Pulsed Magnetic Field Treatment Effects on Undissolved Carbides in Continuous Casting Billets of GCr15 Bearing Steel
Abstract
1. Introduction
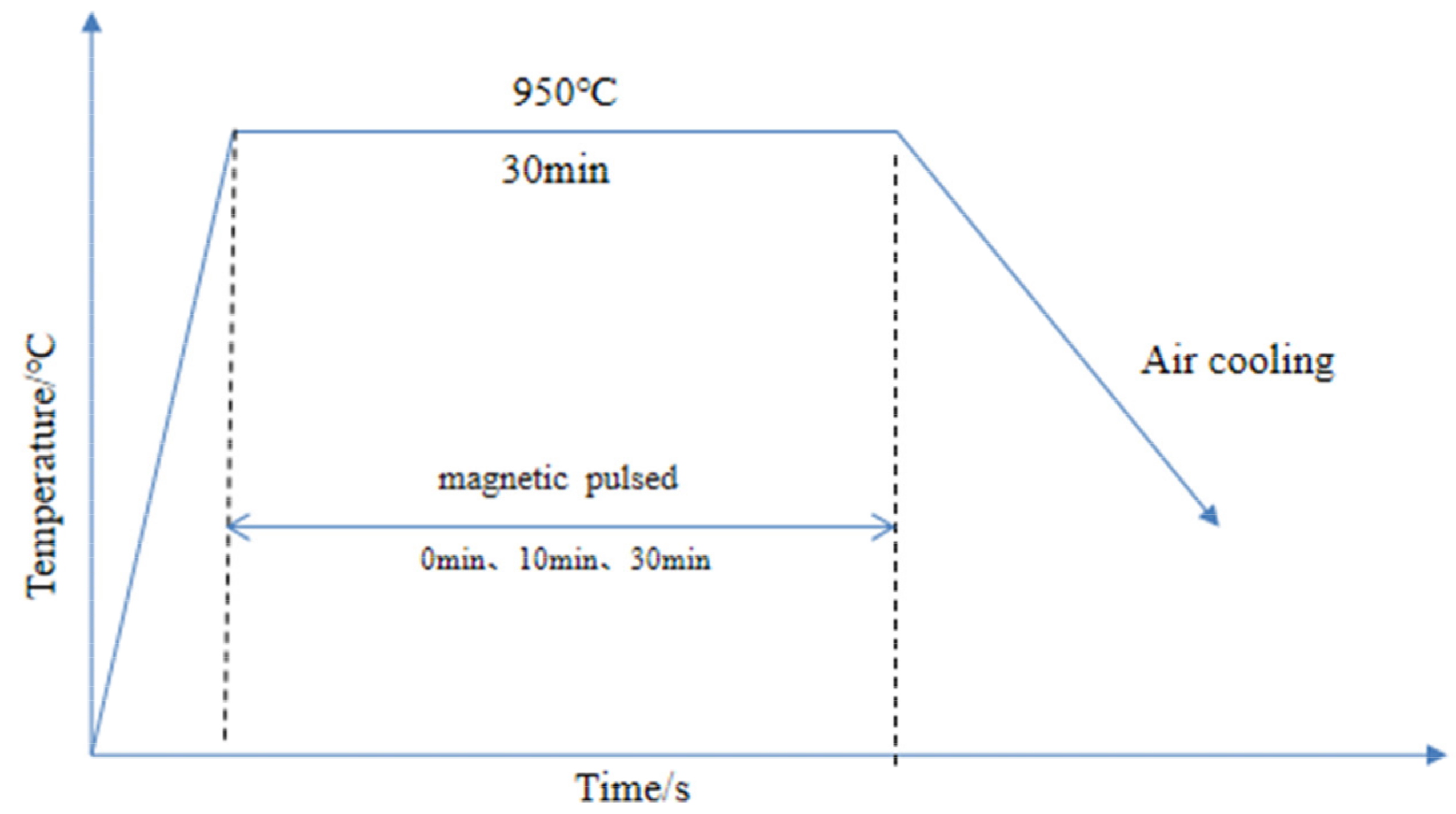
2. Experimental Methods
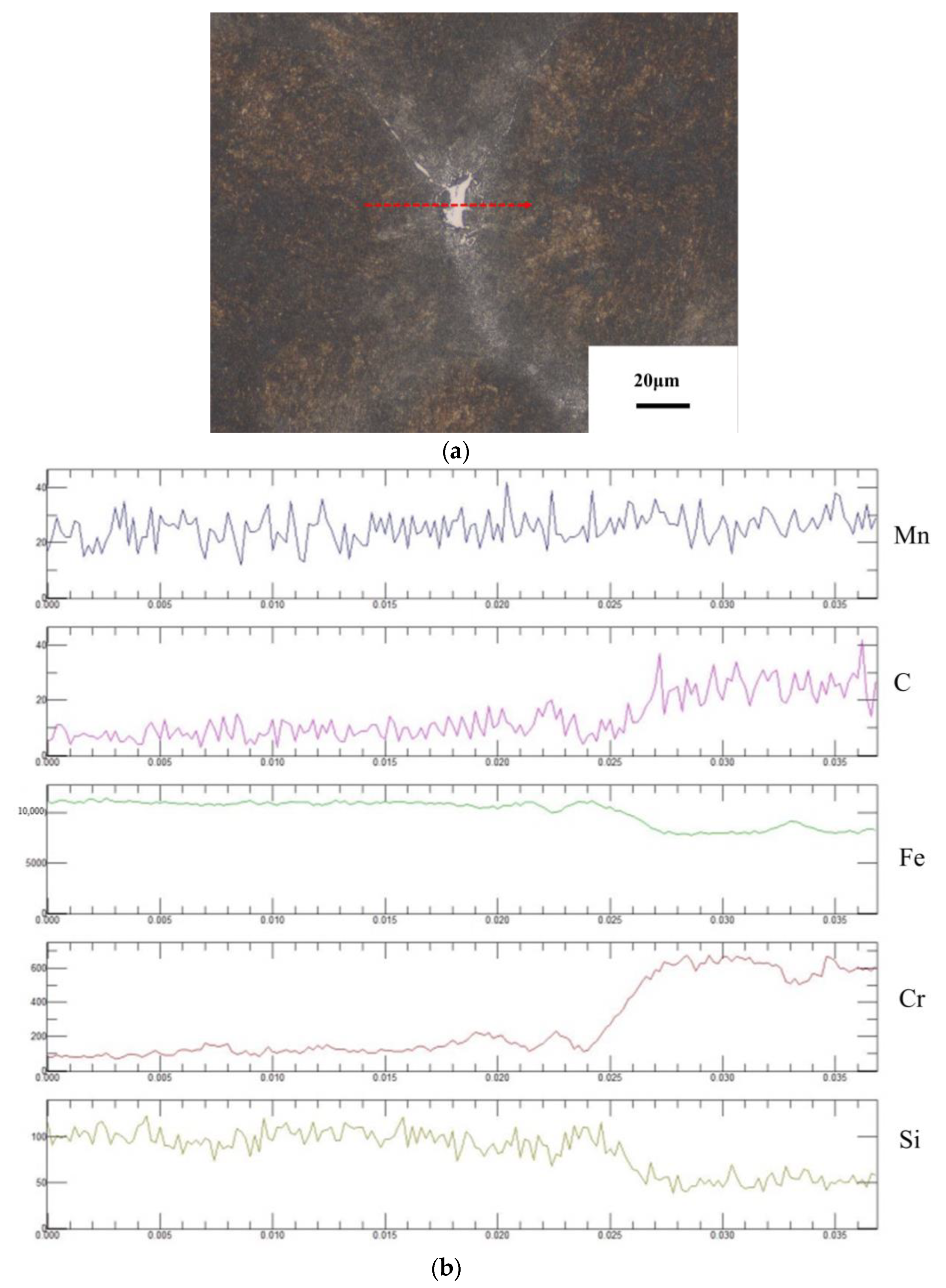
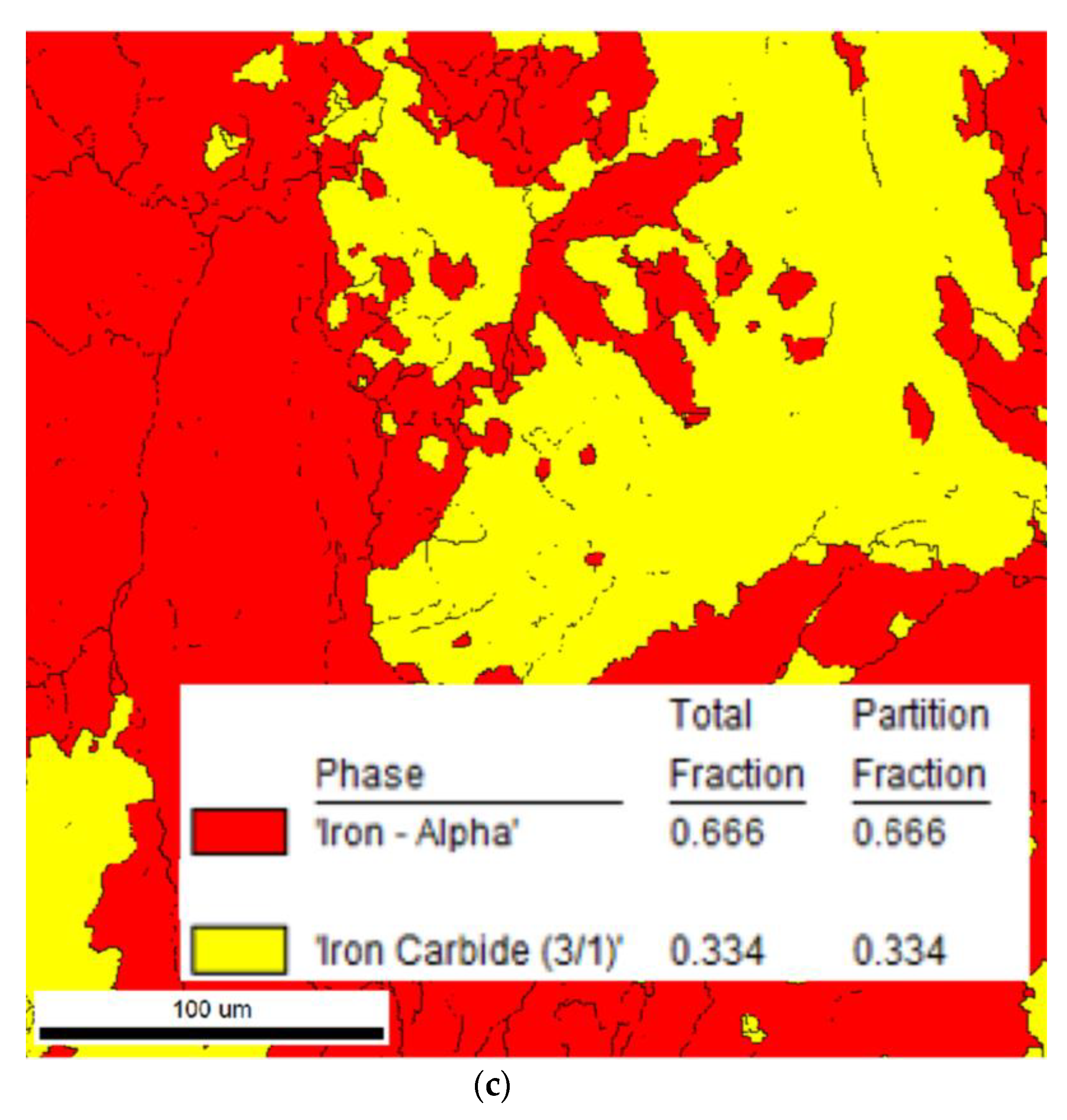
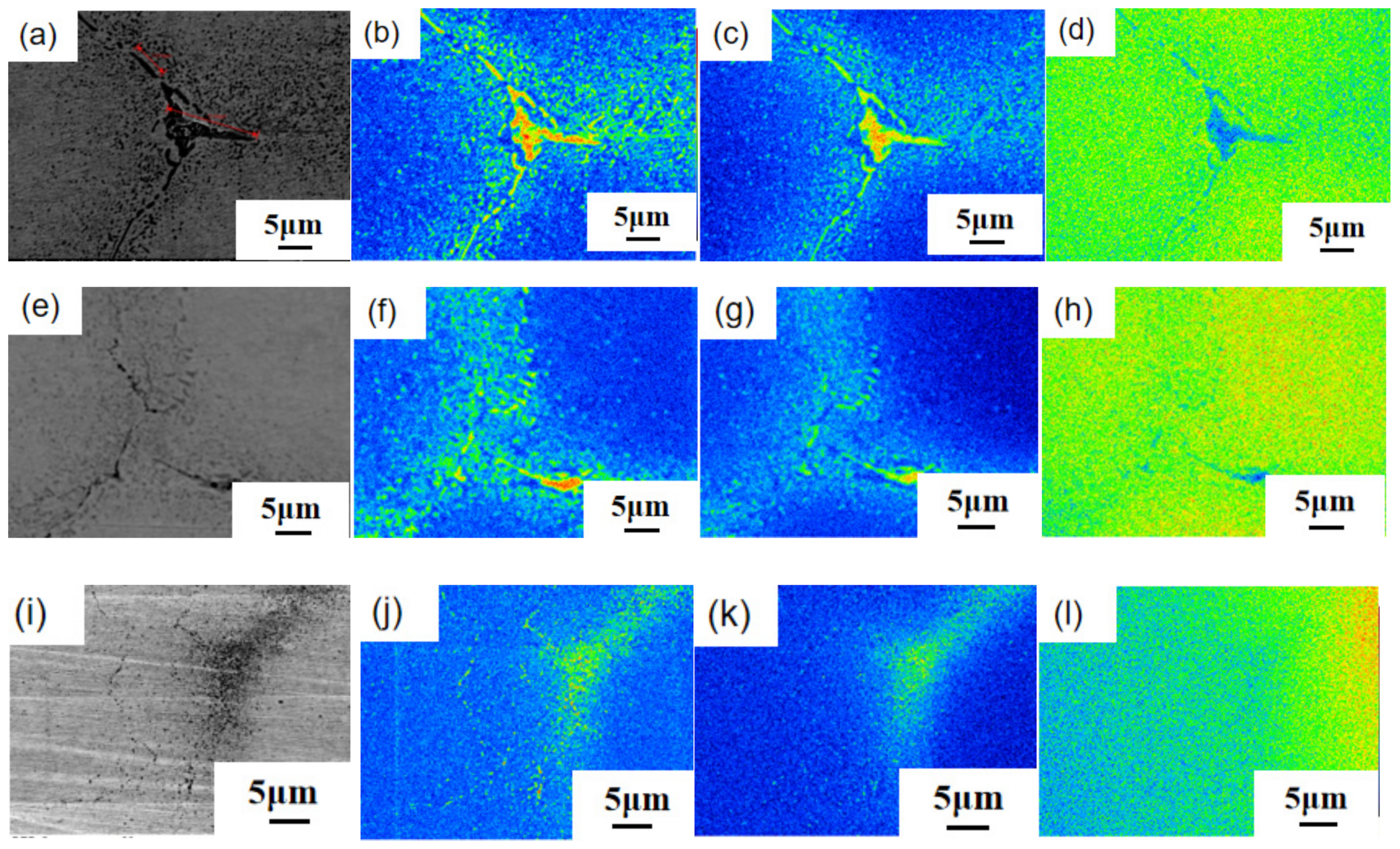
3. Experimental Results
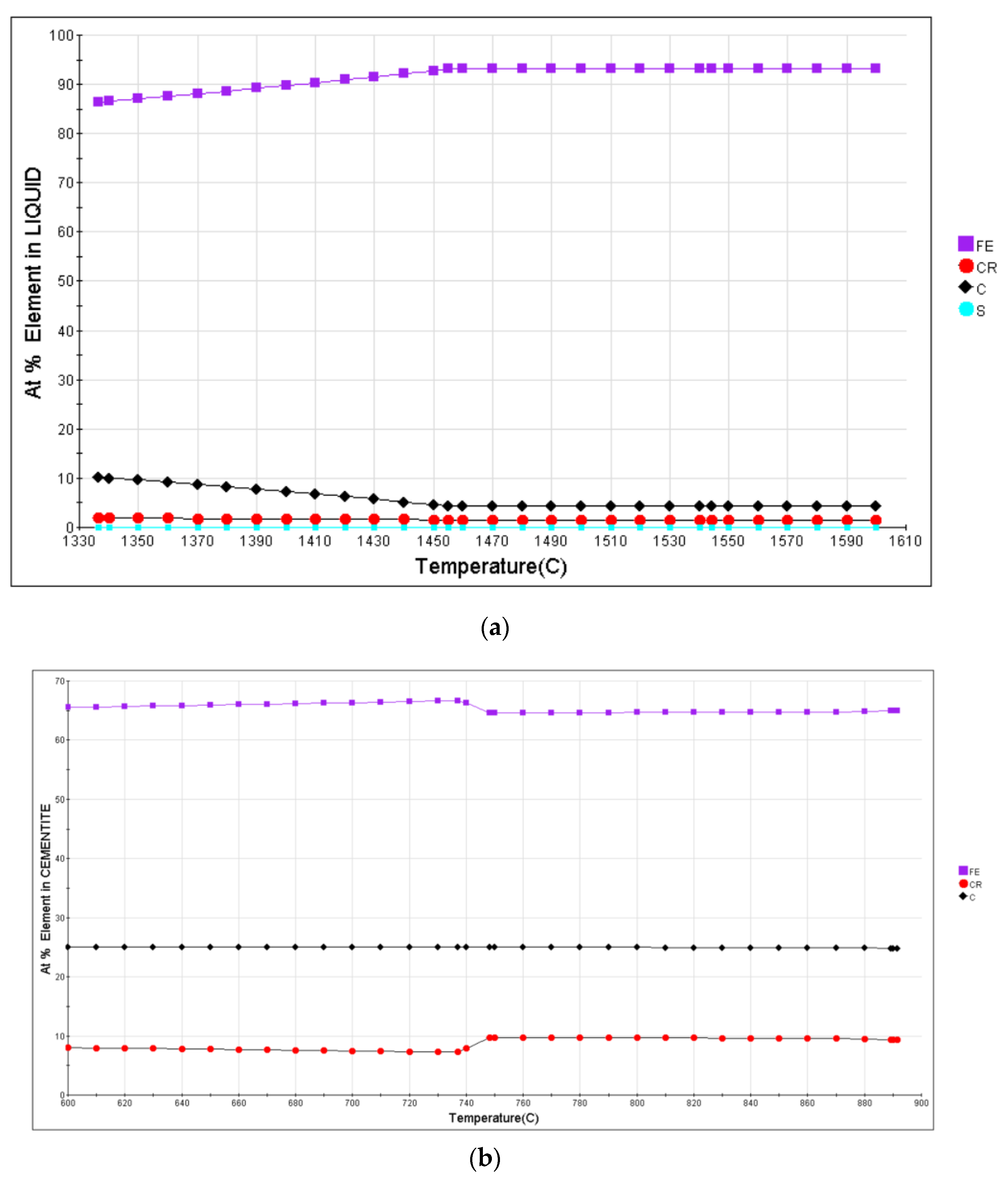
3.1. Calculation and Analysis
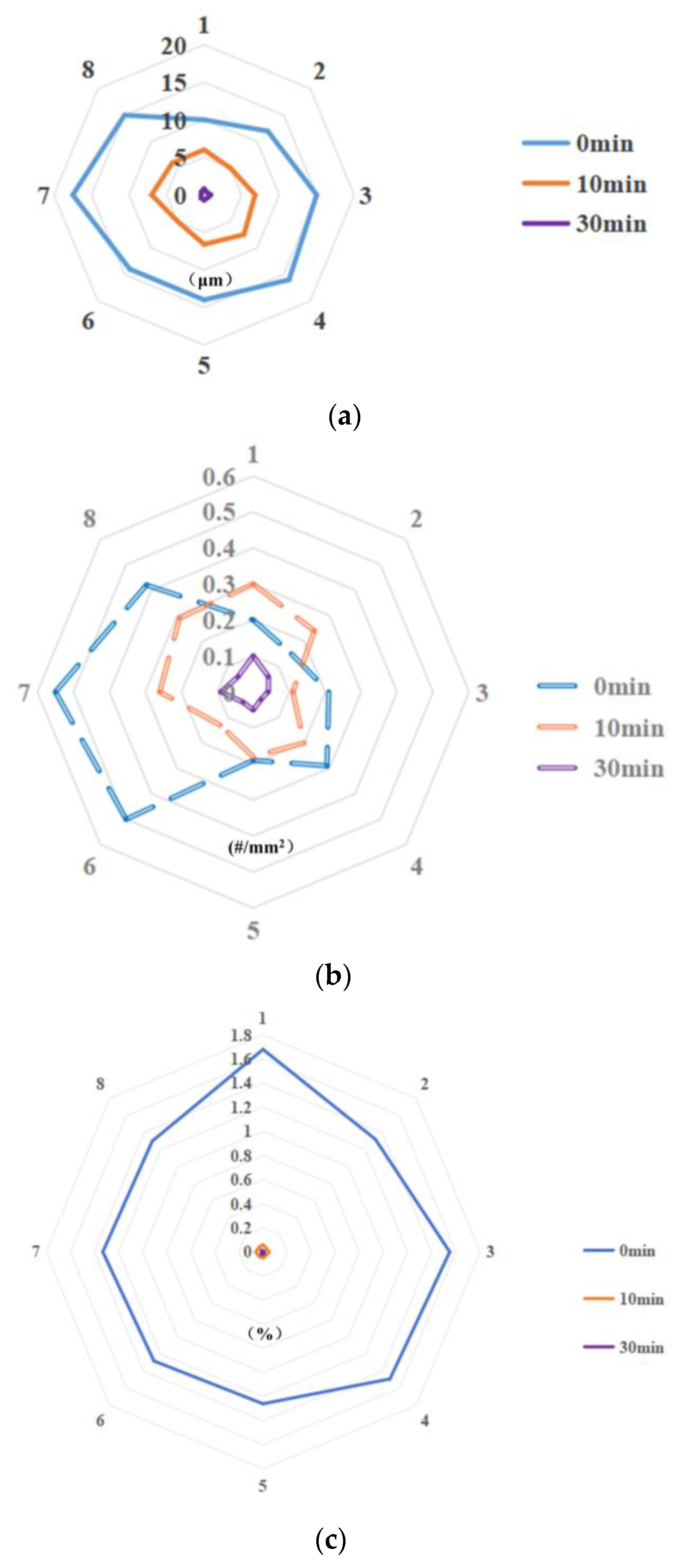
3.2. Effect of Pulsed Magnetic Field Parameters on Undissolved Carbides
4. Analysis and Discussion
4.1. Thermodynamics of Dissolution of Undissolved Carbides under Pulsed Magnetic Field Conditions
4.2. Dissolution Kinetics of Undissolved Carbides under Pulsed Magnetic Field Conditions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Q.; Wang, X.; Pan, Z.; Wei, Y.; Cheng, H.; Ma, Y.; Luo, H.; Li, X. Effects of rare earth elements addition on mechanical properties and corrosion behavior of GCr15 bearing steel under different heat treatment conditions. Corros. Commun. 2023, 9, 65–76. [Google Scholar] [CrossRef]
