Obtention of Suitable Pregnant Leach Solution (PLS) for Copper Solvent Extraction Plants from Copper Concentrate Using Hydrogen Peroxide and Iodine in a Sulfuric Acid–Chloride Medium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Copper Concentrate Characterization
2.2. Experimental Methods
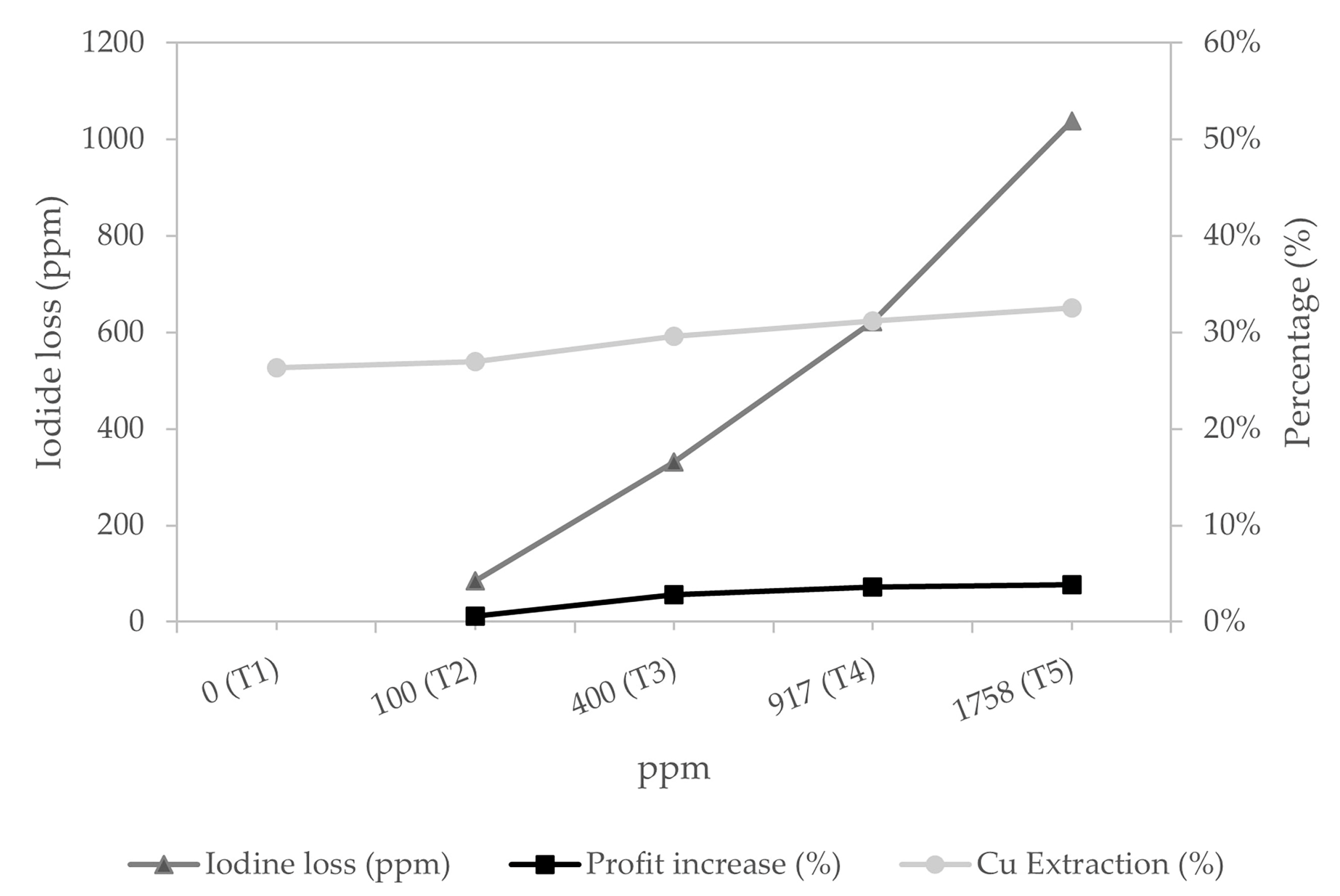
2.2.1. Determination of Optimal Concentration of Iodide Salt
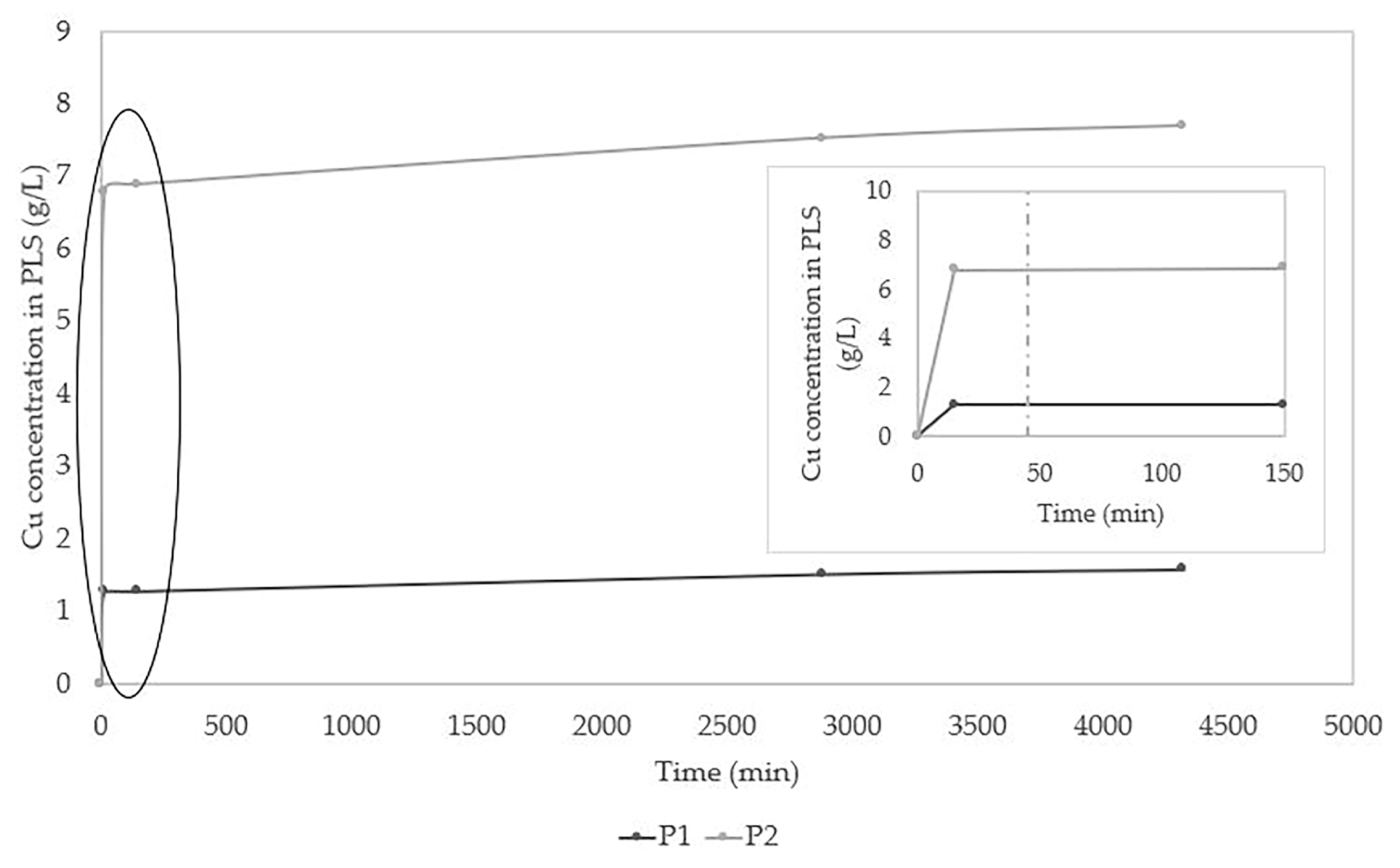
2.2.2. Determination of Residence Time
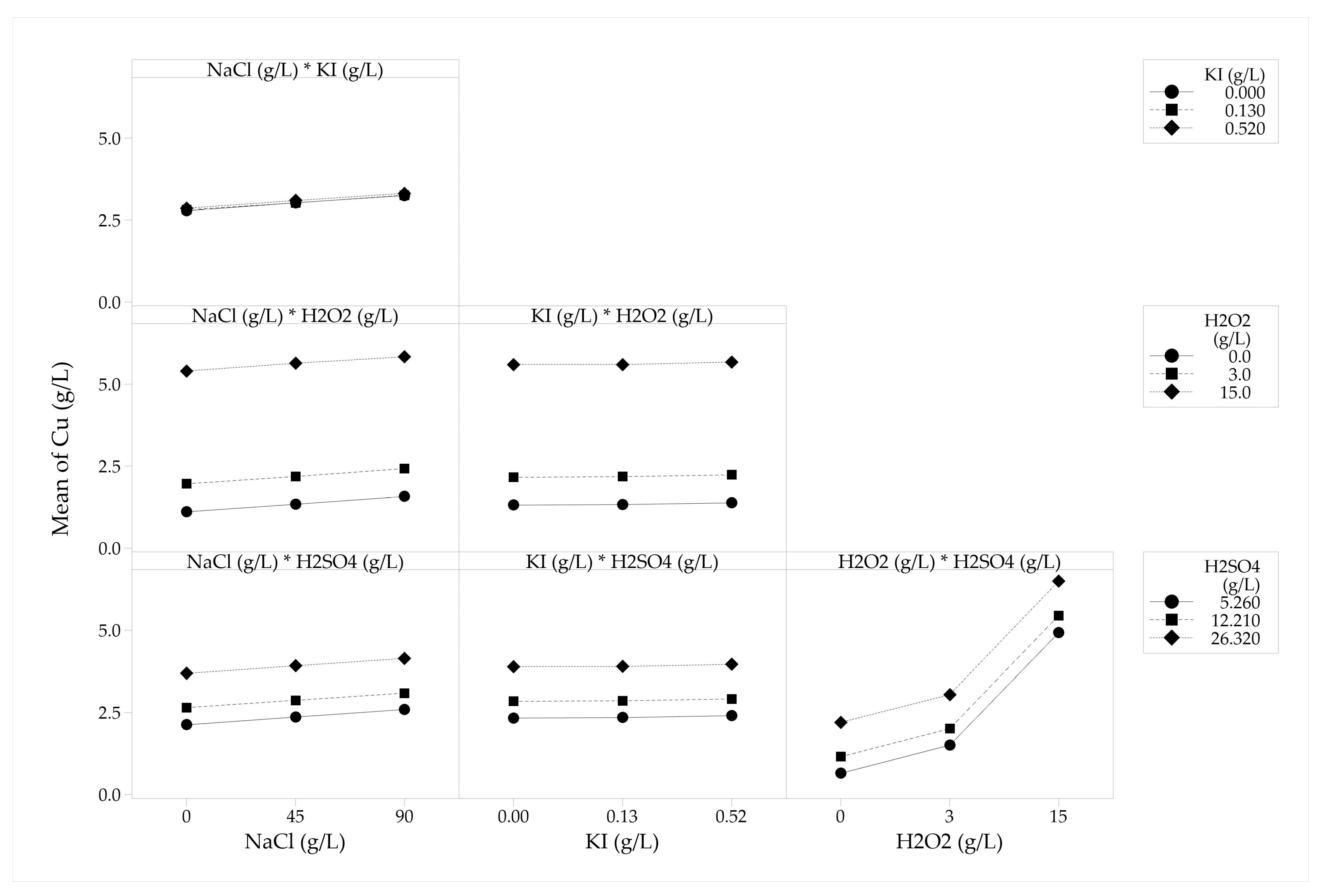
2.2.3. Evaluation of Reagent Concentration Effect on Copper PLS
3. Results
3.1. Optimal Iodide Salt Dosage
3.2. Study of Leaching Kinetics and Determination of Optimal Residence Time
3.3. Species Concentration Effect on the Concentrate Leaching
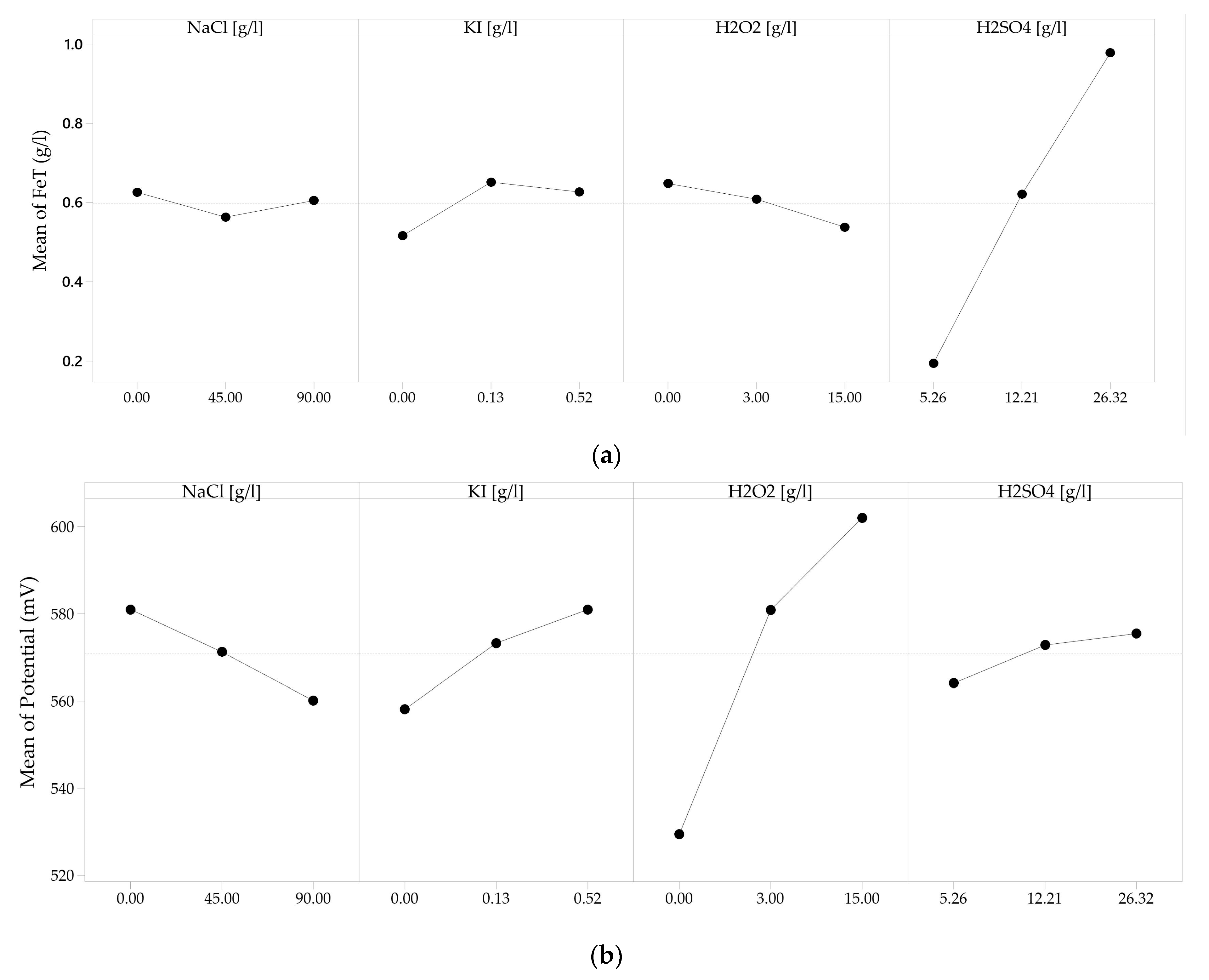
3.4. Leaching Reagents Effect on Iron Concentration and PLS Redox Potential
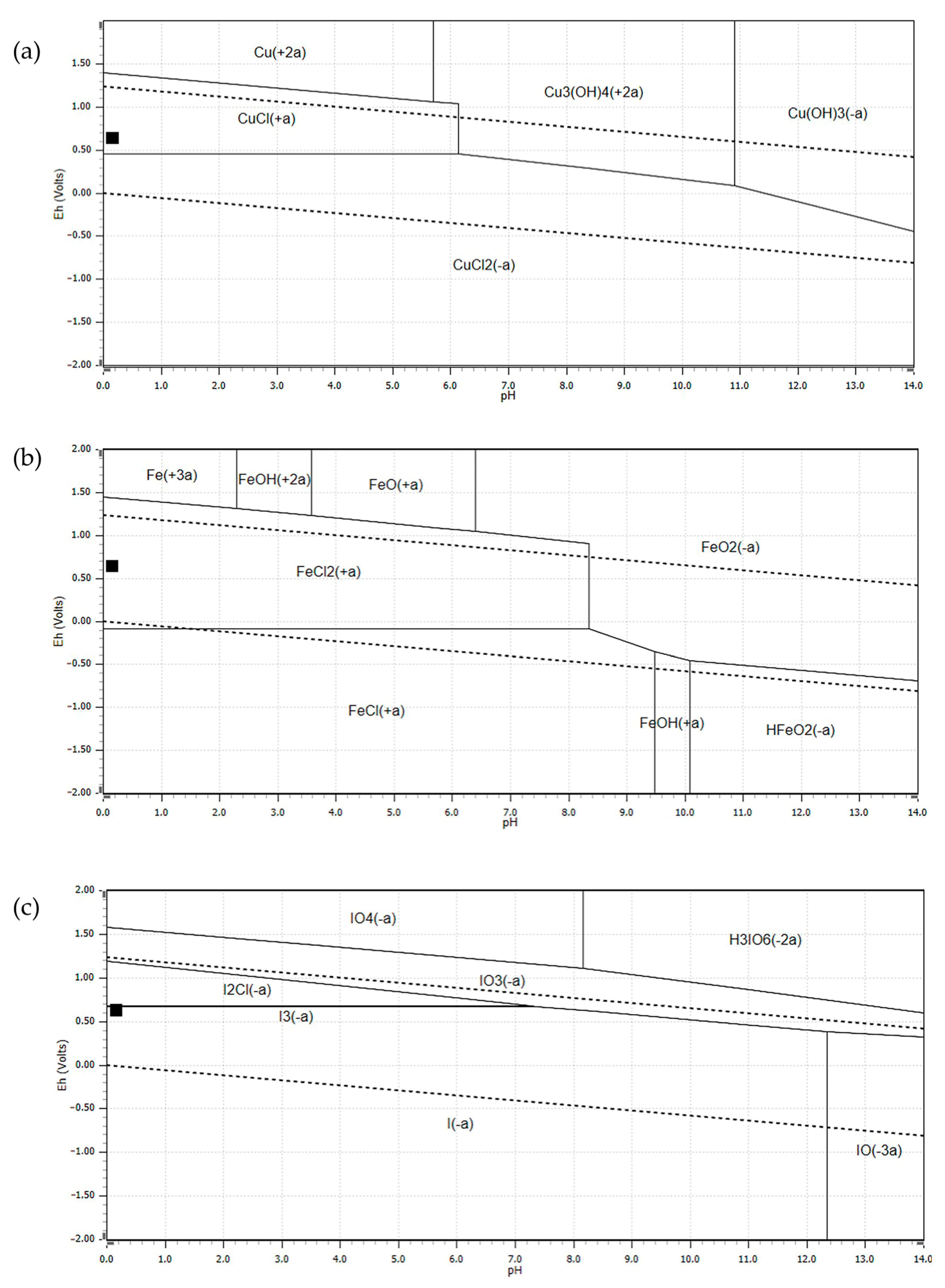
3.5. Electrochemistry Analysis of the Leaching Tests
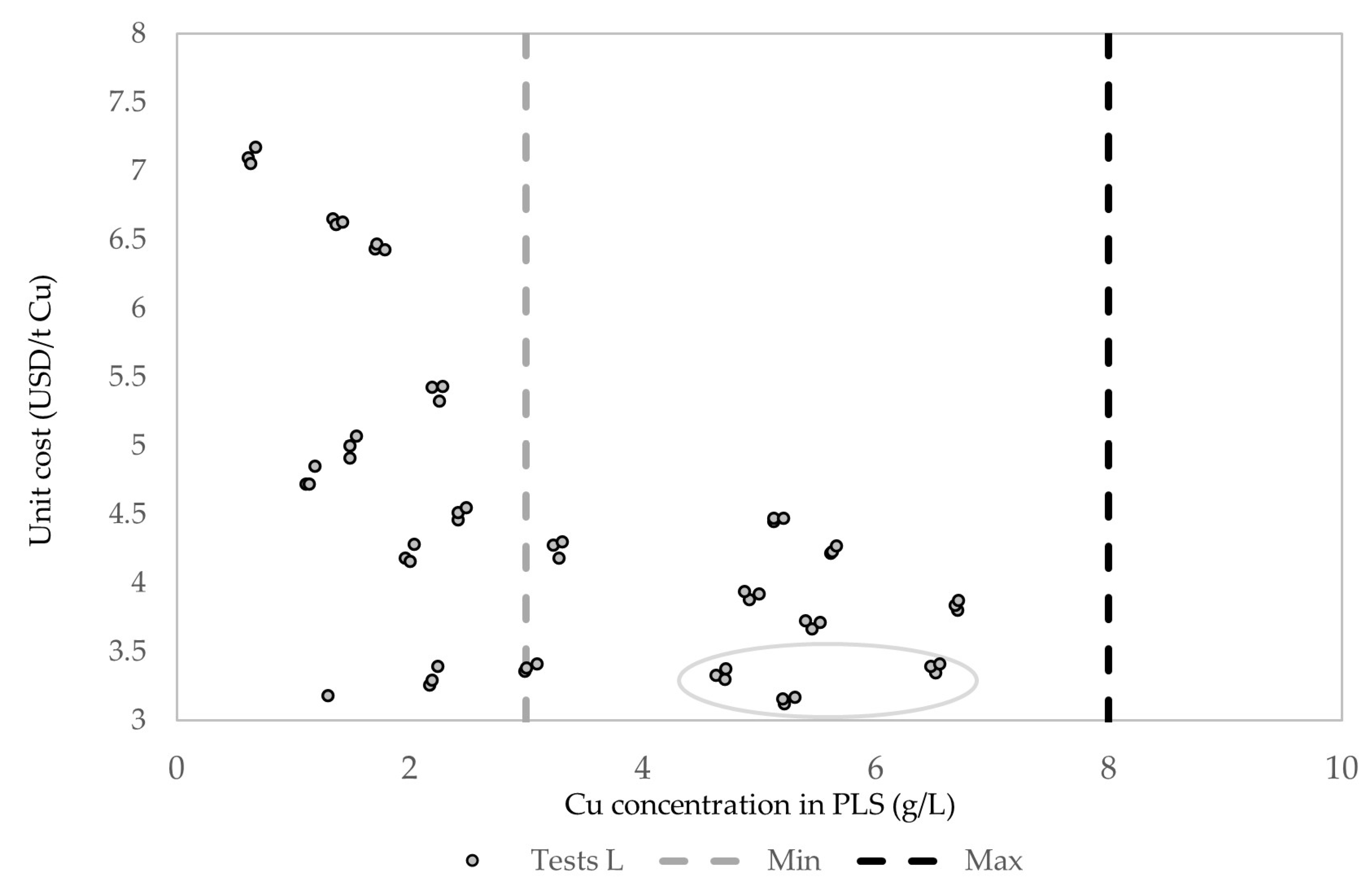
4. Economic Analysis
5. Discussion and Future Prospects
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vidal, O.; Le Boulzec, H.; Andrieu, B.; Verzier, F. Modelling the demand and access of mineral resources in a changing world. Sustainability 2022, 14, 11. [Google Scholar] [CrossRef]
- Jena, S.; Tripathy, S.K.; Mandre, N.R.; Venugopal, R.; Farrokhpay, S. Sustainable Use of Copper Resources: Beneficiation of Low-Grade Copper Ores. Minerals 2022, 12, 545. [Google Scholar] [CrossRef]
