Phase Transformations after Heat Treating an As-Cast Fe-30Mn-8.8Al-0.3Si-0.15C Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Numerical Methodology
2.2. Experimental Methodology
3. Results and Discussion
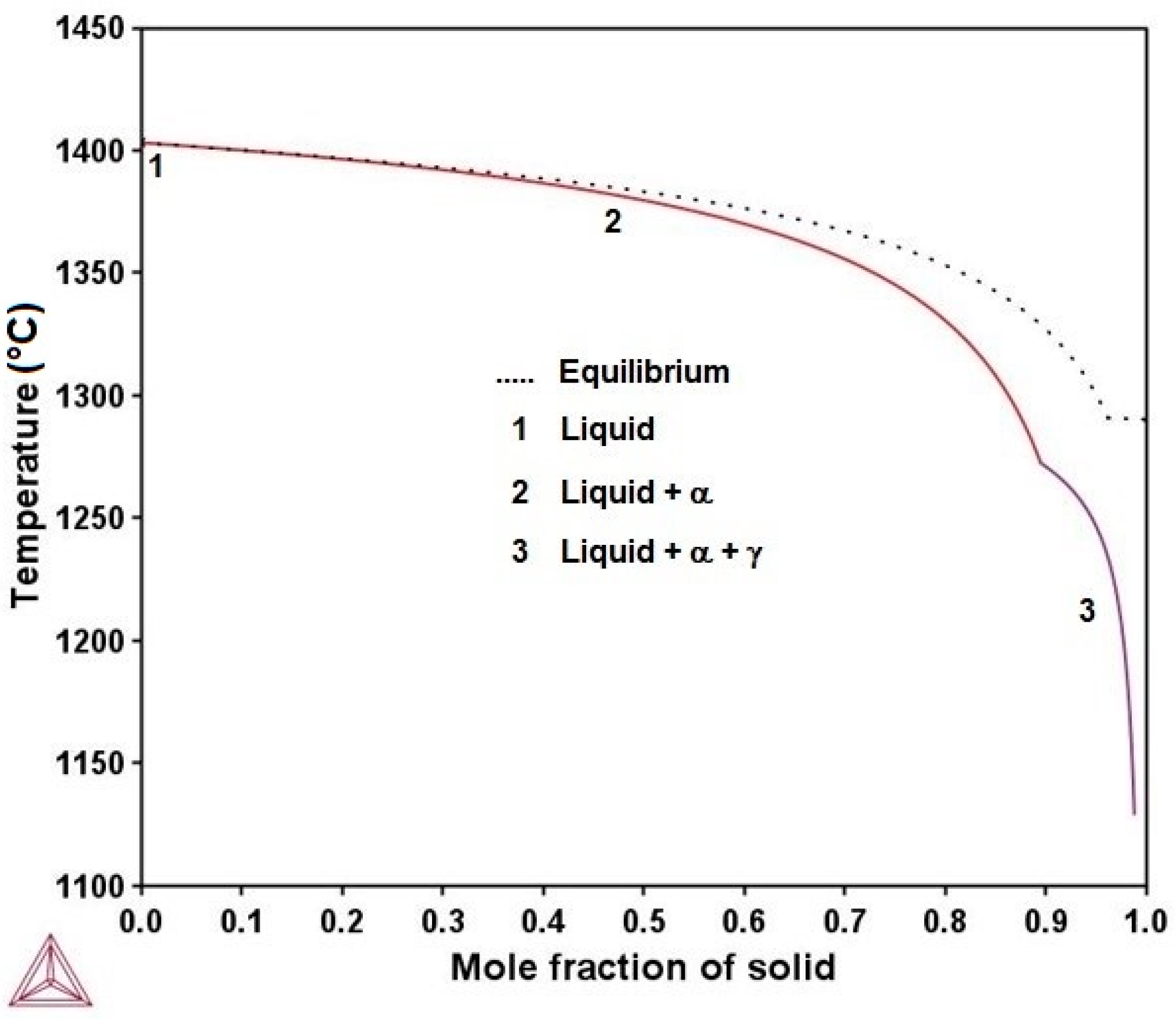
