Role of the Biogenic Carbon Physicochemical Properties in the Manufacturing and Industrial Transferability of Mill Scale-Based Self-Reducing Briquettes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
2.2. Powder Briquetting
2.3. Mechanical Characterization
2.4. Metallurgical Performances Characterization
2.4.1. Reduction Behavior
2.4.2. Smelting Behavior and Iron Recovery
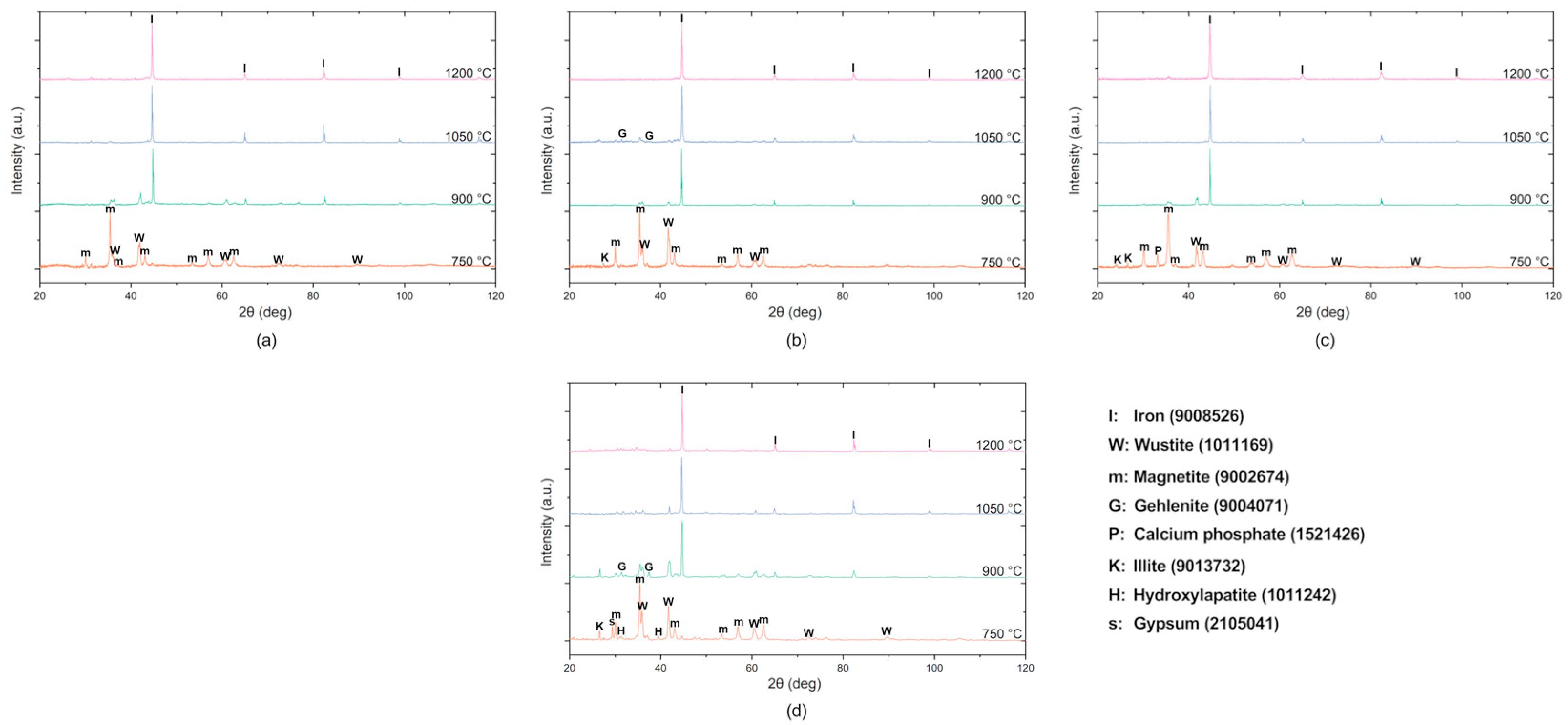
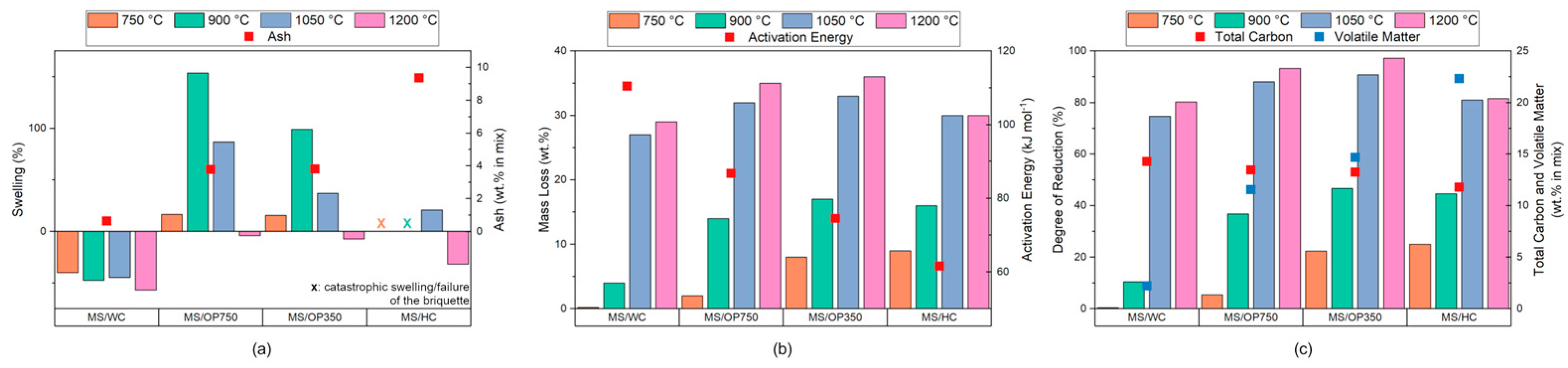
3. Results
3.1. Briquette Mechanical Performances
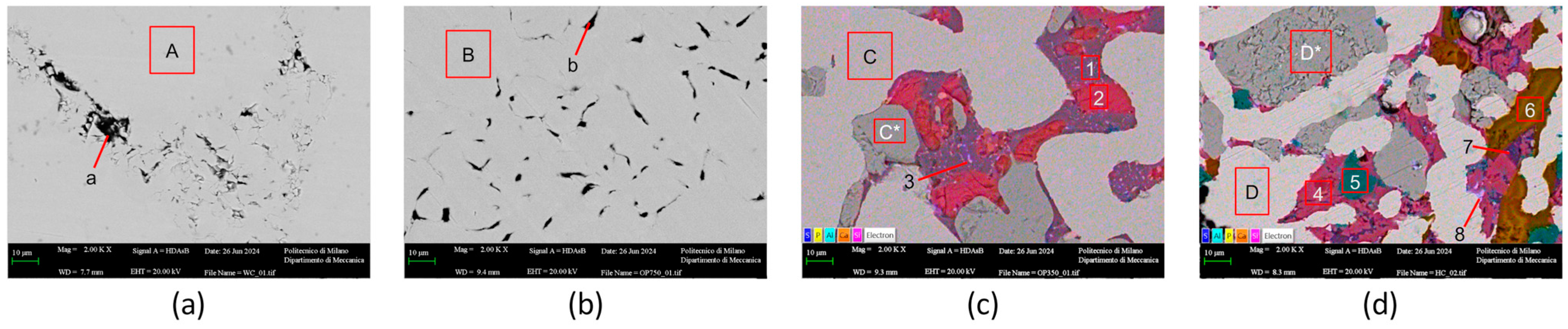
3.2. Metallurgical Performance Characterization
Smelting Behavior, Iron Recovery, and Industrial Transferability
4. Conclusion
- The mechanical performance are indirectly controlled by the hydrophilicity or hydrophobicity of the reducing agents used. Hydrophilic biogenic carbon allows the obtaining of agglomerates characterized by smooth surfaces and are highly packed, whereas hydrophobic biogenic carbon enhances the water expulsion from the agglomerate during the curing period leading to the formation of several cracks that inhibit the overall mechanical resistance, acting as low-resistance points. Still, the values achieved were comparable to or even better than that of pellets typically used in shaft furnaces (e.g., survival of 7 drops, CCS of 9.5 MPa, bulk density of 2 g cm−3).
- The iron recovery is regulated by the amount of fixed carbon and volatile matter in the agglomerate. Specifically, though the briquetting is able to exploit the reduction of the iron oxides from the volatiles at 750 °C onward, even in the