Nodular Graphite Dissolution and Nucleus Observation: High-Temperature Dynamics of Ductile Iron Recycling
Abstract
1. Introduction
2. Materials and Methods
2.1. High-Temperature Molten Metal Holding
2.2. Dissolution Behavior of Nodular Graphite
3. Experimental Results
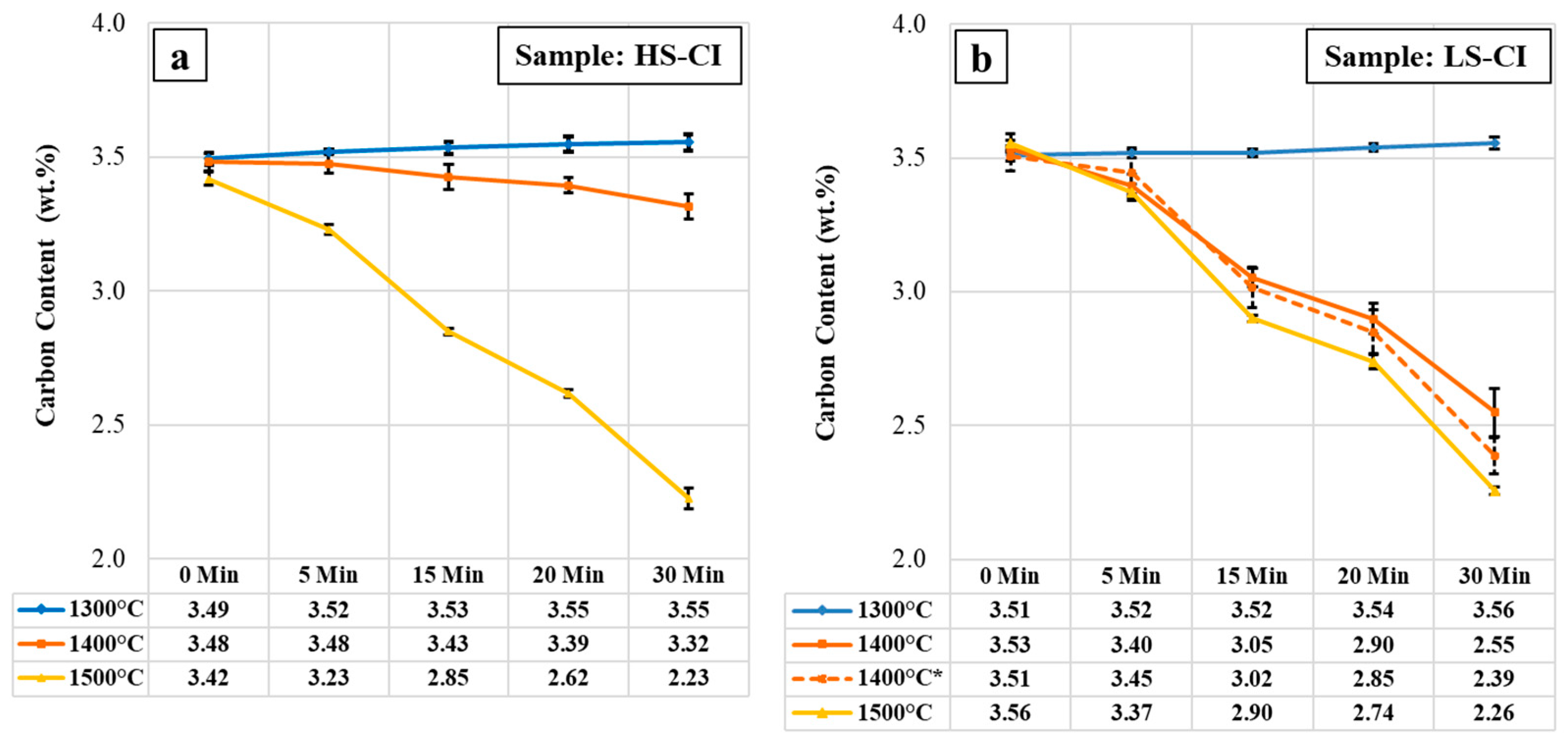
