Parametric Study of Vanadium Extraction Process from Refining Tailings of Crude Titanium Tetrachloride
Abstract
:1. Introduction
2. Experimental Section
2.1. Raw Materials
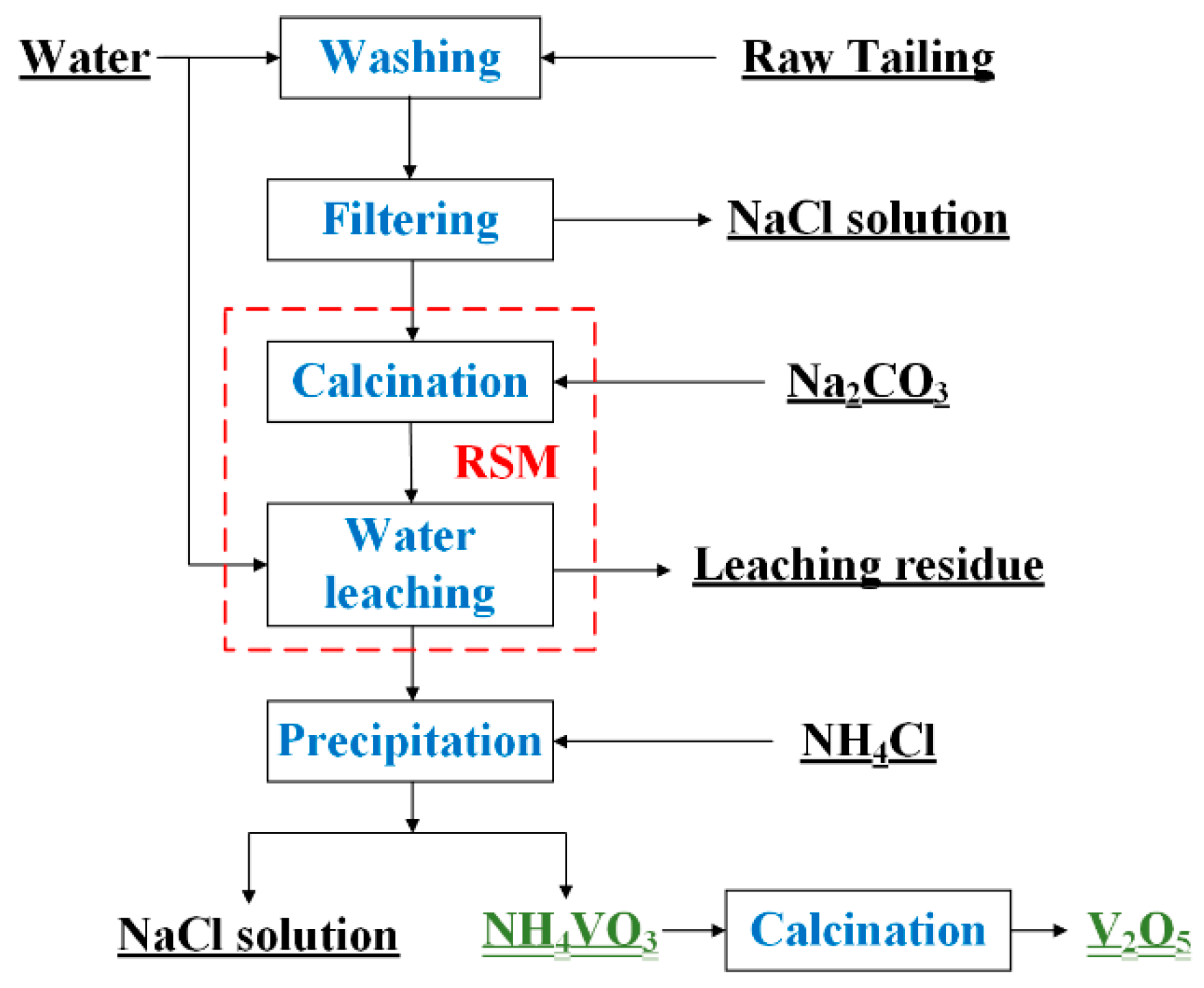
2.2. Experimental Procedures
2.3. Experimental Design and Data Analysis
