Corrosion of Carbon Steel in a Tropical Marine Environment Enhanced by H2S from Sargassum Seaweed Decomposition
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Sites
- Type 1: A chloride-rich atmosphere with a minimal concentration of H2S (Diamant);
- Type 2: A tropical atmosphere with low levels of both H2S and chloride (Vert-Pré);
- Type 3: An atmosphere abundant in H2S with minimal chloride concentration (Frégate Est);
- Type 4: An atmosphere abundant in H2S (though less than type 3) and also containing high levels of Cl− possibly at concentrations similar to type 1 (Vauclin).
2.2. Preparation and Sample Exposure
2.3. Gravimetric Measurements
2.4. Characterization and Identification of the Corrosion Product
3. Results and Discussion
3.1. Assessment of Carbon Steel Thickness Loss after One-Year Exposure
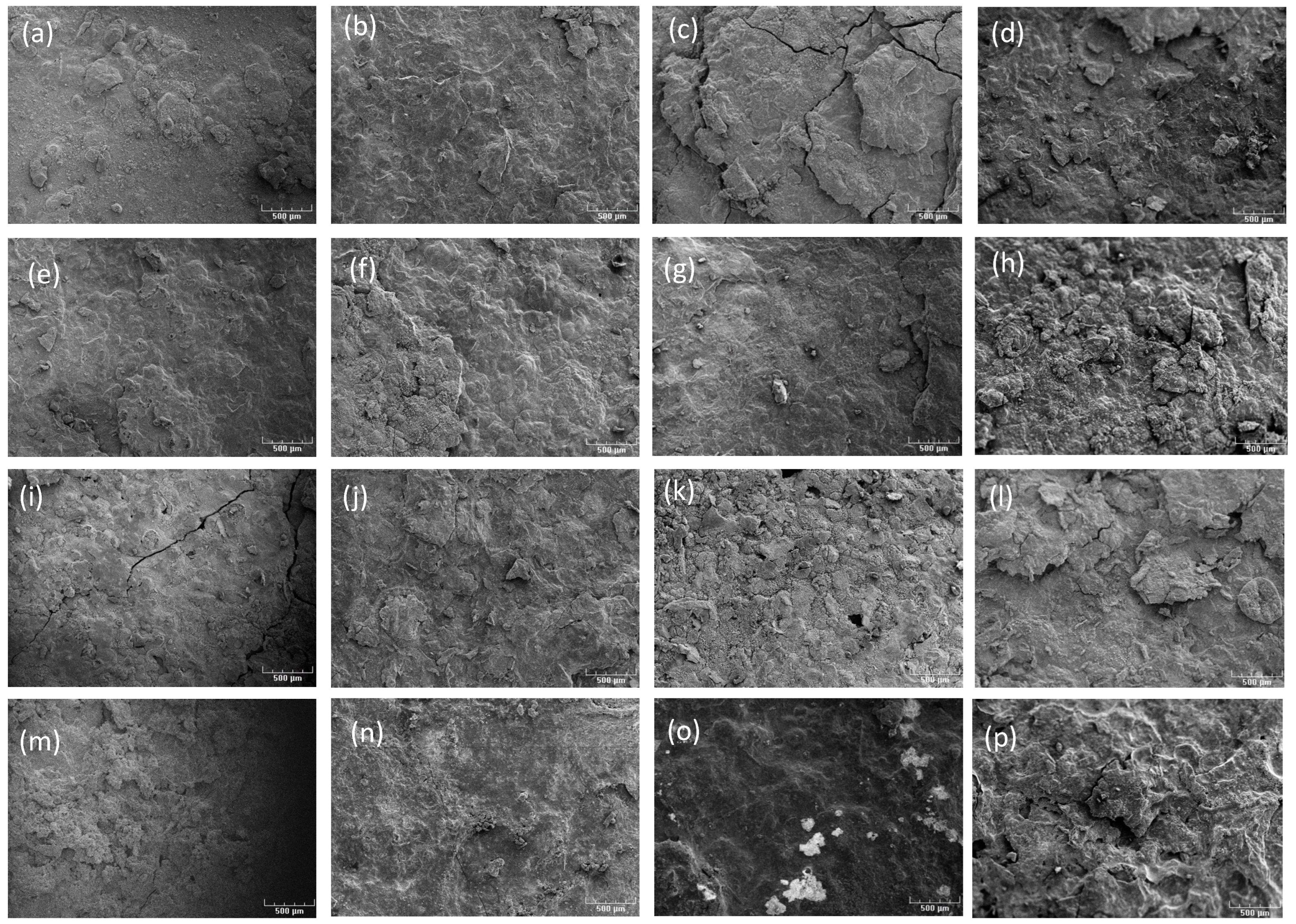
3.2. Characterization of Corrosion Products by SEM/EDS Analysis
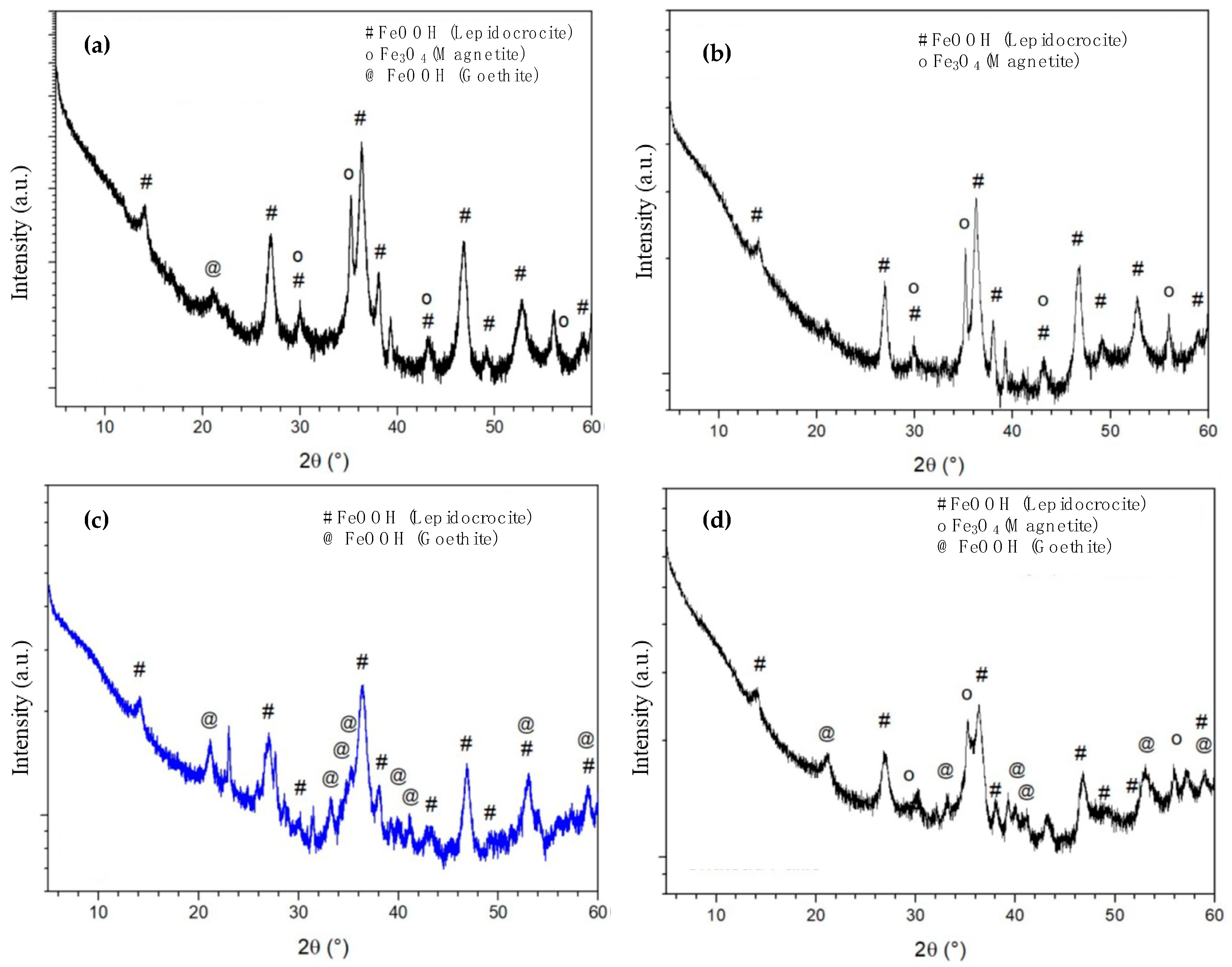
3.3. XRD Characterization of Corrosion Products
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Evans, U.R. The Corrosion and Oxidation of Metals: Scientific Principles and Practical Applications; Edward Arnold Ltd.: London, UK, 1960. [Google Scholar]
- Tomashov, N.D. Theory of Corrosion and Protection of Metals. The Science of Corrosion; The MacMillan Co.: New York, NY, USA, 1966. [Google Scholar]
- Rozenfeld, I.L. Atmospheric Corrosion of Metals; NACE: Houston, TX, USA, 1972. [Google Scholar]
- Feliu, S.; Morcillo, M.; Feliu, S., Jr. The prediction of atmospheric corrosion from meteorological and pollution parameters—II. Long-term forecasts. Corros. Sci. 2005, 34, 415–422. [Google Scholar] [CrossRef]
