Application of an Artificial Neural Network for Efficient Computation of Chemical Activities within an EAF Process Model
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Steel Association. World Steel in Figures: Concise Version; World Steel Association: Brussels, Belgium, 2023; Available online: https://worldsteel.org (accessed on 6 February 2024).
- Demus, T.; Reichel, T.; Schulten, M.; Echterhof, T.; Pfeifer, H. Increasing the sustainability of steel production in the electric arc furnace by substituting fossil coal with biochar agglomerates. Ironmak. Steelmak. 2016, 43, 564–570. [Google Scholar] [CrossRef]
- Kirschen, M.; Hay, T.; Echterhof, T. Process Improvements for Direct Reduced Iron Melting in the Electric Arc Furnace with Emphasis on Slag Operation. Processes 2021, 9, 402. [Google Scholar] [CrossRef]
- Hay, T.; Visuri, V.-V.; Aula, M.; Echterhof, T. A Review of Mathematical Process Models for the Electric Arc Furnace Process. Steel Res. Int. 2021, 92, 2000395. [Google Scholar] [CrossRef]
- Carlsson, L.S.; Samuelsson, P.B.; Jönsson, P.G. Predicting the Electrical Energy Consumption of Electric Arc Furnaces Using Statistical Modeling. Metals 2019, 9, 959. [Google Scholar] [CrossRef]
- Meier, T. Modellierung und Simulation des Elektrolichtbogenofens; Verlagshaus Mainz: Mainz, Germany, 2016; ISBN 978-3-95886-129-9. [Google Scholar]
- Logar, V.; Dovžan, D.; Škrjanc, I. Modeling and Validation of an Electric Arc Furnace: Part 1, Heat and Mass Transfer. ISIJ Int. 2012, 52, 402–412. [Google Scholar] [CrossRef]
- Logar, V.; Dovžan, D.; Škrjanc, I. Modeling and Validation of an Electric Arc Furnace: Part 2, Thermo-chemistry. ISIJ Int. 2012, 52, 413–423. [Google Scholar] [CrossRef]
- Pfeifer, H.; Nacke, B.; Beneke, F. Praxishandbuch Thermoprozesstechnik: Band II: Anlagen-Komponenten-Sicherheit, 3rd ed.; Vulkan: Essen, Germany, 2022; ISBN 9783802731228. [Google Scholar]
- Ben Bowman, K.K. Arc Furnace Physics, 1st ed.; Stahleisen: Düsseldorf, Germany, 2009; ISBN 978-3514007680. [Google Scholar]
- Graham, K.J. Integrated Ladle Metallurgy Control. Ph.D. Thesis, McMaster University, Hamilton, ON, Canada, 2008. [Google Scholar]
- MacRosty, R.D.M.; Swartz, C.L.E. Dynamic Modeling of an Industrial Electric Arc Furnace. Ind. Eng. Chem. Res. 2005, 44, 8067–8083. [Google Scholar] [CrossRef]
- Hay, T.; Reimann, A.; Echterhof, T. Improving the Modeling of Slag and Steel Bath Chemistry in an Electric Arc Furnace Process Model. Metall. Mater. Trans. B 2019, 50, 2377–2388. [Google Scholar] [CrossRef]
- Shampine, L.F.; Reichelt, M.W. The MATLAB ODE Suite. SIAM J. Sci. Comput. 1997, 18, 1–22. [Google Scholar] [CrossRef]
- Lupis, C.; Elliott, J. Generalized interaction coefficients: Part II: Free energy terms and the quasi-chemical theory. Acta Metall. 1966, 14, 1019–1032. [Google Scholar] [CrossRef]
- Pelton, A.D.; Bale, C.W. A modified interaction parameter formalism for non-dilute solutions. Metall. Trans. A 1986, 17, 1211–1215. [Google Scholar] [CrossRef]
- Basu, S.; Seetharaman, S.; Lahiri, A.K. Thermodynamics of Phosphorus and Sulphur Removal during Basic Oxygen Steelmaking. Steel Res. Int. 2010, 81, 932–939. [Google Scholar] [CrossRef]
