Study on the Influence of Surface Roughness and Temperature on the Interface Void Closure and Microstructure Evolution of Stainless Steel Diffusion Bonding Joints
Abstract
:1. Introduction
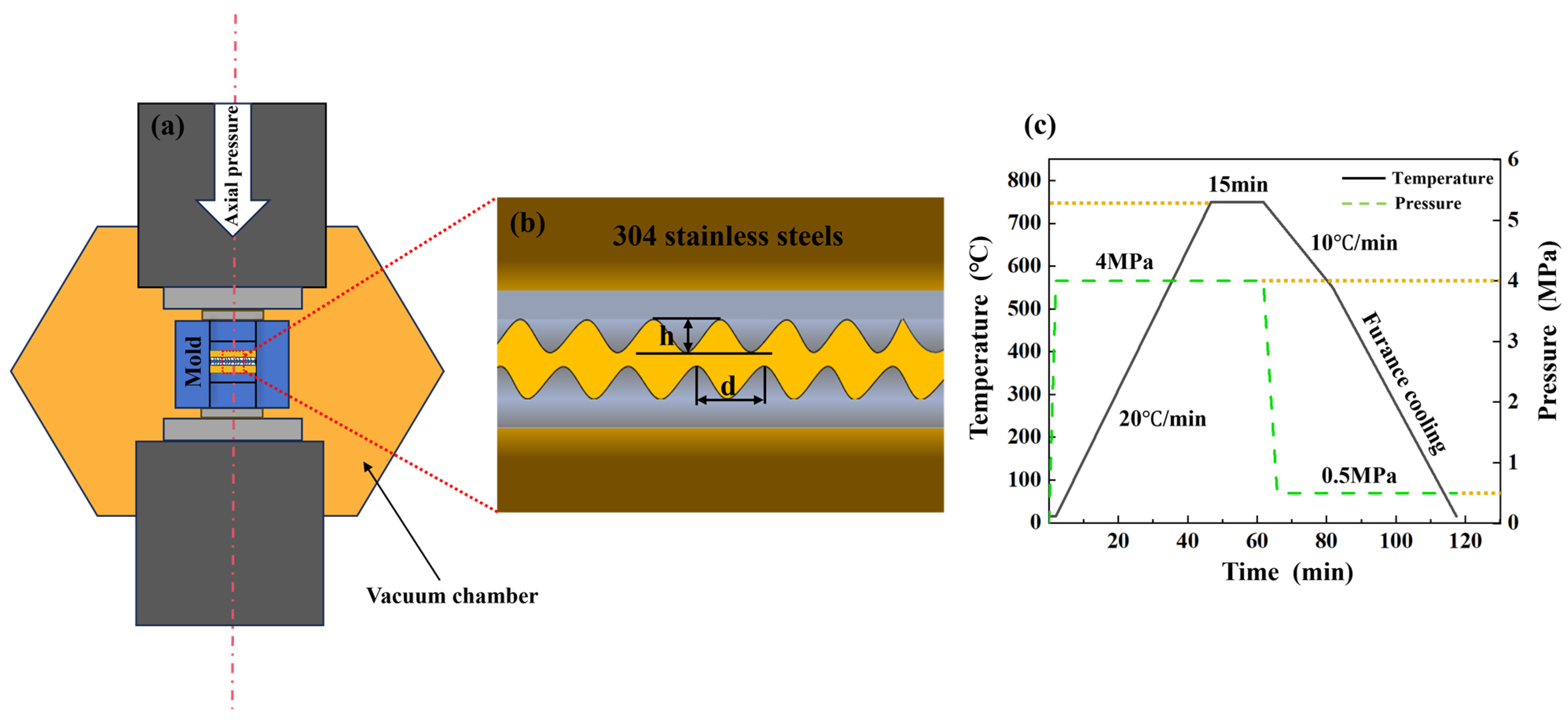
2. Experimental and Procedures
2.1. Materials and Experimental Parameters
2.2. Microstructure Characterization
3. Results and Discussion
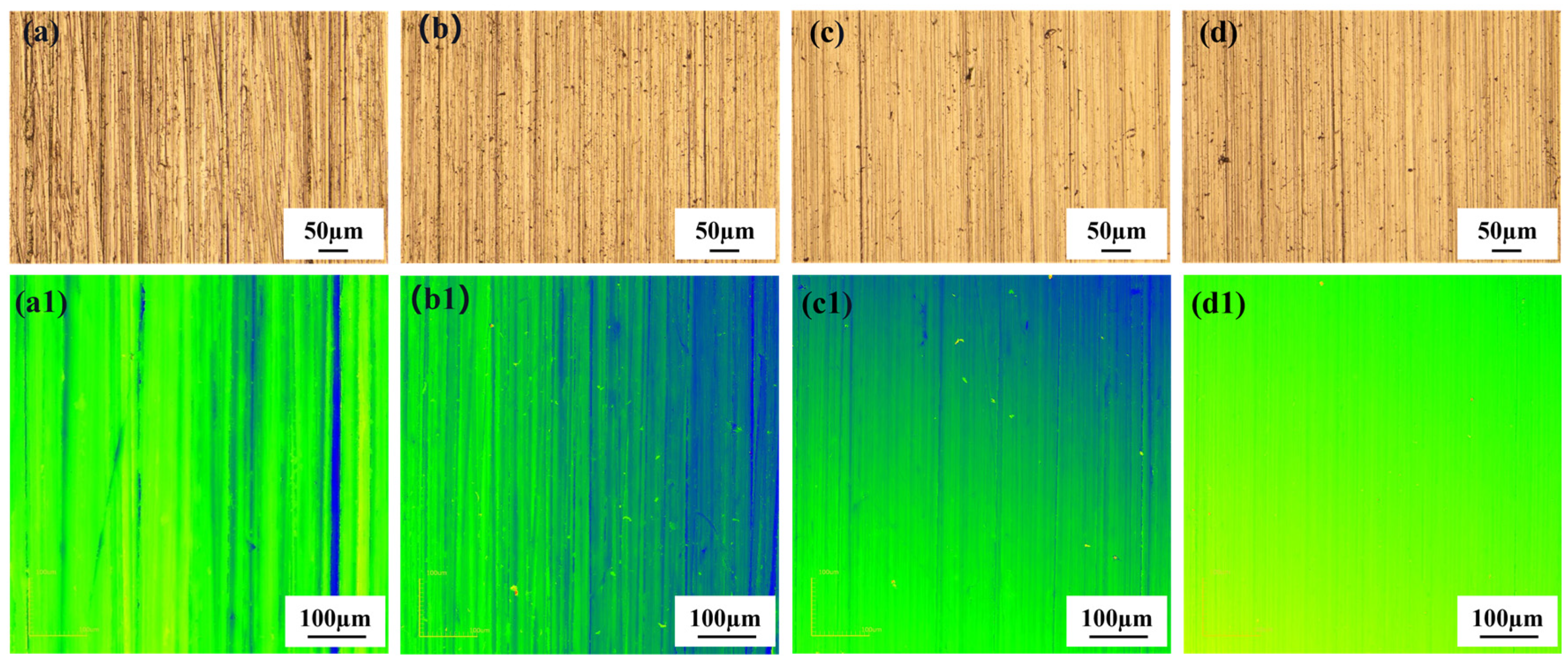
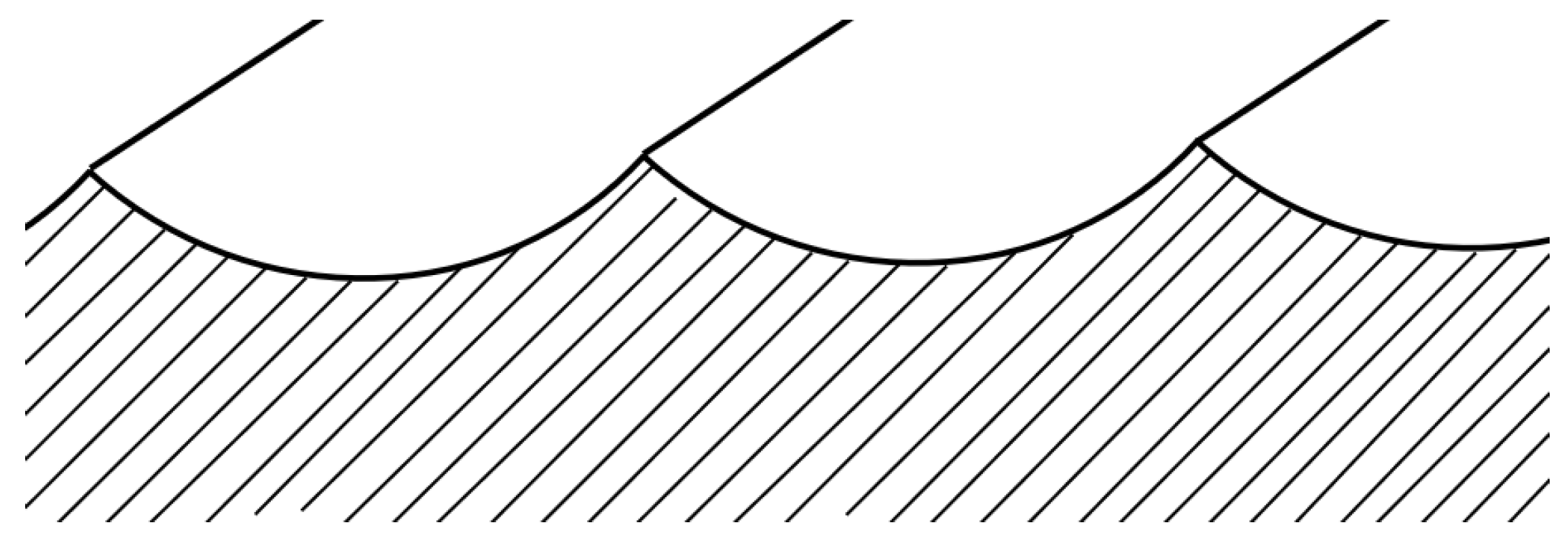
3.1. Initial Surface Characterization
3.2. Microstructure of the Diffusion Bonding Interface
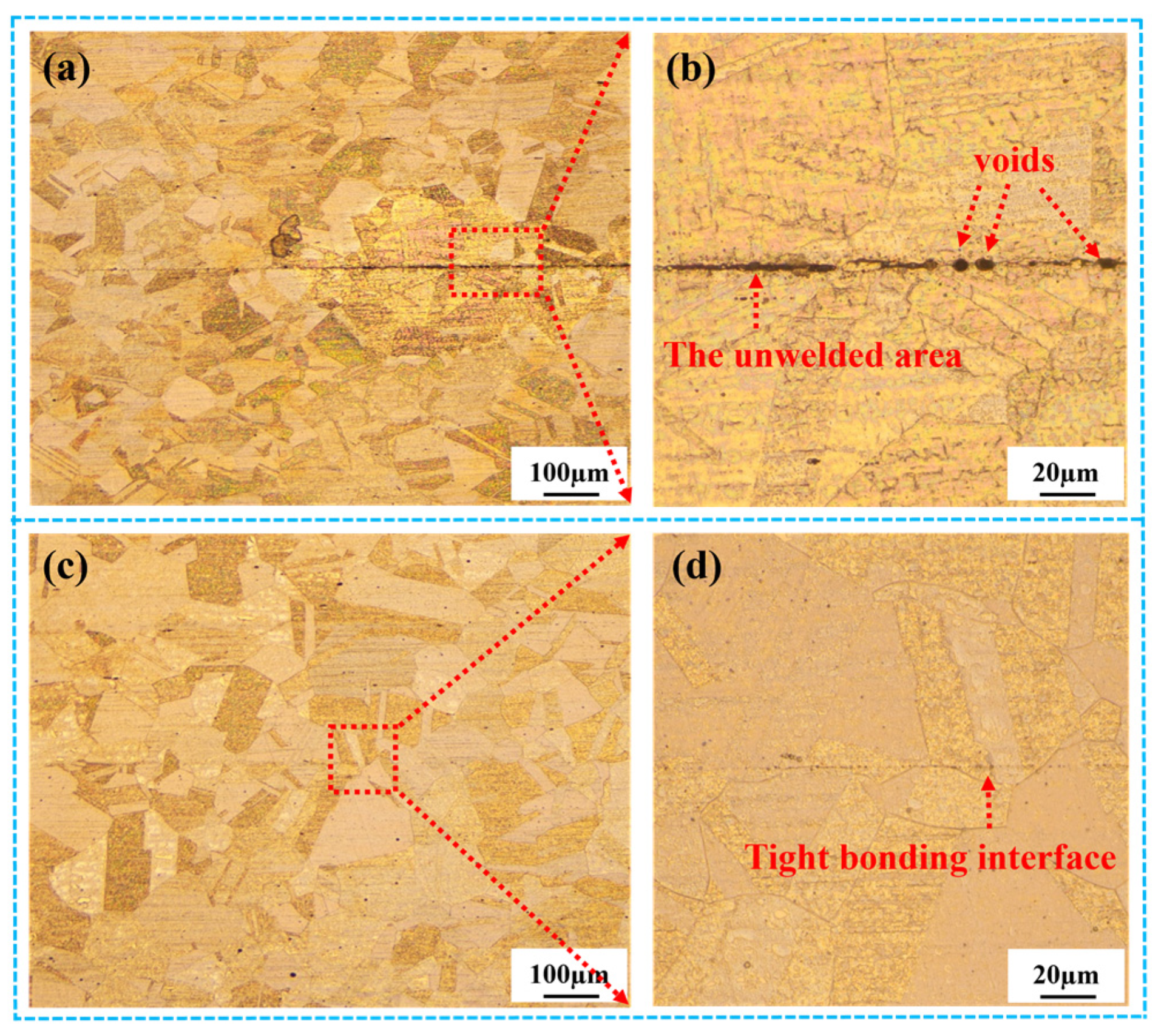
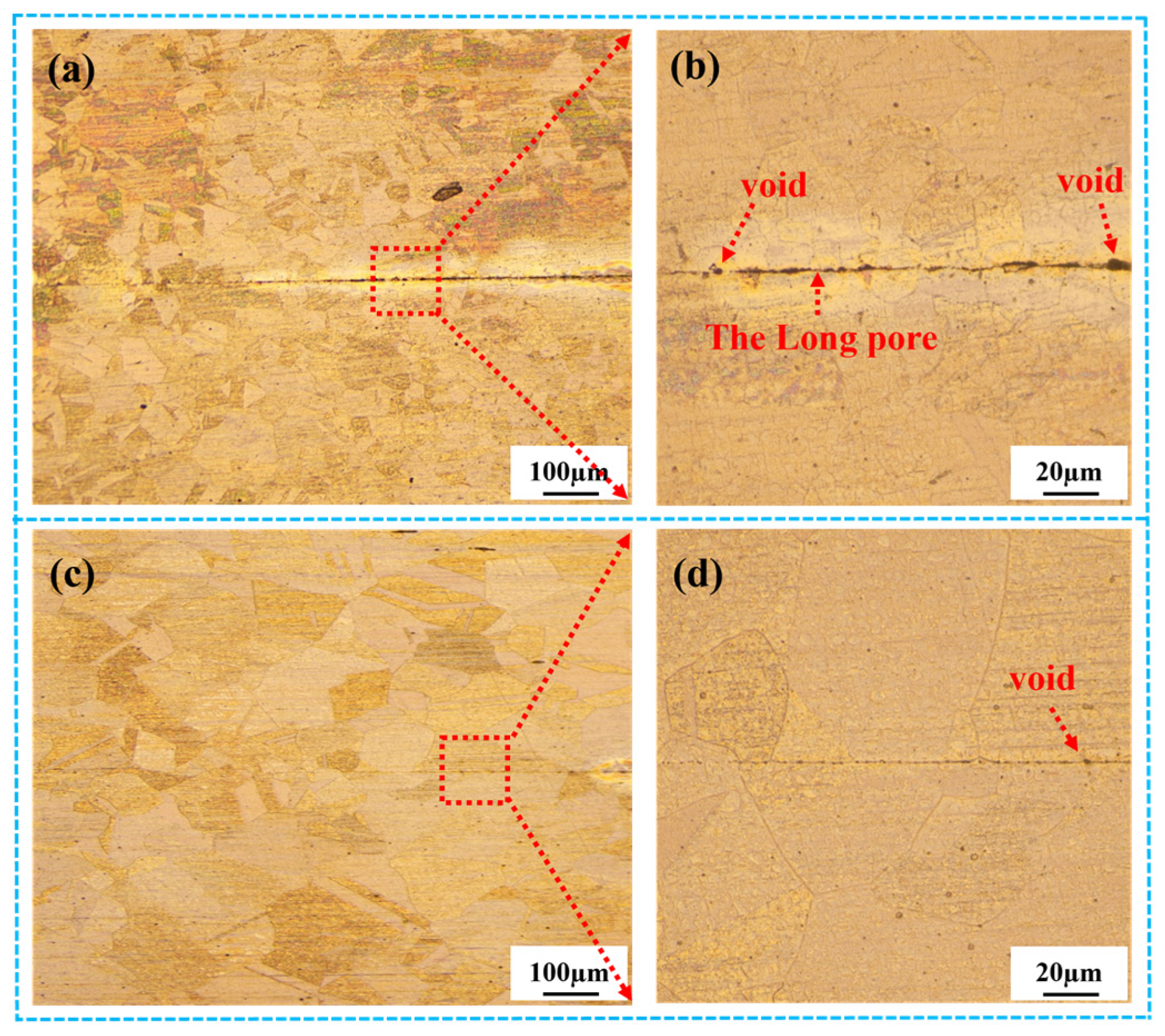
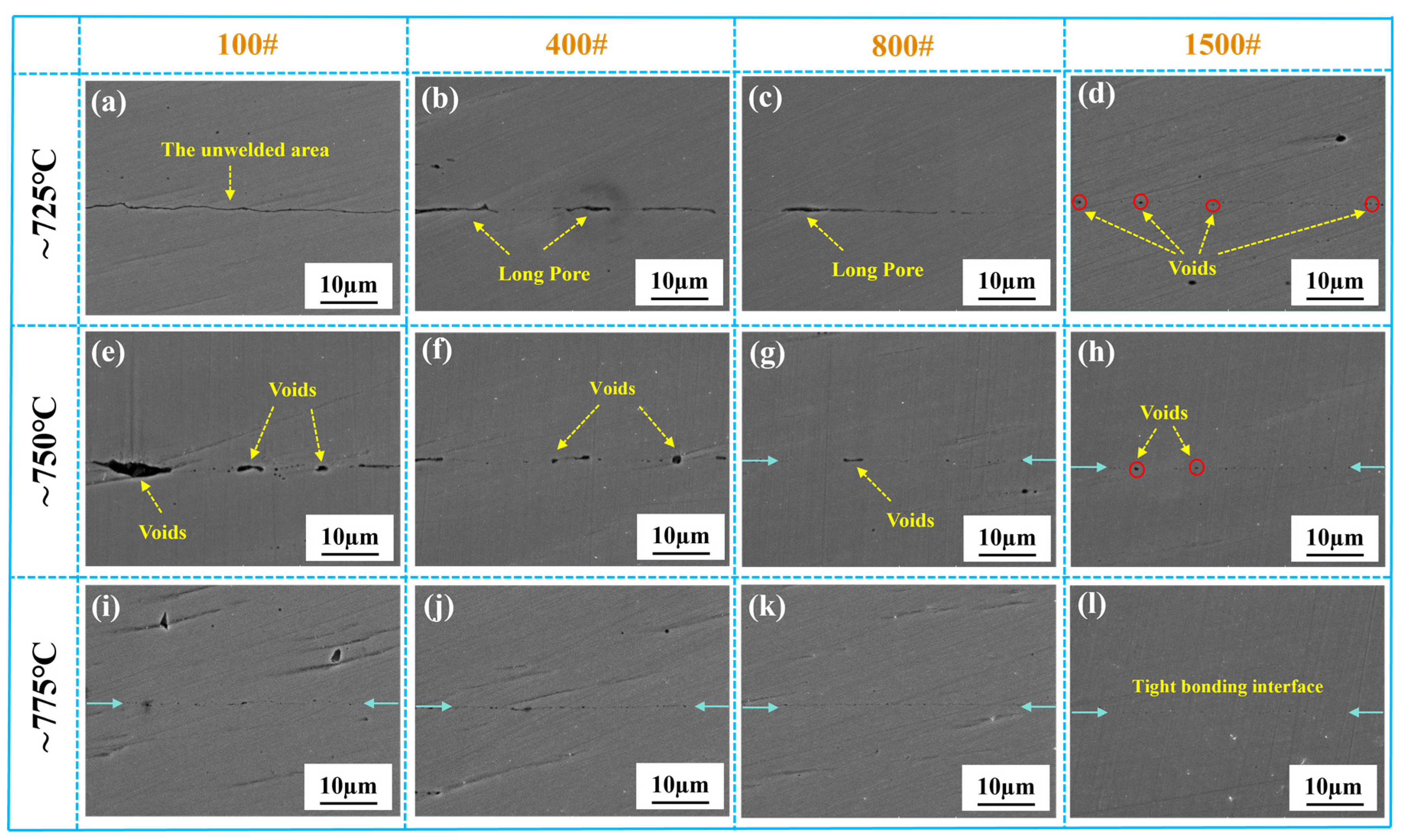
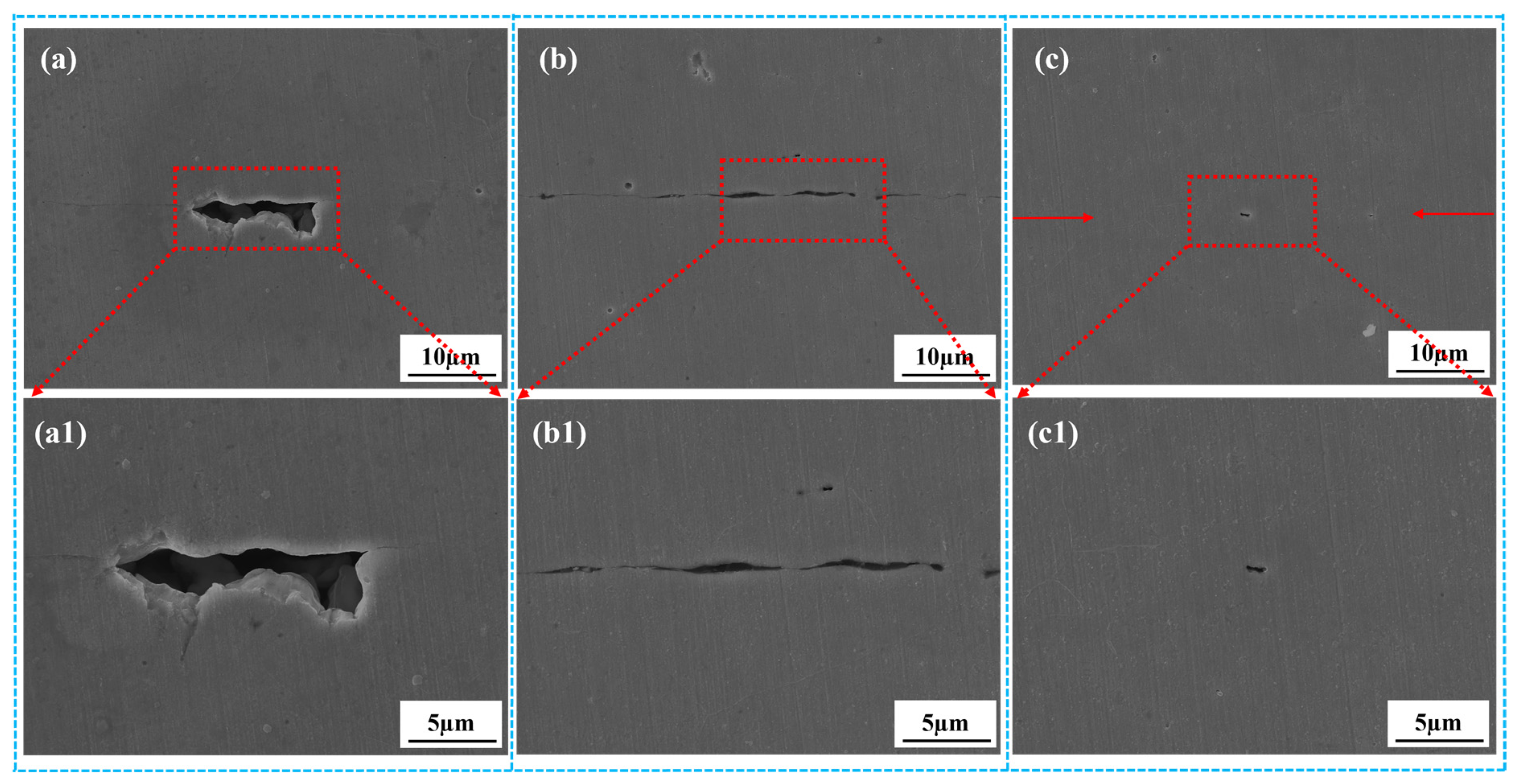
3.2.1. Optical Microstructure of the Stainless Steel Bonding Interface
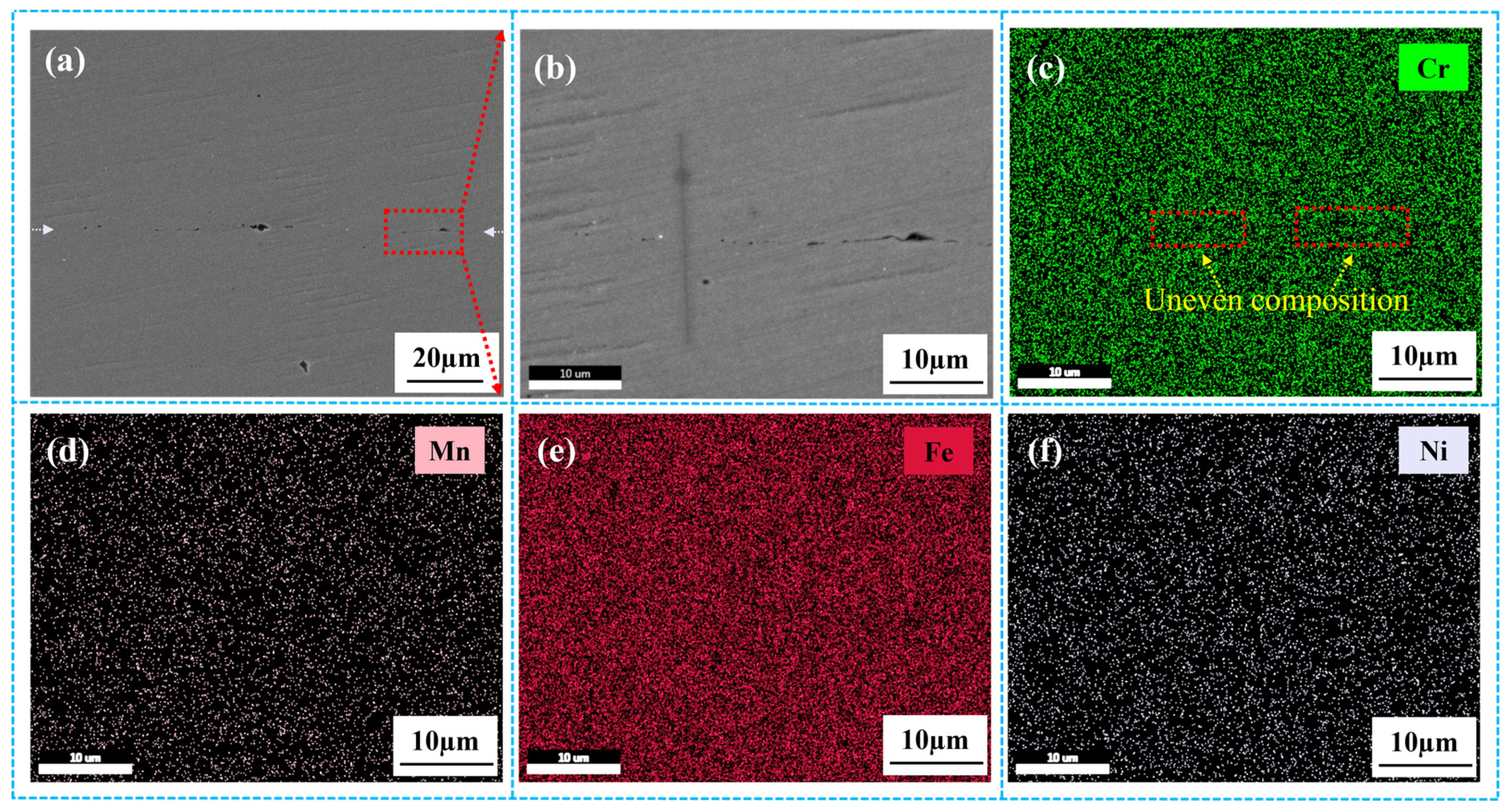
3.2.2. Microstructure and Elemental Analysis
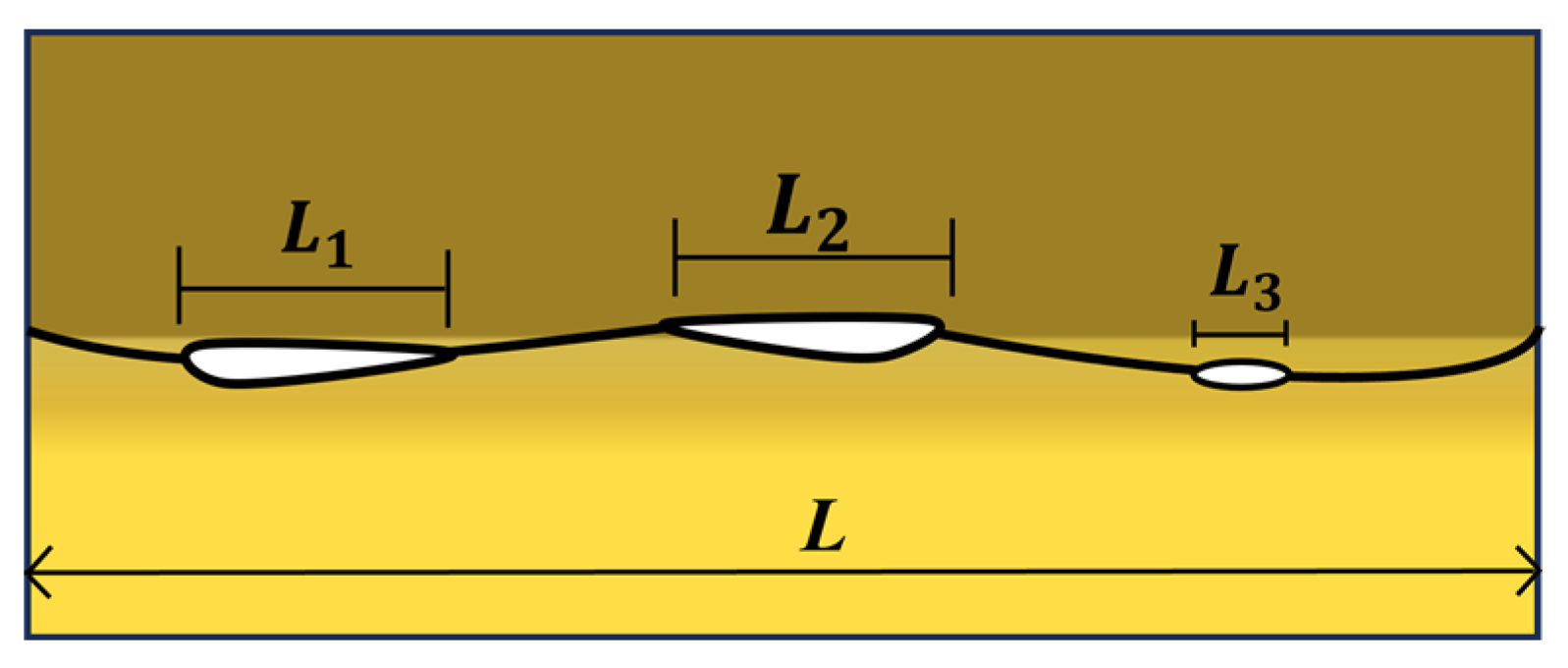
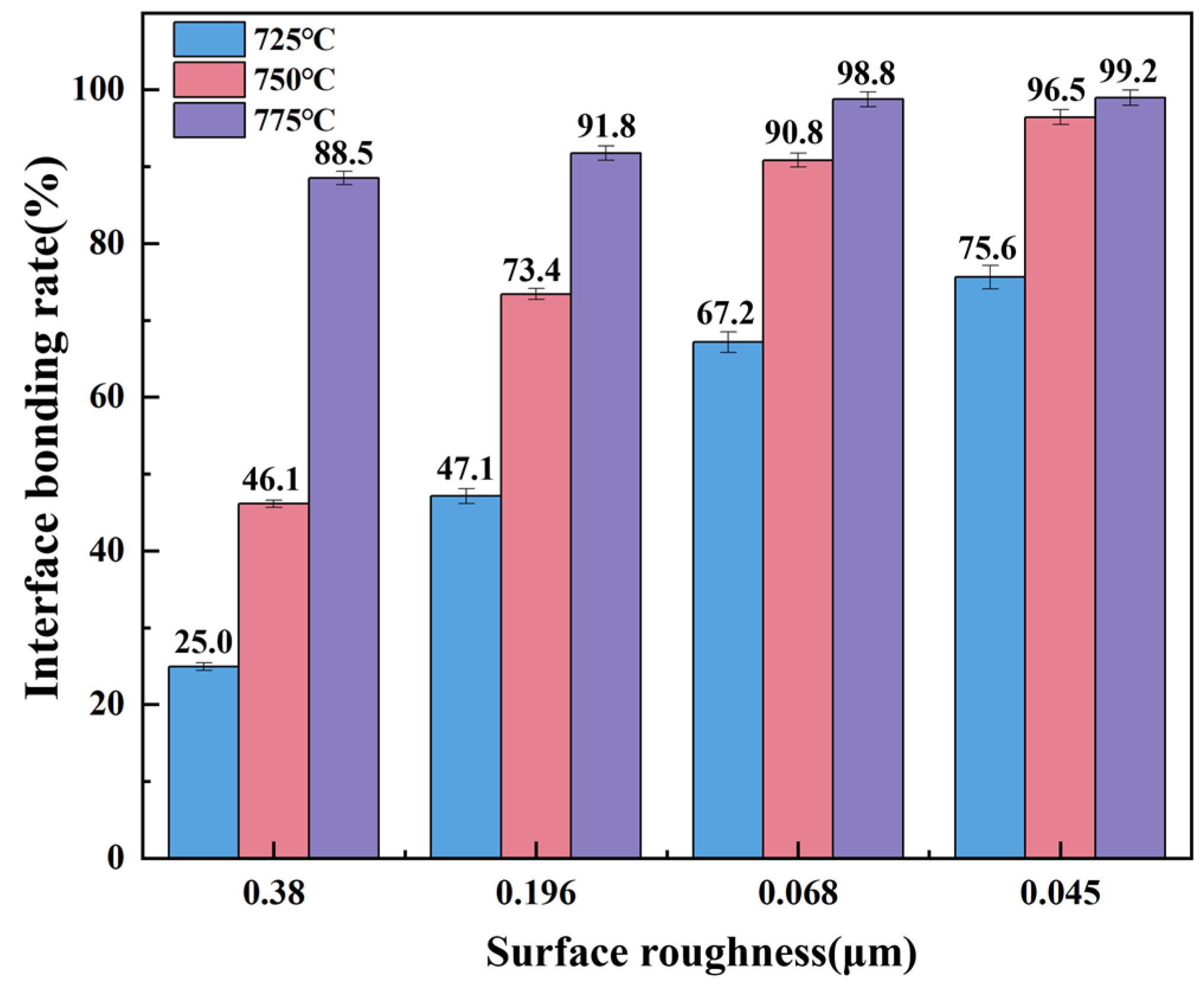
3.2.3. Interface Bonding Rate under Different Bonding Parameters
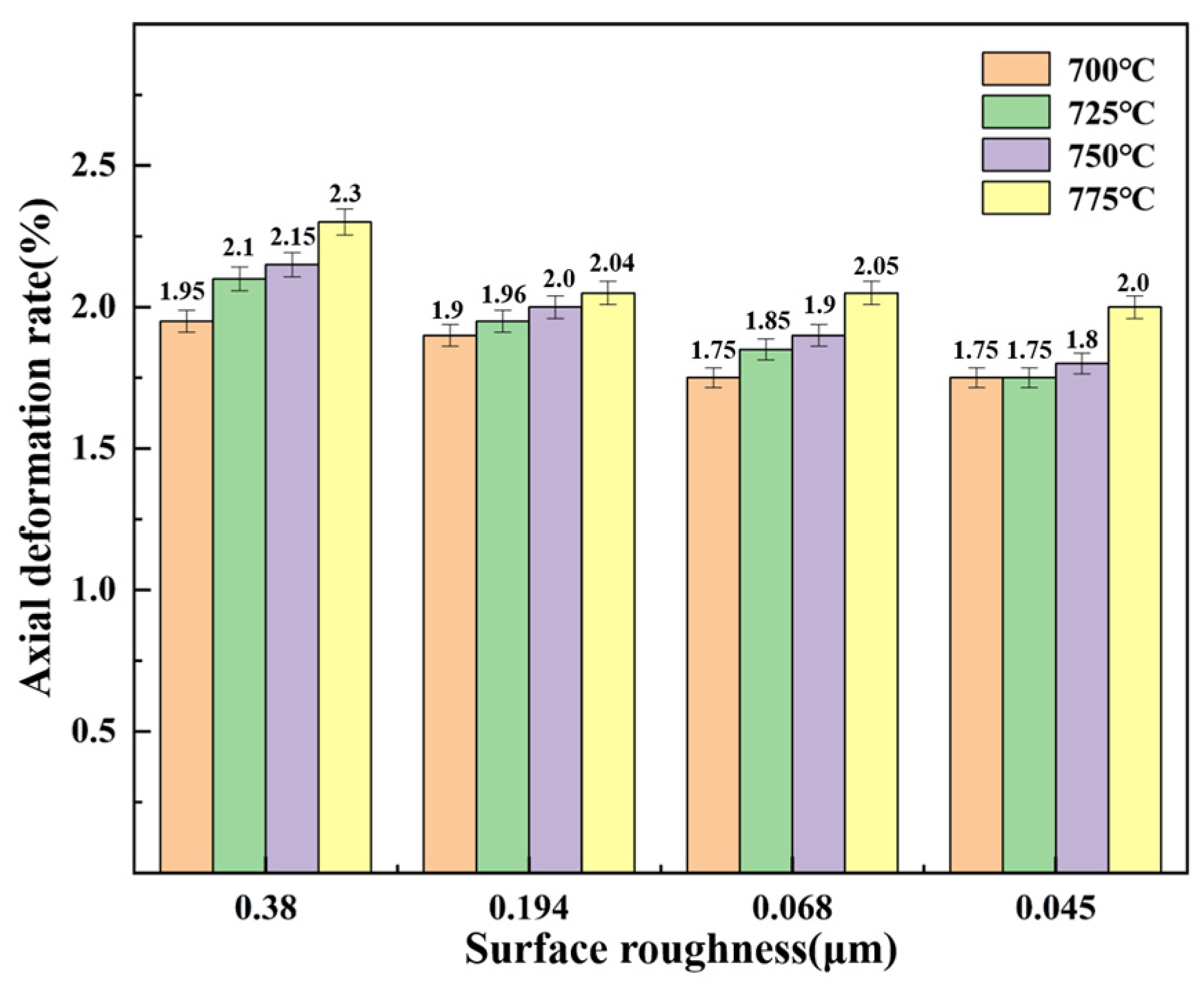
3.3. Axial Deformation Rate of Stainless Steel Diffusion-Bonded Joint
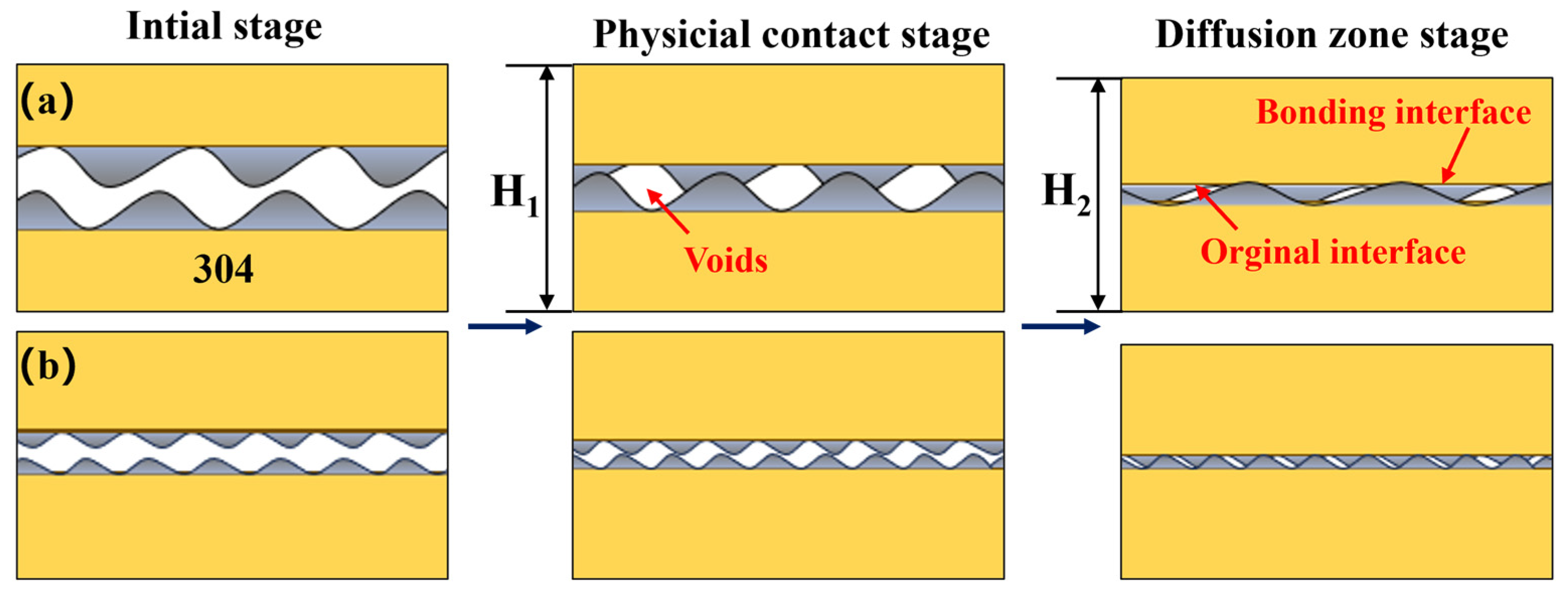
3.4. Mechanism of Hole Closure at Diffusion Bonding Interfaces
3.4.1. Mechanisms of Plastic Deformation
3.4.2. Creep Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saranam, V.R.; Mullany, B.; Tabei, A.; Srenevas, S.; Evans, C.; Paul, B.K. Surface topographical effects in the diffusion bonding of 316 stainless steel. J. Mater. Process. Technol. 2021, 296, 117173. [Google Scholar] [CrossRef]