case of nearly null fixed carbon, an amount higher than 6.93% of fixed carbon and lower than 11.55% of volatile matter is required to fully recover the iron oxides of the agglomerate at 1400 °C, with a final microstructure associable to that of cast irons. Lower amounts of fixed carbon and higher volatile matter lead to the maintenance of the agglomerate morphology with the coexistence of reduced and unreduced iron as well as slag.
- The presence of alkali in the ash is highly detrimental for the agglomerates once immersed due to their dissolution and catalyzing effect towards the calcite hydration which leads to briquette failure within 600 s. Furthermore, during heating, even a small amount of alkali can enhance the swelling in the 750–1050 °C range up to catastrophic values. Nevertheless, the presence of Ca-, Al-, and Si- compounds in the reducing agent ash allows the creation of a steelmaking close slag at 1400 °C, able to trap most of the phosphorus and sulfur, and protecting the recovered iron.
- Among the four biogenic carbon matrices, the use of wood chips or olive pomace pyrolyzed at 750 °C appears to be the most promising to recover the iron from mill scale and directly reintroduce it as secondary raw material in metallurgical furnaces when reduced at 1400 °C. On the contrary, slag separation processes and/or higher treatment temperatures are required for the recipes exploiting olive pomace pyrolyzed at 350 °C and hydrothermally carbonized sewage sludge to enhance the degree of metallization achieved (82.32% and 66.79%) up to industrial usable levels.