- Calvo, G.; Mudd, G.; Valero, A.; Valero, A. Decreasing ore grades in global metallic mining: A theoretical issue or a global reality? Resources 2016, 5, 36. [Google Scholar] [CrossRef]
- Alvear Flores, G.; Risopatron, C.; Pease, J. Processing of Complex Materials in the Copper Industry: Challenges and Opportunities Ahead. Jom 2020, 72, 3447–3461. [Google Scholar] [CrossRef] [PubMed]
- Cacciuttolo, C.; Atencio, E. Past, Present, and Future of Copper Mine Tailings Governance in Chile (1905–2022): A Review in One of the Leading Mining Countries in the World. Int. J. Environ. Res. Public Health 2022, 19, 13060. [Google Scholar] [CrossRef]
- COCHILCO Proyección de la Producción de Cobre en Chile 2020–2031; 2020. Available online: https://www.cochilco.cl/Mercado%20de%20Metales/Proyeccion%20de%20la%20produccion%20esperada%20de%20cobre%202020%20-%202031.pdf (accessed on 15 July 2024).
- Cháidez, J.; Parga, J.; Valenzuela, J.; Carrillo, R.; Almaguer, I. Leaching chalcopyrite concentrate with oxygen and sulfuric acid using a low-pressure reactor. Metals 2019, 9, 189. [Google Scholar] [CrossRef]
- Dakkoune, A.; Bourgeois, F.; Po, A.; Joulian, C.; Hubau, A.; Touzé, S.; Julcour, C.; Guezennec, A.-G.; Cassayre, L. Hydrometallurgical Processing of Chalcopyrite by Attrition-Aided Leaching. ACS Eng. Au 2023, 3, 195–209. [Google Scholar] [CrossRef]
- Nkuna, R.; Ijoma, G.N.; Matambo, T.S.; Chimwani, N. Accessing Metals from Low-Grade Ores and the Environmental Impact Considerations: A Review of the Perspectives of Conventional versus Bioleaching Strategies. Minerals 2022, 12, 506. [Google Scholar] [CrossRef]