- Lin, T.; Li, C.; Chen, Y.; Chen, L.; Si, X.; Qi, J.; Cao, J. Role of nanostructured Ni surface layer in enhanced Hastelloy alloy diffusion bonding at temperatures far beyond recrystallization. Scr. Mater. 2024, 239, 115826. [Google Scholar] [CrossRef]
- Li, W.; Liang, Y.; Bai, Y.; Lin, T.; Li, B.; Fu, Z.; Feng, J. Realizing high-strength diffusion bonding of copper at ultra-low temperatures via single point diamond turning and spark plasma sintering. Mater. Sci. Eng. A 2022, 857, 144116. [Google Scholar] [CrossRef]
- Yang, J.; He, Y.; Zhang, X.; Yang, W.; Li, Y.; Li, X.; Chen, Q.; Chen, X.; Du, K.; Yan, Y. Improving the electrical conductivity of copper/graphene composites by reducing the interfacial impurities using spark plasma sintering diffusion bonding. J. Mater. Res. Technol. 2021, 15, 3005–3015. [Google Scholar] [CrossRef]
- Huang, W.C.; Tsai, C.H.; Lee, P.T.; Kao, C. Effects of bonding pressures on microstructure and mechanical properties of silver–tin alloy powders synthesized by ball milling for high-power electronics packaging. J. Mater. Res. Technol.-JMRT 2022, 19, 3828–3841. [Google Scholar] [CrossRef]
- Dunkerton, S.B. Diffusion bonding—An overview. In Diffusion Bonding 2; Springer: Berlin/Heidelberg, Germany, 1991; pp. 1–12. [Google Scholar] [CrossRef]
