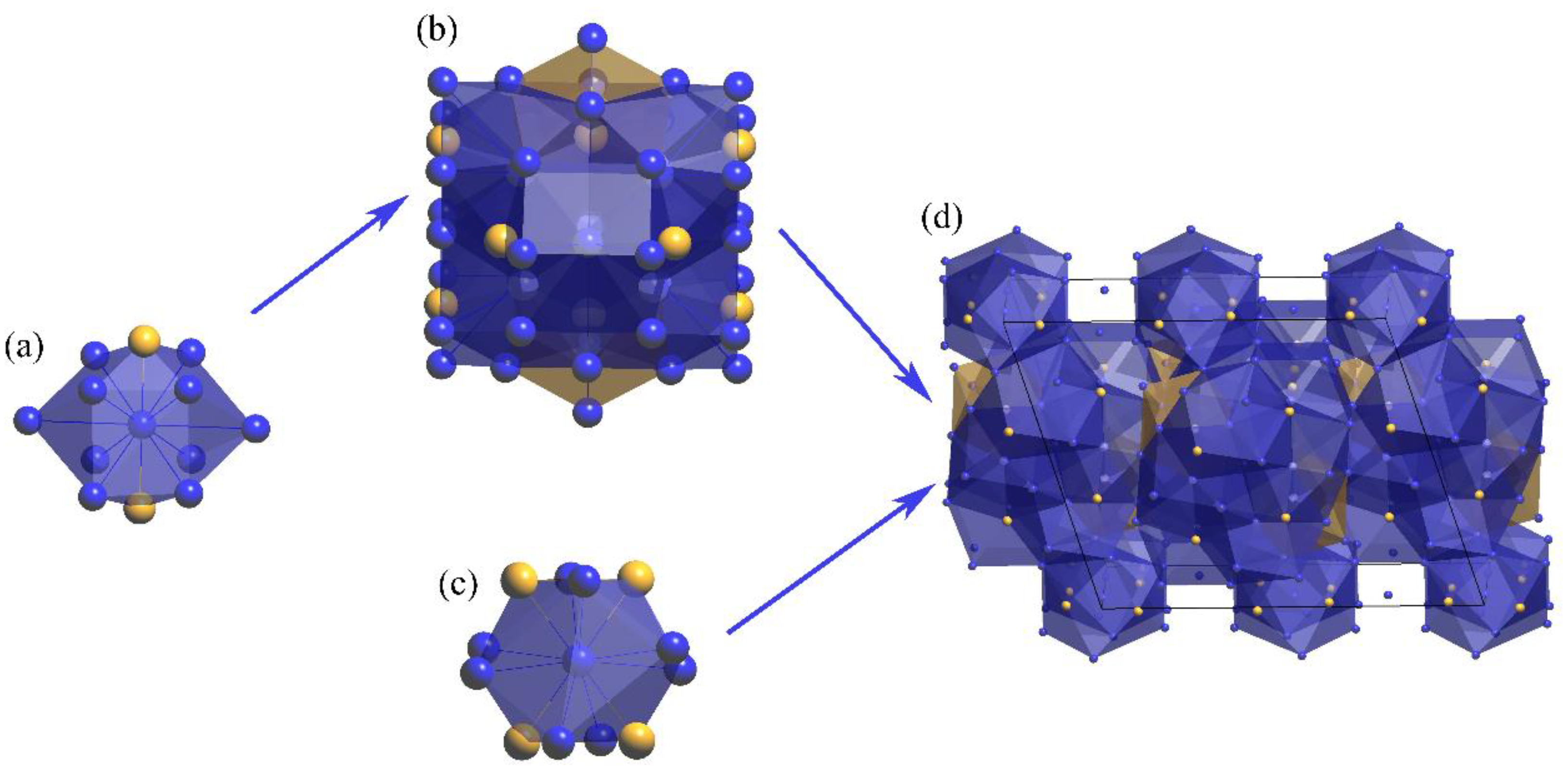
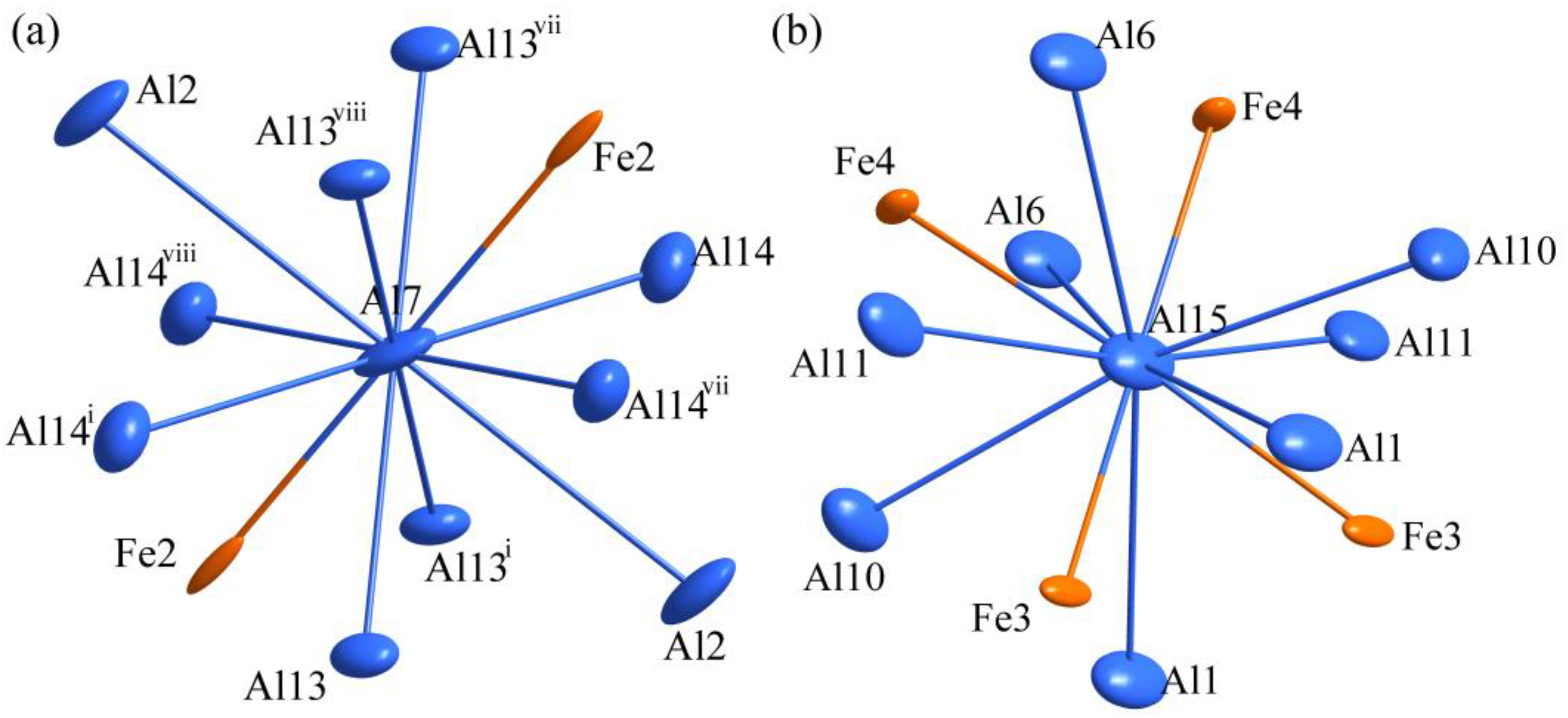
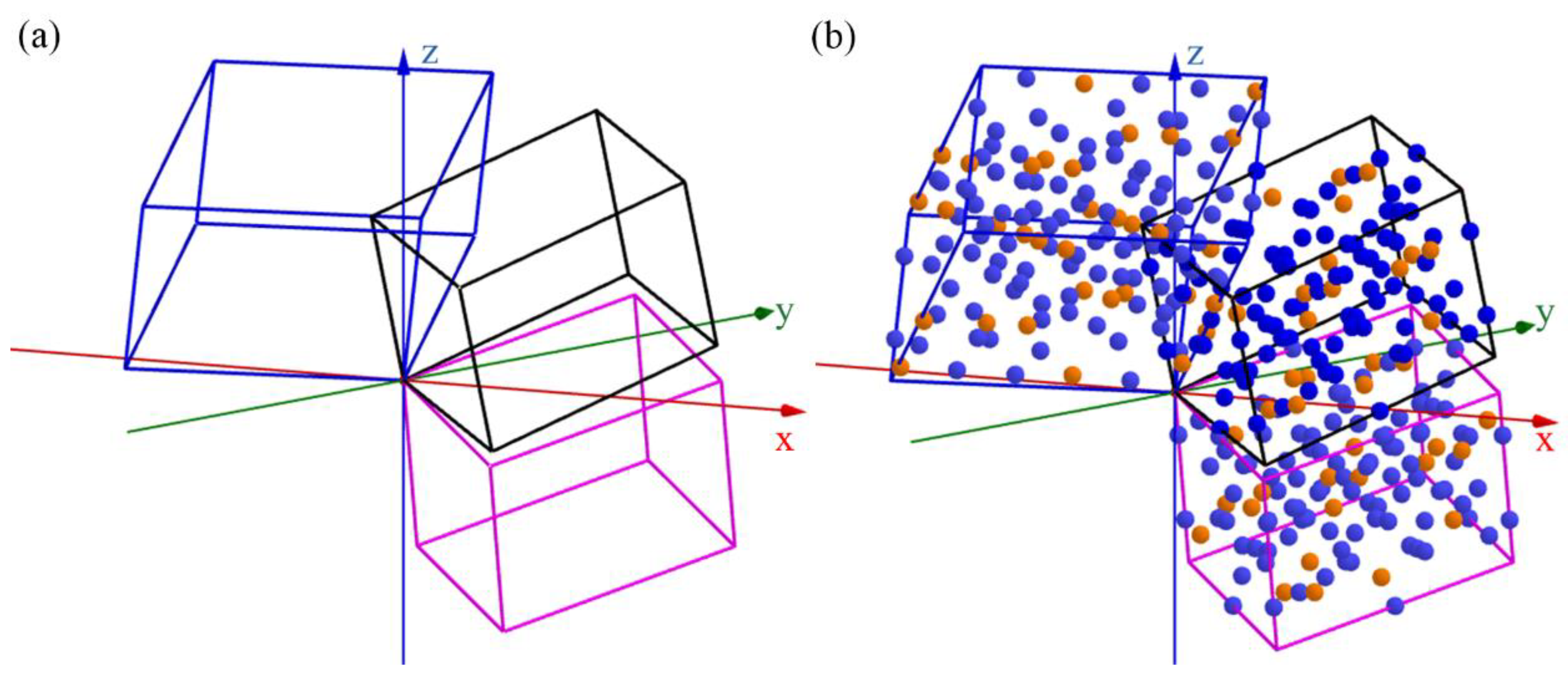
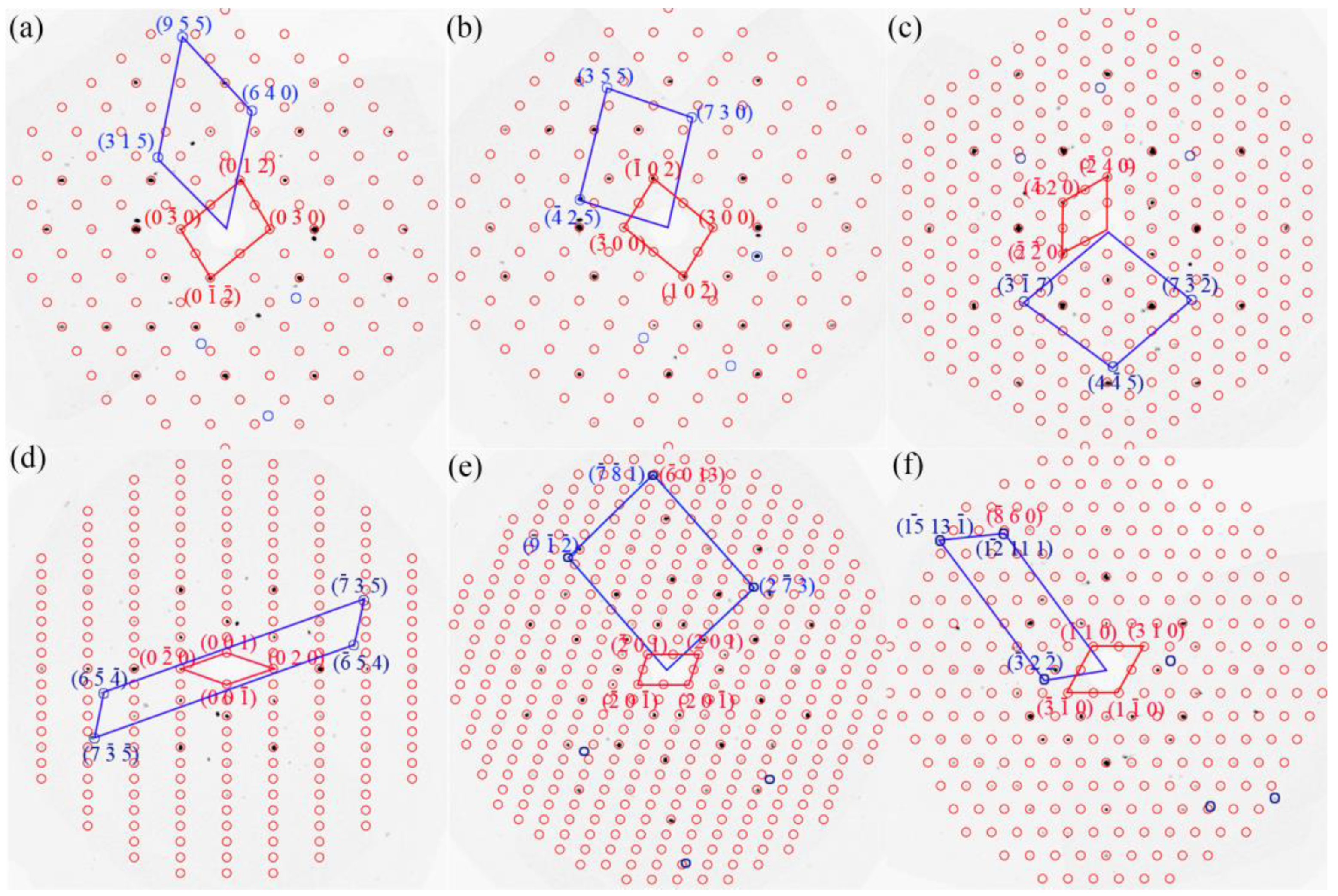
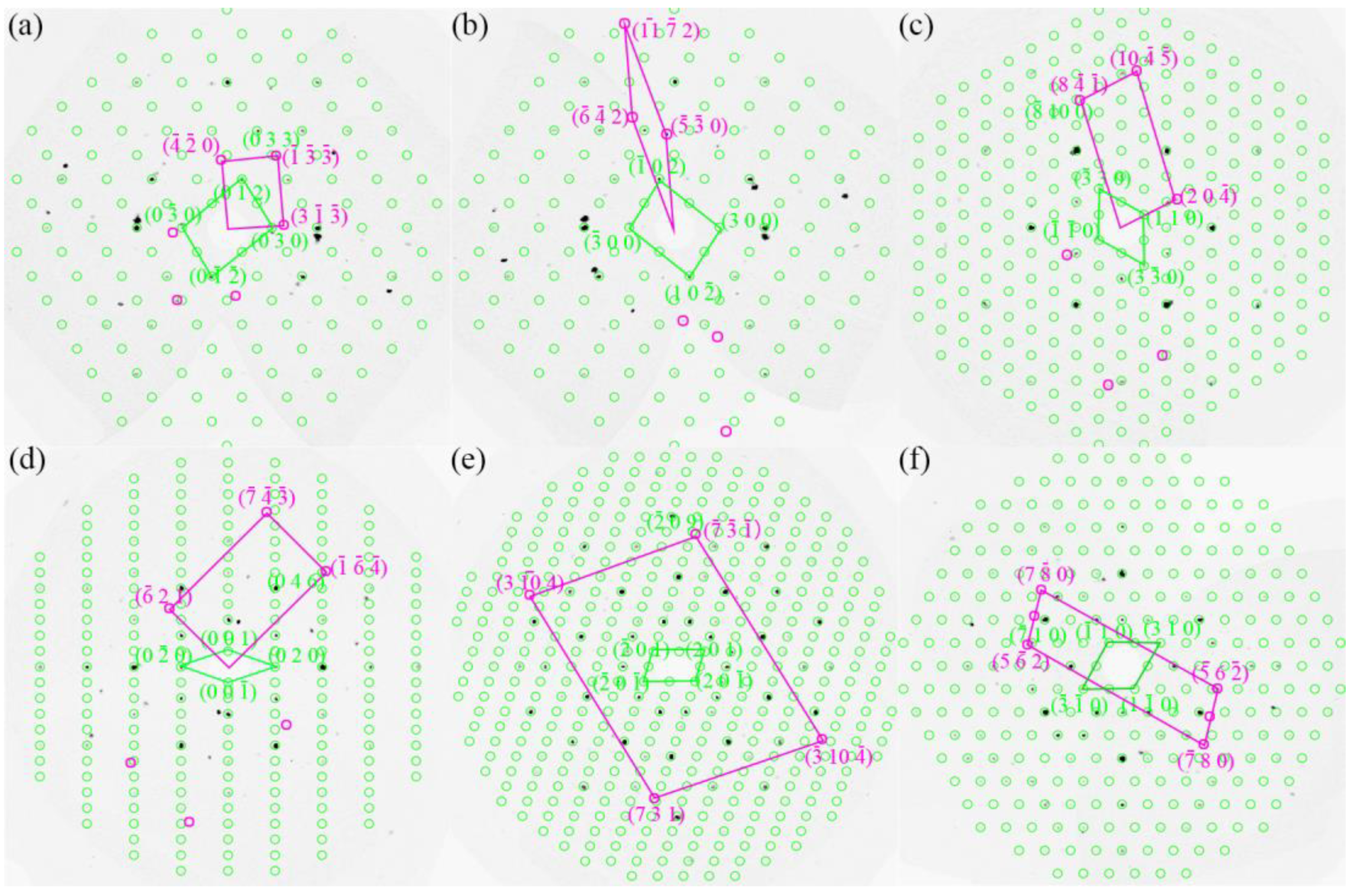
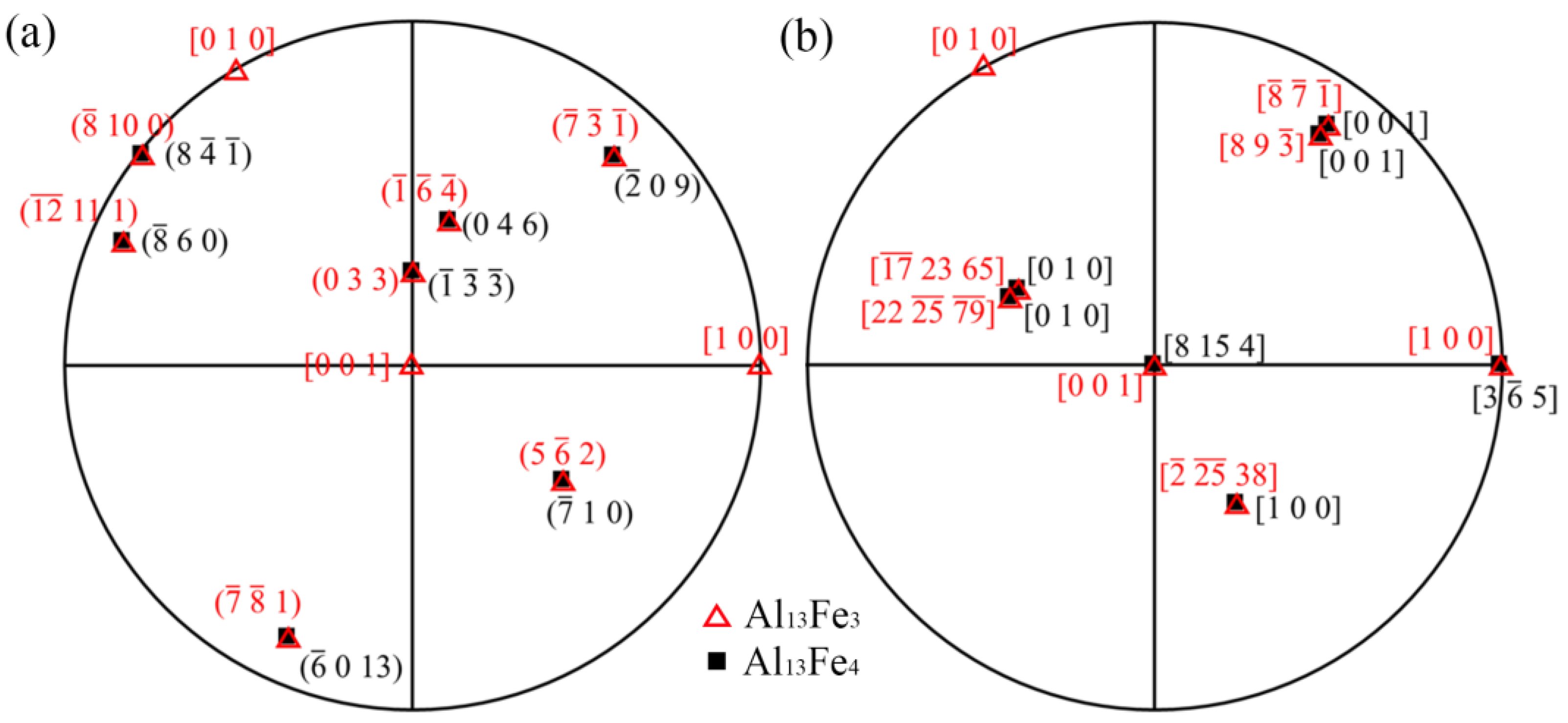

| Chemical Formula | Al13Fe3 | Al13Fe4 |
|---|---|---|
| a, b, c/Å | 14.5956(9), 14.5956(9), 7.6929(4) | 15.498(4), 8.0814(17), 12.488(3) |
| α, β, γ/° | 90, 90, 120 | 90, 107.790(8), 90 |
| V/Å3 | 1419.27(19) | 1489.2(6) |
| Z | 1 | 24 |
| Space group | R | C2/m |
| Crystal system | Trigonal | Monoclinic |
| Diffractometer | Bruker D8 Venture Photon 100 COMS | |
| Monochromator | Graphite | |
| Tmeas/K | 300(2) | |
| Radiation | Mo-Kα, λ = 0.71073 (Å) | |