- Ji, G.; Liao, Y.; Wu, Y.; Xi, J.; Liu, Q. A Review on the Research of Hydrometallurgical Leaching of Low—Grade Complex Chalcopyrite. J. Sustain. Metall. 2022, 8, 964–977. [Google Scholar] [CrossRef]
- Harichandan, B.; Mandre, N.R. Studies on the potential recovery of copper from low-grade mixed sulfide-oxide ore and optimization of the process parameters. Sep. Sci. Technol. 2022, 57, 719–732. [Google Scholar] [CrossRef]
- Torres, C.M.; Taboada, M.E.; Graber, T.A.; Herreros, O.O.; Ghorbani, Y.; Watling, H.R. The effect of seawater based media on copper dissolution from low-grade copper ore. Miner. Eng. 2015, 71, 139–145. [Google Scholar] [CrossRef]
- Watling, H.R. Chalcopyrite hydrometallurgy at atmospheric pressure: 2. Review of acidic chloride process options. Hydrometallurgy 2014, 146, 96–110. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Montes, K.S.; Padilla, R. Chalcopyrite leaching in sulfate-chloride media at ambient pressure. Hydrometallurgy 2011, 109, 37–42. [Google Scholar] [CrossRef]
- Bogdanović, G.D.; Petrović, S.; Sokić, M.; Antonijević, M.M. Chalcopyrite leaching in acid media: A review. Metall. Mater. Eng. 2020, 26, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.M.; Ghorbani, Y.; Hernández, P.C.; Justel, F.J.; Aravena, M.I.; Herreros, O.O. Cupric and chloride ions: Leaching of chalcopyrite concentrate with low chloride concentration media. Minerals 2019, 9, 639. [Google Scholar] [CrossRef]