- Leygraf, C.; Odnevall, W.; Tidblad, I. Atmospheric Corrosion, 2nd ed.; The Electrochemical Society Series; John Wiley and Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Béranger, G.; Henry, G.; Sanz, G. Le livre de l’acier; Lavoisier-Technique et Documentation: Paris, France, 1994. [Google Scholar]
- Yamashita, M.; Konishi, H.; Kozakura, T.; Mizuki, J.; Uchida, H. In situ observation of initial rust formation process on carbon steel under Na2SO4 and NaCl solution films with wet/dry cycles using synchrotron radiation X-rays. Corros. Sci. 2005, 47, 2492–2498. [Google Scholar] [CrossRef]
- Leidheiser, H.; Musić, S. The atmospheric corrosion of iron as studied by Mössbauer spectroscopy. Corros. Sci. 1982, 22, 1089–1096. [Google Scholar] [CrossRef]
- Maréchal, L.; Perrin, S.; Dillmann, P.; Santarini, G. Experimental atmospheric ageing archaeological artefacts and contemporary low-alloy steel in climatic chamber. In Proceedings of the European Corrosion Congress, Lisbonne, Portugal, 4–8 September 2005. [Google Scholar]
- Okada, H.; Hosoi, Y.; Yukawa, K.; Naito, H. Structure of the protective and decirative rust formed on low-alloy steels in the atmosphere. Trans. ASM 1969, 62, 278–281. [Google Scholar]
- Keiser, J.T.; Brown, C.W.; Heidersbach, R.H. Characterization of the passive film formed on weathering steels. Corros. Sci. 1983, 23, 251–259. [Google Scholar] [CrossRef]
- Bellot-Gurlet, L. Raman imaging of ancient trust scales on archaelogical iron artefacts for long term atmospheric corrosion mechanisms study. J. Raman Spectrosc. 2006, 37, 1228–1237. [Google Scholar]