| Scan mode | φ and ω scan | |
| Time per step/s | 6 | |
| Absorption correction | Multi-scan | |
| F(000) | 1482 | 1638 |
| θ range/° | 4.84~27.33 | 3.12~27.49 |
| μ/mm−1 | 5.69 | 6.82 |
| No. measured reflections | 19200 | 3922 |
| No. unique reflections | 367 | 1423 |
| No. observed reflections (I > 2σ(I)) | 341 | 756 |
| No. reflections used in refinement | 367 | 1423 |
| No. parameters used in refinement | 28 | 128 |
| Reflection range | −18 ≤ h ≤ 18, −18 ≤ k ≤ 18, −9 ≤ l ≤ 9 | −18 ≤ h ≤ 11, −9 ≤ k ≤ 9, −16 ≤ l ≤ 13 |
| Rint | 0.077 | 0.102 |
| R(σ) | 0.0152 | 0.178 |
| Final R indices (Fobs > 4σ(Fobs)) | R1 = 0.033, ωR2 = 0.0710 | R1 = 0.080, ωR2 = 0.223 |
| R indices (all data) | R1 = 0.034, ωR2 = 0.0710 | R1 = 0.169, ωR2 = 0.223 |
| Goodness of fit | 1.326 | 1.07 |
| The maximum residual electron densities | 0.52 (1.43 Å from Al3) | 1.71 (0.94 Å from Al14) |
| The minimum residual electron densities | −0.71 (0.72 Å from Al3) | −2.04 (0.89 Å from Al3) |
| Label | Site | x | y | z | Occ. | Ueq |
|---|---|---|---|---|---|---|
| Fe1 | 18e | 0.333333 | 0.49355 (6) | 0.416667 | 1 | 0.0061 (2) |
| Al1 | 18e | 0.35988 (13) | 0.35988 (13) | 0.250000 | 1 | 0.0110 (4) |
| Al2 | 6a | 0.333333 | 0.666667 | 0.416667 | 1 | 0.0180 (8) |
| Al3 | 36f | 0.48065 (11) | 0.63616 (12) | 0.24732 (18) | 1 | 0.0188 (4) |
| Al4 | 18d | 0.166667 | 0.333333 | 0.333333 | 1 | 0.0183 (5) |
| Label | Site | x | y | z | Occ. | Ueq |