- Lu, J.; Dreisinger, D. Copper leaching from chalcopyrite concentrate in Cu(II)/Fe(III) chloride system. Miner. Eng. 2013, 45, 185–190. [Google Scholar] [CrossRef]
- Barton, I.F.; Hiskey, J.B. Chalcopyrite leaching in novel lixiviants. Hydrometallurgy 2022, 207, 105775. [Google Scholar] [CrossRef]
- Hernández, P.; Dorador, A.; Martínez, M.; Toro, N.; Castillo, J.; Ghorbani, Y. Use of seawater/brine and caliche’s salts as clean and environmentally friendly sources of chloride and nitrate ions for chalcopyrite concentrate leaching. Minerals 2020, 10, 477. [Google Scholar] [CrossRef]
- Hernández, P.; Gahona, G.; Martínez, M.; Toro, N.; Castillo, J. Caliche and seawater, sources of nitrate and chloride ions to chalcopyrite leaching in acid media. Metals 2020, 10, 551. [Google Scholar] [CrossRef]
- Castellón, C.I.; Hernández, P.C.; Velásquez-Yévenes, L.; Taboada, M.E. An alternative process for leaching chalcopyrite concentrate in nitrate-acid-seawater media with oxidant recovery. Metals 2020, 10, 518. [Google Scholar] [CrossRef]
- Winarko, R. Iodine Assisted Heap Leaching of Chalcopirite: Laboratory and Modelling Studies. Ph.D. Thesis, The University of Brithish Columbia, Vancouver, BC, Canada, 2022. [Google Scholar]
- Corporación Alta Ley Hoja de Ruta 2.0 de la Minería Chilena. Actualización y Consensos Para una Mirada Renovada. 2019. Available online: https://www.corporacionaltaley.cl/roadmap-de-la-mineria-2/ (accessed on 15 July 2024).
