Fluid Dynamics Studies on Bottom Liquid Detachment from a Rising Bubble Crossing a Liquid–Liquid Interface
Abstract
:1. Introduction
2. Experimental Methodology
2.1. Experimental Setup
2.2. Materials
2.3. Image Processing
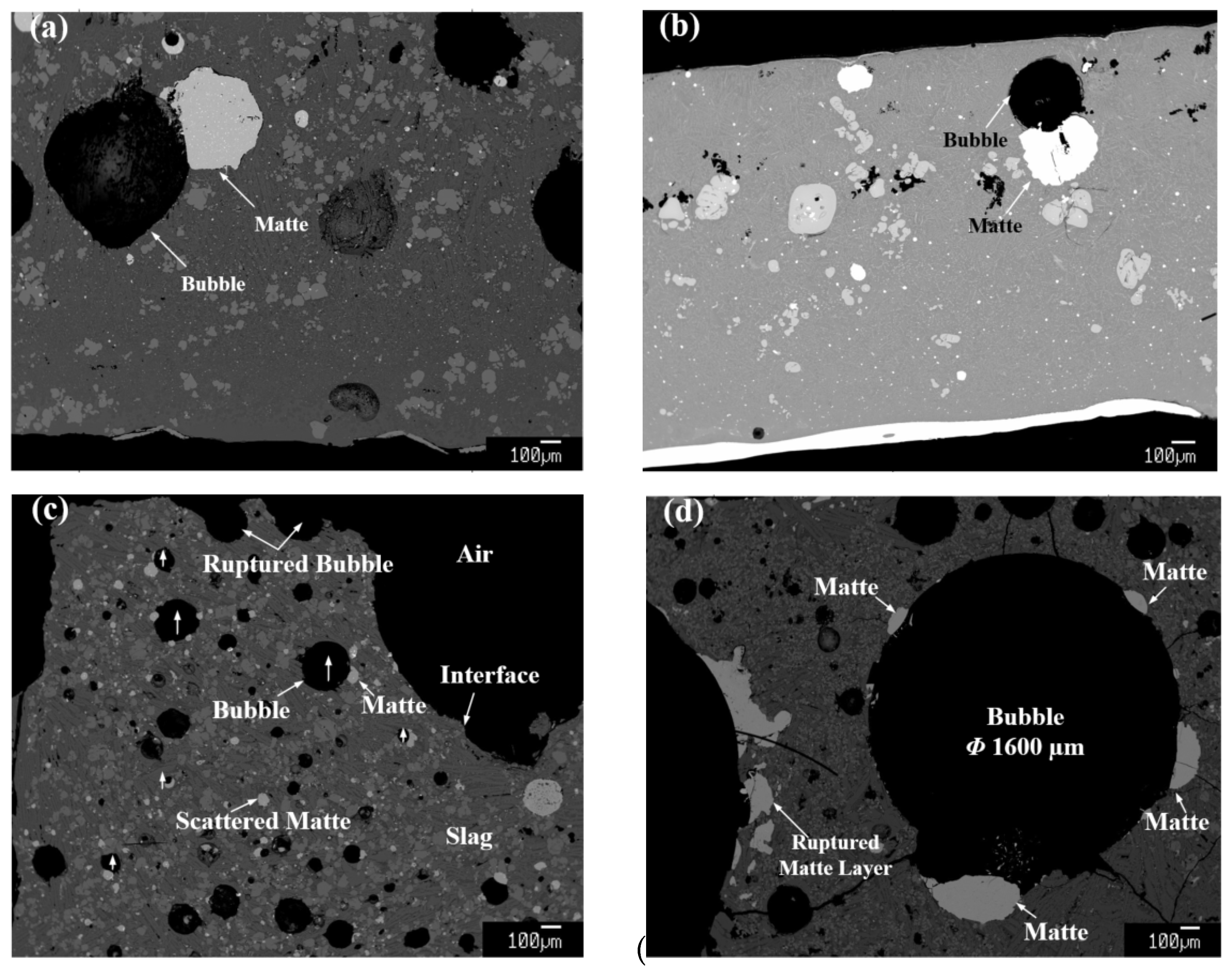
3. Results
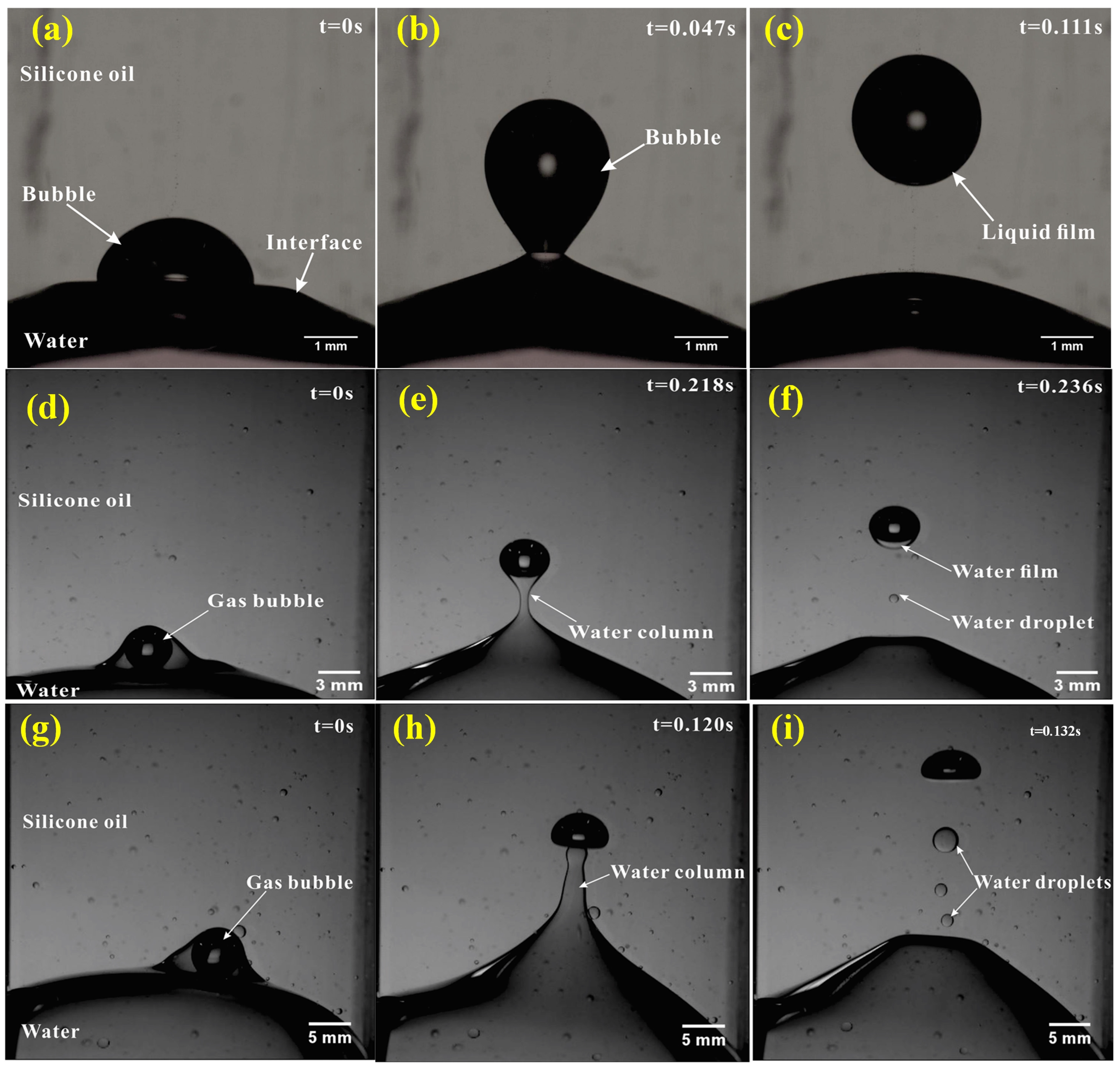
3.1. Bubble Passages at the Interface between Immiscible Liquids
3.1.1. Thin Liquid Film Detachment from Small Bubbles