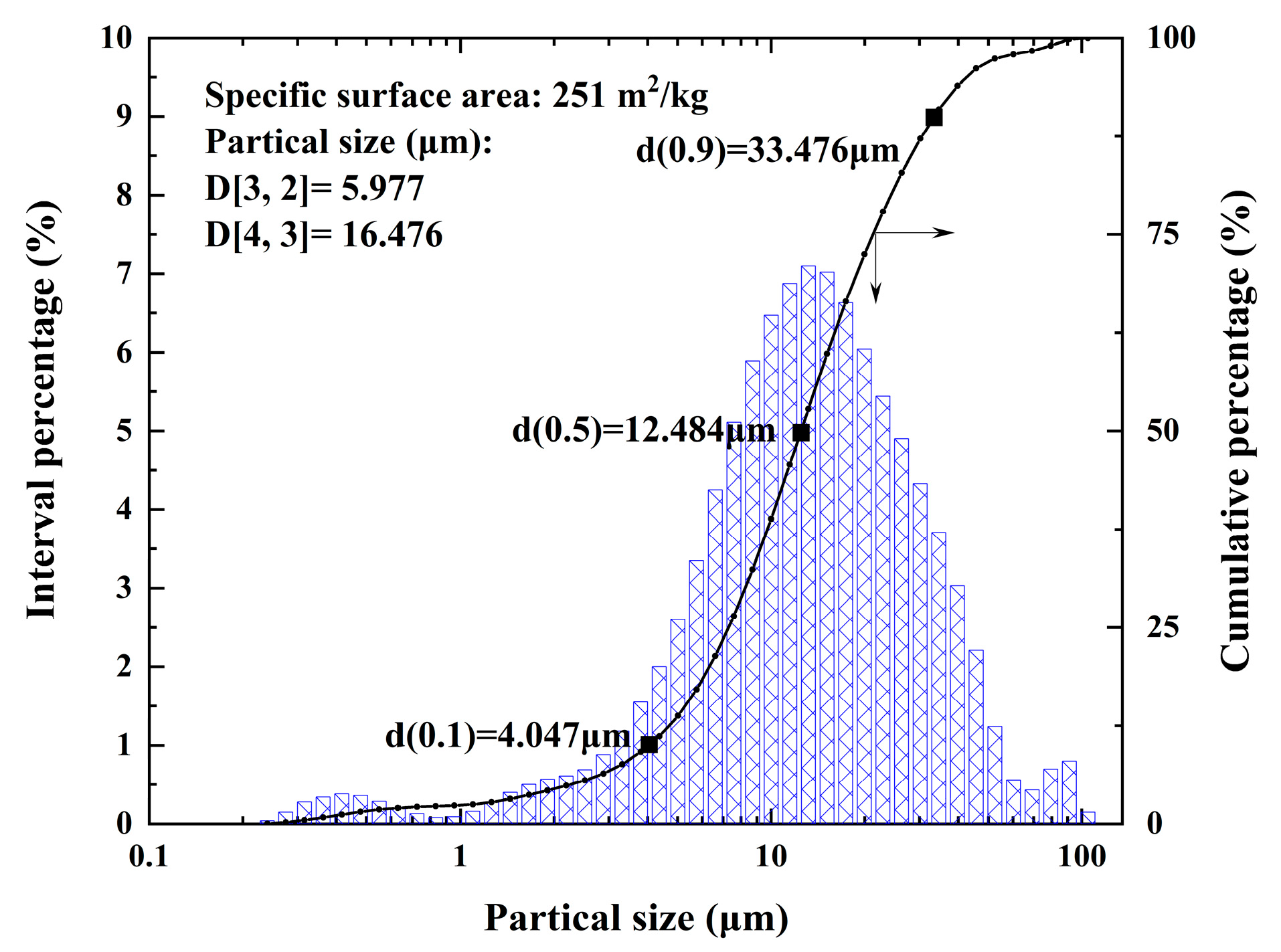
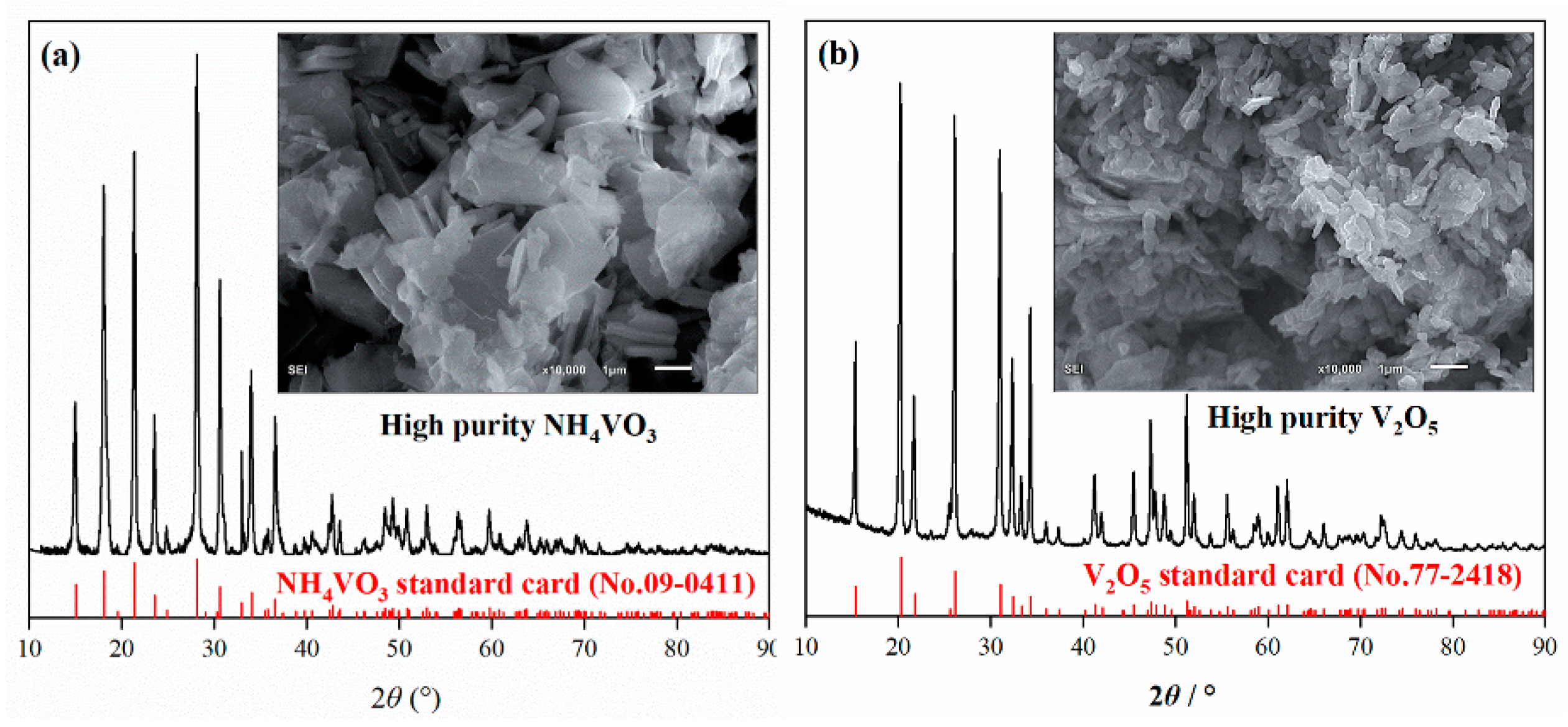
2.4. Characterization
3. Results and Discussions
3.1. Mineralogical Analysis of Titanium Tetrachloride Tail Residue
3.2. Evolution of the Phase of the Vanadium Tailing at Different Calcination Temperatures