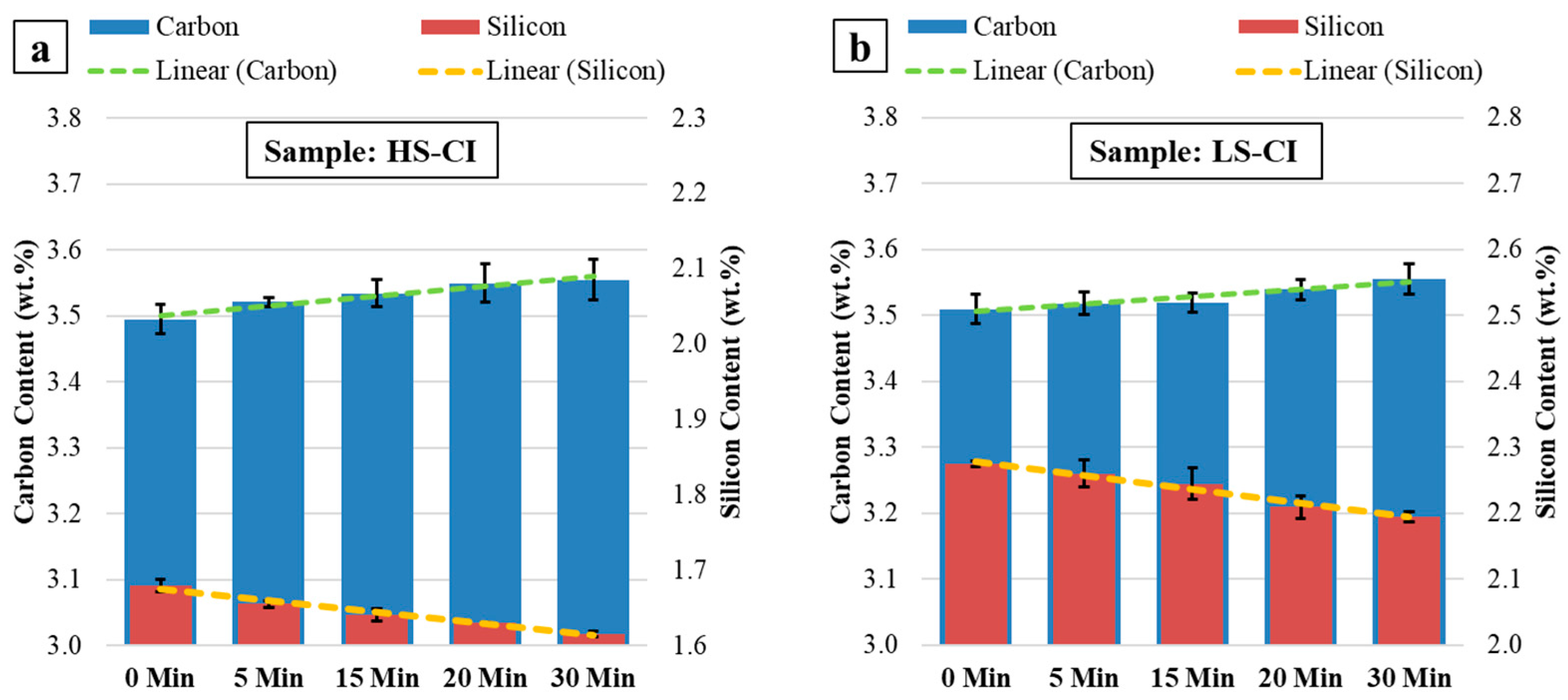
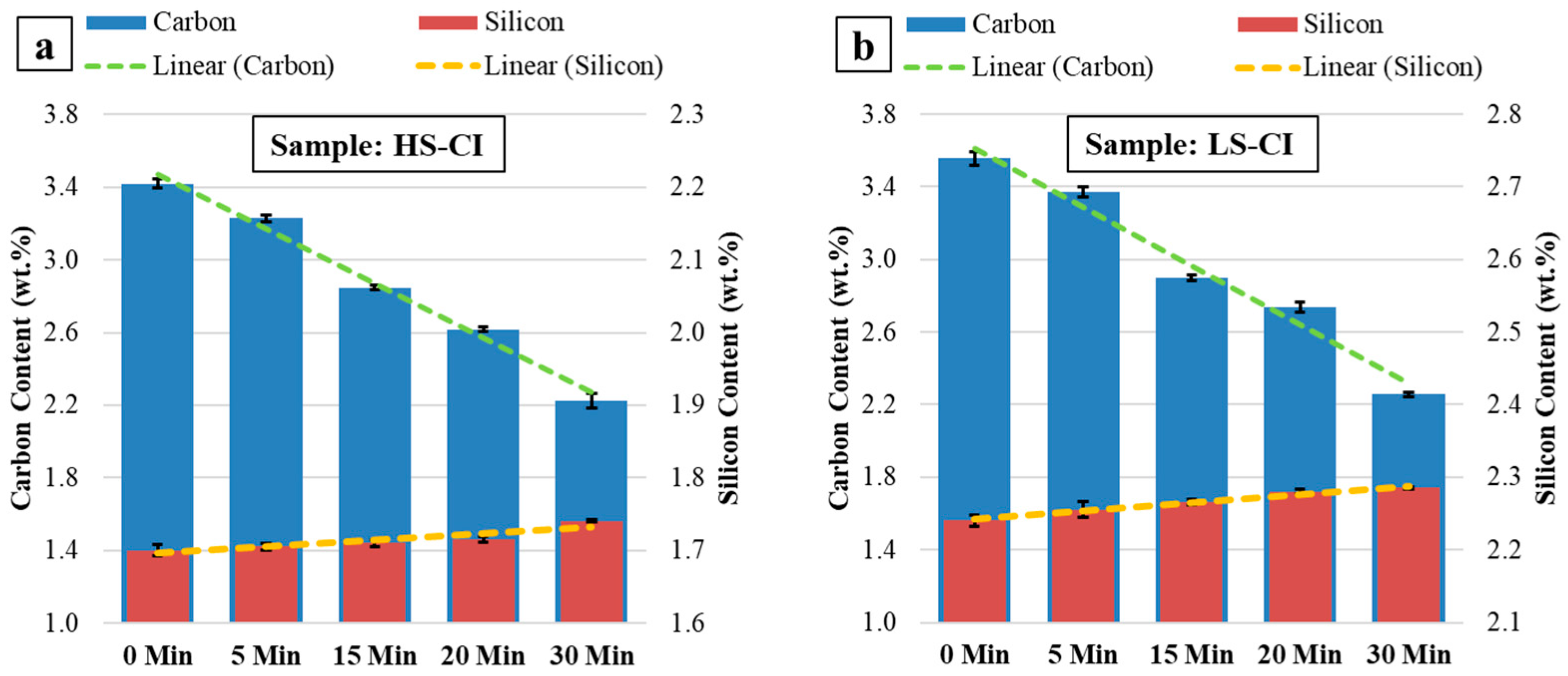
3.1. Concentration Development of Carbon and Silicon during MH-Exp
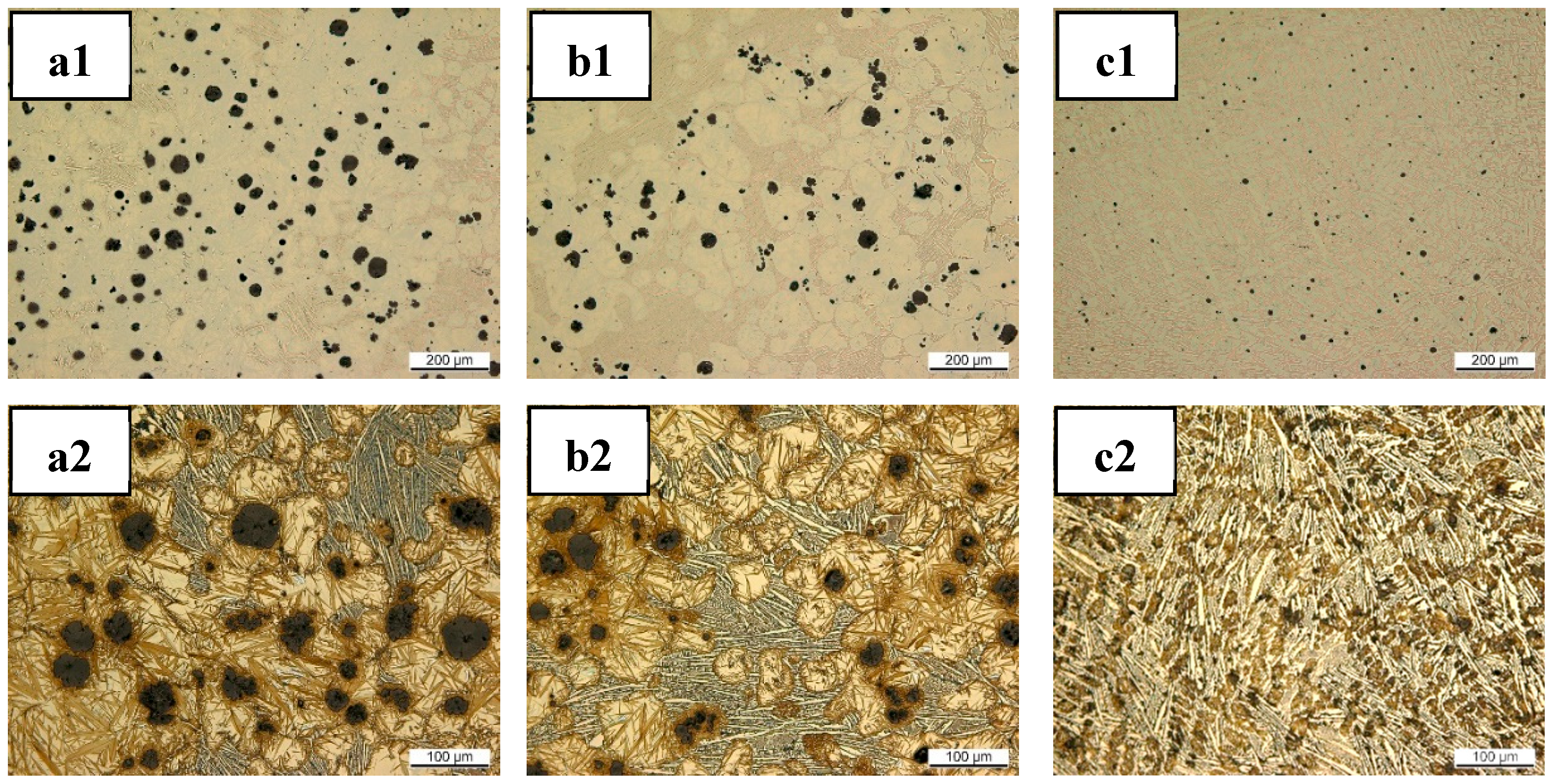
3.2. Matrix Development and Graphite Structures after ET-Exp
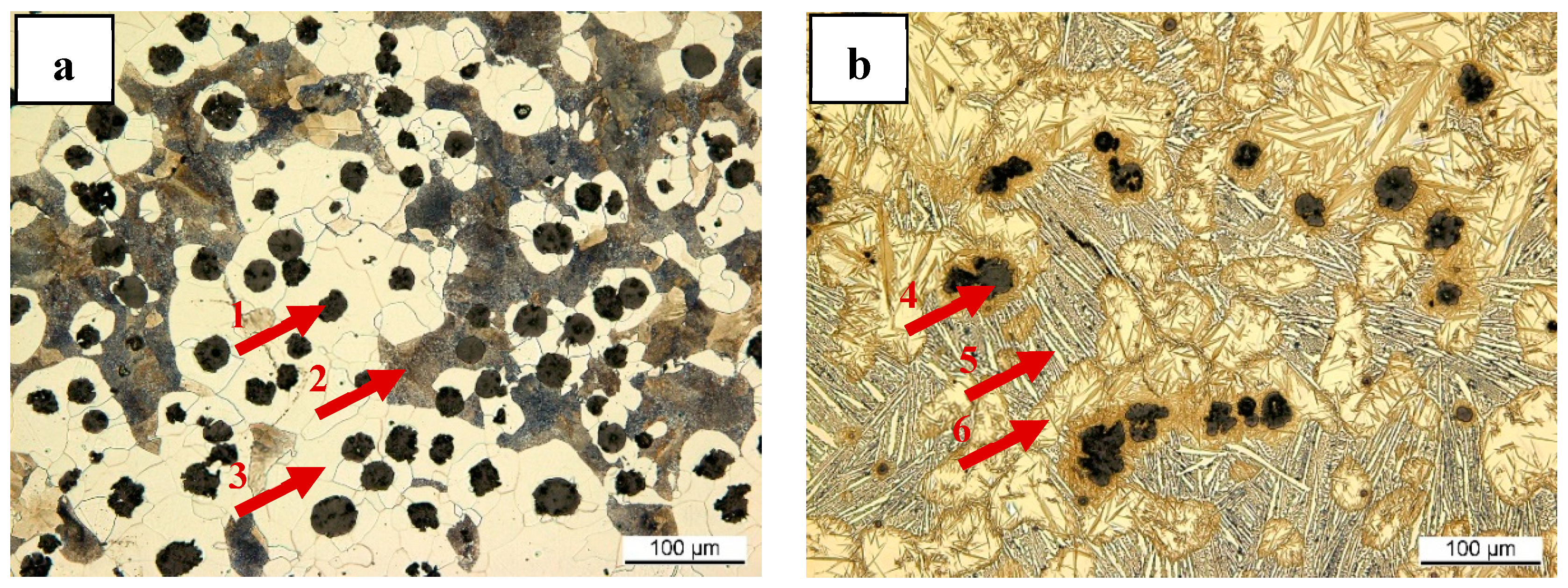