- Zeng, Q.; Hui, W.; Zhang, Y.; Liu, X.; Yao, Z. Very high-cycle fatigue performance of high carbon-chromium bearing steels with different metallurgical qualities. Int. J. Fatigue 2023, 172, 107632. [Google Scholar] [CrossRef]
- Zhang, W.; Li, W.; Liu, H.; He, T.; Deng, S.; Tian, H.; Cao, W. Microstructure effects on improving rolling contact fatigue by a double-quenching heat treatment process for GCr15 steel. Tribol. Int. 2024, 194, 109519. [Google Scholar] [CrossRef]
- Wang, P.; Wang, B.; Liu, Y.; Zhang, P.; Luan, Y.; Li, D.; Zhang, Z. Effects of inclusion types on the high-cycle fatigue properties of high-strength steel. Scr. Mater. 2022, 206, 114232. [Google Scholar] [CrossRef]
- Yu, F.; Chen, X.; Xu, H.; Dong, H.; Weng, Y.; Cao, W. Current Status of Metallurgical Quality and FatiguePerformance of Rolling Bearing Steeland Development Direction ofHigh-End Bearing Steel. Acta Metall. Sin. 2020, 56, 513–522. [Google Scholar]
- Pang, J.C.; Wang, G.D.; Yi, H.L. Effect of isothermal annealing on microstructure and hardness of low-density bearing steel. J. Iron Steel Res. 2020, 32, 1077–1083. [Google Scholar]
- Pang, J. The Structure and Performance of Low Density Steel 1.2C-1.5Cr-5Al Designed for Bearings. Master’s Thesis, Northeastern University, Shenyang, China, 2015. [Google Scholar]
- Cao, Z.; Shi, Z.; Yu, F.; Sugimoto, K.-I.; Cao, W.; Weng, Y. Effects of double quenching on fatigue properties of high carbon bearing steel with extra-high purity. Int. J. Fatigue 2019, 128, 105176. [Google Scholar] [CrossRef]
- Luo, T.F.; Wang, W.L.; Liu, Z.H.; Luo, S.; Zhu, M. In-situ observarion on solidification of GCr15 bearing steel at cooling rates of continuous castion. Iron Steel 2022, 57, 73–84. [Google Scholar]
- Hou, T.P.; Li, Z.H.; Wu, K.M.; Lin, H.F.; Li, Y.; Zhang, G.H.; Liu, W.M. Role of external magnetic fields in determining the thermodynamic properties of iron carbides in steel. Acta Mater. 2019, 167, 71–79. [Google Scholar] [CrossRef]