3.3. Statistical Results and Regression Model Equation
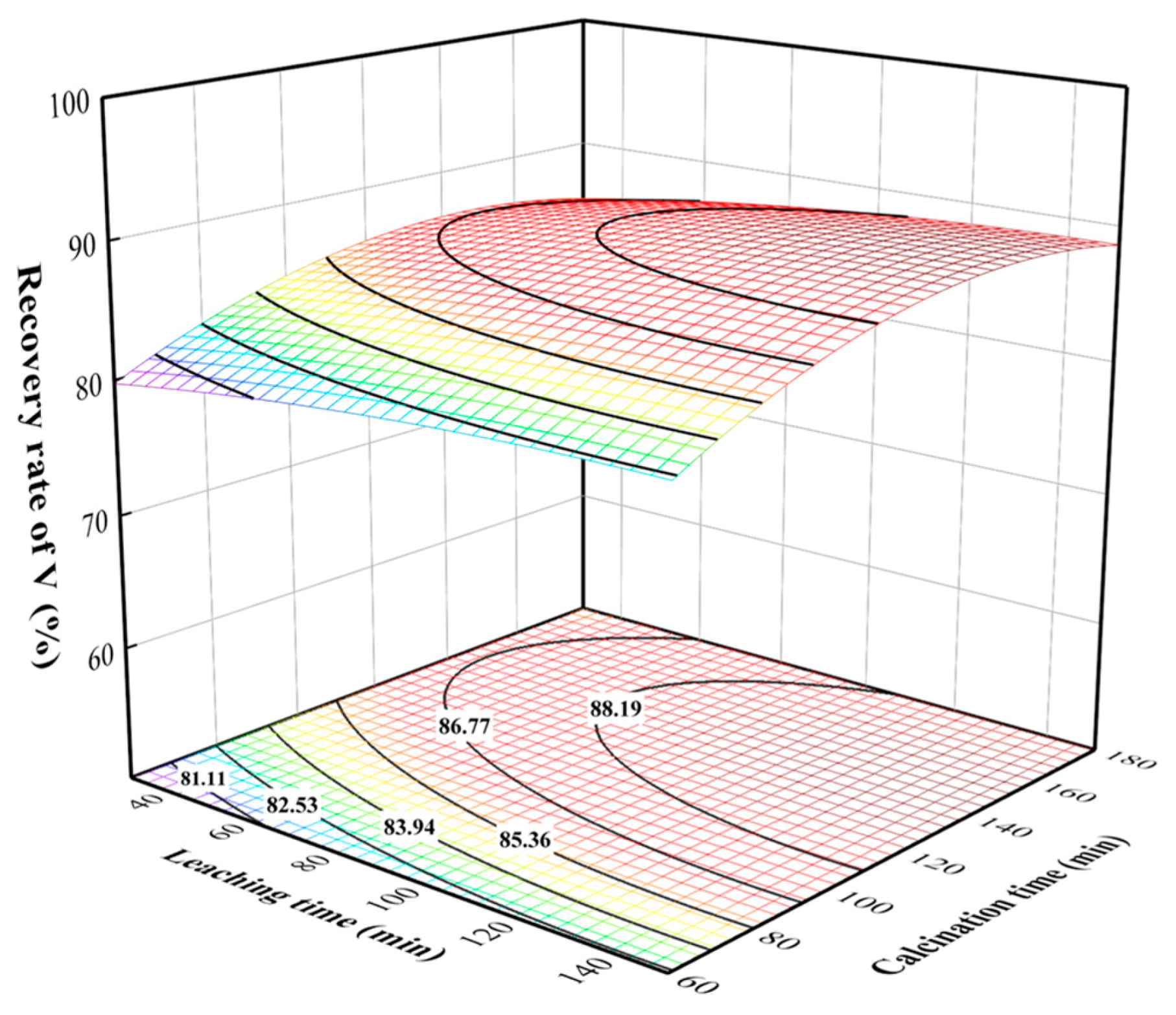
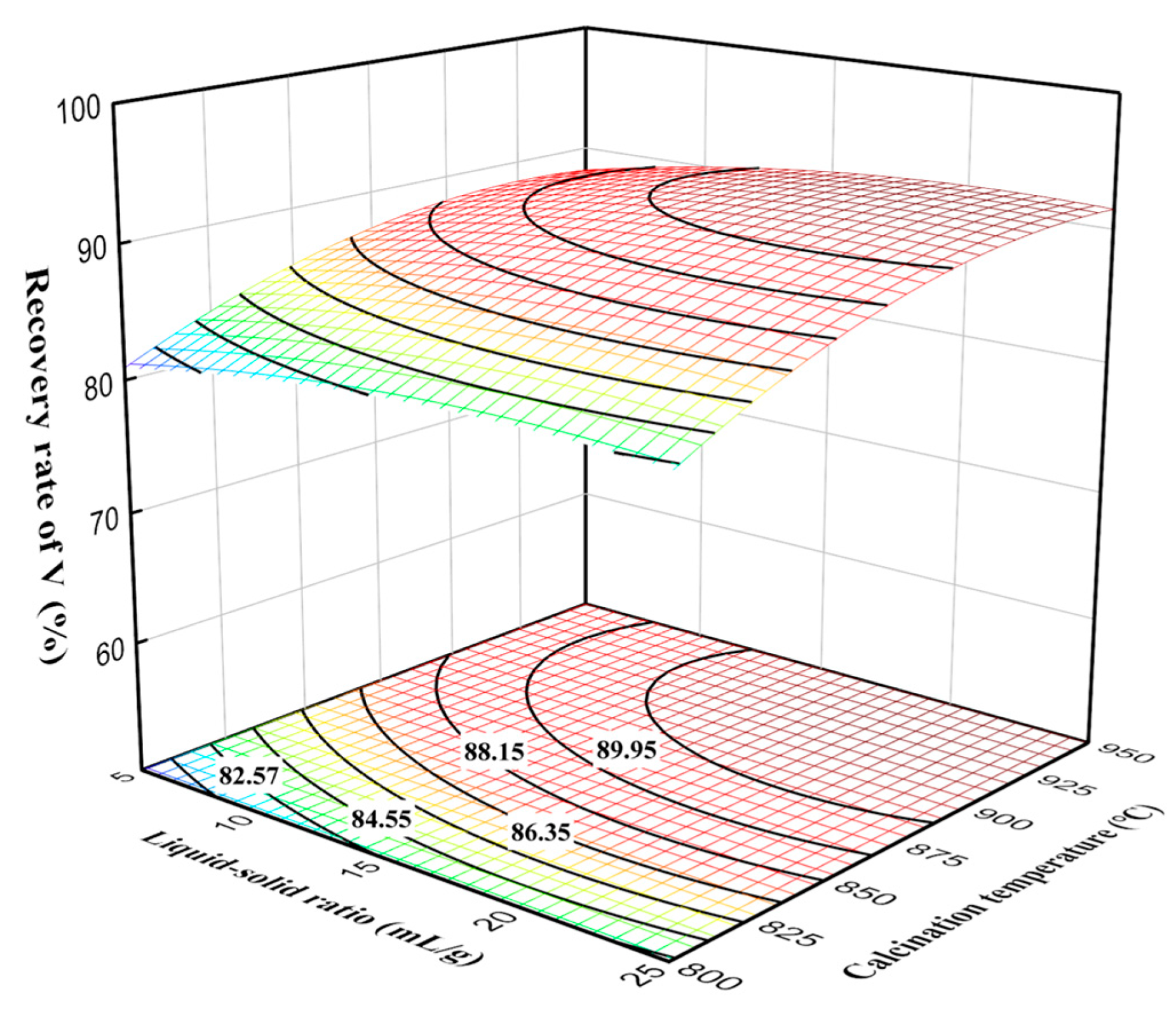
3.4. Processes Analysis of Calcination and Leaching Process
3.5. Process Optimization
3.6. Comparison of Processes for Extracting Vanadium from Secondary Sources
4. Conclusions
- (1)
- Titanium tetrachloride vanadium-containing tailings with high NaCl contents were found to be suitable for the application of the water washing process to remove nearly 80% of the NaCl.