3.1.2. Lower Liquid Shell Detachment from Medium Bubbles
3.1.3. Lower Liquid Column Detachment from Large Bubbles
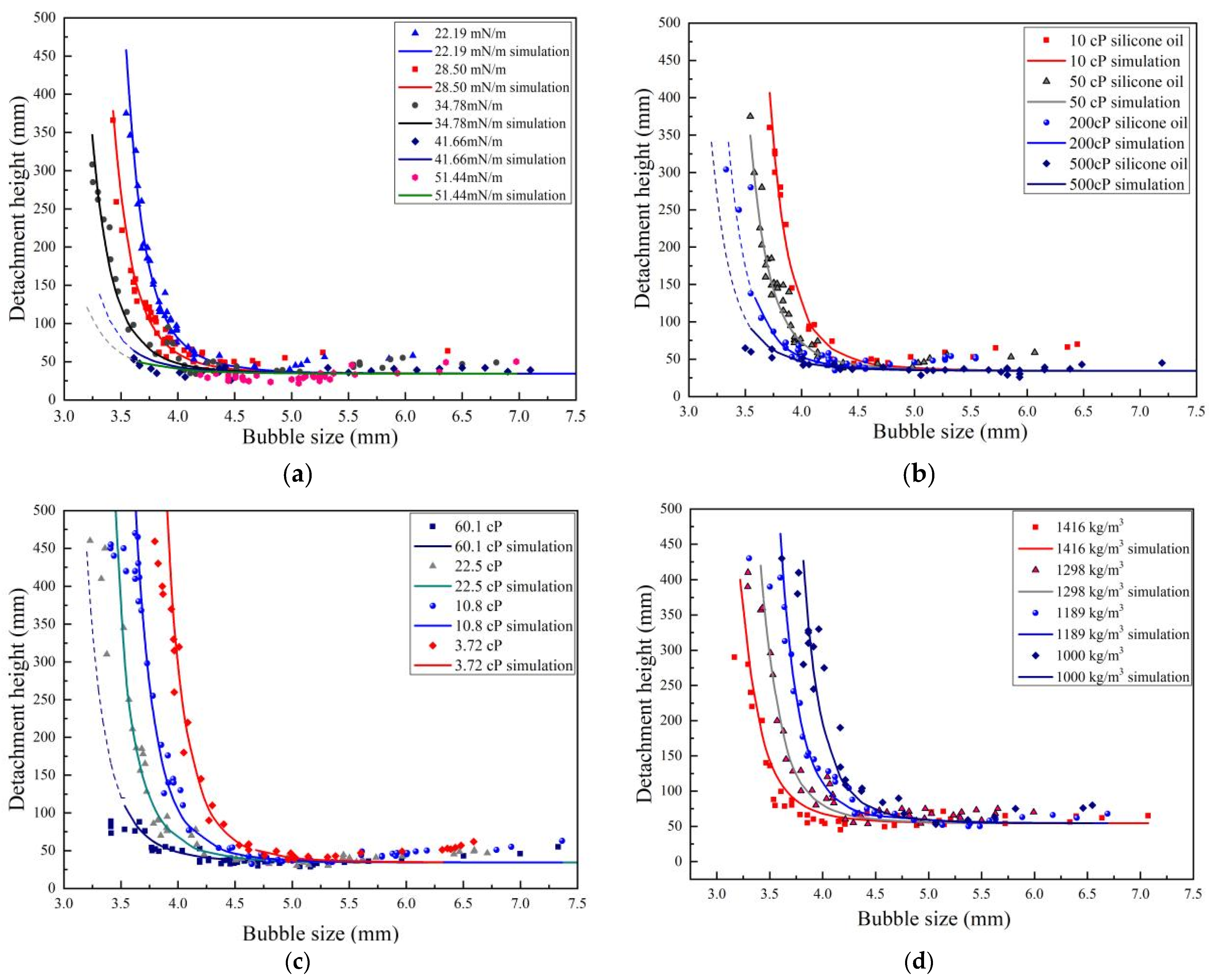
3.2. Detachment Height of Lower Liquid from a Rising Bubble
3.2.1. Influence of Interfacial Tension