- Singh, A.K.; Quraishi, M. The effect of some bis-thiadiazole derivatives on the corrosion of mild steel in hydrochloric acid. Corros. Sci. 2010, 52, 1373–1385. [Google Scholar] [CrossRef]
- Ghelichkhah, Z.; Dehkharghani, F.K.; Sharifi-Asl, S.; Obot, I.; Macdonald, D.D.; Farhadi, K.; Avestan, M.S.; Petrossians, A. The inhibition of type 304LSS general corrosion in hydrochloric acid by the New Fuchsin compound. Corros. Sci. 2021, 178, 109072. [Google Scholar] [CrossRef]
- Li, Q.; Li, J.; He, G. Compressive properties and damping capacities of magnesium reinforced with continuous steel wire. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2017, 680, 92–96. [Google Scholar] [CrossRef]
- Hinojos, A.; Mireles, J.; Reichardt, A.; Frigola, P.; Hosemann, P.; Murr, L.E.; Wicker, R.B. Joining of Inconel 718 and 316 Stainless Steel using electron beam melting additive manufacturing technology. Mater. Des. 2016, 94, 17–27. [Google Scholar] [CrossRef]
- Gietzelt, T.; Toth, V.; Huell, A.; Messerschmidt, F.; Dittmeyer, R. Systematic Investigation of the Diffusion Welding Behavior of the Austenitic Stainless Steel 304. Adv. Eng. Mater. 2014, 16, 1381–1390. [Google Scholar] [CrossRef]
- Kurt, B.; Orhan, N.; Kaya, M. Interface characterisation of diffusion bonded Ti–6Al–4V alloy and austenitic stainless steel couple. Mater. Sci. Technol. 2009, 25, 556–560. [Google Scholar] [CrossRef]