- Michalek, K.; Čamek, L.; Gryc, K.; Tkadlečková, M.; Huczala, T.; Troszok, V. Desulphurization of the high-alloy and middle-alloy steels under the conditions of an eaf by means of synthetic slag based on CaO-Al2O3. Mater. Technol. 2012, 46, 297–303. [Google Scholar]
- Schrama, F.N.H.; Beunder, E.M.; van den Berg, B.; Yang, Y.; Boom, R. Sulphur removal in ironmaking and oxygen steelmaking. Ironmak. Steelmak. 2017, 44, 333–343. [Google Scholar] [CrossRef]
- Kirschen, M. Visualization of Slag Data for Efficient Monitoring and Improvement of Steelmaking Slag Operation in Electric Arc Furnaces, with a Focus on MgO Saturation. Metals 2021, 11, 17. [Google Scholar] [CrossRef]
- Ban-Ya, S. Mathematical Expression of Slag-Metal Reactions in Steelmaking Process by Quadratic Formalism Based on the Regular Solution Model. ISIJ Int. 1993, 33, 2–11. [Google Scholar] [CrossRef]
- Gaye, H.; Lehmann, J.; Matsumiya, T.; Yamada, W. A statistical thermodynamics model of slags: Applications to systems containing S, F, P2O5 and Cr oxides. In Proceedings of the 4th International Conference on Molten Slags and Fluxes, Sendai, Japan, 8–11 June 1992; ISIJ: Sendai, Japan, 1992; pp. 103–108. [Google Scholar]
- Hay, T. Mathematische Modellierung des Elektrostahlverfahrens. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2021. [Google Scholar]
- Xiao, Y.; Holappa, L. Thermodynamics of slags containing chromium oxides. In Proceedings of the INFACON 7, Trondheim, Norway, 11–14 June 1995; Norwegian Ferroalloy Research Organization (FFF): Trondheim, Norway, 1995. [Google Scholar]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J.; et al. FactSage thermochemical software and databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- GTT, C. FactSage Database Overview. Available online: https://www.factsage.com/facthelp/FSNew_Databases.htm (accessed on 31 January 2024).
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep Learning; MIT Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Szandała, T. Review and Comparison of Commonly Used Activation Functions for Deep Neural Networks. In Bio-Inspired Neurocomputing; Bhoi, A., Mallick, P., Liu, C.M., Balas, V., Eds.; Springer: Singapore, 2021; pp. 203–224. ISBN 978-981-15-5495-7. [Google Scholar]
- Rumelhart, D.E.; Hinton, G.E.; Williams, R.J. Learning representations by back-propagating errors. Nature 1986, 323, 533–536. [Google Scholar] [CrossRef]
- Renaud, O.; Victoria-Feser, M.-P. A robust coefficient of determination for regression. J. Stat. Plan. Inference 2010, 140, 1852–1862. [Google Scholar] [CrossRef]
- Romer, T.H.; Lee, D.; Voelker, G.M.; Wolman, A.; Wong, W.A.; Baer, J.-L.; Bershad, B.N.; Levy, H.M. The structure and performance of interpreters. In Proceedings of the Seventh International Conference on Architectural Support for Programming Languages and Operating Systems, Cambridge, MA, USA, 1–4 October 1996; Association for Computing Machinery: New York, NY, USA, 1996; pp. 150–159, ISBN 0897917677. [Google Scholar]
- Barany, G. Python Interpreter Performance Deconstructed. In Proceedings of the Workshop on Dynamic Languages and Applications, Edinburgh, UK, 12 June 2014; Association for Computing Machinery: New York, NY, USA, 2014; pp. 1–9, ISBN 9781450329163. [Google Scholar]
- Anaconda. Numba: User Manual. Available online: https://numba.readthedocs.io/en/stable/user/index.html (accessed on 3 June 2024).