- Moraga, G.A.; Jamett, N.E.; Hernández, P.C.; Graber, T.A.; Taboada, M.E. Chalcopyrite leaching with hydrogen peroxide and iodine species in acidic chloride media at room temperature: Technical and economic evaluation. Metals 2021, 11, 1567. [Google Scholar] [CrossRef]
- Petrovic, S.J.; Bogdanovic, G.D.; Antonijevic, M.M. Leaching of chalcopyrite with hydrogen peroxide in hydrochloric acid solution. Trans. Nonferrous Met. Soc. China Engl. Ed. 2018, 28, 1444–1455. [Google Scholar] [CrossRef]
- Hernández, P.C.; Taboada, M.E.; Herreros, O.O.; Torres, C.M.; Ghorbani, Y. Chalcopyrite dissolution using seawater-based acidic media in the presence of oxidants. Hydrometallurgy 2015, 157, 325–332. [Google Scholar] [CrossRef]
- Hirato, T.; Majima, H.; Awakura, Y. The leaching of chalcopyrite with cupric chloride. Metall. Trans. B 1987, 18, 31–39. [Google Scholar] [CrossRef]
- Winarko, R.; Dreisinger, D.B.; Miura, A.; Tokoro, C.; Liu, W. Kinetic modelling of chalcopyrite leaching assisted by iodine in ferric sulfate media. Hydrometallurgy 2020, 197, 105481. [Google Scholar] [CrossRef]
- INEN Ecuatorian Technical Standard Norm NTE INEN 1446 1985. Available online: https://law.resource.org/pub/ec/index.html (accessed on 13 July 2024).
- Schlesinger, M.E.; Sole, K.C.; Davenport, W.G.; Alvear, G. Extractive Metallurgy of Copper, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780128219034. [Google Scholar]
- Hirato, T.; Majima, H.; Awakura, Y. The Leaching of Chalcopyrite with Ferric Sulfate. Met. Trans. B 1987, 18, 489–496. [Google Scholar] [CrossRef]
- Howard, D.; Crundwell, F.K. A Kinetic Study of the Leaching of Chalcopyrite with Sulfolobus metallicus. Process Metall. 1999, 209–217. [Google Scholar] [CrossRef]
- Kaplun, K.; Li, J.; Kawashima, N.; Gerson, A.R. Cu and Fe chalcopyrite leach activation energies and the effect of added Fe3+. Geochim. Cosmochim. Acta 2011, 75, 5865–5878. [Google Scholar] [CrossRef]
- Li, J.; Kawashima, N.; Kaplun, K.; Absolon, V.J.; Gerson, A.R. Chalcopyrite leaching: The rate controlling factors. Geochim. Cosmochim. Acta 2010, 74, 2881–2893. [Google Scholar] [CrossRef]
- Córdoba, E.M.; Muñoz, J.A.; Blázquez, M.L.; González, F.; Ballester, A. Leaching of chalcopyrite with ferric ion. Part I: General aspects. Hydrometallurgy 2008, 93, 81–87. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Nicol, M.; Miki, H. The dissolution of chalcopyrite in chloride solutions: Part 1. the effect of solution potential. Hydrometallurgy 2010, 103, 108–113. [Google Scholar] [CrossRef]
- Outotec-Metso HSC Chemistry Version 9.
- Hashemzadeh, M.; Liu, W. The response of sulfur chemical state to different leaching conditions in chloride leaching of chalcocite. Hydrometallurgy 2020, 192, 105245. [Google Scholar] [CrossRef]
- Shakibania, S.; Mahmoudi, A.; Mokmeli, M.; Rashchi, F. The effect of chloride ions on copper solvent extraction from sulfate-chloride medium using LIX 984N. Miner. Eng. 2020, 156, 106498. [Google Scholar] [CrossRef]
- Ren, Z.; Chao, C.; Krishnamoorthy, P.; Asselin, E.; Dixon, D.G.; Mora, N. The overlooked mechanism of chalcopyrite passivation. Acta Mater. 2022, 236, 118111. [Google Scholar] [CrossRef]