- Gao, D.; Li, C.; Zhang, C.; Yang, B.; Lin, T.; Chen, L.; Si, X.; Qi, J.; Cao, J. Microstructure and wettability of the micro-laminated Ti6Al4V/304 stainless steel composite fabricated by diffusion bonding. J. Mater. Res. Technol. 2023, 27, 3788–3796. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, M.; Zhang, B.; Li, H. Growth behavior of interface microstructure between stainless steel and carbon steel during bonding process. Mater. Today Commun. 2022, 33, 104647. [Google Scholar] [CrossRef]
- Li, P.; Li, C.; Dong, H.; Wu, B.; Ma, Y.; Zou, C.; Yang, Y. Vacuum diffusion bonding of TC4 titanium alloy to 316L stainless steel with AlCoCrCuNi2 high-entropy alloy interlayer. J. Alloys Compd. 2022, 909, 164698. [Google Scholar] [CrossRef]
- Gietzelt, T.; Walter, M.; Toth, V.; Messerschmidt, F.; Blem, M. Comprehensive study of the influence of the bonding temperature and contact pressure regimes during diffusion bonding on the deformation and mechanical properties of AISI 304. Adv. Eng. Mater. 2021, 23, 2100188. [Google Scholar] [CrossRef]
- Li, H.; Li, M.Q.; Kang, P.J. Void shrinking process and mechanisms of the diffusion bonded Ti–6Al–4V alloy with different surface roughness. Appl. Phys. A 2016, 122, 18. [Google Scholar] [CrossRef]
- Ma, R.F.; Li, M.Q.; Li, H.; Yu, W.X. Modeling of void closure in diffusion bonding process based on dynamic conditions. Sci. China Technol. Sci. 2012, 55, 2420–2431. [Google Scholar] [CrossRef]