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Li, H.; Huang, S.; An, H.; Santagata, R.; Ulgiati, S. Environmental and Economic-Related Impact Assessment of Iron and Steel Production. A Call for Shared Responsibility in Global Trade. J. Clean. Prod. 2020, 269, 122239. [Google Scholar] [CrossRef]
- World Steel Association. World Steel in Figures 2023; World Steel Association: Bruxelles, Belgium, 2023. [Google Scholar]
- International Energy Agency. Iron and Steel Technology Roadmap; International Energy Agency: Paris, France, 2020. [Google Scholar]
- Dall’Osto, G.; Mombelli, D.; Mapelli, C. Consequences of the Direct Reduction and Electric Steelmaking Grid Creation on the Italian Steel Sector. Metals 2024, 14, 311. [Google Scholar] [CrossRef]
- Colla, V.; Branca, T.A.; Pietruck, R.; Wölfelschneider, S.; Morillon, A.; Algermissen, D.; Rosendahl, S.; Granbom, H.; Martini, U.; Snaet, D. Future Research and Developments on Reuse and Recycling of Steelmaking By-Products. Metals 2023, 13, 676. [Google Scholar] [CrossRef]
- BAT. Best Available Techniques—Reference Document for Iron and Steel Production: Industrial Emissions Directive 2010/75/EU: Integrated Pollution Prevention and Control; Joint Research Centre of the European Commission: Brussels, Belgium, 2012. [Google Scholar]
- Iluțiu-Varvara, D.-A.; Aciu, C.; Tintelecan, M.; Sas-Boca, I.-M. Assessment of Recycling Potential of the Steel Mill Scale in the Composition of Mortars for Sustainable Manufacturing. Procedia Manuf. 2020, 46, 131–135. [Google Scholar] [CrossRef]
- Spiliotis, X.; Ntampegliotis, K.; Kasiteropoulou, D.; Lamprakopoulos, S.; Lolos, K.; Karayannis, V.; Papapolymerou, G. Valorization of Mill Scale Waste by Its Incorporation in Fired Clay Bricks. Key Eng. Mater. 2014, 608, 8–13. [Google Scholar] [CrossRef]
- Touzi, N.; Horchani-Naifer, K. A Study on the Preparation and Characterization of Pigment Quality from Mill Scale Steel Wastes. Environ. Sci. Pollut. Res. 2023, 31, 40538–40553. [Google Scholar] [CrossRef]
- Iluţiu-Varvara, D.-A.; Aciu, C.; Maria Mârza, C.; Sas-Boca, I.-M.; Tintelecan, M. Assessment of Recycling Potential of the Oily Mill Scale in the Steelmaking Industry. Procedia Manuf. 2018, 22, 228–232. [Google Scholar] [CrossRef]
- Khodakovskii, V.R.; Zhornyak, A.F. Estimate of the Supply of Mill Scale for the Manufacture of Iron Powders. Sov. Powder Metall. Met. Ceram. 1965, 4, 505–510. [Google Scholar] [CrossRef]
- Manukyan, N.V. Carbide-Thermic Method of Preparation of Iron Powder. Sov. Powder Metall. Met. Ceram. 1967, 6, 260–263. [Google Scholar] [CrossRef]
- Eissa, M.; Ahmed, A.; El-Fawkhry, M. Conversion of Mill Scale Waste into Valuable Products via Carbothermic Reduction. J. Metall. 2015, 2015, 1–9. [Google Scholar] [CrossRef][Green Version]
- Martín, M.I.; López, F.A.; Torralba, J.M. Production of Sponge Iron Powder by Reduction of Rolling Mill Scale. Ironmak. Steelmak. 2012, 39, 155–162. [Google Scholar] [CrossRef]
- Cho, S.; Lee, J. Metal Recovery from Stainless Steel Mill Scale by Microwave Heating. Met. Mater. Int. 2008, 14, 193–196. [Google Scholar] [CrossRef]
- Bugdayci, M.; Alkan, M.; Turan, A.; Yücel, O. Production of Iron Based Alloys from Mill Scale through Metallothermic Reduction. High Temp. Mater. Process. 2018, 37, 889–898. [Google Scholar] [CrossRef]
- Kallio, M. Use of the Aluminothermic Reaction in the Treatment of Steel Industry By-Products. J. Mater. Synth. Process. 2000, 8, 87–92. [Google Scholar] [CrossRef]
- Khanna, R.; Konyukhov, Y.; Li, K.; Jayasankar, K.; Maslennikov, N.; Zinoveev, D.; Kargin, J.; Burmistrov, I.; Leybo, D.; Kravchenko, M.; et al. Innovative Transformation and Valorisation of Red Mill Scale Waste into Ferroalloys: Carbothermic Reduction in the Presence of Alumina. Sustainability 2023, 15, 16810. [Google Scholar] [CrossRef]
- Benchiheub, O.; Mechachti, S.; Serrai, S.; Khalifa, M.G. Elaboration of Iron Powder from Mill Scale. J. Mater. Environ. Sci. 2010, 1, 267–276. [Google Scholar]
- Bagatini, M.C.; Zymla, V.; Osório, E.; Vilela, A.C.F. Characterization and Reduction Behavior of Mill Scale. ISIJ Int. 2011, 51, 1072–1079. [Google Scholar] [CrossRef]
- Gaballah, N.M.; Zikry, A.F.; Khalifa, M.G.; Farag, A.B.; El-Hussiny, N.A.; Shalabi, M.E.H. Production of Iron from Mill Scale Industrial Waste via Hydrogen. Open J. Inorg. Non-Met. Mater. 2013, 03, 23–28. [Google Scholar] [CrossRef][Green Version]
- Domalski, E.S.; MacCrehan, W.A.; Moody, J.R.; Tewari, Y.B.; Walker, J.A. Characterization of Millscale Steel Wastes. National Bureau of Standards: Washington, DC, USA, 1983.