|---|---|---|---|---|---|---|
| Fe1 | 4i | 0.0855 (3) | 0.000000 | 0.3823 (3) | 1 | 0.0043 (9) |
| Fe2 | 4i | 0.4019 (3) | 0.000000 | 0.6228 (3) | 1 | 0.0065 (9) |
| Fe3 | 4i | 0.0908 (3) | 0.000000 | 0.9885 (3) | 1 | 0.0056 (10) |
| Fe4 | 4i | 0.4028 (3) | 0.000000 | 0.9863 (3) | 1 | 0.0046 (9) |
| Fe5 | 8j | 0.32011(18) | 0.2933 (3) | 0.27793(18) | 1 | 0.0068 (7) |
| Al1 | 4i | 0.0670 (6) | 0.000000 | 0.1738 (6) | 1 | 0.0113 (18) |
| Al2 | 4i | 0.3213 (6) | 0.000000 | 0.2812 (6) | 1 | 0.0112 (11) |
| Al3 | 4i | 0.2381 (6) | 0.000000 | 0.5339 (6) | 1 | 0.0112 (11) |
| Al4 | 4i | 0.0734 (6) | 0.000000 | 0.5801 (6) | 1 | 0.0111 (18) |
| Al5 | 4i | 0.2415 (6) | 0.000000 | 0.9614 (7) | 1 | 0.0104 (17) |