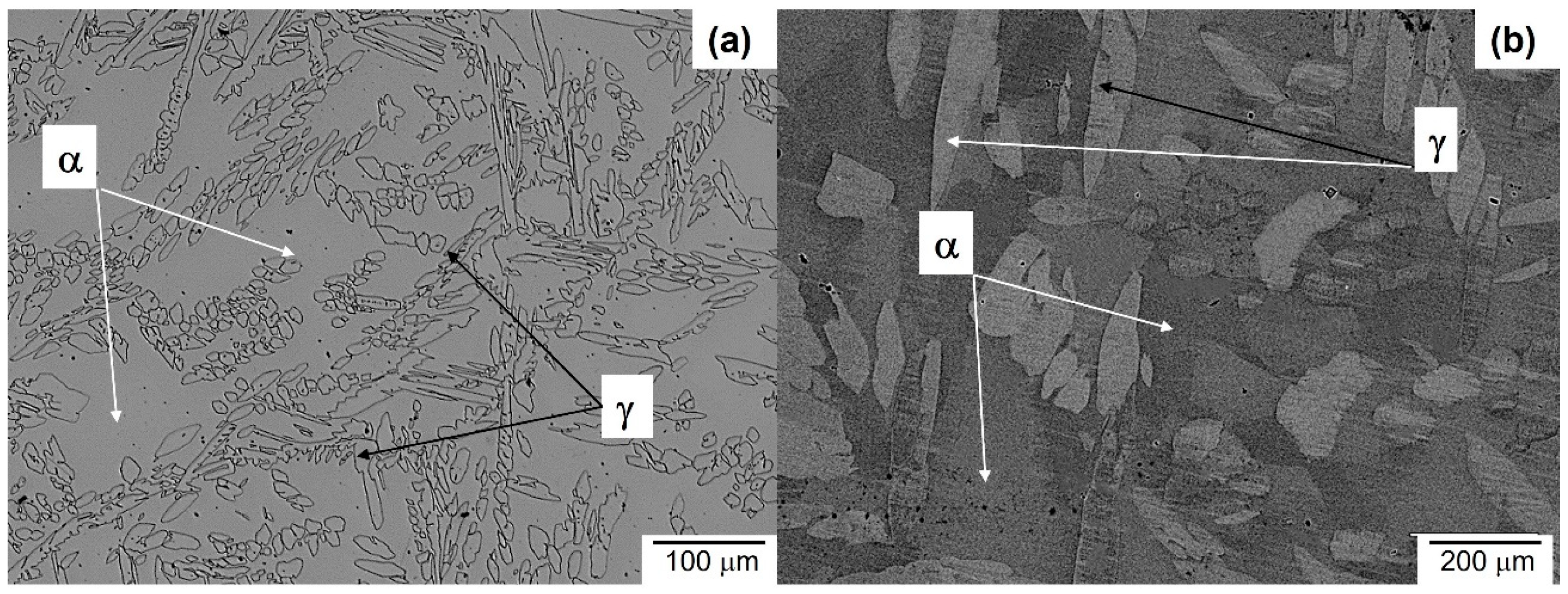
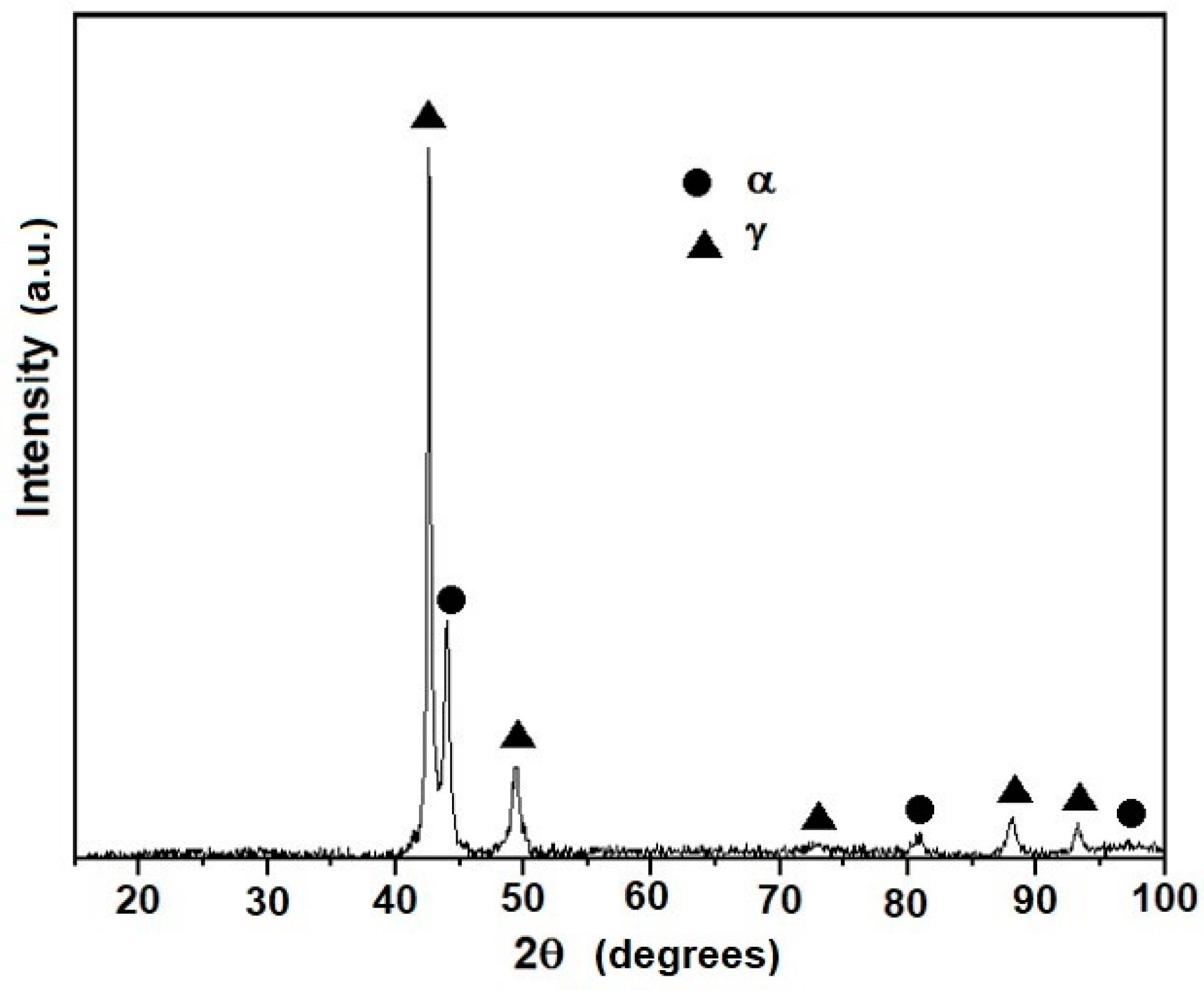
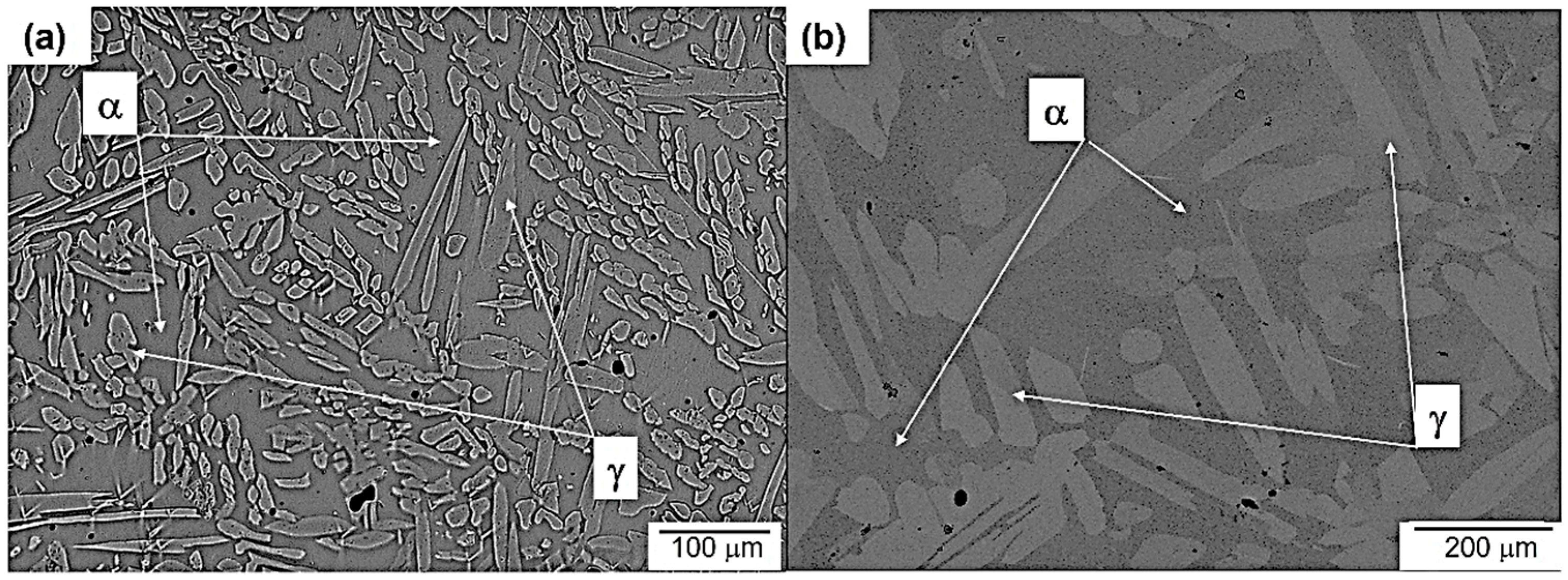
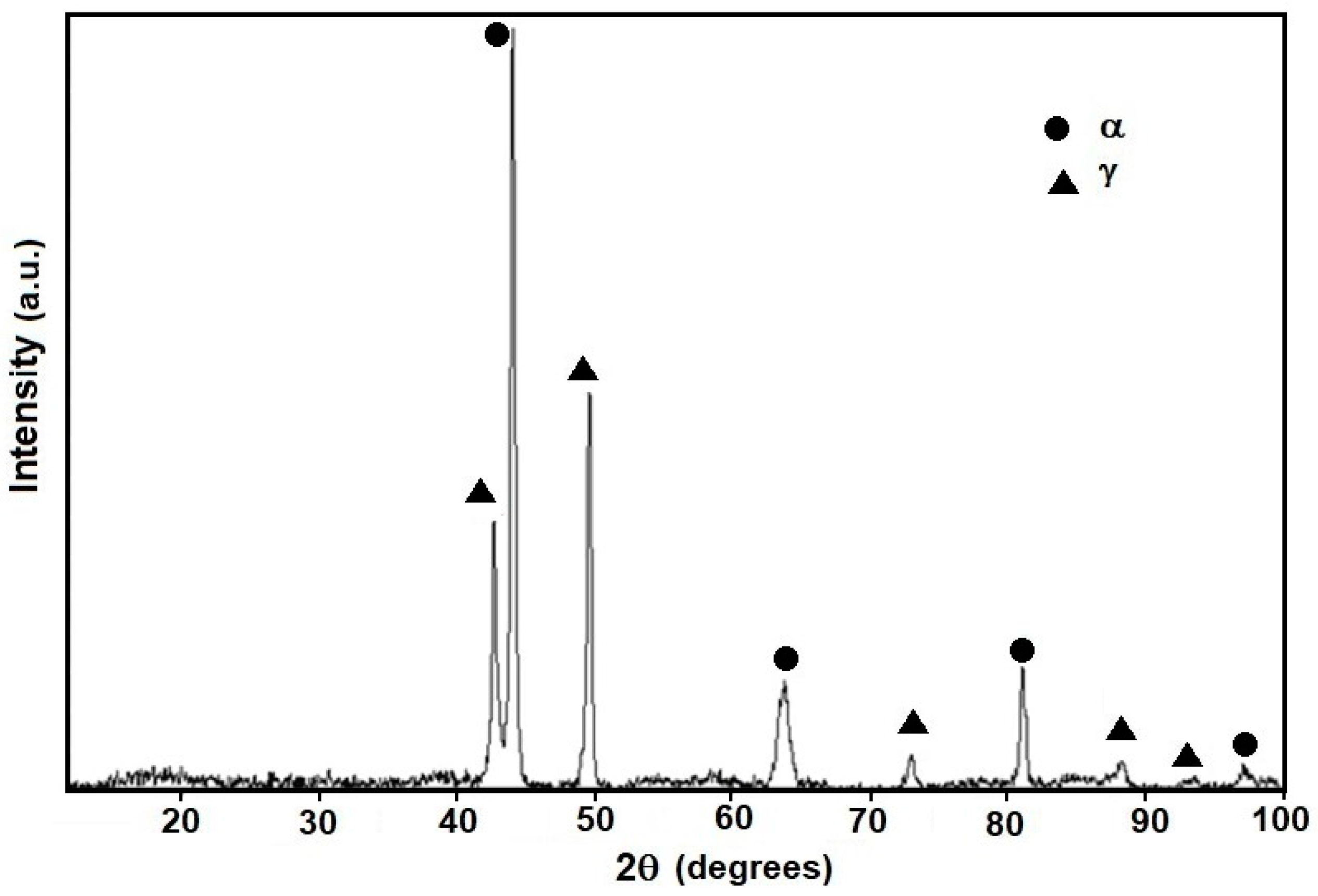
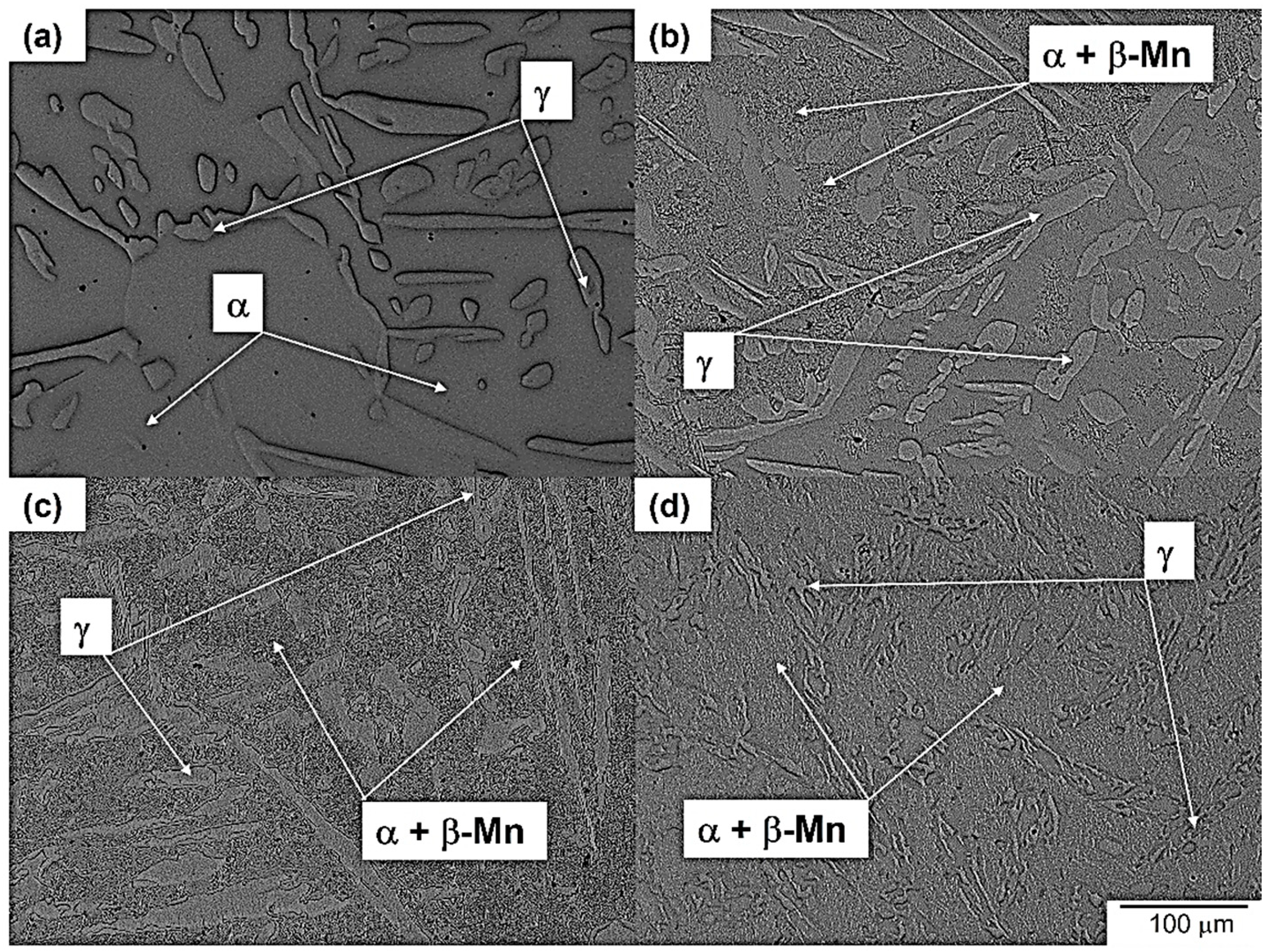