- Umadevi, T.; Brahmacharyulu, A.; Karthik, P.; Mahapatra, P.C.; Prabhu, M.; Ranjan, M. Recycling of Steel Plant Mill Scale via Iron Ore Sintering Plant. Ironmak. Steelmak. 2012, 39, 222–227. [Google Scholar] [CrossRef]
- Biochar for a Sustainable EAF Steel Production (GREENEAF2)—Publications Office of the EU. Available online: https://op.europa.eu/en/publication-detail/-/publication/7198c147-22b2-11e9-8d04-01aa75ed71a1/language-en (accessed on 6 July 2023).
- Sustainable EAF Steel Production (GREENEAF)—Publications Office of the EU. Available online: https://op.europa.eu/en/publication-detail/-/publication/e7dc500c-82de-4c2d-8558-5e24a2d335fb/language-en (accessed on 6 July 2023).
- Meier, T.; Hay, T.; Echterhof, T.; Pfeifer, H.; Rekersdrees, T.; Schlinge, L.; Elsabagh, S.; Schliephake, H. Process Modeling and Simulation of Biochar Usage in an Electric Arc Furnace as a Substitute for Fossil Coal. Steel Res. Int. 2017, 88, 1600458. [Google Scholar] [CrossRef]
- He, X.-M.; Yi, S.; Fu, P.-R.; Zeng, X.-C.; Zhang, D.; Cheng, X.-H. Combustion Reactivity of Biochar and Char Generated from Co-Pyrolysis of Coal and Four Additives: Application in Blast Furnace. J. Energy Eng. 2017, 143, 04016023. [Google Scholar] [CrossRef]
- Robinson, R.; Brabie, L.; Pettersson, M.; Amovic, M.; Ljunggren, R. An Empirical Comparative Study of Renewable Biochar and Fossil Carbon as Carburizer in Steelmaking. ISIJ Int. 2022, 62, 2522–2528. [Google Scholar] [CrossRef]
- Cardarelli, A.; De Santis, M.; Cirilli, F.; Barbanera, M. Computational Fluid Dynamics Analysis of Biochar Combustion in a Simulated Ironmaking Electric Arc Furnace. Fuel 2022, 328, 125267. [Google Scholar] [CrossRef]
- DiGiovanni, C.; Li, D.; Ng, K.W.; Huang, X. Ranking of Injection Biochar for Slag Foaming Applications in Steelmaking. Metals 2023, 13, 1003. [Google Scholar] [CrossRef]
- Echterhof, T. Review on the Use of Alternative Carbon Sources in EAF Steelmaking. Metals 2021, 11, 222. [Google Scholar] [CrossRef]
- Safarian, S. To What Extent Could Biochar Replace Coal and Coke in Steel Industries? Fuel 2023, 339, 127401. [Google Scholar] [CrossRef]
- Konishi, H.; Ichikawa, K.; Usui, T. Effect of Residual Volatile Matter on Reduction of Iron Oxide in Semi-Charcoal Composite Pellets. ISIJ Int. 2010, 50, 386–389. [Google Scholar] [CrossRef]
- Wei, R.; Cang, D.; Bai, Y.; Huang, D.; Liu, X. Reduction Characteristics and Kinetics of Iron Oxide by Carbon in Biomass. Ironmak. Steelmak. 2016, 43, 144–152. [Google Scholar] [CrossRef]
- Bagatini, M.C.; Kan, T.; Evans, T.J.; Strezov, V. Iron Ore Reduction by Biomass Volatiles. J. Sustain. Metall. 2021, 7, 215–226. [Google Scholar] [CrossRef]
- El-Tawil, A.; Ahmed, H.M.; El-Geassy, A.A.; Bjorkman, B. Effect of Volatile Matter on Reduction of Iron Oxide-Containing Carbon Composite. In Proceedings of the 54th Annual Conference of Metallurgists (COM 2015), Fairmont Royal York, Toronto, ON, Canada, 8 August 2015; pp. 1–14. [Google Scholar]
- Das, D.; Anand, A.; Gautam, S.; Rajak, V.K. Assessment of Utilization Potential of Biomass Volatiles and Biochar as a Reducing Agent for Iron Ore Pellets. Environ. Technol. 2024, 45, 158–169. [Google Scholar] [CrossRef]
- Sönmez, İ.; Şahbudak, K. Optimization of Sponge Iron (Direct Reduced Iron) Production with Box-Wilson Experimental Design by Using Iron Pellets and Lignite as Reductant. Rev. Metal. 2023, 59, e241. [Google Scholar] [CrossRef]