| Al6 | 4i | 0.4786 (6) | 0.000000 | 0.8297 (6) | 1 | 0.0112 (11) |
| Al7 | 2d | 0.500000 | 0.000000 | 0.500000 | 1 | 0.011 (2) |
| Al8 | 4i | 0.3060 (6) | 0.000000 | 0.7729 (6) | 1 | 0.0109 (17) |
| Al9 | 4i | 0.0862 (6) | 0.000000 | 0.7888 (6) | 1 | 0.0098 (18) |
| Al10 | 8j | 0.1859 (4) | 0.2172 (7) | 0.1112 (4) | 1 | 0.0096 (14) |
| Al11 | 8j | 0.3673 (4) | 0.2116 (7) | 0.1101 (4) | 1 | 0.0103 (13) |
| Al12 | 8j | 0.1772 (4) | 0.2211 (7) | 0.3343 (4) | 1 | 0.0118 (13) |
| Al13 | 8j | 0.4920 (4) | 0.2328 (6) | 0.3296 (4) | 1 | 0.0082 (13) |
| Al14 | 8j | 0.3636 (4) | 0.2198 (7) | 0.4781 (4) | 1 | 0.0108 (13) |
| Al15 | 4g | 0.000000 | 0.2495 (9) | 0.000000 | 1 | 0.0086 (19) |
| [uvw] Al13Fe3//[uvw] Al13Fe4 | (hkl) Al13Fe3//(hkl) Al13Fe4 | |