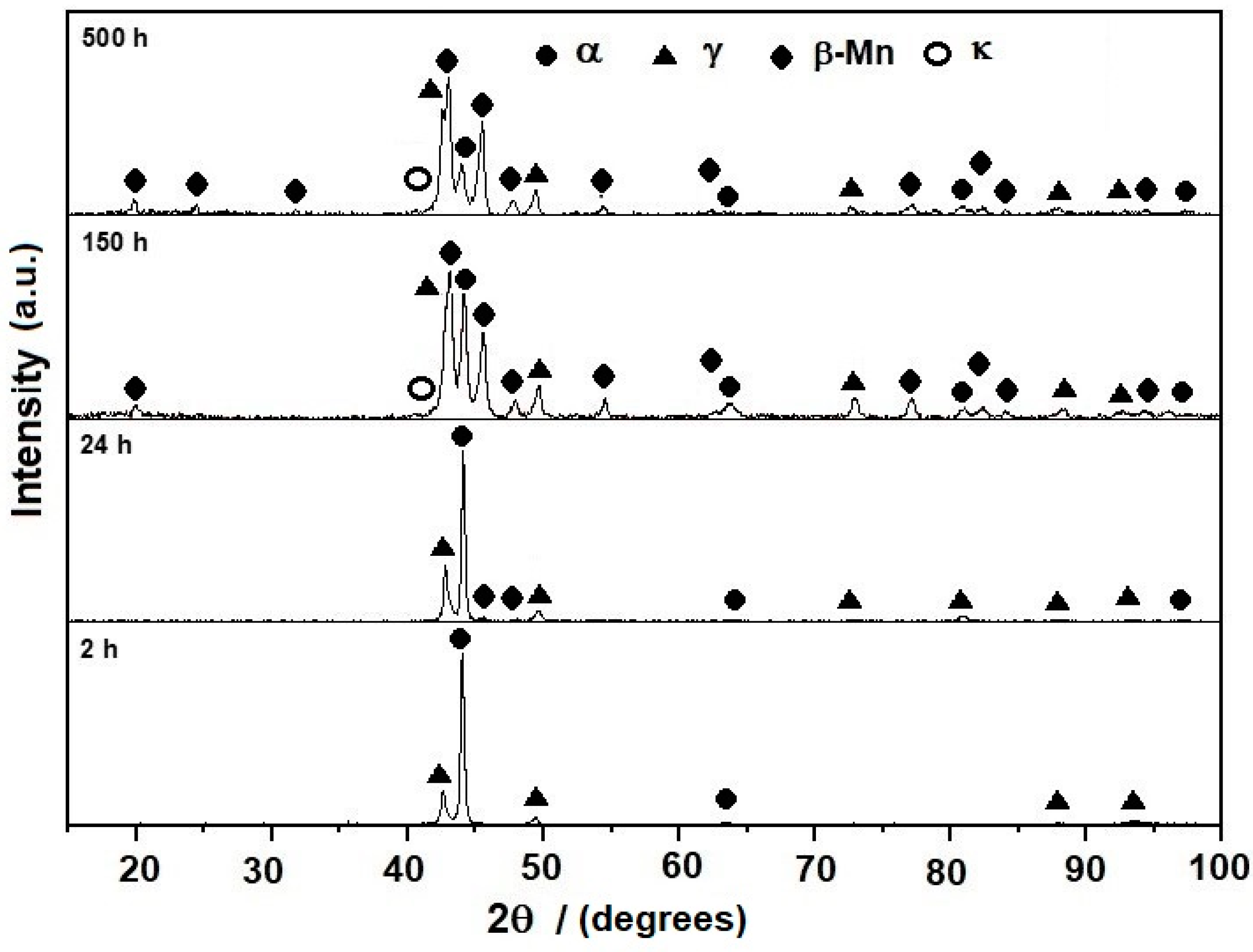
3.1. Microstructure Evolution of As-Cast Steel
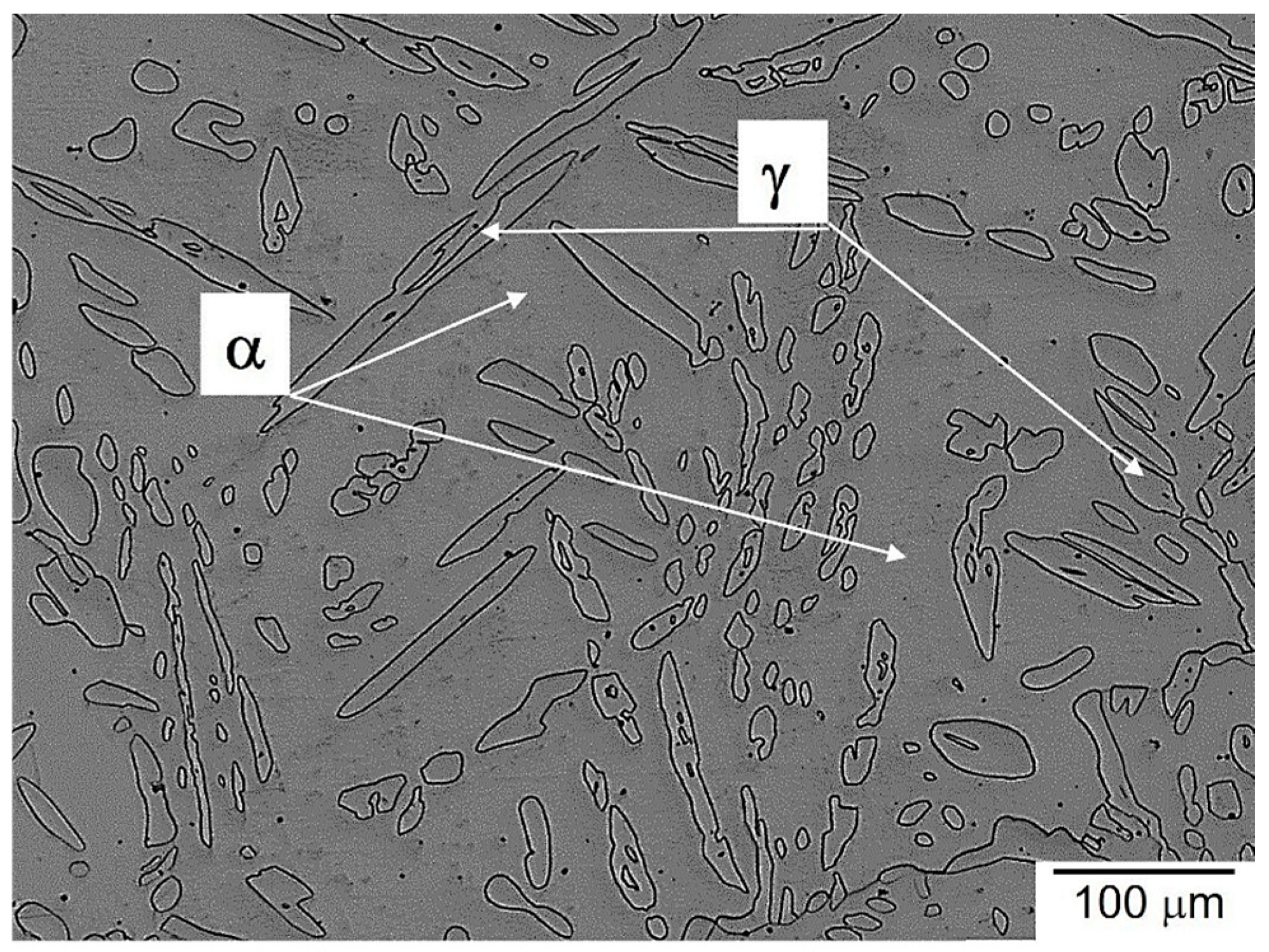
3.2. Microstructure Evolution of Homogenized Steel
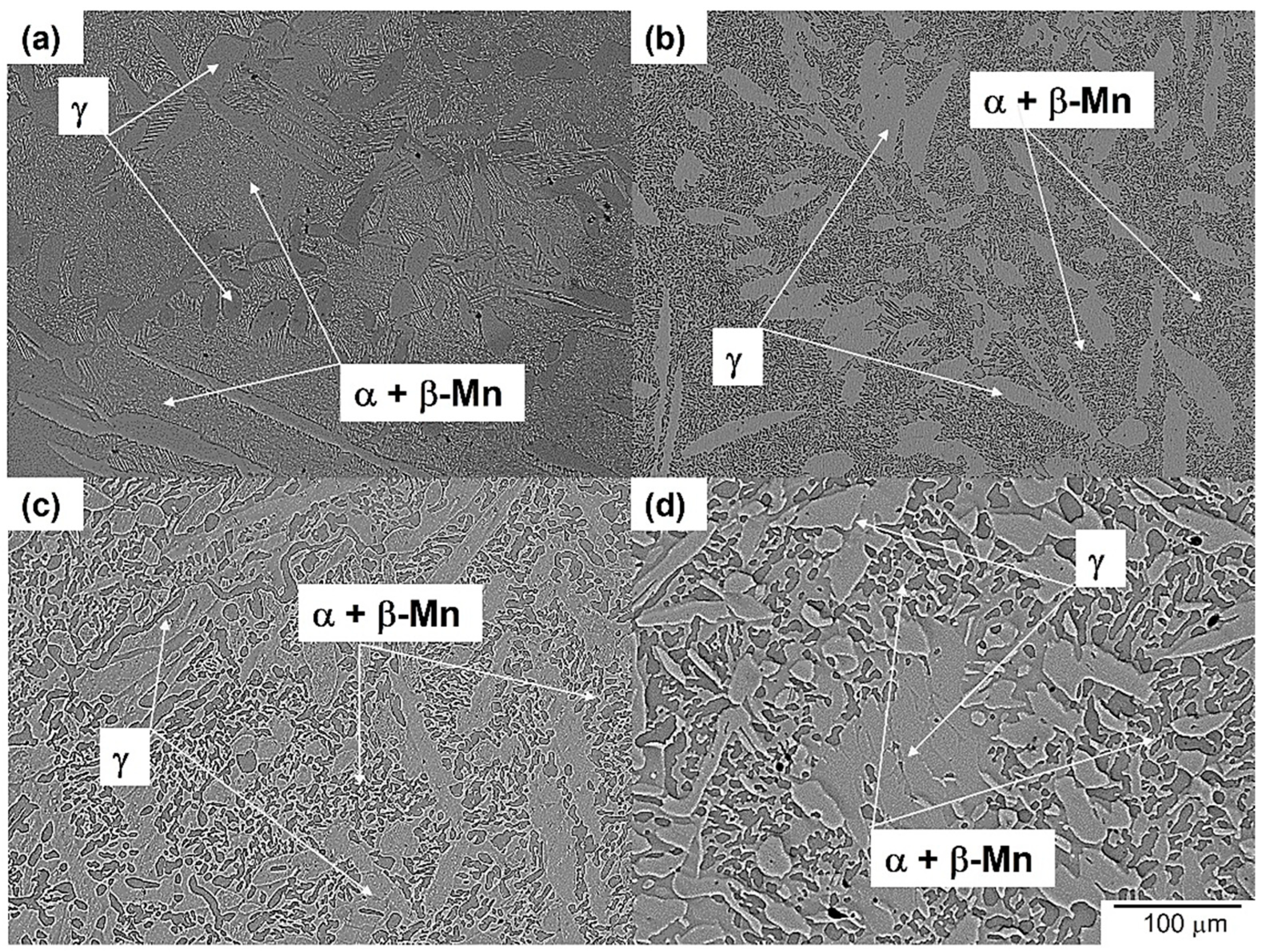
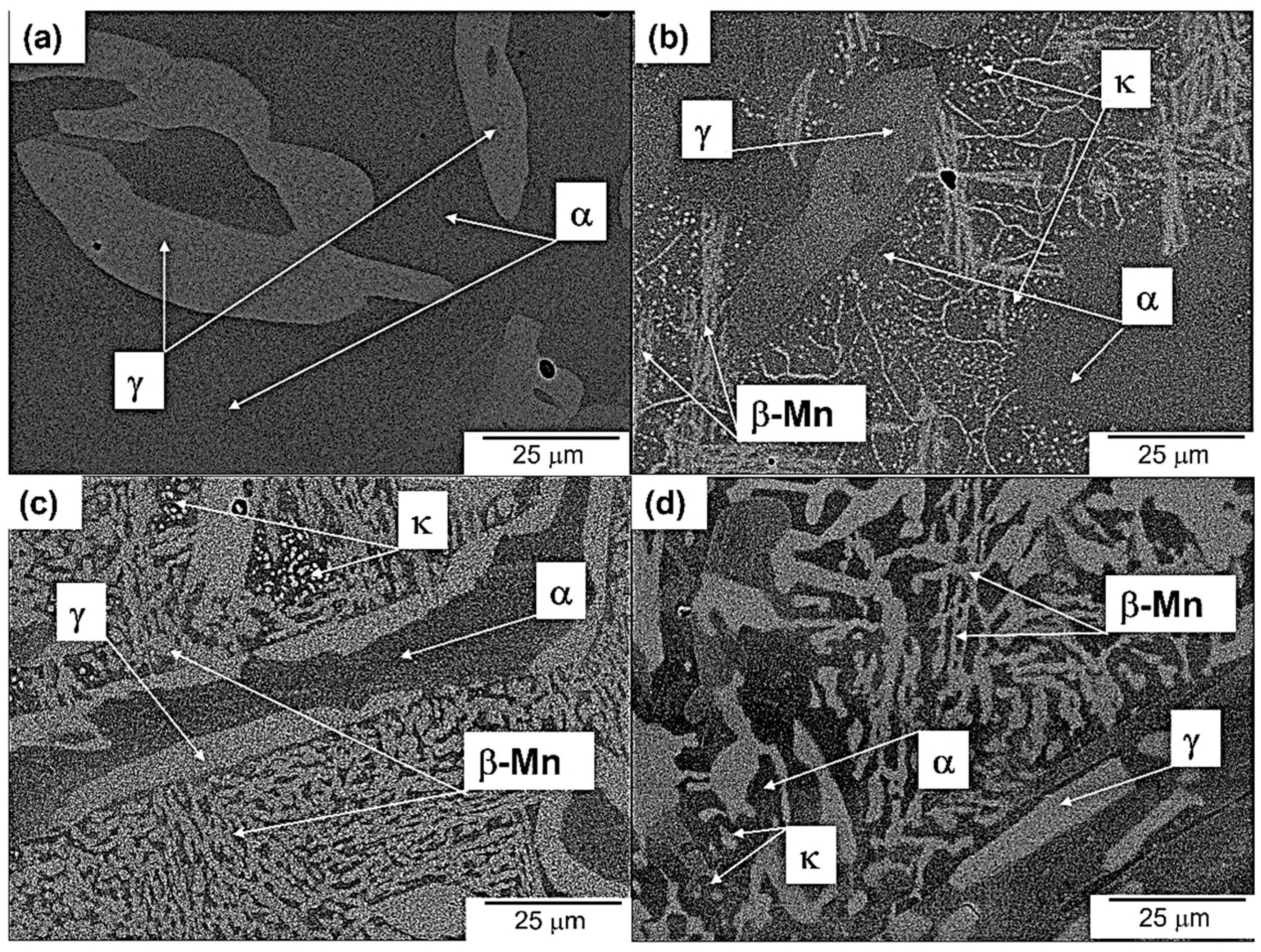