- Murakami, T.; Takahashi, T.; Fuji, S.; Maruoka, D.; Kasai, E. Development of Manufacturing Principle of Porous Iron by Carbothermic Reduction of Composite of Hematite and Biomass Char. Mater. Trans. 2017, 58, 1742–1748. [Google Scholar] [CrossRef]
- Liu, Z.; Bi, X.; Gao, Z.; Liu, W. Carbothermal Reduction of Iron Ore in Its Concentrate-Agricultural Waste Pellets. Adv. Mater. Sci. Eng. 2018, 2018, 1–6. [Google Scholar] [CrossRef]
- Chuanchai, A.; Wu, K.-T. Potential of Pinewood Biochar as an Eco-Friendly Reducing Agent in Iron Ore Reduction. ACS Omega 2024, 9, 14279–14286. [Google Scholar] [CrossRef] [PubMed]
- Ubando, A.T.; Chen, W.-H.; Ong, H.C. Iron Oxide Reduction by Graphite and Torrefied Biomass Analyzed by TG-FTIR for Mitigating CO2 Emissions. Energy 2019, 180, 968–977. [Google Scholar] [CrossRef]
- Vitikka, O.; Iljana, M.; Heikkilä, A.; Tkalenko, I.; Kovtun, O.; Koriuchev, N.; Shehovsov, D.; Fabritius, T. Effect of Biocarbon Addition on Metallurgical Properties of Mill Scale-Based Auger Pressing Briquettes. ISIJ Int. 2024, 64, 964–977. [Google Scholar] [CrossRef]
- Khaerudini, D.S.; Chanif, I.; Insiyanda, D.R.; Destyorini, F.; Alva, S.; Pramono, A. Preparation and Characterization of Mill Scale Industrial Waste Reduced by Biomass-Based Carbon. J. Sustain. Metall. 2019, 5, 510–518. [Google Scholar] [CrossRef]
- Bagatini, M.C.; Zymla, V.; Osório, E.; Vilela, A.C.F. Scale Recycling Through Self-Reducing Briquettes to Use in EAF. ISIJ Int. 2017, 57, 2081–2090. [Google Scholar] [CrossRef]
- Dall’Osto, G.; Mombelli, D.; Pittalis, A.; Mapelli, C. Biochar and Other Carbonaceous Materials Used in Steelmaking: Possibilities and Synergies for Power Generation by Direct Carbon Fuel Cell. Biomass Bioenergy 2023, 177, 106930. [Google Scholar] [CrossRef]
- ASTM D1762-84; American Society for Testing and Materials International Standard Test Method for Chemical Analysis of Wood Charcoal. ASTM: Conshohocken, PA, USA, 2009.
- Lim, A.C.R.; Chin, B.L.F.; Jawad, Z.A.; Hii, K.L. Kinetic Analysis of Rice Husk Pyrolysis Using Kissinger-Akahira-Sunose (KAS) Method. Procedia Eng. 2016, 148, 1247–1251. [Google Scholar] [CrossRef]
- Hussain, R.; Ghosh, K.K.; Garg, A.; Ravi, K. Effect of Biochar Produced from Mesquite on the Compaction Characteristics and Shear Strength of a Clayey Sand. Geotech. Geol. Eng. 2021, 39, 1117–1131. [Google Scholar] [CrossRef]
- Mombelli, D.; Dall’Osto, G.; Trombetta, V.; Mapelli, C. Comparison of the Reduction Behavior through Blast Furnace Sludge of Two Industrial Jarosites. J. Environ. Chem. Eng. 2023, 11, 109360. [Google Scholar] [CrossRef]
- Dall’Osto, G.; Mombelli, D.; Trombetta, V.; Mapelli, C. Effect of Particle Size and Starch Gelatinization on the Mechanical and Metallurgical Performance of Jarosite Plus Blast Furnace Sludge Self-Reducing Briquettes. J. Sustain. Metall. 2024, 10, 759–774. [Google Scholar] [CrossRef]
- Mombelli, D.; Gonçalves, D.L.; Mapelli, C.; Barella, S.; Gruttadauria, A. Processing and Characterization of Self-Reducing Briquettes Made of Jarosite and Blast Furnace Sludges. J. Sustain. Metall. 2021, 7, 1603–1626. [Google Scholar] [CrossRef]
- ASTM D440-07; American Society for Testing and Materials International Standard Test Method of Drop Shatter Test for Coal. ASTM: Conshohocken, PA, USA, 2019.
- BS ISO 4700:2015; Iron Ore Pellets for Blast Furnace and Direct Reduction Feedstocks—Determination of the Crushing Strength. ISO: Geneva, Switzerland, 2015.