- Hao, J.Q.; Qin, S.Y.; Yan, L.G.; Zhang, X.F. Realising carbide ultrafast homogenisation under pulsed electric current. Mater. Sci. Technol. MST: A Publ. Inst. Met. 2022, 38, 689–702. [Google Scholar] [CrossRef]
- He, J.C. Improving Steel Quality by Electromagnetic Field. Iron Steel 2005, 40, 24–30. [Google Scholar]
- Qin, S.; Hao, J.; Yan, L.; Zhang, X. Ultrafast solution treatment to improve the comprehensive mechanical properties of superalloy by pulsed electric current. Scr. Mater. 2021, 199, 113879. [Google Scholar] [CrossRef]
- Hao, J.; Qin, S.; Yan, L.; Zhang, X. Breaking thermodynamic and kinetic barriers in superalloy homogenization process by electropulsing to improve mechanical properties. J. Alloys Compd. 2021, 873, 159854. [Google Scholar] [CrossRef]
- Zhu, F.; Jiang, D.; Sun, S.; Wu, H.; Zhang, Z.; Wang, J.; Ren, Z. Effect of alternating magnetic field on microstructure evolution and mechanical properties of M50 bearing steel during tempering process. J. Mater. Res. Technol. 2023, 26, 4516–4525. [Google Scholar] [CrossRef]