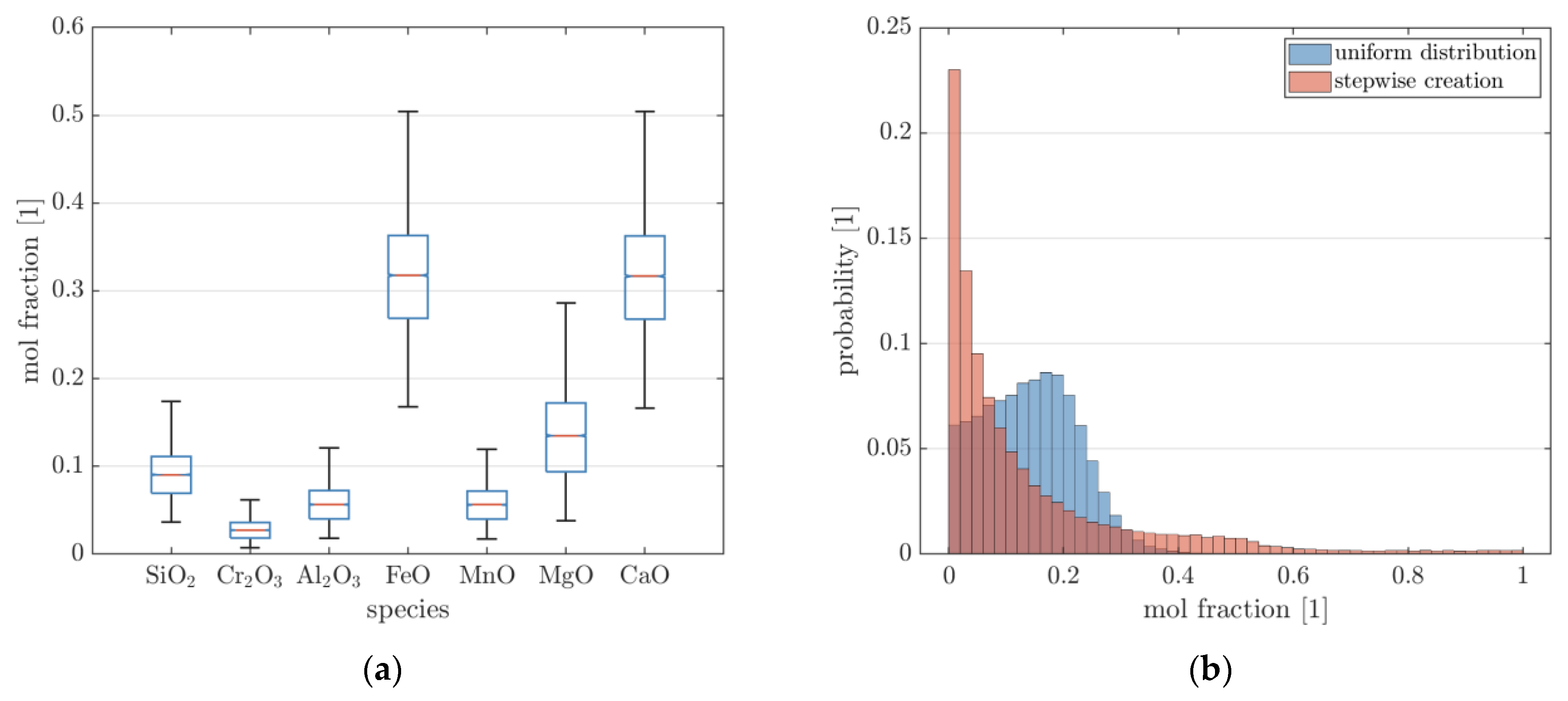
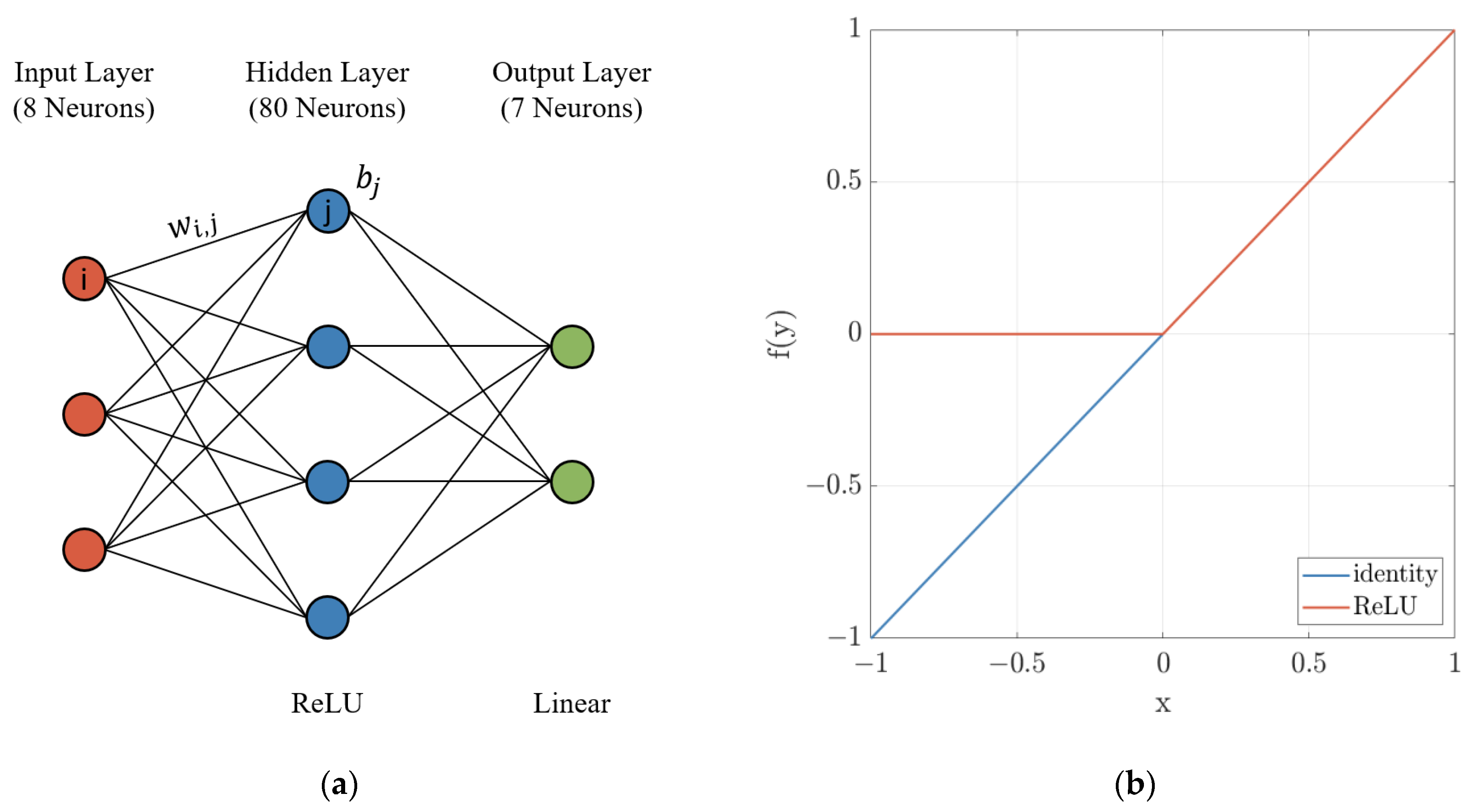
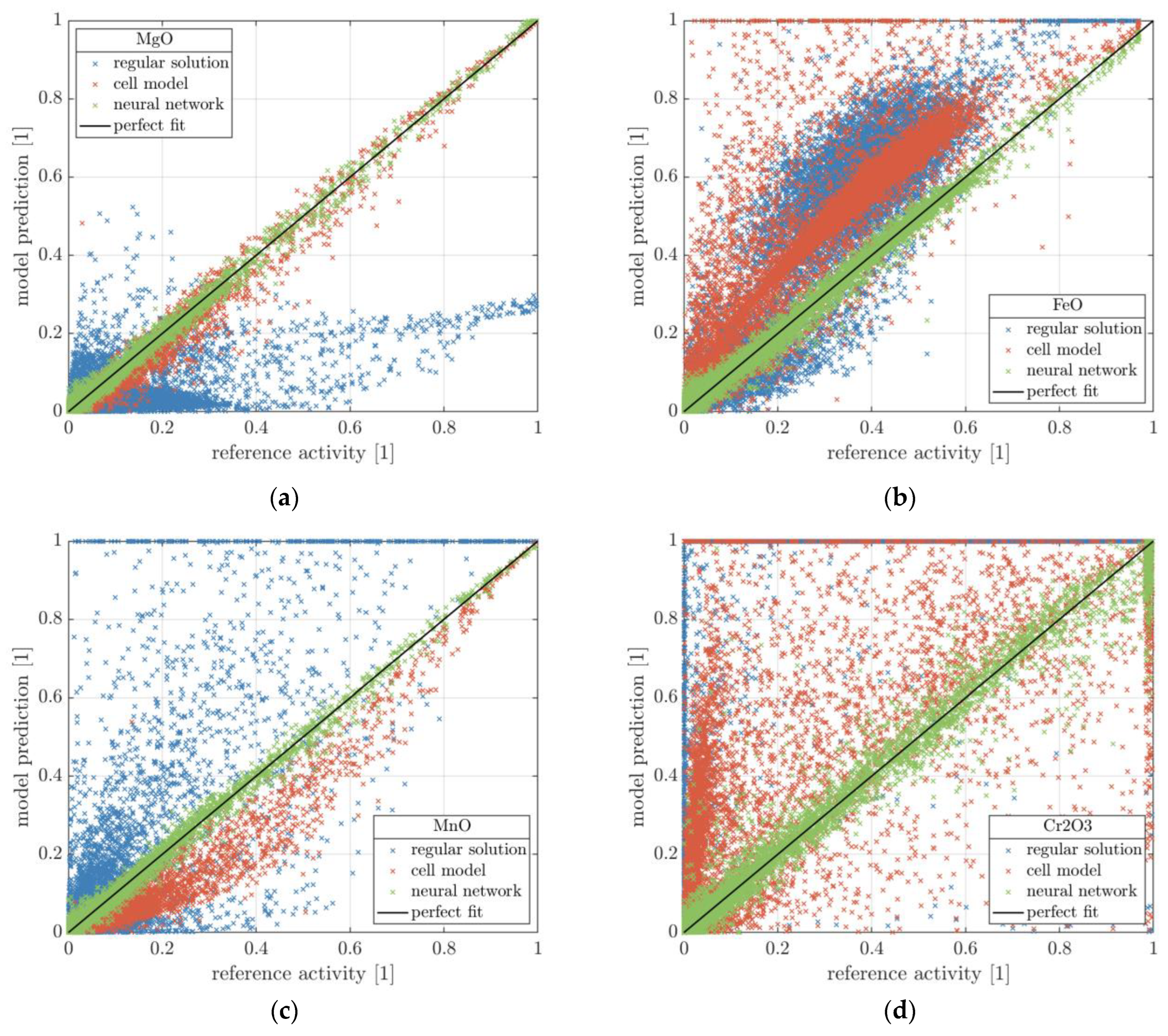
| Temperature | FeO | Cr2O3 | Al2O3 | MnO | P2O5 | SiO2 | MgO | CaO | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Unit | K | wt.% | ||||||||
| Slag * | 35.6 | 2.5 | 6.1 | 6.4 | 0.3 | 11.9 | 9.6 | 25.8 | ||
| Scenario 1: tapping | LL | 1700 | 20 | 1 | 2.5 | 2.5 | 0 | 5 | 5 | 20 |
| UL | 2000 | 50 | 5 | 10 | 10 | 0 | 15 | 25 | 50 | |
| Scenario 2: process | LL | 1700 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| UL | 2000 | 100 | 100 | 100 | 100 | 0 | 100 | 100 | 100 | |
| Model | Execution Time for 1000 Samples | Species | MAE | R2 | 95% Percentile |
|---|---|---|---|---|---|
| RS | 0.037 s | SiO2 | 0.0279 | 0.71 | 0.1483 |
| Cr2O3 | 0.8059 | −10.1 | 0.9995 | ||
| Al2O3 | 0.0089 | 0.95 | 0.0563 | ||
| FeO | 0.1523 | −0.09 | 0.3543 | ||