- Cerda, C.P.; Taboada, M.E.; Jamett, N.E.; Ghorbani, Y.; Hernández, P.C. Effect of pretreatment on leaching primary copper sulfide in acid-chloride media. Minerals 2018, 8, 1. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, W.; Zhao, S.; Lu, D.; Xie, F.; Dreisinger, D. Effect of Mechanical Activation on Leaching Behavior and Mechanism of Chalcopyrite. Miner. Process. Extr. Metall. Rev. 2022, 43, 440–452. [Google Scholar] [CrossRef]
- Misra, M.; Fuerstenau, M.C. Chalcopyrite leaching at moderate temperature and ambient pressure in the presence of nanosize silica. Miner. Eng. 2005, 18, 293–297. [Google Scholar] [CrossRef]

| Mineral Specie | Formula | %Base Material | %Base Sulfide | %Base Sulfide Cu | %Cu |
|---|---|---|---|---|---|
| Chalcopyrite | CuFeS2 | 63.7 | 68.8 | 84.4 | 22.0 |
| Bornite | Cu5FeS4 | 6.4 | 17.8 | 8.5 | 4.1 |
| Digenite | Cu9S5 | 3.3 | 3.6 | 4.4 | 2.6 |
| Enargite | Cu3AsS4 | 1.2 | 1.3 | 1.6 | 0.6 |
| Covellite | CuS | 0.8 | 0.9 | 1.0 | 0.5 |
| Pyrite | FeS2 | 16.4 | 6.9 | ||
| Molybdenite | MoS2 | 0.3 | 0.3 | ||
| Sphalerite | ZnS | 0.2 | 0.2 | ||
| Pyrargyrite | Ag3SbS3 | 0.1 | 0.1 | ||
| Magnetite | Fe3O4 | 0.1 | |||
| Hematite | Fe2O3 | 0.1 | |||
| Gangue | 7.30 | ||||
| Total | 100 | 100 | 100 | 29.8 |
| Test | KI (ppm) |
|---|---|
| T1 | 0.00 |
| T2 | 100 |
| T3 | 400 |
| T4 | 917 |
| T5 | 1758 |
| Compound | Test | |
|---|---|---|
| Low P1 (g/L) | High P2 (g/L) | |
| NaCl | 0.00 | 90.00 |
| KI | 0.00 | 0.52 |
| H2O2 | 3.00 | 15.00 |
| H2SO4 | 5.26 | 26.32 |
| Variable | Levels | ||
|---|---|---|---|
| Lower (g/L) | Medium (g/L) | Upper (g/L) | |
| NaCl | 0.00 | 45.00 | 90.00 |
| KI | 0.00 | 0.13 | 0.52 |
| H2O2 | 0.00 | 3.00 | 15.00 |
| H2SO4 | 5.26 | 12.21 | 26.32 |
| Test L | (g/L) | ||||
|---|---|---|---|---|---|
| NaCl | KI | H2O2 | H2SO4 | Cu in PLS | |
| L1 | 0.00 | 0.00 | 0.00 | 5.26 | 0.39 |
| L2 | 0.00 | 0.00 | 0.00 | 12.21 | 0.90 |
| L3 | 0.00 | 0.00 | 0.00 | 26.32 | 1.94 |
| L4 | 0.00 | 0.00 | 3.00 | 5.26 | 1.25 |
| L5 | 0.00 | 0.00 | 3.00 | 12.21 | 1.75 |
| L6 | 0.00 | 0.00 | 3.00 | 26.32 | 2.77 |
| L7 | 0.00 | 0.00 | 15.00 | 5.26 | 4.63 |
| L8 | 0.00 | 0.00 | 15.00 | 12.21 | 5.22 |
| L9 | 0.00 | 0.00 | 15.00 | 26.32 | 6.20 |
| L10 | 0.00 | 0.13 | 0.00 | 5.26 | 0.41 |
| L11 | 0.00 | 0.13 | 0.00 | 12.21 | 0.92 |
| L12 | 0.00 | 0.13 | 0.00 | 26.32 | 1.96 |
| L13 | 0.00 | 0.13 | 3.00 | 5.26 | 1.27 |
| L14 | 0.00 | 0.13 | 3.00 | 12.21 | 1.78 |
| L15 | 0.00 | 0.13 | 3.00 | 26.32 | 2.82 |
| L16 | 0.00 | 0.13 | 15.00 | 5.26 | 4.71 |
| L17 | 0.00 | 0.13 | 15.00 | 12.21 | 5.20 |
| L18 | 0.00 | 0.13 | 15.00 | 26.32 | 6.26 |
| L19 | 0.00 | 0.52 | 0.00 | 5.26 | 0.46 |
| L20 | 0.00 | 0.52 | 0.00 | 12.21 | 0.96 |
| L21 | 0.00 | 0.52 | 0.00 | 26.32 | 1.99 |
| L22 | 0.00 | 0.52 | 3.00 | 5.26 | 1.30 |
| L23 | 0.00 | 0.52 | 3.00 | 12.21 | 1.81 |
| L24 | 0.00 | 0.52 | 3.00 | 26.32 | 2.87 |
| L25 | 0.00 | 0.52 | 15.00 | 5.26 | 4.72 |
| L26 | 0.00 | 0.52 | 15.00 | 12.21 | 5.31 |
| L27 | 0.00 | 0.52 | 15.00 | 26.32 | 6.36 |
| L28 | 45.00 | 0.00 | 0.00 | 5.26 | 0.62 |
| L29 | 45.00 | 0.00 | 0.00 | 12.21 | 1.12 |
| L30 | 45.00 | 0.00 | 0.00 | 26.32 | 2.18 |
| L31 | 45.00 | 0.00 | 3.00 | 5.26 | 1.49 |
| L32 | 45.00 | 0.00 | 3.00 | 12.21 | 1.96 |
| L33 | 45.00 | 0.00 | 3.00 | 26.32 | 2.99 |