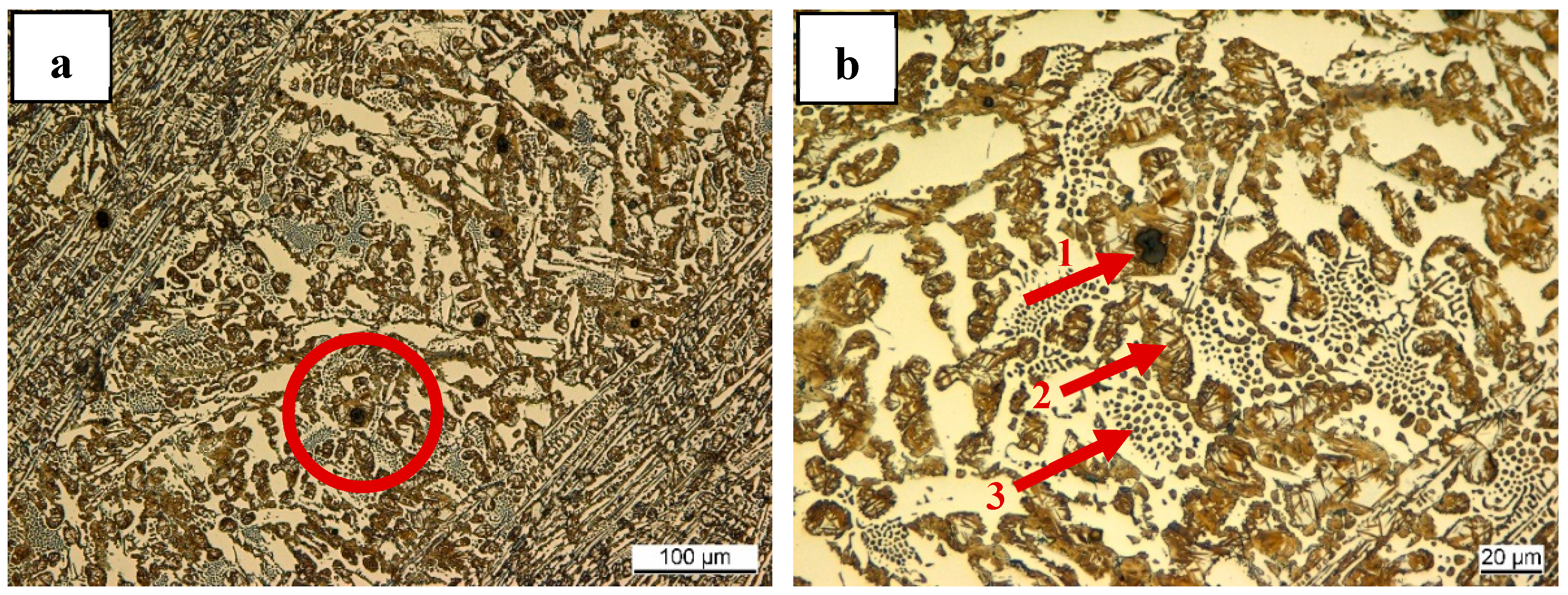
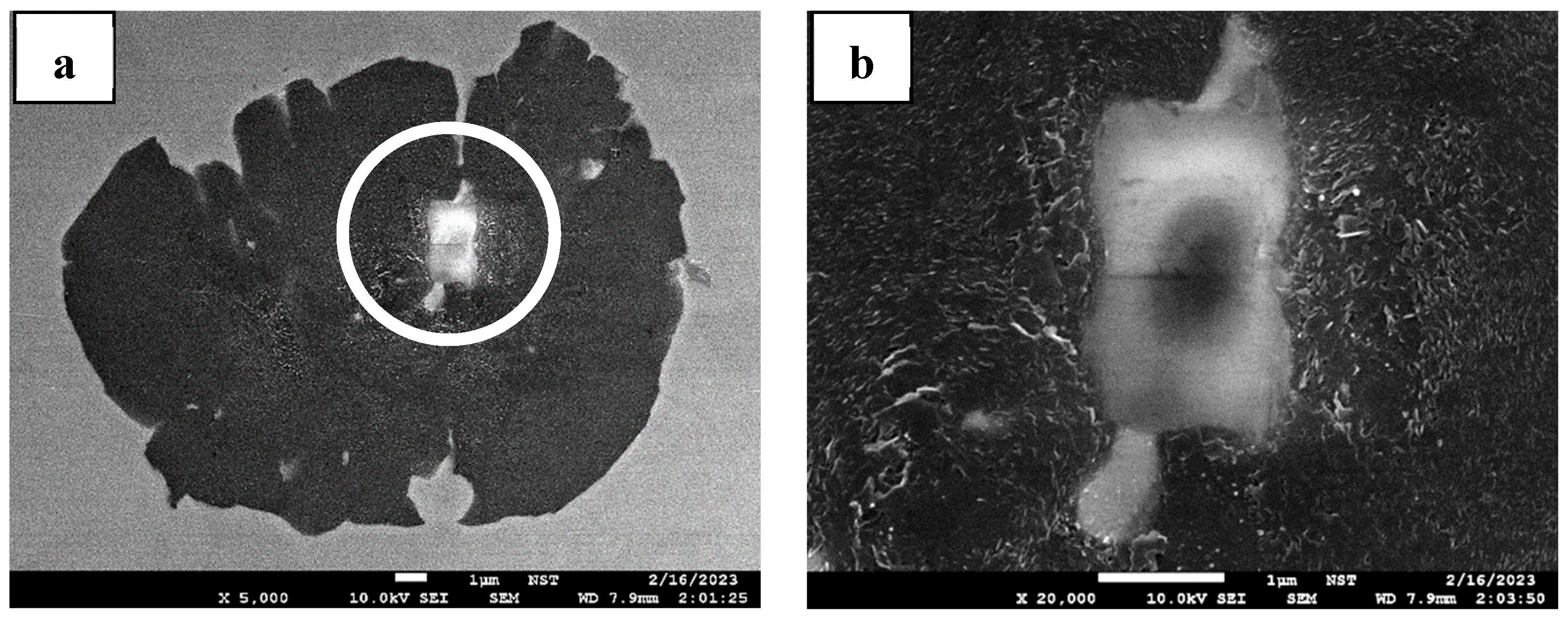
3.3. Observation of the Residual Graphite Structure after ET-Exp
4. Discussion
4.1. Carbon Depletion during High-Temperature Holding
4.2. Graphite Dissolution Mechanism of Nodular Graphite
4.3. Correlation of Homogenization and Oxidation to Fading
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- The European Foundry Association. The European Foundry Industry 2022; CAEF: Düsseldorf, Germany, 2023. [Google Scholar]
- Umweltbundesamt. Emissionen aus Betrieben der Metallindustrie. The Federal Republic of Germany—Ministry for the Environment, Nature Conservation, Nuclear Safety and Consumer Protection (BMUV), 4 March 2024. Available online: https://www.umweltbundesamt.de/daten/umwelt-wirtschaft/industrie/emissionen-aus-betrieben-der-metallindustrie#das-schadstofffreisetzungs-und-verbringungsregister-prtr-in-deutschland (accessed on 8 August 2024).