- (2)
- Among the experimental factors, calcination temperature was the most effective factor in the recovery of vanadium, while the liquid–solid ratio was less effective. The final model established by RSM and BBD in terms of coded factors for the recovery of vanadium can be represented by the following formula: YV = 88.16 + 1.45A + 1.22B + 2.99C + 7.63D − 0.84AB + 0.15AC + 0.34AD − 0.31BC + 0.45BD + 0.27CD − 0.79A2 − 1.27B2 − 3.34C2 − 4.70D2 (R2 = 0.9886).
- (3)
- The optimal conditions for vanadium recovery were determined as a leaching time of 150 min, a solid-to-liquid ratio of 17.4 mL/g, a calcination temperature of 937 °C, and a calcination time of 150 min. Under the optimum conditions, the maximum predicted recovery rate of vanadium reached 93.1% with the experimental recovery rate achieving a 93% and 99.7% V2O5 grade as tested by chemical analysis.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Jiang, T.; Wen, J.; Yu, T.; Li, F. Review of Leaching, Separation and Recovery of Vanadium from Roasted Products of Vanadium Slag. Hydrometallurgy 2024, 226, 106313. [Google Scholar] [CrossRef]
- Wen, J.; Jiang, T.; Yu, T.; Chen, B.; Li, L. Clean and Efficient Extraction of Vanadium from Vanadium Slag: Effect of Manganese on the Phase Composition and Vanadium Extraction Process. J. Clean. Prod. 2022, 367, 133077. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Chen, J.; Ye, L.; Du, J. Research Progress of Vanadium Extraction Processes from Vanadium Slag: A Review. Sep. Purif. Technol. 2024, 342, 127035. [Google Scholar] [CrossRef]
- Du, W.T.; Wang, Y.; Liang, X.P. System Assessment of Carbon Dioxide Used as Gas Oxidant and Coolant in Vanadium-Extraction Converter. JOM 2017, 69, 1785–1789. [Google Scholar] [CrossRef]
- Du, W.-T.; Jiang, Q.; Chen, Z.; Liang, X.-P.; Wang, Y. Experimental Characterization of CO2 and CaCO3 Used in a Pyrometallurgical Vanadium-Extraction Process. JOM 2019, 71, 4925–4930. [Google Scholar] [CrossRef]
- Zhu, F.; Ma, S.; Ma, Z.; Qi, L.; Peng, W.; Li, K.; Qiu, K. Preparation of TiCl4 from Panzhihua Ilmenite Concentrate by Boiling Chlorination. J. Mater. Res. Technol. 2023, 23, 2703–2718. [Google Scholar] [CrossRef]
- Du, G.; Li, Z.; Zhang, J.; Mao, H.; Ma, S.; Fan, C.; Zhu, Q. Chlorination Behaviors for Green and Efficient Vanadium Recovery from Tailing of Refining Crude Titanium Tetrachloride. J. Hazard. Mater. 2021, 420, 126501. [Google Scholar] [CrossRef] [PubMed]
- Vyboishchik, A.; Popov, M. Production of Pigments on the Basis of Titanium Tetrachloride. MATEC Web Conf. 2021, 346, 02037. [Google Scholar] [CrossRef]
- Zhu, F.; Li, L.; Zhang, D.; Ma, S.; Ma, Z.; Qiu, K. Separation and Rectification of Chloroacetyl Chloride from TiCl4. Processes 2021, 9, 287. [Google Scholar] [CrossRef]
- Ma, J.; Ma, B.; An, Y.; Wang, C.; Wang, B.; Gao, M.; Feng, G. Recovery of Vanadium from Rapid-Cooling Converter Vanadium Slag by Sodium Roasting and Water Leaching. JOM 2024. [Google Scholar] [CrossRef]
- Xiang, J.; Luo, M.; Lu, X.; Bai, L.; Zhu, Z.; Huang, Q.; Zhang, S.; Guo, K.; Lv, X. Recovery of Vanadium from Vanadium Slag by Composite Additive Roasting–Acid Leaching Process. J. Iron Steel Res. Int. 2023, 30, 1426–1439. [Google Scholar] [CrossRef]