- Richards, S.R. Physical Testing of Fuel Briquettes. Fuel Process. Technol. 1990, 25, 89–100. [Google Scholar] [CrossRef]
- BS ISO 11258:2015; Iron Ores for Shaft Direct-Reduction Feedstocks. Determination of the Reducibility Index, Final Degree of Reduction and Degree of Metallization. ISO: Geneva, Switzerland, 2015.
- Leino, T.; Taskinen, P.; Eric, R.H. Determination of Metallization Degree of Pre-Reduced Chromite with Image and Rietveld Analysis. J. Min. Metall. Sect. B Metall. 2020, 56, 289–297. [Google Scholar] [CrossRef]
- Li, Z.; Zou, H. Optimization of Biomass Fuel Cold Briquetting Parameters Based on Response Surface Analysis. J. Inst. Eng. (India) Ser. C 2022, 103, 459–472. [Google Scholar] [CrossRef]
- Seetharaman, S.; Teng, L.; Hayashi, M.; Wang, L. Understanding the Properties of Slags. ISIJ Int. 2013, 53, 1–8. [Google Scholar] [CrossRef]
- Seetharaman, S. Treatise on Process Metallurgy, Volume 3: Industrial Processes; Elsevier: Amsterdam, The Netherlands, 2013; Volume 3. [Google Scholar]
- Zhang, C.; Zhang, N.; Pan, D.; Qian, D.; An, Y.; Yuan, Y.; Xiang, Z.; Wang, Y. Experimental Study on Sensitivity of Porosity to Pressure and Particle Size in Loose Coal Media. Energies 2018, 11, 2274. [Google Scholar] [CrossRef]
- Pang, L.; Yang, Y.; Wu, L.; Wang, F.; Meng, H. Effect of Particle Sizes on the Physical and Mechanical Properties of Briquettes. Energies 2019, 12, 3618. [Google Scholar] [CrossRef]
- Richards, S.R. Briquetting Peat and Peat-Coal Mixtures. Fuel. Process. Technol. 1990, 25, 175–190. [Google Scholar] [CrossRef]
- Suarez, J.A.; Luengo, C.A. Coffee Husk Briquettes: A New Renewable Energy Source. Energy Sources 2003, 25, 961–967. [Google Scholar] [CrossRef]
- El-Hussiny, N.A.A.; Shalabi, M.E.H.E.H. A Self-Reduced Intermediate Product from Iron and Steel Plants Waste Materials Using a Briquetting Process. Powder Technol. 2011, 205, 217–223. [Google Scholar] [CrossRef]
- Davies, R.M.; Davies, O.A. Effect of Briquetting Process Variables on Hygroscopic Property of Water Hyacinth Briquettes. J. Renew. Energy 2013, 2013, 429230. [Google Scholar] [CrossRef]
- Finneran, D.W.; Morse, J.W. Calcite Dissolution Kinetics in Saline Waters. Chem. Geol. 2009, 268, 137–146. [Google Scholar] [CrossRef]
- Araújo, J.H.d.; Silva, N.F.d.; Acchar, W.; Gomes, U.U. Thermal Decomposition of Illite. Mater. Res. 2004, 7, 359–361. [Google Scholar] [CrossRef]
- Lu, D.; Chen, Q.; Li, C.; Gong, S. Effect of Potassium Feldspar on the Decomposition Rate of Phosphogypsum. J. Chem. Technol. Biotechnol. 2021, 96, 374–383. [Google Scholar] [CrossRef]
- Reddy, D.S.; Chang, H.-H.; Tsai, M.-Y.; Chen, I.-G.; Wu, K.-T.; Liu, S.-H. Swelling and Softening Behavior of Iron Ore-Spent Mushroom Substrate Composite Pellets during Carbothermal Reduction. J. Mater. Res. Technol. 2023, 22, 1999–2007. [Google Scholar] [CrossRef]
- Singh, M.; Björkman, B. Effect of Reduction Conditions on the Swelling Behaviour of Cement-Bonded Briquettes. ISIJ Int. 2004, 44, 294–303. [Google Scholar] [CrossRef]
- Sarkar, S.B.; Ray, H.S.; Chatterjee, I. Kinetics of Reduction of Iron Ore—Coal Pellets. J. Therm. Anal. 1989, 35, 2461–2469. [Google Scholar] [CrossRef]
- Bagatini, M.C.; Zymla, V.; Osório, E.; Vilela, A.C.F. Carbon Gasification in Self-Reducing Mixtures. ISIJ Int. 2014, 54, 2687–2696. [Google Scholar] [CrossRef]
- Fanfoni, M.; Tomellini, M. The Johnson-Mehl- Avrami-Kohnogorov Model: A Brief Review. Il Nuovo Cimento D 1998, 20, 1171–1182. [Google Scholar] [CrossRef]
- Yuan, X.; Luo, F.; Liu, S.; Zhang, M.; Zhou, D. Comparative Study on the Kinetics of the Isothermal Reduction of Iron Ore Composite Pellets Using Coke, Charcoal, and Biomass as Reducing Agents. Metals 2021, 11, 340. [Google Scholar] [CrossRef]