|---|---|---|
| OR1 | [] Al13Fe3//[0 1 0] Al13Fe4 | (1) Al13Fe3//( Al13Fe4 |
| OR2 | [8 9 ] Al13Fe3//[0 0 1] Al13Fe4 | () Al13Fe3//(0) Al13Fe4 |
| OR3 | [1 0 0] Al13Fe3//[3] Al13Fe4 | (0 3 3) Al13Fe3//() Al13Fe4 |
| OR4 | [0 0 1] Al13Fe3//[8 15 4] Al13Fe4 | ( 10 0) Al13Fe3//(8 ) Al13Fe4 |
| OR5 | [] Al13Fe3//[1 0 0] Al13Fe4 | () Al13Fe3//(0 4 6) Al13Fe4 |
| OR6 | [] Al13Fe3//[0 1 0] Al13Fe4 | ( ) Al13Fe3//( 0 9) Al13Fe4 |
| OR7 | [] Al13Fe3//[0 0 1] Al13Fe4 | (5 2) Al13Fe3//(1 0) Al13Fe4 |
| Orientation Relationship | Conversion Matrix B | Conversion Matrix A | |
|---|---|---|---|
| OR1 | (1) Al13Fe3//( Al13Fe4 [] Al13Fe3//[0 1 0] Al13Fe4 | ||
| OR2 | () Al13Fe3//(0) Al13Fe4 [8 9 ] Al13Fe3//[0 0 1] Al13Fe4 | ||