- Salas, B.V.; Wiener, M.S.; Badilla, G.L.; Beltran, M.C.; Zlatev, R.; Stoycheva, M.; Gaynor, J.T. H2S Pollution and Its Effect on Corrosion of Electronic Components, Air Quality-New Perspective; IntechOpen: London, UK, 2012; pp. 263–285. [Google Scholar]
- Becker, J.; Pellé, J.; Rioual, S.; Lescop, B.; Le Bozec, N.; Thierry, D. Atmospheric corrosion of silver, copper and nickel exposed to hydrogen sulphide: A multi-analytical investigation approach. Corros. Sci. 2022, 209, 110726. [Google Scholar] [CrossRef]
- Rioual, S.; Lescop, B.; Pellé, J.; de Alkmim Radicchi, G.; Chaumat, G.; Bruni, M.D.; Becker, J.; Thierry, D. Development of low-cost RFID sensors dedicated to air pollution monitoring for preventive conservation. Herit. Sci. 2022, 10, 124. [Google Scholar] [CrossRef]
- Foster, S.; Maher, W.; Schmeisser, E.; Taylor, A.; Krikowa, F.; Apte, S. Arsenic species in a rocky intertidal marine food chain in NSW, Australia, revisited. Environ. Chem. 2006, 3, 304–315. [Google Scholar] [CrossRef]
- Bertrand, J.-C.; Doumenq, P.; Guyoneaud, R.; Marrot, B.; Martin-Laurent, F.; Matheron, R.; Moulin, P.; Soulas, G. Applied microbial ecology and decontamination—Micro-organisms that play a major role in the elimination of pollution that affects the environment. In Microbial Ecology, Microbiology of Natural and Anthropogenic Environments; Presses de l’Université de Pau et des Pays de l’Adour: Pau Cedex, France, 2011; pp. 705–798. [Google Scholar]
- Said Ahmed, M.; Lebrini, M.; Lescop, B.; Pellé, J.; Rioual, S.; Amintas, O.; Boullanger, C.; Roos, C. Corrosion of Copper in a tropical marine atmosphere rich in H2S Resulting from the decomposition of Sargassum algae. Metals 2023, 13, 982. [Google Scholar] [CrossRef]