- Chai, Y.; Fan, Y.; Li, Z.; Wu, J.; Zhang, Y.; Wang, Y.; Luo, G.; An, S. Kinetics of Reduction in Stages of Pellets Prepared from the Bayan Obo Iron Ore Concentrate. ACS Omega 2022, 7, 7759–7768. [Google Scholar] [CrossRef] [PubMed]
- Friess, J.; Sonntag, U.; Steller, I.; Bührig-Polaczek, A. From Individual Graphite Assignment to an Improved Digital Image Analysis of Ductile Iron. Int. J. Met. 2020, 14, 1090–1104. [Google Scholar] [CrossRef]
- Sohn, I.; Fruehan, R.J. The Reduction of Iron Oxides by Volatiles in a Rotary Hearth Furnace Process: Part III. The Simulation of Volatile Reduction in a Multi-Layer Rotary Hearth Furnace Process. Metall. Mater. Trans. B 2006, 37, 231–238. [Google Scholar] [CrossRef]
- Ghosh, A.; Chatterjee, A. Ironmaking and Steelmaking Theory and Practice; PHI Learning Private Limited: New Delhi, India, 2008; Volume 20, ISBN 812033289X. [Google Scholar]
- Rietmeijer, F.J.M.; Nuth, J.A.; Pun, A. The Formation of Mg,Fe-silicates by Reactions between Amorphous Magnesiosilica Smoke Particles and Metallic Iron Nanograins with Implications for Comet Silicate Origins. Meteorit. Planet. Sci. 2013, 48, 1823–1840. [Google Scholar] [CrossRef]
- Liu, C.; Huang, S.; Blanpain, B.; Guo, M. Effect of Al2O3 Addition on Mineralogical Modification and Crystallization Kinetics of a High Basicity BOF Steel Slag. Metall. Mater. Trans. B 2019, 50, 271–281. [Google Scholar] [CrossRef]
- Bagatini, M.C.; Fernandes, T.; Silva, R.; Galvão, D.F.; Flores, I.V. Mill Scale and Flue Dust Briquettes as Alternative Burden to Low Height Blast Furnaces. J. Clean. Prod. 2020, 276, 124332. [Google Scholar] [CrossRef]
- Böhme, N.; Hauke, K.; Dohrn, M.; Neuroth, M.; Geisler, T. High-Temperature Phase Transformations of Hydroxylapatite and the Formation of Silicocarnotite in the Hydroxylapatite–Quartz–Lime System Studied in Situ and in Operando by Raman Spectroscopy. J. Mater. Sci. 2022, 57, 15239–15266. [Google Scholar] [CrossRef]
- Mohanty, M.K.; Mishra, S.; Mishra, B.; Sarkar, S.; Samal, S.K. A Novel Technique for Making Cold Briquettes for Charging in Blast Furnace. IOP Conf. Ser. Mater. Sci. Eng. 2016, 115, 012020. [Google Scholar] [CrossRef]
- Mombelli, D.; Mapelli, C.; Barella, S.; Gruttadauria, A.; Spada, E. Jarosite Wastes Reduction through Blast Furnace Sludges for Cast Iron Production. J. Environ. Chem. Eng. 2019, 7, 102996. [Google Scholar] [CrossRef]
- Valenti, F.; Arcidiacono, C.; Chinnici, G.; Cascone, G.; Porto, S.M. Quantification of Olive Pomace Availability for Biogas Production by Using a GIS-based Model. Biofuels Bioprod. Biorefining 2017, 11, 784–797. [Google Scholar] [CrossRef]
- CREA Consiglio per la Ricerca in Agricoltura e L’analisi Dell’economia Agraria. Italian Agriculture in Figures 2022; CREA Consiglio per la Ricerca in Agricoltura e L’analisi Dell’economia Agraria: Roma, Italy, 2022. [Google Scholar]
- Vasileiadou, A.; Zoras, S.; Iordanidis, A. Bioenergy Production from Olive Oil Mill Solid Wastes and Their Blends with Lignite: Thermal Characterization, Kinetics, Thermodynamic Analysis, and Several Scenarios for Sustainable Practices. Biomass Convers. Biorefinery 2021, 13, 5325–5338. [Google Scholar] [CrossRef]
- Federacciai. La Siderurgia Italiana in Cifre: The Italian Steel Industry Key Statistics 2022; Federacciai: Milano, Italy, 2023; Available online: https://federacciai.it/wp-content/uploads/2023/05/AssembleaAnnuale2023_Relazione-Annuale-2022.pdf (accessed on 28 July 2024).
- Method for Direct Reduction of Iron Oxide-Based Material for The Production of Steel, Iron Sponge or Cast Iron. WIPO Patent Application WO/2024/013653, 18 January 2024.