- Yu, J.; Ma, B.; Zhao, S.; Yao, Z.; Wang, C.; Wang, B.; Gao, M.; Feng, G. Vanadium Extraction from Water-Cooled Vanadium Converter Slag via Salt-Free Roasting and Acid Leaching. Process Saf. Environ. Prot. 2023, 172, 727–737. [Google Scholar] [CrossRef]
- Xiang, J.; Huang, Q.; Lv, X.; Bai, C. Multistage Utilization Process for the Gradient-Recovery of V, Fe, and Ti from Vanadium-Bearing Converter Slag. J. Hazard. Mater. 2017, 336, 1–7. [Google Scholar] [CrossRef]
- Sun, C.; Li, M.; Gao, G.; Li, L. Crude Titanium Tetrachloride Organic Refining Vanadium Tailings Vanadium Technology. J. Process Eng. 2019, 1, 6. [Google Scholar] [CrossRef]
- Liu, B.; Wang, N.; Yuan, J.; Huang, Y.; Chen, J.; Tian, Y. Comprehensive Utilization of Vanadium Removal Waste from Titanium Tetrachloride Purification. Environ. Prot. Chem. Ind. 2009, 29, 58–61. [Google Scholar] [CrossRef]
- Wang, X.; Xiang, J.; Pei, G.; Li, L.; Huang, Q.; Lv, X. Application of Response Surface Methodology for Roasting Optimization in Composite Roasting—Acid Leaching Vanadium Extraction Process. Chem. Eng. Res. Des. 2021, 172, 254–263. [Google Scholar] [CrossRef]
- Peng, H.; Wang, F.; Li, G.; Guo, J.; Li, B. Highly Efficient Recovery of Vanadium and Chromium: Optimized by Response Surface Methodology. ACS Omega 2019, 4, 904–910. [Google Scholar] [CrossRef]
- Wen, J.; Jiang, T.; Liu, Y.; Xue, X. Extraction Behavior of Vanadium and Chromium by Calcification Roasting-Acid Leaching from High Chromium Vanadium Slag: Optimization Using Response Surface Methodology. Miner. Process. Extr. Metall. Rev. 2019, 40, 56–66. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, L.; Wang, L.; Li, S.; Yang, G.; Cao, C.; Liu, S.; Nie, Y.; Jia, L. Optimization and Kinetic Analysis of Direct Acid Leaching of Vanadium from Converter Vanadium Slag under Atmospheric Pressure. Miner. Eng. 2023, 198, 108091. [Google Scholar] [CrossRef]
- Hout, S.; Salem, Z.; Tassalit, D.; Tigrine, Z.; Aburideh, H.; Boukendakji, H. Assessing Desalination Pretreatment Conditions towards Pilot Scale-up Using Box-Behnken Experimental Design. Water Environ. J. 2021, 35, 473–485. [Google Scholar] [CrossRef]
- Zhou, S.; Dong, M.; Ding, X.; Xue, X.; Yang, H. Application of RSM to Optimize the Recovery of Ammonia Nitrogen from High Chromium Effluent Produced in Vanadium Industry Using Struvite Precipitation. J. Environ. Chem. Eng. 2021, 9, 106318. [Google Scholar] [CrossRef]
- Deng, R.; Xiao, H.; Xie, Z.; Liu, Z.; Yu, Q.; Chen, G.; Tao, C. A Novel Method for Extracting Vanadium by Low Temperature Sodium Roasting from Converter Vanadium Slag. Chin. J. Chem. Eng. 2020, 28, 2208–2213. [Google Scholar] [CrossRef]
- Lee, J.; Kurniawan; Kim, E.; Chung, K.W.; Kim, R.; Jeon, H.-S. A Review on the Metallurgical Recycling of Vanadium from Slags: Towards a Sustainable Vanadium Production. J. Mater. Res. Technol. 2021, 12, 343–364. [Google Scholar] [CrossRef]