- Negemiya, A.A.; Rajakumar, S.; Balasubramanian, V. High-temperature diffusion bonding of austenitic stainless steel to titanium dissimilar joints. Mater. Res. Express 2019, 6, 066572. [Google Scholar] [CrossRef]
- Dwivedi, D.K. Dissimilar Metal Joining; Springer Nature: Dordrecht, The Netherlands, 2023; pp. 245–267. [Google Scholar]
- Leo, J.R.d.O.; Fitzpatrick, M.E. Development of a Diffusion Bonding Route for Joining Oxide-Dispersion-Strengthened (ODS) Steels for Nuclear Applications. Metall. Mater. Trans. A-Phys. Metall. Mater. Sci. 2024, 55, 827–838. [Google Scholar] [CrossRef]
- Sharma, G.; Dwivedi, D.K. Diffusion bonding of 304 austenitic stainless-steel using pressure pulses. Mater. Today 2021, 44, 2135–2141. [Google Scholar] [CrossRef]
- Ferguson, B.; Ramulu, M. Surface tracking of diffusion bonding void closure and its application to titanium alloys. Int. J. Mater. Form. 2020, 13, 517–531. [Google Scholar] [CrossRef]
- Hua, L.; Liu, Y.; Qian, D.; Xie, L.; Wang, F.; Wu, M. Mechanism of void healing in cold rolled aeroengine M50 bearing steel under electroshocking treatment: A combined experimental and simulation study. Mater. Charact. 2022, 185, 111736. [Google Scholar] [CrossRef]
- Derby, B.; Wallach, E.R. Theoretical model for diffusion bonding. Met. Sci. 1982, 16, 49–56. [Google Scholar] [CrossRef]
- Hill, A.; Wallach, E. Modelling solid-state diffusion bonding. Acta Met. 1989, 37, 2425–2437. [Google Scholar] [CrossRef]