- Trianni, A.; Cagno, E.; Thollander, P.; Backlund, S. Barriers to Industrial Energy Efficiency in Foundries: A European Comparison. J. Clean. Prod. 2013, 40, 161–176. [Google Scholar] [CrossRef]
- Yilmaz, O.; Anctil, A.; Karanfil, T. LCA as a Decision Support Tool for Evaluation of Best Available Techniques (BATs) for Cleaner Production of Iron Casting. J. Clean. Prod. 2015, 105, 337–347. [Google Scholar] [CrossRef]
- Zhu, Y.; Keoleian, G.A.; Cooper, D.R. A Parametric Life Cycle Assessment Model for Ductile Cast Iron Components. Resour. Conserv. Recycl. 2023, 189, 106729. [Google Scholar] [CrossRef]
- Riposan, I.; Chisamera, M.; Stan, S. Enhanced Quality in Electric Melt Grey Cast Iron. ISIJ Int. 2013, 53, 1683–1695. [Google Scholar] [CrossRef]
- Fraś, E.; López, H. Eutectic Cells and Nodule Count—An Index of Molten Iron Quality. Int. J. Met. 2010, 4, 35–61. [Google Scholar] [CrossRef]
- Bai, J.-X. Selection of Raw Materials and Control of Trace Elements for Production of High-Quality SG iron. China Foundry 2019, 16, 79–87. [Google Scholar] [CrossRef]
- Salonitis, K.; Jolly, M.; Pagone, E.; Papanikolaou, M. Life-Cycle and Energy Assessment of Automotive Component Manufacturing: The Dilemma Between Aluminum and Cast Iron. Energies 2019, 12, 2557. [Google Scholar] [CrossRef]
- Jhaveri, K.; Lewis, G.M.; Sullivan, J.L.; Keoleian, G.A. Life Cycle Assessment of Thin-Wall Ductile Cast Iron for Automotive Lightweighting Applications. Sustain. Mater. Technol. 2018, 15, 1–8. [Google Scholar] [CrossRef]
- Orths, K.; Weis, W. Bedeutung und Beeinflussung des Kieselsäuregehaltes von Gußeisen. Giessereiforschung 1973, 25, 1–11. [Google Scholar]
- Grachev, V.A. Thermodynamics and Mechanism of Silicon Reduction by Carbon in a Crucible Reaction. Orient. J. Chem. 2016, 32, 2929–2937. [Google Scholar] [CrossRef]
- Orths, K.; Weis, W. Die Beeinflussung des Kieselsäuregehaltes von Gußeisen durch die Schmelztechnik. Giesserei-Forschung 1973, 25, 9–19. [Google Scholar]
- Frost, J.M.; Stefanescu, D.M. Melt Quality Assessment of SG Iron Through Computer-Aided Cooling Curve Analysis. AFS Trans. 1992, 100, 189–200. [Google Scholar]
- Stan, S.; Riposan, I.; Chisamera, M.; Barstow, M. Undesirable Graphite Morphologies Incidence in Inoculated Grey Irons. Adv. Mater. Res. 2007, 23, 307–310. [Google Scholar] [CrossRef]
- Double, D.; Hellawell, A. The nucLeation and Growth of Graphite—The Modification of Cast Iron. Acta Met. Mater. 1995, 43, 2435–2442. [Google Scholar] [CrossRef]
- Amini, S.; Abbaschian, R. Nucleation and Growth Kinetics of Graphene Layers from a Molten Phase. Carbon 2013, 51, 110–123. [Google Scholar] [CrossRef]
- Theuwissen, K.; Lacaze, J.; Laffont, L. Structure of Graphite Precipitates in Cast Iron. Carbon 2016, 96, 1120–1128. [Google Scholar] [CrossRef]
- Sahajwalla, V.; Khanna, R. A Monte Carlo Simulation Study of Dissolution of Graphite in Iron-Carbon Melts. Met. Mater. Trans. B 2000, 31, 1517–1525. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, J.; Li, K.; Liang, W.; Bi, Z. Influence of Graphite Crystalline Orientation on the Carbon Dissolution Reaction in Liquid Iron: A ReaxFF Molecular Dynamics Simulation Study. J. Mol. Liq. 2021, 335, 115688. [Google Scholar] [CrossRef]
- Wade, N.; Ueda, Y. Isothermal Austenitizing of Spheroidal Graphite Cast Iron. Trans. Iron Steel Inst. Jpn. 1980, 20, 849–856. [Google Scholar] [CrossRef]
- Miki, T.; Ishii, K. Decomposition Behavior of Fe3C under Ar Atmosphere. ISIJ Int. 2014, 54, 29–31. [Google Scholar] [CrossRef]
- Wade, N.; Ueda, Y. Continuous Heating Transformation of Spheroidal Graphite Cast Iron. Trans. Iron Steel Inst. Jpn. 1980, 20, 857–861. [Google Scholar] [CrossRef]