| OR3 | (0 3 3) Al13Fe3//() Al13Fe4 [1 0 0] Al13Fe3//[3] Al13Fe4 | ||
| OR4 | () Al13Fe3//() Al13Fe4 [0 0 1] Al13Fe3//[8 15 4] Al13Fe4 | ||
| OR5 | () Al13Fe3//(0 4 6) Al13Fe4 [] Al13Fe3//[1 0 0] Al13Fe4 | ||
| OR6 | ( ) Al13Fe3//( 0 9) Al13Fe4 [] Al13Fe3//[0 1 0] Al13Fe4 | ||
| OR7 | (5 2) Al13Fe3//(1 0) Al13Fe4 [] Al13Fe3//[0 0 1] Al13Fe4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Fan, C.; Xu, Z.; Fu, R.; Wen, B.; Zhang, L. Orientation Relationship of the Intergrowth Al13Fe3 and Al13Fe4 Intermetallics Determined by Single-Crystal X-ray Diffraction. Metals 2024, 14, 463. https://doi.org/10.3390/met14040463
Liu Y, Fan C, Xu Z, Fu R, Wen B, Zhang L. Orientation Relationship of the Intergrowth Al13Fe3 and Al13Fe4 Intermetallics Determined by Single-Crystal X-ray Diffraction. Metals. 2024; 14(4):463. https://doi.org/10.3390/met14040463
Chicago/Turabian StyleLiu, Yibo, Changzeng Fan, Zhefeng Xu, Ruidong Fu, Bin Wen, and Lifeng Zhang. 2024. "Orientation Relationship of the Intergrowth Al13Fe3 and Al13Fe4 Intermetallics Determined by Single-Crystal X-ray Diffraction" Metals 14, no. 4: 463. https://doi.org/10.3390/met14040463
APA StyleLiu, Y., Fan, C., Xu, Z., Fu, R., Wen, B., & Zhang, L. (2024). Orientation Relationship of the Intergrowth Al13Fe3 and Al13Fe4 Intermetallics Determined by Single-Crystal X-ray Diffraction. Metals, 14(4), 463. https://doi.org/10.3390/met14040463