- Qiu, Y.; Shi, J.; Yu, B.; Xiao, P.; Zhao, F.; Ma, W.; Li, J.; Liu, C. Review and Perspective of Vanadium Extraction Techniques from Converter Vanadium-Bearing Slag. Chem. Ind. Eng. Progress. 2021, 40, 5281–5292. [Google Scholar] [CrossRef]
- Li, H.-Y.; Fang, H.-X.; Wang, K.; Zhou, W.; Yang, Z.; Yan, X.-M.; Ge, W.-S.; Li, Q.-W.; Xie, B. Asynchronous Extraction of Vanadium and Chromium from Vanadium Slag by Stepwise Sodium Roasting–Water Leaching. Hydrometallurgy 2015, 156, 124–135. [Google Scholar] [CrossRef]
- Gilligan, R.; Nikoloski, A.N. The Extraction of Vanadium from Titanomagnetites and Other Sources. Miner. Eng. 2020, 146, 106106. [Google Scholar] [CrossRef]
- Yang, Q.; Qing, H.; Zhang, J.; Zhang, Z.; Ju, D.; Zhang, J.; Du, W. Study on the Preparation of V2O5 by Manganization Roasting of Vanadium-Containing Tailings from Titanium Tetrachloride Refining Process. Metal. Mine 2024, 571, 01036. [Google Scholar] [CrossRef]
- Qu, J.; Weng, X.; Li, G.; Hu, Y.; Ma, N.; Dou, Z. Study on Calcified Alkaline Leaching of Vanadium Extraction Tailings from Vanadium Titanium-magnetite Metallurgy. China Nonferrous Metall. 2024, 53, 129–138. [Google Scholar] [CrossRef]
- Li, H.; Ren, Q.; Tian, J.; Tian, S.; Wang, J.; Zhu, X.; Shang, Y.; Liu, J.; Fu, L. Efficient Recovery of Vanadium from Calcification Roasted-Acid Leaching Tailings Enhanced by Ultrasound in H2SO4-H2O2 System. Miner. Eng. 2024, 205, 108492. [Google Scholar] [CrossRef]
- Ma, N.; Chen, L.; Qu, J.; Zhang, Y. Study on Comprehensive Utilization of Titanium Tetrachloride Refined Tailings. Nonferrous Met. 2017, 7, 62–66+71. [Google Scholar] [CrossRef]




| Code | Factors | Levels | ||
|---|---|---|---|---|
| Minimum (−1) | Central (0) | Maximum (1) | ||
| A | Leaching time (min) | 30 | 90 | 150 |
| B | Liquid–solid ratio (mL/g) | 5 | 15 | 25 |
| C | Calcination time (min) | 60 | 120 | 180 |
| D | Calcination temperature (°C) | 750 | 850 | 950 |
| Run no. | Experimental Design Factors | The Recovery Rate of V (%) | ||||
|---|---|---|---|---|---|---|
| A (min) | B (mL/g) | C (min) | D (°C) | Experimental | Predicted | |
| 1 | 150 (1) | 15 (0) | 120 (0) | 950 (1) | 91.59 | 92.08 |
| 2 | 90 (0) | 15 (0) | 180 (1) | 750 (−1) | 75.65 | 75.21 |
| 3 | 90 (0) | 15 (0) | 120 (0) | 850 (0) | 88.17 | 88.16 |
| 4 | 150 (1) | 5 (−1) | 120 (0) | 850 (0) | 87.00 | 87.17 |
| 5 | 150 (1) | 15 (0) | 120 (0) | 750 (−1) | 75.43 | 76.16 |
| 6 | 150 (1) | 15 (0) | 60 (−1) | 850 (0) | 81.90 | 82.35 |
| 7 | 30 (−1) | 5 (−1) | 120 (0) | 850 (0) | 82.40 | 82.59 |
| 8 | 30 (−1) | 15 (0) | 120 (0) | 750 (−1) | 73.11 | 73.93 |
| 9 | 90 (0) | 25 (1) | 180 (1) | 850 (0) | 85.58 | 87.44 |
| 10 | 90 (0) | 5 (−1) | 60 (−1) | 850 (0) | 79.59 | 79.04 |
| 11 | 30−1) | 15 (0) | 60 (−1) | 850 (0) | 79.40 | 79.74 |
| 12 | 90 (0) | 25 (1) | 120 (0) | 750 (−1) | 75.66 | 75.34 |
| 13 | 150 (1) | 25 (1) | 120 (0) | 850 (0) | 89.13 | 87.93 |
| 14 | 90 (0) | 5 (−1) | 180 (1) | 850 (0) | 85.01 | 85.62 |
| 15 | 90 (0) | 15 (0) | 120 (0) | 850 (0) | 88.17 | 88.16 |
| 16 | 90 (0) | 5 (−1) | 120 (0) | 750 (−1) | 74.23 | 73.79 |
| 17 | 30 (−1) | 15 (0) | 120 (0) | 950 (1) | 87.93 | 88.51 |
| 18 | 90 (0) | 15 (0) | 120 (0) | 850 (0) | 88.23 | 88.16 |