3.3. Microstructure Evolution of Aged Steel
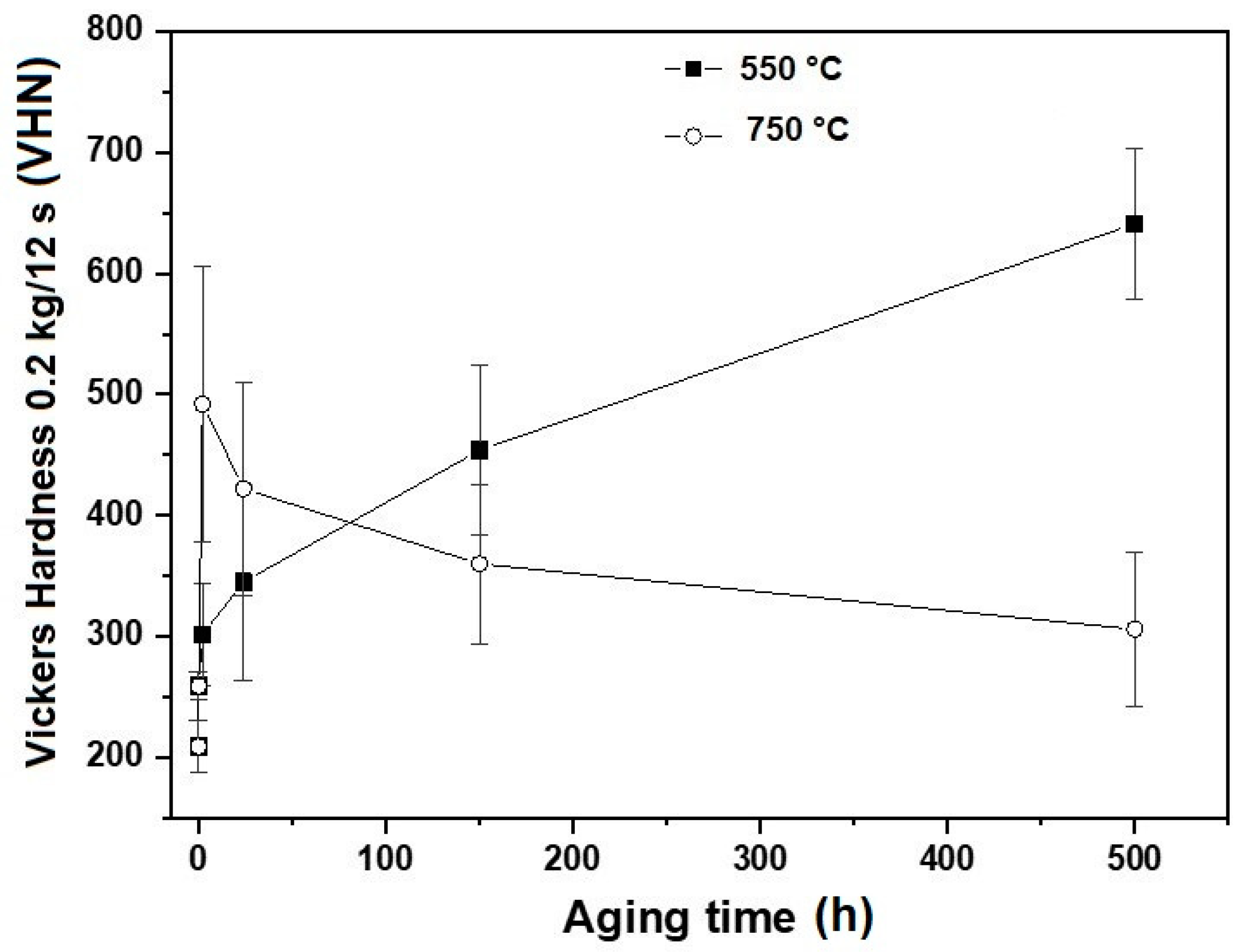
3.4. Relationship beween Mechanical Properties and Microstructure
4. Conclusions
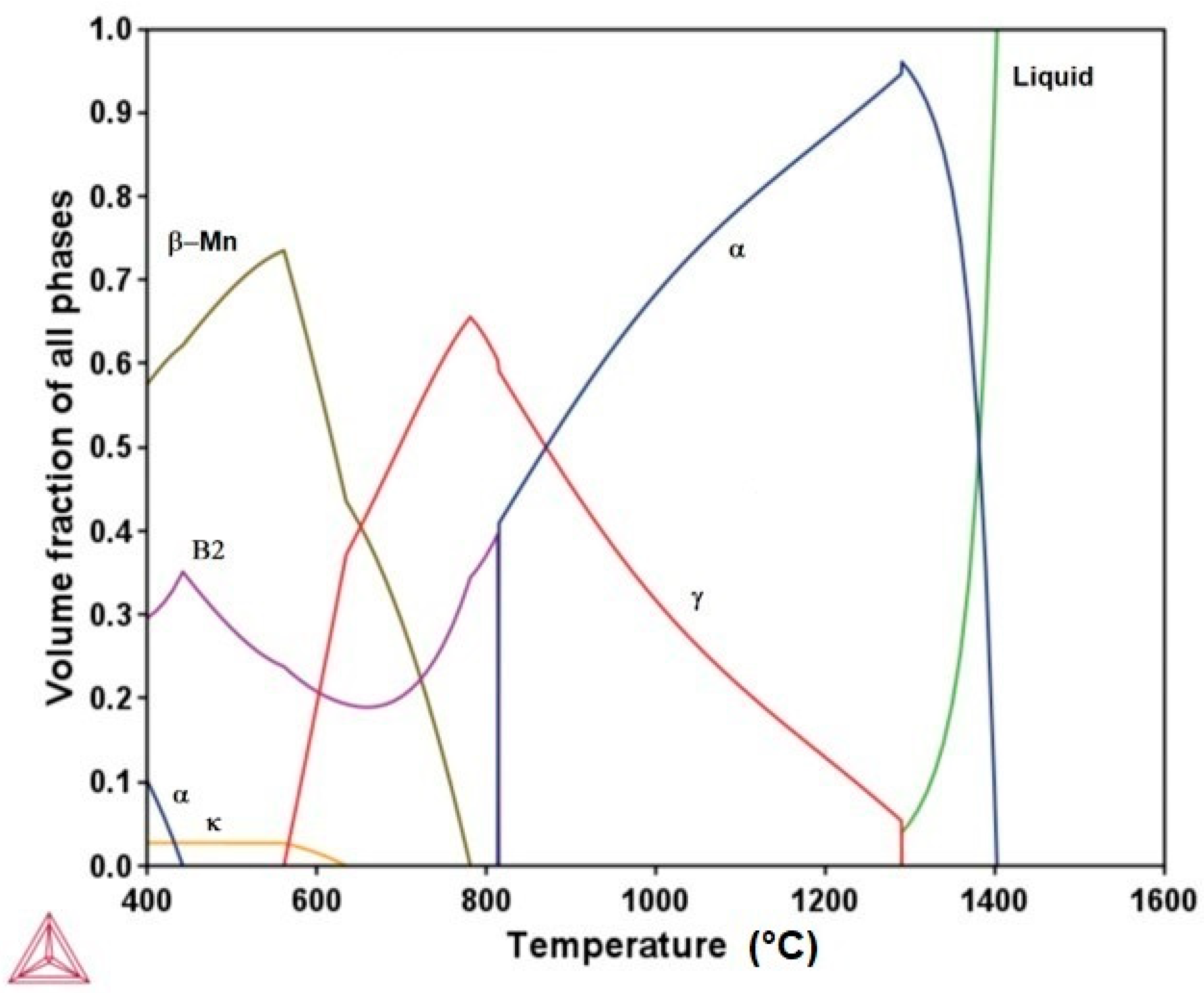
- The microconstituents for the as-cast, homogenizing, and quenching conditions are the α and γ phases. The volume fractions of these phases and their compositions are in good agreement with those predicted by Thermo-Calc. The Vickers hardness increased from 209 VHN in the as-cast condition to 259 VHN in the as-quenched state due to a finer dispersion of the γ phases.
- The aging treatment at 750 °C promoted a decrease in hardness, mainly attributed to an increase in the ductile γ phase, which is softer and tougher than the α phase.
- The aging treatment at 550 °C caused the steel to age-harden because of the formation of fine intragranular κ precipitates, leading to an increase in hardness of up to about 630 VHN.