- Mo, D.-F.; Song, T.-F.; Fang, Y.-J.; Jiang, X.-S.; Luo, C.Q.; Simpson, M.D.; Luo, Z.-P. A Review on Diffusion Bonding between Titanium Alloys and Stainless Steels. Adv. Mater. Sci. Eng. 2018, 2018, 8701890. [Google Scholar] [CrossRef]
- Cao, M.; Wang, C.-J.; Deng, K.-K.; Nie, K.-B.; Liang, W.; Wu, Y.-C. Effect of interface on mechanical properties and formability of Ti/Al/Ti laminated composites. J. Mater. Res. Technol.-JMRT. 2021, 14, 1655–1669. [Google Scholar] [CrossRef]
- Sauvage, X.; Dinda, G.; Wilde, G. Non-equilibrium intermixing and phase transformation in severely deformed Al/Ni multilayers. Scr. Mater. 2007, 56, 181–184. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, J.; Wang, J.; Han, Y.; Yu, Z.; Wang, Q.; Zhang, P.; Yang, S. Effects of solder thickness on interface behavior and nanoindentation characteristics in Cu/Sn/Cu microbumps. Weld. World 2022, 66, 973–983. [Google Scholar] [CrossRef]
- Chen, H.; Cao, J.; Liu, J.; Song, X.; Feng, J. Contributions of atomic diffusion and plastic deformation to the diffusion bonding of metallic glass to crystalline aluminum alloy. Comput. Mater. Sci. 2013, 71, 179–183. [Google Scholar] [CrossRef]
- Johnson, W.; Sowerby, R.; Venter, R.D. Plane-Strain Slip-Line Fields for Metal-Deformation Processes; British Library Cataloguing in Publication Data; British Library: London, UK, 2013. [Google Scholar] [CrossRef]
- Sargent, P.M.; Ashby, M.F. Deformation maps for titanium and zirconium. Scr. Met. 1982, 16, 1415–1422. [Google Scholar] [CrossRef]