| 19 | 90 (0) | 15 (0) | 120 (0) | 850 (0) | 88.08 | 88.16 |
| 20 | 30 (−1) | 25 (1) | 120 (0) | 850 (0) | 87.88 | 86.70 |
| 21 | 150 (1) | 15 (0) | 180 (1) | 850 (0) | 89.24 | 88.61 |
| 22 | 90 (0) | 15 (0) | 60 (−1) | 950 (1) | 85.07 | 84.49 |
| 23 | 90 (0) | 25 (1) | 120 (0) | 950 (1) | 91.33 | 91.48 |
| 24 | 90 (0) | 15 (1) | 180 (1) | 950 (1) | 91.67 | 91.00 |
| 25 | 30 (−1) | 15 (0) | 180 (1) | 850 (0) | 86.15 | 85.41 |
| 26 | 90 (0) | 25 (1) | 60 (−1) | 850 (0) | 81.39 | 82.09 |
| 27 | 90 (0) | 5 (−1) | 120 (0) | 950 (1) | 88.12 | 88.15 |
| 28 | 90 (0) | 15 (0) | 60 (−1) | 750 (−1) | 70.13 | 69.78 |
| 29 | 90 (0) | 15 (0) | 120 (0) | 850 (0) | 88.15 | 88.16 |
| Tailing | Fe | Cl | Na | Si | Ti | Al | V | C | Zr | Nb | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw | 32.74 | 35.44 | 6.67 | 5.38 | 5.60 | 3.64 | 3.78 | 1.47 | 1.30 | 0.90 | 3.07 |
| Washed | 47.54 | 7.82 | 1.52 | 9.96 | 11.36 | 4.66 | 7.26 | 2.20 | 2.65 | 1.54 | 3.47 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | P-Value |
|---|---|---|---|---|---|
| Model | 1037.76 | 8 | 74.13 | 86.50 | <0.0001 |
| A | 25.29 | 1 | 25.29 | 29.51 | <0.0001 |
| B | 17.81 | 1 | 17.81 | 20.79 | 0.0004 |
| C | 106.92 | 1 | 106.92 | 124.77 | <0.0001 |
| D | 697.69 | 1 | 697.69 | 814.16 | <0.0001 |
| A2 | 4.08 | 1 | 4.08 | 4.76 | 0.0467 |
| B2 | 10.51 | 1 | 10.51 | 12.26 | 0.0035 |
| C2 | 72.38 | 1 | 72.38 | 84.46 | <0.0001 |
| D2 | 143.16 | 1 | 143.16 | 167.06 | <0.0001 |
| Lack of fit | 11.99 | 10 | 1.20 | 413.29 | <0.0001 |
| Residual | 12.00 | 14 | 0.86 | - | - |
| Pure error | 0.012 | 4 | 0.0029 | R2 | 0.9886 |
| Cor total | 1049.76 | 28 | - | R2adj | 0.9771 |
| Raw Material | Method | Calcination | Leaching | ƞ (%) | Ref. | |
|---|---|---|---|---|---|---|
| Temperature/ Time (°C/min) | Reagent | Temperature/ Time/L:S (°C/min) | ||||
| VCTR | Blank calcination- alkaline leaching | 650/120 | Na2CO3 | 80/60/6:1 | 85 | [14] |
| VCTR | Combined acid–alkali method leaching | — | NaOH + HCl | 60/60/— | 75 | [15] |
| VCTR | Manganese calcination–acid leaching | 850/120 | H2C2O4 | 50/30/10:1 | 82.16 | [27] |
| PVCT | Ultrasound H2O2 synergistic enhanced leaching | — | H2O2 | 50/30/10:1 | 63.64 | [29] |
| VCTR | Direct alkali leaching | — | NaOH | 120/180/5:1 | 95 | [28] |
| CVCT | Direct calcified alkali leaching | — | CaO + NaOH | 160/60/6:1 | 82.25 | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, W.; Yao, T.; Cheng, H.; Ju, D.; Chen, Z. Parametric Study of Vanadium Extraction Process from Refining Tailings of Crude Titanium Tetrachloride. Metals 2024, 14, 912. https://doi.org/10.3390/met14080912
Du W, Yao T, Cheng H, Ju D, Chen Z. Parametric Study of Vanadium Extraction Process from Refining Tailings of Crude Titanium Tetrachloride. Metals. 2024; 14(8):912. https://doi.org/10.3390/met14080912
Chicago/Turabian StyleDu, Weitong, Tingfeng Yao, Haiming Cheng, Dianchun Ju, and Zhuo Chen. 2024. "Parametric Study of Vanadium Extraction Process from Refining Tailings of Crude Titanium Tetrachloride" Metals 14, no. 8: 912. https://doi.org/10.3390/met14080912