| Wavelength-Dispersive X-ray Fluorescence | Rietveld Analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | CaO | Cr2O3 | CuO | Fe2O3 | MgO | MnO | NiO | SiO2 | Wustite | Magnetite | Hematite |
| 0.30 | 0.32 | 0.45 | 0.10 | 96.95 | 0.07 | 1.13 | 0.08 | 0.60 | 60 | 30 | 10 |
| Proximate Analysis (wt.%db) | Ctot (wt.%) | S (wt.%) | Activation Energy (kJ mol−1) | Contact Angle (deg.) | |||
|---|---|---|---|---|---|---|---|
| Cfix | VM | Ash | |||||
| WC | 83.81 | 12.58 | 3.61 | 93.51 | 0.001 | 110.45 | 71.75 |
| OP750 | 31.14 | 51.9 | 16.96 | 69.87 | 0.109 | 86.76 | 52.02 |
| OP350 | 21.22 | 62.55 | 16.22 | 65.23 | 0.059 | 74.52 | 146.98 |
| HC | 0.73 | 69.95 | 29.32 | 42.67 | 0.589 | 61.61 | 157.84 |
| Mill Scale | Cfix | VM | Ash | |
|---|---|---|---|---|
| (wt.%db) | ||||
| MS/WC | 82.38 | 14.77 | 2.22 | 0.64 |
| MS/OP750 | 77.75 | 6.93 | 11.55 | 3.77 |
| MS/OP350 | 76.53 | 4.98 | 14.68 | 3.81 |
| MS/HC | 68.08 | 0.23 | 22.32 | 9.36 |
| MS/WC | MS/OP750 | MS/OP350 | MS/HC | |
|---|---|---|---|---|
| Apparent density, green (g cm−3) | 2.48 (0.06) | 2.69 (0.04) | 2.33 (0.06) | 2.35 (0.06) |
| Apparent density, cured (g cm−3) | 2.34 (0.04) | 2.57 (0.05) | 2.16 (0.05) | 2.24 (0.07) |
| Number of drop/1.63 m | 10.0 (0.0) | 9.5 (0.5) | 3.0 (2.0) | 7.7 (1.2) |
| Impact resistance index | 1000.0 (0.0) | 766.7 (230.2) | 75.0 (22.5) | 230.0 (4.8) |
| Size stability (%) | 99.60 (3.64) | 96.02 (1.63) | 76.01 (10.52) | 86.33 (6.11) |
| Ultimate compressive strength (MPa) | 15.39 (0.19) | 12.56 (0.74) | 3.91 (0.56) | 6.33 (0.74) |
| Water resistance index (%) | 90.41 | Failed before 600 s | Failed before 600 s | 80.45 |
| Spectrum | C | O | Al | Si | P | S | Ca | Fe | Phase |
|---|---|---|---|---|---|---|---|---|---|
| A–D | 3.57 a | 96.43 | Iron | ||||||
| C*–D* | 24.24 | 75.76 | Wustite | ||||||
| a–b | 75.99 | 1.20 | 22.81 | Graphite | |||||
| 1 | 38.83 | 14.05 | 11.54 | 27.55 | 8.03 | Gehlenite | |||
| 2 | 38.29 | 15.95 | 39.71 | 6.05 | Calcio-olivine | ||||
| 3 | 37.39 | 2.82 | 15.56 | 0.2 | 0.71 | 24.41 | 18.91 | Ferri-gehlenite | |
| 4 | 38.52 | 14.52 | 1.16 | 19.01 | 26.8 | Kirschenite | |||
| 5 | 34.17 | 22.87 | 42.96 | Spinel | |||||
| 6 | 44.66 | 19.45 | 33.73 | 2.16 | Calcium phosphate | ||||
| 7 | 44.66 | 18.12 | 10.33 | 4.18 | 11.49 | 11.23 | Gehlenite | ||
| 8 | 4.27 | 1.21 | 35 | 1.02 | 58.51 | Iron sulfide |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dall’Osto, G.; Mombelli, D.; Scolari, S.; Mapelli, C. Role of the Biogenic Carbon Physicochemical Properties in the Manufacturing and Industrial Transferability of Mill Scale-Based Self-Reducing Briquettes. Metals 2024, 14, 882. https://doi.org/10.3390/met14080882
Dall’Osto G, Mombelli D, Scolari S, Mapelli C. Role of the Biogenic Carbon Physicochemical Properties in the Manufacturing and Industrial Transferability of Mill Scale-Based Self-Reducing Briquettes. Metals. 2024; 14(8):882. https://doi.org/10.3390/met14080882
Chicago/Turabian StyleDall’Osto, Gianluca, Davide Mombelli, Sara Scolari, and Carlo Mapelli. 2024. "Role of the Biogenic Carbon Physicochemical Properties in the Manufacturing and Industrial Transferability of Mill Scale-Based Self-Reducing Briquettes" Metals 14, no. 8: 882. https://doi.org/10.3390/met14080882
APA StyleDall’Osto, G., Mombelli, D., Scolari, S., & Mapelli, C. (2024). Role of the Biogenic Carbon Physicochemical Properties in the Manufacturing and Industrial Transferability of Mill Scale-Based Self-Reducing Briquettes. Metals, 14(8), 882. https://doi.org/10.3390/met14080882