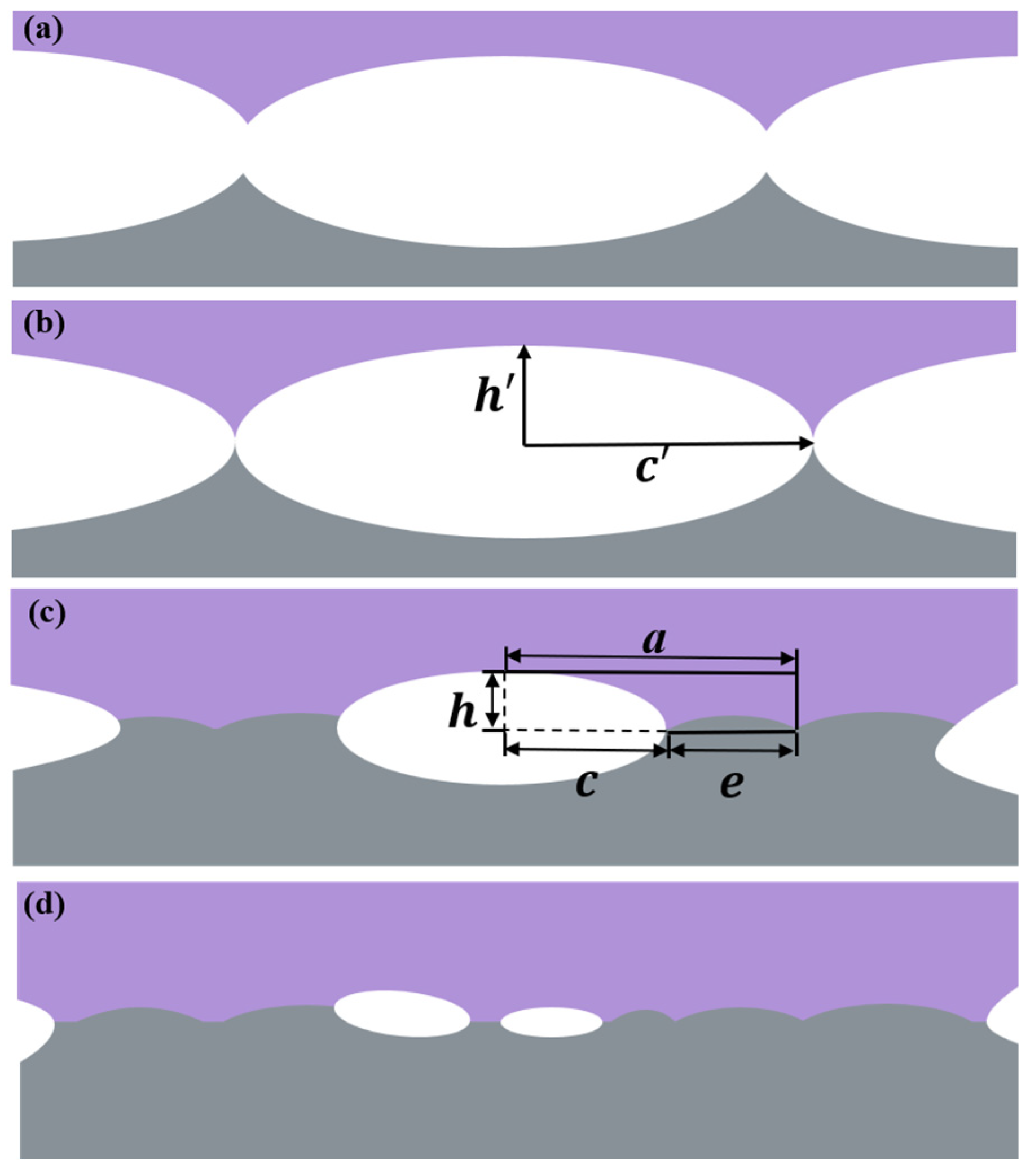
| Chemical Elements | Fe | Cr | Ni | Mn | Si | C | P | S |
|---|---|---|---|---|---|---|---|---|
| Concentration | Bal. | 18.3 | 8.5 | 2.0 | 1.0 | 0.08 | 0.045 | 0.030 |
| Standard ranges | Bal. | 18.00~20.00 | 8.00~11.00 | 2.0 | 1.0 | 0.08 | 0.045 | 0.030 |
| Sample | Grit of SiC Paper | ) | ) |
|---|---|---|---|
| 1 | 100# | 0.38 | 0.52 |
| 2 | 400# | 0.194 | 0.235 |
| 3 | 800# | 0.068 | 0.101 |
| 4 | 1500# | 0.045 | 0.07 |
| Nomenclature | |||
|---|---|---|---|
| Ra | ε | the deformation rate, % | |
| Sq | Rλq | square surface profile wavelength | |
| # | grit of SiC paper | the yield strength of the material, MPa | |
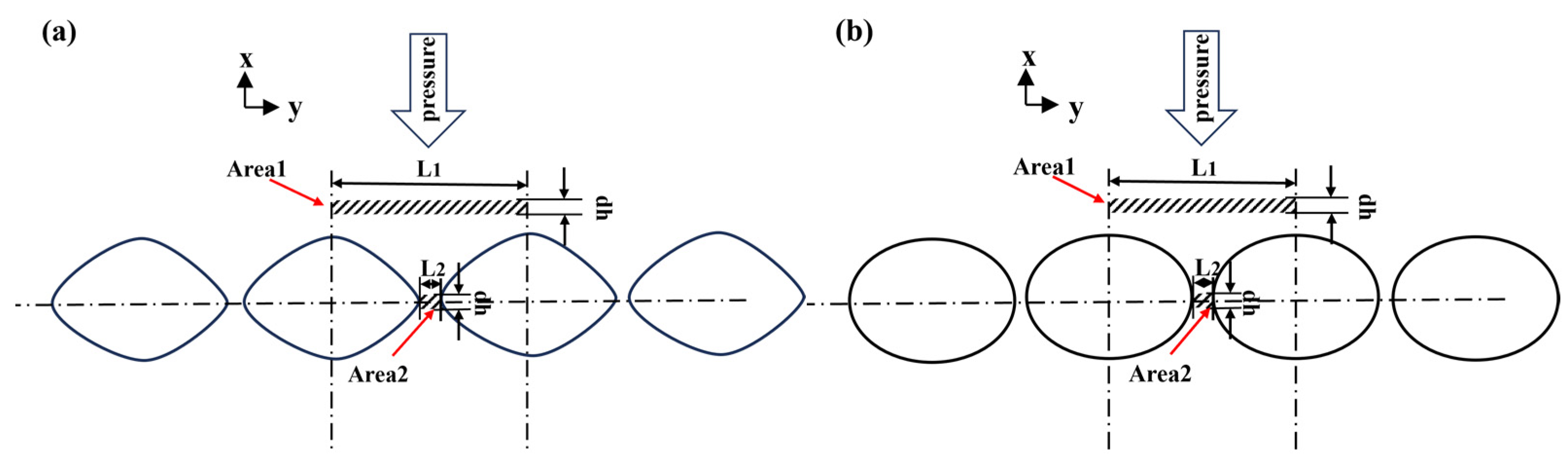
| Void length | horizontal length of pores along the interface | the stress at the joint interface, MPa | |
| H1 | sample axial height before bonding, mm | the average stress at the joint interface, MPa | |
| H2 | sample axial height after bonding, mm | h | half the height of the pore |
| e | half the bond length in the joint interface | c | half the length of the pore |
| a | equal to the sum of e and c | h’ | half-height of the elliptical pore |
| γ | the surface energy, (J/m2) | p | bonding load, MPa |
| K | the shear yield strength of the material, MPa | ||
| A | a constant related to the material | Young’s modulus | |
| b | Platt’s vector | k | Boltzmann’s constant |
| T | the Kelvin temperature | σ | the Kelvin temperature |