| MnO | 0.0651 | −0.11 | 0.2815 | ||
| MgO | 0.0846 | −0.05 | 0.2424 | ||
| CaO | 0.0380 | −0.09 | 0.1097 | ||
| Cell | 9.848 s | SiO2 | 0.0289 | 0.65 | 0.1558 |
| Cr2O3 | 0.1483 | 0.15 | 0.5462 | ||
| Al2O3 | 0.0142 | 0.53 | 0.0480 | ||
| FeO | 0.1582 | −0.18 | 0.3823 | ||
| MnO | 0.0376 | 0.81 | 0.1210 | ||
| MgO | 0.0145 | 0.97 | 0.0425 | ||
| CaO | 0.0058 | 0.97 | 0.0191 | ||
| ANN | 0.115 s | SiO2 | 0.0086 | 0.97 | 0.0379 |
| Cr2O3 | 0.0163 | 0.98 | 0.0655 | ||
| Al2O3 | 0.0046 | 0.99 | 0.0201 | ||
| FeO | 0.0112 | 0.99 | 0.0354 | ||
| MnO | 0.0071 | 0.99 | 0.0232 | ||
| MgO | 0.0068 | 0.99 | 0.0222 | ||
| CaO | 0.0041 | 0.99 | 0.0137 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reinicke, A.; Engbrecht, T.-N.; Schüttensack, L.; Echterhof, T. Application of an Artificial Neural Network for Efficient Computation of Chemical Activities within an EAF Process Model. Metals 2024, 14, 736. https://doi.org/10.3390/met14060736
Reinicke A, Engbrecht T-N, Schüttensack L, Echterhof T. Application of an Artificial Neural Network for Efficient Computation of Chemical Activities within an EAF Process Model. Metals. 2024; 14(6):736. https://doi.org/10.3390/met14060736
Chicago/Turabian StyleReinicke, Alexander, Til-Niklas Engbrecht, Lilly Schüttensack, and Thomas Echterhof. 2024. "Application of an Artificial Neural Network for Efficient Computation of Chemical Activities within an EAF Process Model" Metals 14, no. 6: 736. https://doi.org/10.3390/met14060736
APA StyleReinicke, A., Engbrecht, T. -N., Schüttensack, L., & Echterhof, T. (2024). Application of an Artificial Neural Network for Efficient Computation of Chemical Activities within an EAF Process Model. Metals, 14(6), 736. https://doi.org/10.3390/met14060736