- TC-PRISMA simulation enabled us to follow the intragranular precipitation growth kinetics of the κ phase based on nucleation, growth, and coarsening mechanisms.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zambrano, O.A. A General perspective of Fe-Mn-Al-C steels. J. Mater. Sci. 2018, 53, 14003–140062. [Google Scholar] [CrossRef]
- Ding, H.; Liu, D.; Cai, M.; Zhang, Y. Austenite-based Fe-Mn-Al-C lightweight steels: Research and Prospective. Metals 2022, 12, 1572. [Google Scholar] [CrossRef]
- Chen, S.; Rana, R.; Haldar, A.; Ray, R.K. Current state of Fe-Mn-Al-C low density steels. Prog. Mater. Sci. 2017, 89, 345–391. [Google Scholar] [CrossRef]
- Frommeyer, G.; Brux, U. Microstructures and mechanical properties of high-strength Fe-Mn-Al-C light-weight TRIPLEX steels. Steel Res. Int. 2006, 77, 627–633. [Google Scholar] [CrossRef]
- Bai, S.; Chen, Y.; Liu, X.; Lu, H.; Bai, P.-K.; Li, D.-Z.; Huang, Z.-Q.; Li, J.-Y. Research status and development prospect of Fe-Mn-C-Al system low-density steels. J. Mater. Sci. Technol. 2023, 25, 1537–1559. [Google Scholar] [CrossRef]
- Ren, P.; Chen, X.P.; Yang, M.J.; Liu, S.M.; Cao, W.Q. Effect of early stage of κ-carbides precipitation on tensile properties and deformation mechanism in high Mn–Al–C austenitic low-density steel. Mater. Sci. Eng. A 2022, 857, 144132. [Google Scholar] [CrossRef]
- An, Y.F.; Chen, X.P.; Mei, L.; Ren, P.; Wei, D.; Cao, W.Q. Precipitation transformation pathway and mechanical behavior of nanoprecipitation strengthened Fe–Mn–Al–C–Ni austenitic low-density steel. J. Mater. Sci. Technol. 2024, 174, 157–167. [Google Scholar] [CrossRef]
- Baniz, A.; Gomez, A.; Dutta, A.; Savirov, I.; Petrov, R.H. The Effect of nano-sized κ-carbides on the mechanical properties of an Fe-Mn-Al-C alloy. Mater. Charact. 2023, 205, 113364. [Google Scholar] [CrossRef]
- Gutierrez-Urrutia, I. Low density Fe–Mn–Al–C steels: Phase structures, mechanisms, and properties. ISIJ Int. 2021, 61, 16–25. [Google Scholar] [CrossRef]
- Cheng, P.; Li, X.; Yi, H. The κ-carbides in low-density Fe-Mn-Al-C steels: A review on their structure, precipitation and deformation mechanism. Metals 2020, 10, 1021. [Google Scholar] [CrossRef]
- Palm, M.; Inden, G. Experimental determination of phase equilibria in the Fe-Al-C system. Intermetall 1995, 3, 443–454. [Google Scholar] [CrossRef]
- Chin, K.-G.; Lee, H.-J.; Kwak, J.-H.; Kang, Y.-H.; Lee, B.-Y. Thermodynamic calculation on the stability of (Fe,Mn)3AlC carbide in high aluminum steels. J. Alloys Compd. 2010, 55, 217–223. [Google Scholar] [CrossRef]
- Man, T.; Wang, W.; Zhou, Y.; Liu, T.; Lu, H.; Zhao, X.; Zhang, M.; Dong, H. Effect of cooling rate on the precipitation behavior of κ-carbide in Fe-32Mn-11Al-0.9C low density steel. Mater. Lett. 2002, 314, 131778. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Liang, J.; Godfrey, A.; Wang, Y. Effect of alloying content on microstructure and mechanical properties of Fe-Mn-Al-C low density steels. Mater. Si Eng. 2023, 876, 145675. [Google Scholar] [CrossRef]
- Lv, X.; Wang, X.; Lang, A.; Qiao, J. Synergistic effect of Al and Ni on microstructure evolutions and mechanical properties of Fe-Mn-Al-C low density steels. Metals 2024, 14, 495. [Google Scholar] [CrossRef]
- Shi, P.; Engstrom, A.; Hoglund, l.; Sundman, B.; Agren, J. Thermo-calc and DICTRA Enhance Materials Design and Processing. Mater. Sci. For. 2005, 475–479, 3339–3347. [Google Scholar]
- Thermo-Calc, Thermo-Calc Versión 2023b. Available online: https://thermocalc.com/products/thermo-calc/ (accessed on 20 June 2024).
- PRISMA, Thermo-Calc versión 2023b. Available online: https://thermocalc.com/products/add-on-modules/precipitation-module-tc-prisma/ (accessed on 20 June 2024).
- Chang, S.C.; Hsiau, Y.H.; Hang, M.T. Tensile and fatigue properties of Fe-Mn-AI-C alloys. J. Mater. Sci. 1989, 24, 1117–1128. [Google Scholar] [CrossRef]
- Sato, K.; Tagawa, K.; Inoue, Y. Modulated properties structure and magnetic of age-hardenable Fe-Mn-AI-C alloys. Met. Trans. A 1990, 24, 5–11. [Google Scholar] [CrossRef]
- Kostorz, G. Phase Transformations in Materials, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2001; pp. 309–408. [Google Scholar]
- Cheng, W.-C. PhasetTransformations of an Fe-0.85C-17.9Mn-7.1Al austenitic steel after quenching and annealing. JOM 2014, 66, 1809–1820. [Google Scholar] [CrossRef]



| Element | Fe | Mn | Al | Si | C |
|---|---|---|---|---|---|
| mass % | 60.69 | 30.31 | 8.84 | 0.31 | 0.15 |
| Method | Phase | Vol. % | % Al | % C | % Mn | % Fe |
|---|---|---|---|---|---|---|
| Thermo-Calc | α | 89.4 | 7.98 | 0.13 | 44.99 | 46.88 |
| γ | 10.6 | 6.28 | 0.61 | 42.97 | 49.07 | |
| Experimental | α | 80.0 | 8.25 | --- | 32.29 | 58.09 |
| γ | 20.0 | 7.25 | --- | 35.44 | 57.45 |
| Method | Phase | Vol. % | % Al | % Mn | % Fe |
|---|---|---|---|---|---|
| Thermo-Calc | α | 78.6 | 9.53 | 28.44 | 61.74 |
| γ | 21.4 | 7.36 | 34.28 | 57.50 | |
| Experimental | α | 70.0 | 7.37 | 32.29 | 58.09 |
| γ | 30.0 | 9.06 | 29.73 | 56.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Hirata, V.M.; Perez-Badillo, E.; Saucedo-Muñoz, M.L.; Hernandez-Santiago, F.; Villegas-Cardenas, J.D. Phase Transformations after Heat Treating an As-Cast Fe-30Mn-8.8Al-0.3Si-0.15C Steel. Metals 2024, 14, 748. https://doi.org/10.3390/met14070748
Lopez-Hirata VM, Perez-Badillo E, Saucedo-Muñoz ML, Hernandez-Santiago F, Villegas-Cardenas JD. Phase Transformations after Heat Treating an As-Cast Fe-30Mn-8.8Al-0.3Si-0.15C Steel. Metals. 2024; 14(7):748. https://doi.org/10.3390/met14070748
Chicago/Turabian StyleLopez-Hirata, Victor M., Eduardo Perez-Badillo, Maribel Leticia Saucedo-Muñoz, Felipe Hernandez-Santiago, and Jose David Villegas-Cardenas. 2024. "Phase Transformations after Heat Treating an As-Cast Fe-30Mn-8.8Al-0.3Si-0.15C Steel" Metals 14, no. 7: 748. https://doi.org/10.3390/met14070748
APA StyleLopez-Hirata, V. M., Perez-Badillo, E., Saucedo-Muñoz, M. L., Hernandez-Santiago, F., & Villegas-Cardenas, J. D. (2024). Phase Transformations after Heat Treating an As-Cast Fe-30Mn-8.8Al-0.3Si-0.15C Steel. Metals, 14(7), 748. https://doi.org/10.3390/met14070748