- Zhou, P.W.; Yang, W.L.; Wu, Y.C.; Zong, Y.Y. Characterization of microstructural evolution with pre-strain in BG801 bearing steel: Grain, carbides, retained austenite and martensite. Vacuum 2023, 216, 112354. [Google Scholar] [CrossRef]
- Zheng, K.; Zhong, Z.; Wang, H.; Xu, H.; Yu, F.; Wang, C.; Wu, G.; Liang, J.; Godfrey, A.; Cao, W. Obtaining Excellent Mechanical Properties in an Ultrahigh-Strength Stainless Bearing Steel via Solution Treatment. Metals 2023, 13, 1824. [Google Scholar] [CrossRef]
- Xie, G.; Shen, L.; Xing, S.; Ma, Y.; Liu, Y.; Chen, Z. Effect of Pulsed Magnetic Field on Quenched Heat Treatment of GCr15 Steel. JOM 2023, 75, 2256–2264. [Google Scholar] [CrossRef]
- Hao, J.Q. Controlling the Microstructure of Bearing Steel Based on the Diffusion Behavior of Solute Atoms under Pulsed Electric Cerrent. Ph.D. Thesis, University of Sience and Technology Beijing, Beijing, China, 2022. [Google Scholar]
- Li, C.-S.; Li, Z.-X.; Ren, J.-Y.; Tu, X.-Y.; Li, B.-Z. Microstructure and Properties of 1.0C-1.5Cr Bearing Steel in Processes of Hot Rolling, Spheroidization, Quenching, and Tempering. Steel Res. Int. 2019, 90, 1800470. [Google Scholar] [CrossRef]
- Li, Q.; Wen, G.; Chen, F.; Tang, P.; Hou, Z.; Mo, X. Irregular initial solidification by mold thermal monitoring in the continuous casting of steels: A review. Int. J. Miner. Metall. Mater. 2024, 31, 1003–1015. [Google Scholar] [CrossRef]
- Wang, E.; Zhai, Z. Growth and transition of dendrites in steel strands under different electromagnetic stirring methods. J. Cryst. Growth 2024, 642, 127789. [Google Scholar] [CrossRef]
- Li, S.S.; Chen, Y.; Gong, T.Z.; Chen, X.; Fu, P.; Li, D. Effect of Cooling Rate on the Precipitation Mechanism ofPrimary Carbide During Solidification in HighCarbon-Chromium Bearing Steel. Acta Metall. Sin. 2022, 58, 1024. [Google Scholar]