- Chou, J.-M.; Hon, M.-H.; Lee, J.-L. The Austenite Transformation in Ferritic Ductile Cast Iron. Mater. Sci. Eng. A 1992, 158, 241–249. [Google Scholar] [CrossRef]
- Palkanoglou, E.N.; Baxevanakis, K.P.; Silberschmidt, V.V. Thermal Debonding of Inclusions in Compacted Graphite Iron: Effect of Matrix Phases. Eng. Fail. Anal. 2022, 139, 106476. [Google Scholar] [CrossRef]
- Warmuzek, M.; Polkowska, A. Micromechanism of Damage of the Graphite Spheroid in the Nodular Cast Iron During Static Tensile Test. J. Manuf. Mater. Process. 2020, 4, 22. [Google Scholar] [CrossRef]
- Darken, L.S. Diffusion of Carbon in Austenite with a Discontinuity in Composition. Met. Technol. 1948, 430–438. [Google Scholar] [CrossRef][Green Version]
- Franzen, D.; Weiß, P.; Pustal, B.; Bührig-Polaczek, A. Modification of Silicon Microsegregation in Solid-Solution-Strengthened Ductile Iron by Alloying with Aluminum. Int. J. Met. 2020, 14, 1105–1114. [Google Scholar] [CrossRef]
- Arshad, W.; Mehmood, A.; Hashmi, M.F.; Rauf, O.u. The Effect of Increasing Silicon on Mechanical Properties of Ductile Iron. J. Physic IOP Conf. Ser. 2018, 1082, 012059. [Google Scholar] [CrossRef]
- Alhussein, A.; Risbet, M.; Bastien, A.; Chobaut, J.; Balloy, D.; Favergeon, J. Influence of Silicon and Addition Elements on the Mechanical Behavior of Ferritic Ductile Cast Iron. Mater. Sci. Eng. A 2014, 605, 222–228. [Google Scholar] [CrossRef]
- Ahn, S.H.; Kim, Y.H.; Shin, J.-P.; Lee, Y.E. Thermodynamic Assessment of Liquid Fe-Si-C System by Unified Interaction Parameter Model. ISIJ Int. 2014, 54, 750–755. [Google Scholar] [CrossRef]
- Domeij, B.; Diószegi, A. The Distribution of Carbon in Austenite Studied on a Water-Quenched Compacted Graphite Iron using Electron Probe Microanalysis. Int. J. Met. 2020, 14, 782–793. [Google Scholar] [CrossRef]
- Kosaka, M.; Minowa, S. On the Rate of Dissolution of Carbon into Molten Fe-C Alloy. Trans. Iron Steel Inst. Jpn. 1968, 8, 392–400. [Google Scholar] [CrossRef]
- Sun, H. Factors influencing dissolution of carbonaceous materials in liquid iron. Met. Mater. Trans. B 2005, 36, 893–894. [Google Scholar] [CrossRef]
- Sun, M.-M.; Zhang, J.-L.; Li, K.-J.; Ren, S.; Wang, Z.-M.; Jiang, C.-H.; Li, H.-T. Dissolution Behaviours of Various Carbonaceous Materials in Liquid Iron: Interaction Between Graphite and Iron. JOM 2019, 71, 4305–4310. [Google Scholar] [CrossRef]
- Mercier, J.C.; Paton, R.; Margerie, J.C.; Mascre, C. Inclusions dans les spheroides de graphite. Fonderie 1969, 277, 191–200. [Google Scholar]
- Igarashi, Y.; Okada, S. Observation and Analysis of the Nucleus of Spheroidal Graphite in Magnesium-Treated Ductile Iron. Int. J. Cast Met. Res. 1998, 11, 83–88. [Google Scholar] [CrossRef]
- Solberg, J.; Onsøien, M. Nuclei for Heterogeneous Formation of Graphite Spheroids in Ductile Cast Iron. Mater. Sci. Technol. 2001, 17, 1238–1242. [Google Scholar] [CrossRef]
- Laffont, L.; Pugliara, A.; Hungria, T.; Lacaze, J. STEM Observation of a Multiphase Nucleus of Spheroidal Graphite. J. Mater. Res. Technol. 2020, 9, 4665–4671. [Google Scholar] [CrossRef]
- Alonso, G.; Tokarski, T.; Stefanescu, D.; Górny, M.; Cios, G.; Suarez, R. On the Crystallography of the Mg-Si-Al Nitride Nuclei and of the Graphite/Nitride Interface in Spheroidal Graphite Iron. Carbon 2022, 199, 170–180. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Q.; Chen, D. Dissolution Equilibrium of Magnesium V Por in Liquid Iron. Met. Trans. B 1991, 22, 918–921. [Google Scholar] [CrossRef]
- Mikhailov, G.G.; Samoilova, O.V.; Makrovets, L.A.; Smirnov, L.A. Thermodynamic Modeling of Isotherms of Oxygen Solubility in Liquid Metal of the Fe–Mg–Al–O System. Steel Transl. 2019, 49, 522–527. [Google Scholar] [CrossRef]