| L34 | 45.00 | 0.00 | 15.00 | 5.26 | 4.92 |
| L35 | 45.00 | 0.00 | 15.00 | 12.21 | 5.46 |
| L36 | 45.00 | 0.00 | 15.00 | 26.32 | 6.52 |
| L37 | 45.00 | 0.13 | 0.00 | 5.26 | 0.64 |
| L38 | 45.00 | 0.13 | 0.00 | 12.21 | 1.14 |
| L39 | 45.00 | 0.13 | 0.00 | 26.32 | 2.19 |
| L40 | 45.00 | 0.13 | 3.00 | 5.26 | 1.49 |
| L41 | 45.00 | 0.13 | 3.00 | 12.21 | 2.01 |
| L42 | 45.00 | 0.13 | 3.00 | 26.32 | 3.01 |
| L43 | 45.00 | 0.13 | 15.00 | 5.26 | 4.88 |
| L44 | 45.00 | 0.13 | 15.00 | 12.21 | 5.40 |
| L45 | 45.00 | 0.13 | 15.00 | 26.32 | 6.47 |
| L46 | 45.00 | 0.52 | 0.00 | 5.26 | 0.68 |
| L47 | 45.00 | 0.52 | 0.00 | 12.21 | 1.19 |
| L48 | 45.00 | 0.52 | 0.00 | 26.32 | 2.24 |
| L49 | 45.00 | 0.52 | 3.00 | 5.26 | 1.55 |
| L50 | 45.00 | 0.52 | 3.00 | 12.21 | 2.04 |
| L51 | 45.00 | 0.52 | 3.00 | 26.32 | 3.10 |
| L52 | 45.00 | 0.52 | 15.00 | 5.26 | 5.00 |
| L53 | 45.00 | 0.52 | 15.00 | 12.21 | 5.53 |
| L54 | 45.00 | 0.52 | 15.00 | 26.32 | 6.55 |
| L55 | 90.00 | 0.00 | 0.00 | 5.26 | 0.84 |
| L56 | 90.00 | 0.00 | 0.00 | 12.21 | 1.35 |
| L57 | 90.00 | 0.00 | 0.00 | 26.32 | 2.42 |
| L58 | 90.00 | 0.00 | 3.00 | 5.26 | 1.71 |
| L59 | 90.00 | 0.00 | 3.00 | 12.21 | 2.19 |
| L60 | 90.00 | 0.00 | 3.00 | 26.32 | 3.28 |
| L61 | 90.00 | 0.00 | 15.00 | 5.26 | 5.13 |
| L62 | 90.00 | 0.00 | 15.00 | 12.21 | 5.62 |
| L63 | 90.00 | 0.00 | 15.00 | 26.32 | 6.71 |
| L64 | 90.00 | 0.13 | 0.00 | 5.26 | 0.87 |
| L65 | 90.00 | 0.13 | 0.00 | 12.21 | 1.38 |
| L66 | 90.00 | 0.13 | 0.00 | 26.32 | 2.42 |
| L67 | 90.00 | 0.13 | 3.00 | 5.26 | 1.72 |
| L68 | 90.00 | 0.13 | 3.00 | 12.21 | 2.26 |
| L69 | 90.00 | 0.13 | 3.00 | 26.32 | 3.24 |
| L70 | 90.00 | 0.13 | 15.00 | 5.26 | 5.13 |
| L71 | 90.00 | 0.13 | 15.00 | 12.21 | 5.63 |
| L72 | 90.00 | 0.13 | 15.00 | 26.32 | 6.68 |
| L73 | 90.00 | 0.52 | 0.00 | 5.26 | 0.93 |
| L74 | 90.00 | 0.52 | 0.00 | 12.21 | 1.43 |
| L75 | 90.00 | 0.52 | 0.00 | 26.32 | 2.49 |
| L76 | 90.00 | 0.52 | 3.00 | 5.26 | 1.79 |
| L77 | 90.00 | 0.52 | 3.00 | 12.21 | 2.29 |
| L78 | 90.00 | 0.52 | 3.00 | 26.32 | 3.32 |
| L79 | 90.00 | 0.52 | 15.00 | 5.26 | 5.21 |
| L80 | 90.00 | 0.52 | 15.00 | 12.21 | 5.67 |
| L81 | 90.00 | 0.52 | 15.00 | 26.32 | 6.72 |
| Reagent | Price (USD/t) |
|---|---|
| H2SO4 98% w/w | 250 |
| H2O2 50% w/w | 500 |
| KI | 800 |
| NaCl | 50 |
| Tests L | Concentration (g/L) | (USD/t) | ||||
|---|---|---|---|---|---|---|
| NaCl | KI | H2O2 | H2SO4 | Cu in PLS | Cost | |
| L8 | 0.00 | 0.00 | 15.00 | 12.21 | 5.22 | 3.46 |
| L27 | 0.00 | 0.52 | 15.00 | 26.32 | 6.36 | 3.46 |
| L18 | 0.00 | 0.13 | 15.00 | 26.32 | 6.26 | 3.46 |
| L26 | 0.00 | 0.52 | 15.00 | 12.21 | 5.31 | 3.48 |
| L9 | 0.00 | 0.00 | 15.00 | 26.32 | 6.20 | 3.48 |
| L16 | 0.00 | 0.13 | 15.00 | 5.26 | 4.71 | 3.49 |
| L17 | 0.00 | 0.13 | 15.00 | 12.21 | 5.20 | 3.49 |
| L7 | 0.00 | 0.00 | 15.00 | 5.26 | 4.63 | 3.52 |
| L25 | 0.00 | 0.52 | 15.00 | 5.26 | 4.72 | 3.55 |
| L36 | 45.00 | 0.00 | 15.00 | 26.32 | 6.52 | 3.66 |
| L45 | 45.00 | 0.13 | 15.00 | 26.32 | 6.47 | 3.70 |
| L54 | 45.00 | 0.52 | 15.00 | 26.32 | 6.55 | 3.70 |
| L35 | 45.00 | 0.00 | 15.00 | 12.21 | 5.46 | 3.72 |
| L53 | 45.00 | 0.52 | 15.00 | 12.21 | 5.53 | 3.75 |
| L34 | 45.00 | 0.00 | 15.00 | 5.26 | 4.92 | 3.77 |
| L44 | 45.00 | 0.13 | 15.00 | 12.21 | 5.40 | 3.78 |