- Said Ahmed, M.; Lebrini, M.; Pellé, J.; Rioual, S.; Amintas, O.; Boullanger, C.; Lescop, B.; Roos, C. Study of atmospheric corrosion of zinc in a tropical marine environment rich in H2S resulting from the decomposition of sargassum algae. Mater. Corros. 2024, 1–9. [Google Scholar]
- EN 10130; Cold rolled low carbon steel flat products for cold forming Technical delivery conditions. iTeh STANDARD PREVIEW: Brussels, Belgium, 2006.
- EN10139; Cold rolled uncoated low carbon steel narrow strip cold forming—Technical delivery conditions. ILNAS: Brussels, Belgium, 2020.
- EN 13523-19; Coil Coated Metals—Test Methods—Part 19: Panel Design and Method of Atmospheric Exposure Testing. iTeh Standard Preview: Brussels, Belgium, 2019.
- EN ISO-8407; Removal of Corrosion Products from Corrosion Test Specimens. iTech Standard Preview: Rotkreuz, Switzerland, 2009.
- Knotkova, D.; Kreislova, D.; Dean, W.S. ISOCORRAG International Atmospheric Exposure Program: Summary of Results; ASTM Data Serie 71; ASTM International: West Conshohocken, PA, USA, 2010; ISBN 978-0-8031-7011-7. [Google Scholar]
- UNECE. International Cooperative Programme on Effects on Materials Including Historic and Cultural Monuments; Technical Manual, No. 1; Swedish Corrosion Institute: Gothenburg, Sweden, 1988. [Google Scholar]
- Morcillo, M.; Almeida, E.; Rosales, B.; Uruchurtu, J.; Marrocos, M. Corrosion y Protección de Metales en las Atmósferas de Iberoamérica. Parte I—Mapas de Iberoamérica de Corrosividad Atmosférica (Proyecto MICAT,XV.1/CYTED); CYTED: Madrid, Spain, 1998. [Google Scholar]
- ISO 9223:2012; Corrosion of Metals and Alloys—Corrosivity of atmospheres—Classification, Determination and Estimation. iTech Standard Preview: Rotkreuz, Switzerland, 2012.