- Jiang, Y.; Tang, G.; Shek, C.; Zhu, Y.; Xu, Z. On the thermodynamics and kinetics of electropulsing induced dissolution of β-Mg17Al12 phase in an aged Mg-9Al-1Zn alloy. Acta Mater. 2009, 57, 4797–4808. [Google Scholar] [CrossRef]
- Yan, C.L.; Tian, M.S.; Shen, L. Effect of electromagnetic energy on the microstructure and compositional segregation of DC-casting Al-Si-Cu-Mg-Ni piston alloy. Chin. J. Rare Met. 2023, 47, 203–209. [Google Scholar]
- Peskir, G. On the Diffusion Coefficient: The Einstein Relation and Beyond. Stoch. Models 2003, 19, 383–405. [Google Scholar] [CrossRef]
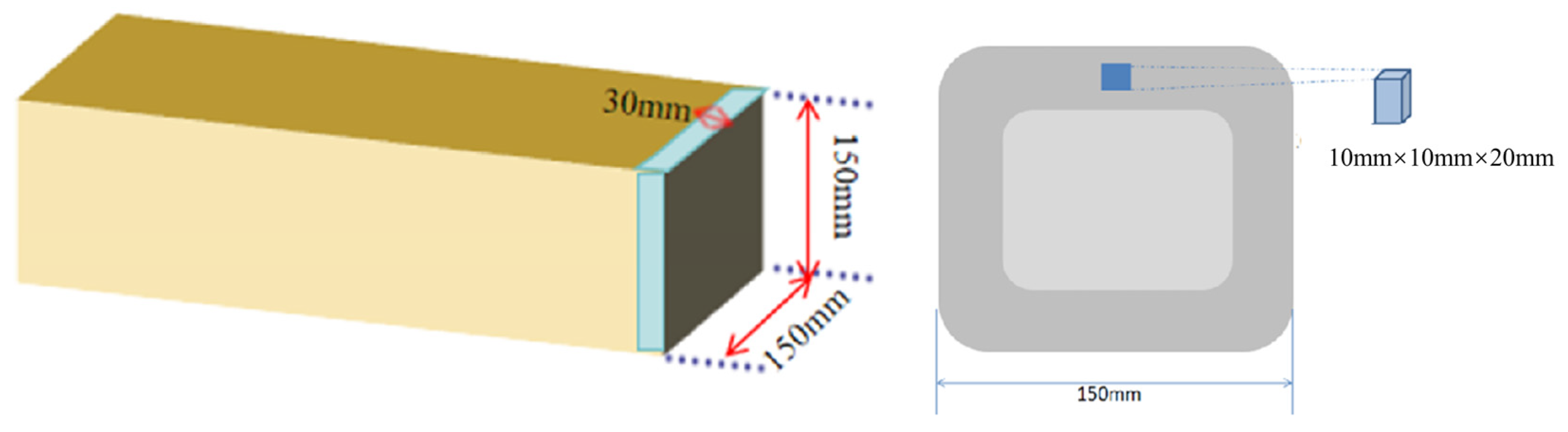
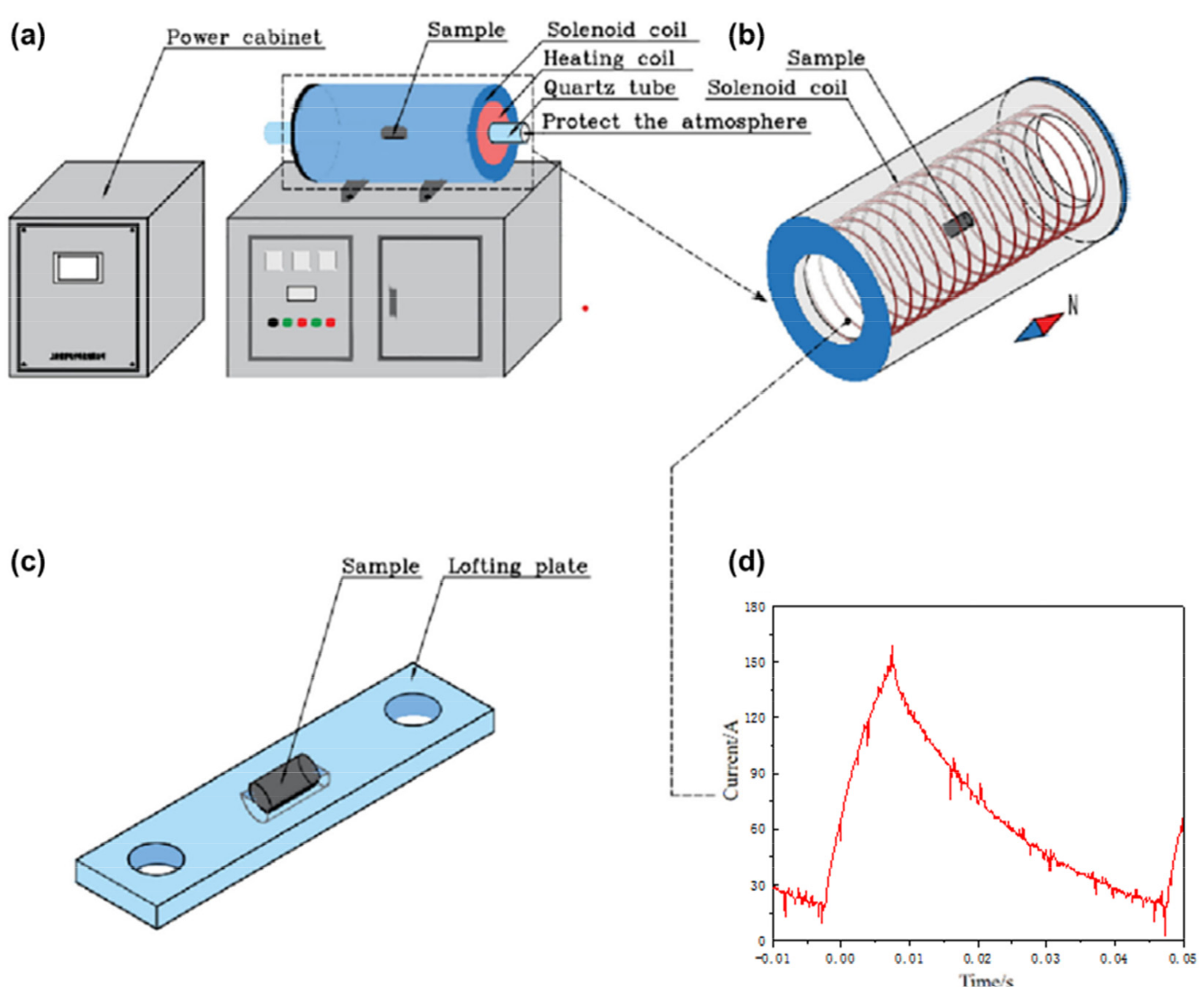





| C | Cr | Mn | Si | O | Al |
|---|---|---|---|---|---|
| 0.98 | 1.49 | 0.32 | 0.27 | 0.0005 | 0.022 |
| Temperature/°C | Heat Field Time/min | Magnetic Field Time/min | Intensity of Magnetization/mT | Duty Cycle/% | Frequency/Hz | Average Current/A |
|---|---|---|---|---|---|---|
| 950 | 30 | 0 | 0 | 0 | 0 | 0 |
| 950 | 30 | 10 | 34 | 20 | 20 | 20 |
| 950 | 30 | 30 | 34 | 20 | 20 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, L.; Lang, R.; Xing, S.; Ma, Y. Pulsed Magnetic Field Treatment Effects on Undissolved Carbides in Continuous Casting Billets of GCr15 Bearing Steel. Metals 2024, 14, 818. https://doi.org/10.3390/met14070818
Shen L, Lang R, Xing S, Ma Y. Pulsed Magnetic Field Treatment Effects on Undissolved Carbides in Continuous Casting Billets of GCr15 Bearing Steel. Metals. 2024; 14(7):818. https://doi.org/10.3390/met14070818
Chicago/Turabian StyleShen, Lijuan, Ruiqing Lang, Shuqing Xing, and Yonglin Ma. 2024. "Pulsed Magnetic Field Treatment Effects on Undissolved Carbides in Continuous Casting Billets of GCr15 Bearing Steel" Metals 14, no. 7: 818. https://doi.org/10.3390/met14070818
APA StyleShen, L., Lang, R., Xing, S., & Ma, Y. (2024). Pulsed Magnetic Field Treatment Effects on Undissolved Carbides in Continuous Casting Billets of GCr15 Bearing Steel. Metals, 14(7), 818. https://doi.org/10.3390/met14070818