| L52 | 45.00 | 0.52 | 15.00 | 5.26 | 5.00 | 3.80 |
| L43 | 45.00 | 0.13 | 15.00 | 5.26 | 4.88 | 3.83 |
| L63 | 90.00 | 0.00 | 15.00 | 26.32 | 6.71 | 3.89 |
| L72 | 90.00 | 0.13 | 15.00 | 26.32 | 6.68 | 3.92 |
| L81 | 90.00 | 0.52 | 15.00 | 26.32 | 6.72 | 3.95 |
| L51 | 45.00 | 0.52 | 3.00 | 26.32 | 3.10 | 3.95 |
| L42 | 45.00 | 0.13 | 3.00 | 26.32 | 3.01 | 3.97 |
| L62 | 90.00 | 0.00 | 15.00 | 12.21 | 5.62 | 4.02 |
| L71 | 90.00 | 0.13 | 15.00 | 12.21 | 5.63 | 4.02 |
| L80 | 90.00 | 0.52 | 15.00 | 12.21 | 5.67 | 4.05 |
| L61 | 90.00 | 0.00 | 15.00 | 5.26 | 5.13 | 4.06 |
| L79 | 90.00 | 0.52 | 15.00 | 5.26 | 5.21 | 4.07 |
| L70 | 90.00 | 0.13 | 15.00 | 5.26 | 5.13 | 4.08 |
| L60 | 90.00 | 0.00 | 3.00 | 26.32 | 3.28 | 4.29 |
| L78 | 90.00 | 0.52 | 3.00 | 26.32 | 3.32 | 4.37 |
| L69 | 90.00 | 0.13 | 3.00 | 26.32 | 3.24 | 4.38 |
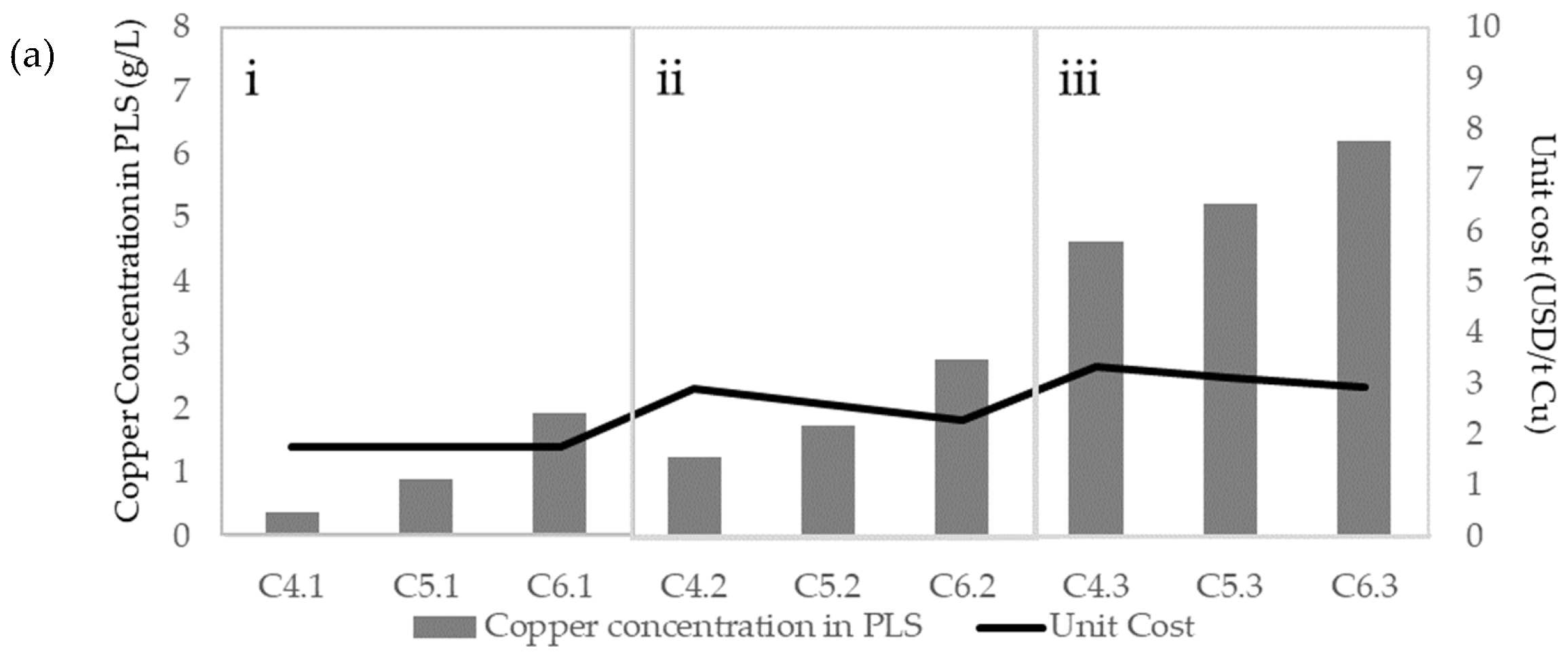
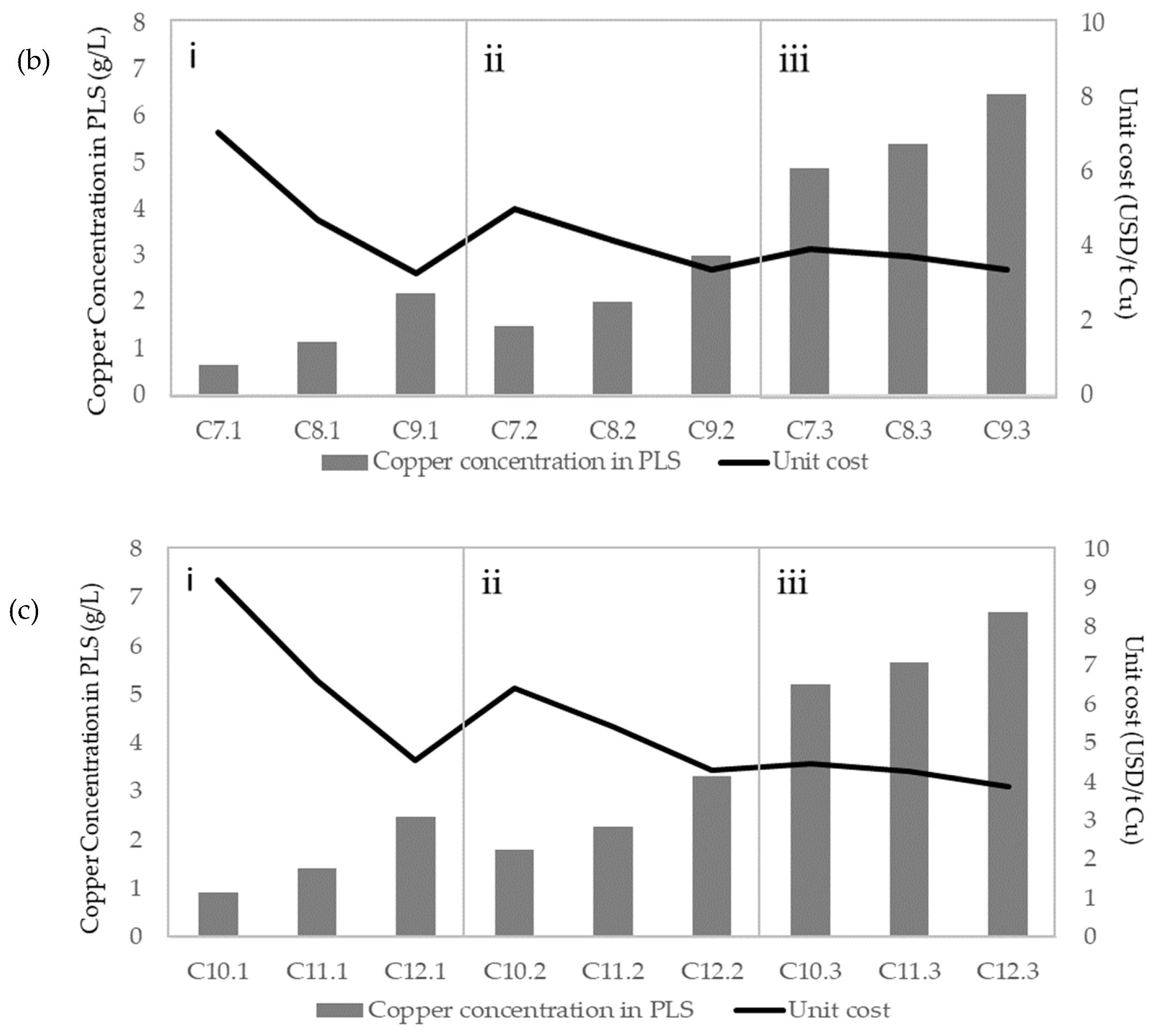
| Figures | Concentration (g/L) | ||||
|---|---|---|---|---|---|
| Test | KI | NaCl | H2SO4 | H2O2 | |
| Figure 8a | C4.1 | 0.00 | 0.00 | 5.26 | 0.00 |
| C4.2 | 0.00 | 0.00 | 5.26 | 3.00 | |
| C4.3 | 0.00 | 0.00 | 5.26 | 15.00 | |
| C5.1 | 0.00 | 0.00 | 12.21 | 0.00 | |
| C5.2 | 0.00 | 0.00 | 12.21 | 3.00 | |
| C5.3 | 0.00 | 0.00 | 12.21 | 15.00 | |
| C6.1 | 0.00 | 0.00 | 26.32 | 0.00 | |
| C6.2 | 0.00 | 0.00 | 26.32 | 3.00 | |
| C6.3 | 0.00 | 0.00 | 26.32 | 15.00 | |
| Figure 8b | C7.1 | 0.13 | 45.00 | 5.26 | 0.00 |
| C7.2 | 0.13 | 45.00 | 5.26 | 3.00 | |
| C7.3 | 0.13 | 45.00 | 5.26 | 15.00 | |
| C8.1 | 0.13 | 45.00 | 12.21 | 0.00 | |
| C8.2 | 0.13 | 45.00 | 12.21 | 3.00 | |
| C8.3 | 0.13 | 45.00 | 12.21 | 15.00 | |
| C9.1 | 0.13 | 45.00 | 26.32 | 0.00 | |
| C9.2 | 0.13 | 45.00 | 26.32 | 3.00 | |
| C9.3 | 0.13 | 45.00 | 26.32 | 15.00 | |
| Figure 8c | C10.1 | 0.52 | 90.00 | 5.26 | 0.00 |
| C10.2 | 0.52 | 90.00 | 5.26 | 3.00 | |
| C10.3 | 0.52 | 90.00 | 5.26 | 15.00 | |
| C11.1 | 0.52 | 90.00 | 12.21 | 0.00 | |
| C11.2 | 0.52 | 90.00 | 12.21 | 3.00 | |
| C11.3 | 0.52 | 90.00 | 12.21 | 15.00 | |
| C12.1 | 0.52 | 90.00 | 26.32 | 0.00 | |
| C12.2 | 0.52 | 90.00 | 26.32 | 3.00 | |
| C12.3 | 0.52 | 90.00 | 26.32 | 15.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taboada, M.E.; Jamett, N.E.; Moraga, G.A.; Hernández, P.C.; Graber, T.A. Obtention of Suitable Pregnant Leach Solution (PLS) for Copper Solvent Extraction Plants from Copper Concentrate Using Hydrogen Peroxide and Iodine in a Sulfuric Acid–Chloride Medium. Metals 2024, 14, 817. https://doi.org/10.3390/met14070817
Taboada ME, Jamett NE, Moraga GA, Hernández PC, Graber TA. Obtention of Suitable Pregnant Leach Solution (PLS) for Copper Solvent Extraction Plants from Copper Concentrate Using Hydrogen Peroxide and Iodine in a Sulfuric Acid–Chloride Medium. Metals. 2024; 14(7):817. https://doi.org/10.3390/met14070817
Chicago/Turabian StyleTaboada, María E., Nathalie E. Jamett, German A. Moraga, Pia C. Hernández, and Teófilo A. Graber. 2024. "Obtention of Suitable Pregnant Leach Solution (PLS) for Copper Solvent Extraction Plants from Copper Concentrate Using Hydrogen Peroxide and Iodine in a Sulfuric Acid–Chloride Medium" Metals 14, no. 7: 817. https://doi.org/10.3390/met14070817