| d | the grain diameter | p | the exponent of the reciprocal of a grain size |
| D0 | the frequency factor | D | the diffusion coefficient |
| R | the universal gas constant | Q | the diffusion activation energy |
| η | the rheological constant | δ | the interfacial bonding ratio |
| f | (variable) interface bonding rate | the compressive strain rate of region i | |
| the compressive stress in region 1 | the initial strain rate | ||
| σT | material’s high-temperature yield strength | the constant of integration | |
| n | the material’s own constant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Zhang, S.; Jia, L.; Li, Q.; Ma, M. Study on the Influence of Surface Roughness and Temperature on the Interface Void Closure and Microstructure Evolution of Stainless Steel Diffusion Bonding Joints. Metals 2024, 14, 812. https://doi.org/10.3390/met14070812
Wei Y, Zhang S, Jia L, Li Q, Ma M. Study on the Influence of Surface Roughness and Temperature on the Interface Void Closure and Microstructure Evolution of Stainless Steel Diffusion Bonding Joints. Metals. 2024; 14(7):812. https://doi.org/10.3390/met14070812
Chicago/Turabian StyleWei, Yanni, Shuyuan Zhang, Lei Jia, Quanning Li, and Mengfan Ma. 2024. "Study on the Influence of Surface Roughness and Temperature on the Interface Void Closure and Microstructure Evolution of Stainless Steel Diffusion Bonding Joints" Metals 14, no. 7: 812. https://doi.org/10.3390/met14070812