- Belén, C.; De la Fuente, D.; Iván, D. Annual Atmospheric Corrosion of Carbon SteelWorldwide. An Integration of ISOCO RAGICP/UNECE and MICAT Databases. Materials 2017, 10, 601. [Google Scholar]
- Panchenko, M.; Marshakov, A.; Igonin, T.N.; Kovtanyuk, V.V.; Nikolaeva, L.A. Long-term forecast of corrosion mass losses of technically important metals in various world regions using a power function. Corros. Sci. 2014, 88, 306–316. [Google Scholar] [CrossRef]
- Rodríguez, J.J.S.; Hernández, F.J.S.; González, J.E. The effect of environmental and meteorological variables on atmospheric corrosion of carbon steel, copper, zinc and aluminium in a limited geographic zone with different types of environmen. Corros. Sci. 2003, 45, 799–815. [Google Scholar] [CrossRef]
- Morcillo, M.; Chico, B.; Diaz, I.; Cano, H.; De la Fuente, D. Atmospheric corrosion data of weathering steels. Corros. Sci. 2013, 77, 6–24. [Google Scholar] [CrossRef]
- Morcillo, M.; Feliu, S.; Simancas, J. Deviation from bilogarithmic law for atmospheric corrosion of steel. Br. Corros. J. 1993, 28, 50–52. [Google Scholar] [CrossRef]
- Alcántara, J.; De la Fuente, D.; Chico, B. Marine Atmospheric Corrosion of Carbon Steel:A Review. Materials 2017, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Seechurn, Y.; Wharton, J.A.; Surnam, B.Y.R. Mechanistic modelling of atmospheric corrosion of carbon steel in Port-Louis by electrochemical characterisation of rust layers. Mater. Chem. Phys. 2022, 291, 126694. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Wang, F. Corrosion of low carbon steel in atmospheric environments of different chloride content. Corros. Sci. 2009, 51, 997–1006. [Google Scholar] [CrossRef]
- Rezel, D.; Genin, J.M.R. The substitution of chloride ions to OH− ions in the akaganeite beta ferric oxyhydroxide studied by Mössbauer effect. Hyperfine Interact 1990, 57, 2067–2075. [Google Scholar] [CrossRef]
- Feitknecht, W. The breakdown of oxide films on metal surfaces in acidic vapors and the mechanism of atmospheric corrosion. Chimia 1952, 6, 3–13. [Google Scholar]
- Henriksen, J.F. Distribution of NaCl on Fe during atmospheric corrosionw. Corros. Sci. 1969, 9, 573–577. [Google Scholar] [CrossRef]
- Misawa, T.; Kyuno, T.; Shimodaira, S. The mechanism of atmospheric rusting and the effect of Cu and P on the rust formation of low-alloy steels. Corros. Sci. 1971, 11, 35–48. [Google Scholar] [CrossRef]
- Misawa, T.; Hashimoto, K.; Shimodaira, S. The mechanism of formation of iron oxide and oxyhydroxides in aqueous solutions at room temperature. Corros. Sci. 1974, 14, 131–149. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses, 2nd ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2003. [Google Scholar]
- Ma, Y.; Li, Y.; Wang, F. The effect of β-FeOOH on the corrosion behavior of low carbon steel exposed in tropic marine environment. Mater. Chem. Phys. 2008, 112, 844–852. [Google Scholar] [CrossRef]