- Qing, J.; Lekakh, S.; Xu, M.; Field, D. Formation of Complex Nuclei in Graphite Nodules of Cast Iron. Carbon 2021, 171, 276–288. [Google Scholar] [CrossRef]
- Uchida, H.; Itatani, K.; Aizawa, M.; Howell, F.S.; Kishioka, A. Synthesis of Magnesium Silicon Nitride by the Nitridation of Powders in the Magnesium-Silicon System. J. Ceram. Soc. Jpn. 1997, 105, 934–939. [Google Scholar] [CrossRef]
- Maekawa, S.; Nakagawa, Y. Solubility of Nitrogen in Liquid Iron and Effect of Carbon, Silicon and Manganese on the Solubility. Tetsu-to-Hagane 1960, 46, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-M.; Kim, D.-H.; Paek, M.-K.; Pak, J.-J. Nitrogen Solubility in Cast Iron Containing C, Si and Mn. ISIJ Int. 2018, 58, 1185–1190. [Google Scholar] [CrossRef]
- Paek, M.-K.; Jeon, J.; Lindberg, D. Thermodynamic Behaviour of Nitrogenin the Carbon Saturated Fe-Mn-Si Alloy during Casting. Int. J. Cast Met. Res. 2020, 33, 226–232. [Google Scholar] [CrossRef]
- Prijanovič, M.T.; Mrvar, P.; Burja, J.; Donik, Č.; Petrič, M. Study on Dissolution of Ba-Containing Inoculant in Ductile Cast Iron Melt and Nucleation of Graphite. Int. J. Met. 2023, 1–11. [Google Scholar] [CrossRef]
- Hwang, S.-M.; Park, S.-J.; Kim, G.-T.; Kim, H.-N.; Ko, J.-W.; Park, Y.-H.; Lee, D.-W. Metallic Silicon Powder Produced by Vacuum Decomposition of Magnesium Silicide Prepared by Magnesiothermic Reduction. Mater. Trans. 2021, 62, 1285–1290. [Google Scholar] [CrossRef]
- Cochard, V.; Harding, R.; Campbell, J.; Hérold, R. Inoculation of Spheroidal Graphite Cast Iron. Adv. Mater. Res. 1997, 4–5, 277–284. [Google Scholar] [CrossRef]
- Fras, E.; López, H.F.; Podrzucki, C. The Influence of Oxygen on the Inoculation Process of Cast Iron. Int. J. Cast Met. Res. 2000, 13, 107–121. [Google Scholar] [CrossRef]
- Stathokostopoulos, D.; Chaliampalias, D.; Pavlidou, E.; Paraskevopoulos, K.M.; Chrissafis, K.; Vourlias, G. Oxidation resistance of magnesium silicide under high-temperature air exposure. J. Therm. Anal. Calorim. 2015, 121, 169–175. [Google Scholar] [CrossRef]
- Stefanaki, E.-C.; Hatzikraniotis, E.; Vourlias, G.; Chrissafis, K.; Kitis, G.; Paraskevopoulos, K.M.; Polymeris, G.S. Thermal Stability Study from Room Temperature to 1273 K (1000 °C) in Magnesium Silicide. Met. Mater. Trans. A 2016, 47, 5146–5158. [Google Scholar] [CrossRef]
- Skaland, T.; Grong, Ø.; Grong, T. A Model for the Graphite Formation in Ductile Cast Iron: Part I. Inoculation Mechanisms. Met. Trans. A 1993, 24, 2321–2345. [Google Scholar] [CrossRef]


| C | Si | S | P | Mn | Fe | |
|---|---|---|---|---|---|---|
| LS-CI | 3.4–3.6 | 2.2–2.3 | <0.005 | 0.02–0.03 | 0.10–0.15 | 93–94 |
| HS-CI | 3.4–3.5 | 1.6–1.7 | 0.10–0.12 | 0.06–0.07 | 0.5–0.6 | 93–94 |
| C | Si | Mg | N | Al | Fe | |
|---|---|---|---|---|---|---|
| Nucleus | 11–17 | 36–37 | 24–25 | 34–38 | 1.0–1.5 | 1.0–3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adhiwiguna, I.; Nobakht, N.; Deike, R. Nodular Graphite Dissolution and Nucleus Observation: High-Temperature Dynamics of Ductile Iron Recycling. Metals 2024, 14, 915. https://doi.org/10.3390/met14080915
Adhiwiguna I, Nobakht N, Deike R. Nodular Graphite Dissolution and Nucleus Observation: High-Temperature Dynamics of Ductile Iron Recycling. Metals. 2024; 14(8):915. https://doi.org/10.3390/met14080915
Chicago/Turabian StyleAdhiwiguna, I., N. Nobakht, and R. Deike. 2024. "Nodular Graphite Dissolution and Nucleus Observation: High-Temperature Dynamics of Ductile Iron Recycling" Metals 14, no. 8: 915. https://doi.org/10.3390/met14080915
APA StyleAdhiwiguna, I., Nobakht, N., & Deike, R. (2024). Nodular Graphite Dissolution and Nucleus Observation: High-Temperature Dynamics of Ductile Iron Recycling. Metals, 14(8), 915. https://doi.org/10.3390/met14080915