- Schwarz, H. Über die Wirkung des magnetits beim atmosphärischen Rosten und beim Unterrosten von Anstrichen. Mater. Corros. 1972, 23, 648–663. [Google Scholar] [CrossRef]
- Singh, A.K. Mössbauer and X-ray diffraction phase analysis of rusts from atmospheric test sites with different environments in Sweden. Corros. Sci. 1985, 25, 931–945. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kondo, Y.; Yasukawa, A.; Kandori, K. Formation of magnetite in the presence of ferric oxyhydroxides. Corros. Sci. 1998, 40, 1239–1251. [Google Scholar] [CrossRef]
- Tanaka, H.; Mishima, R.; Hatanaka, N.; Ishikawa, T.; Nakayama, T. Formation of magnetite rust particles by reacting iron powder with artificial α-, β- and γ-FeOOH in aqueous media. Corros. Sci. 2014, 78, 384–387. [Google Scholar] [CrossRef]
- Sun, W. Kinetics of Iron Carbonate and Iron Sulfide Scale Formation in CO2/H2S Corrosion; The Russ College of Engineering and Technology of Ohio University: Athens, OH, USA, 2006. [Google Scholar]
- Cayard, M.S.; Maldonado, J.G. Use and Misuse of Laboratory Tests; NACE International: Orlando, FL, USA, 2000. [Google Scholar]
- Ning, J.; Zheng, Y.; Young, D.; Brown, B.; Nesic, S. Thermodynamic Study of Hydrogen Sulfide Corrosion of Mild Steel. Corrosion 2014, 70, 335–389. [Google Scholar] [CrossRef]
- Zittlau, A.H.; Shi, Q.; Boerio-Goates, J.; Woodfield, B.F.; Majzlan, J. Thermodynamics of the basic copper sulfate antlerite, posnjakite and brochantite. Geochemistry 2013, 73, 39–50. [Google Scholar] [CrossRef]

| Site | H2S (ppb) | Cl− (mg m−2 day−1) |
|---|---|---|
| Diamant | 7 | 481 |
| Vert-Pré | 15.5 | 46 |
| Frégate Est | 2995 | 60.5 |
| Vauclin | 223 |
| Chemical Composition (%) | |||
|---|---|---|---|
| C | Mn | p | S |
| ≤0.12 | ≤0.60 | ≤0.045 | ≤0.045 |
| Site | Fe | O | Cl | S |
|---|---|---|---|---|
| Vert-Pré | 20.3 | 79.2 | 0.3 | 0.2 |
| Diamant | 30.5 | 69.0 | 0.4 | 0.2 |
| Frégate Est | 28.2 | 64.5 | / | 7.3 |
| Vauclin | 20.8 | 77.3 | 1.1 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Said Ahmed, M.; Lescop, B.; Pellé, J.; Rioual, S.; Roos, C.; Lebrini, M. Corrosion of Carbon Steel in a Tropical Marine Environment Enhanced by H2S from Sargassum Seaweed Decomposition. Metals 2024, 14, 676. https://doi.org/10.3390/met14060676
Said Ahmed M, Lescop B, Pellé J, Rioual S, Roos C, Lebrini M. Corrosion of Carbon Steel in a Tropical Marine Environment Enhanced by H2S from Sargassum Seaweed Decomposition. Metals. 2024; 14(6):676. https://doi.org/10.3390/met14060676
Chicago/Turabian StyleSaid Ahmed, Mahado, Benoit Lescop, Julien Pellé, Stéphane Rioual, Christophe Roos, and Mounim Lebrini. 2024. "Corrosion of Carbon Steel in a Tropical Marine Environment Enhanced by H2S from Sargassum Seaweed Decomposition" Metals 14, no. 6: 676. https://doi.org/10.3390/met14060676
APA StyleSaid Ahmed, M., Lescop, B., Pellé, J., Rioual, S., Roos, C., & Lebrini, M. (2024). Corrosion of Carbon Steel in a Tropical Marine Environment Enhanced by H2S from Sargassum Seaweed Decomposition. Metals, 14(6), 676. https://doi.org/10.3390/met14060676