3.2.2. Influence of Upper Liquid Viscosity
3.2.3. Influence of Lower Liquid Viscosity
3.2.4. Influence of Lower Liquid Density
3.3. Bubble Terminal Rising Velocities
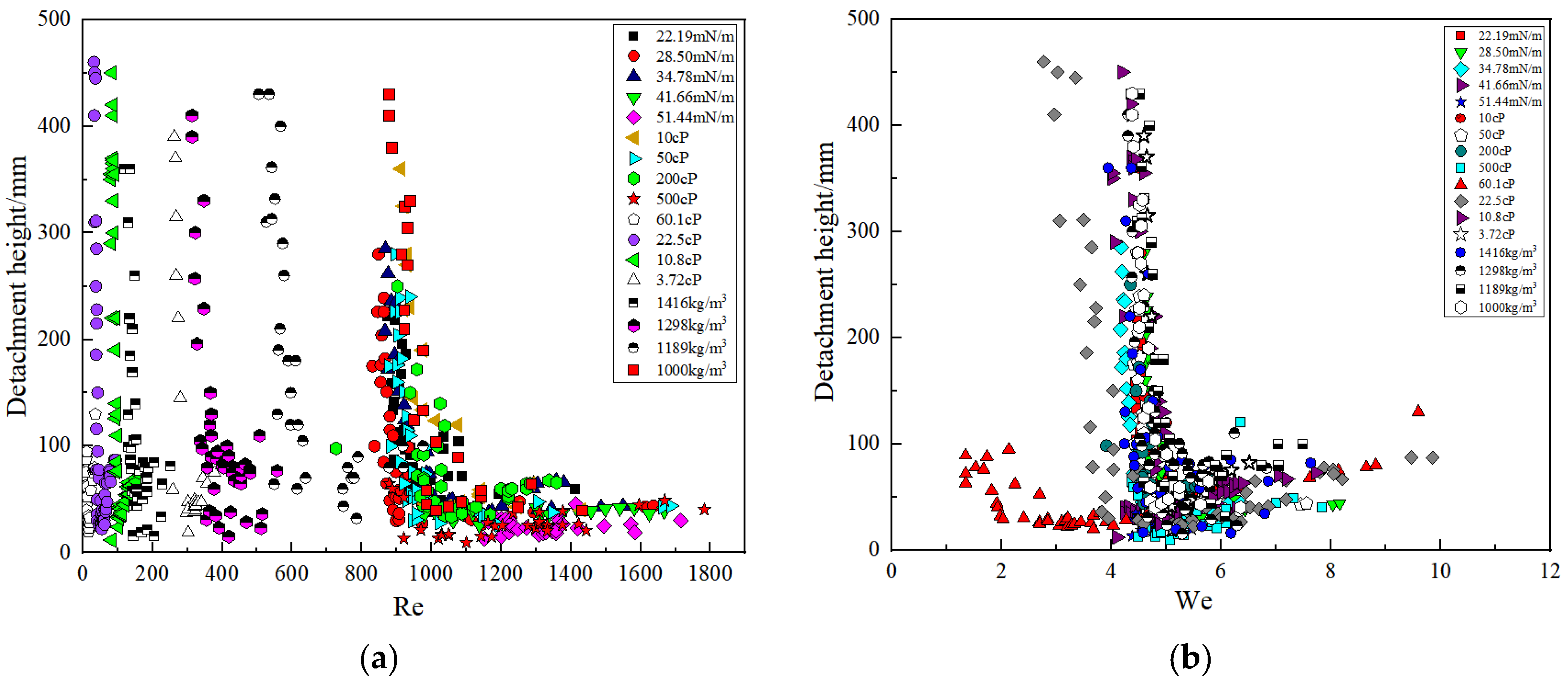
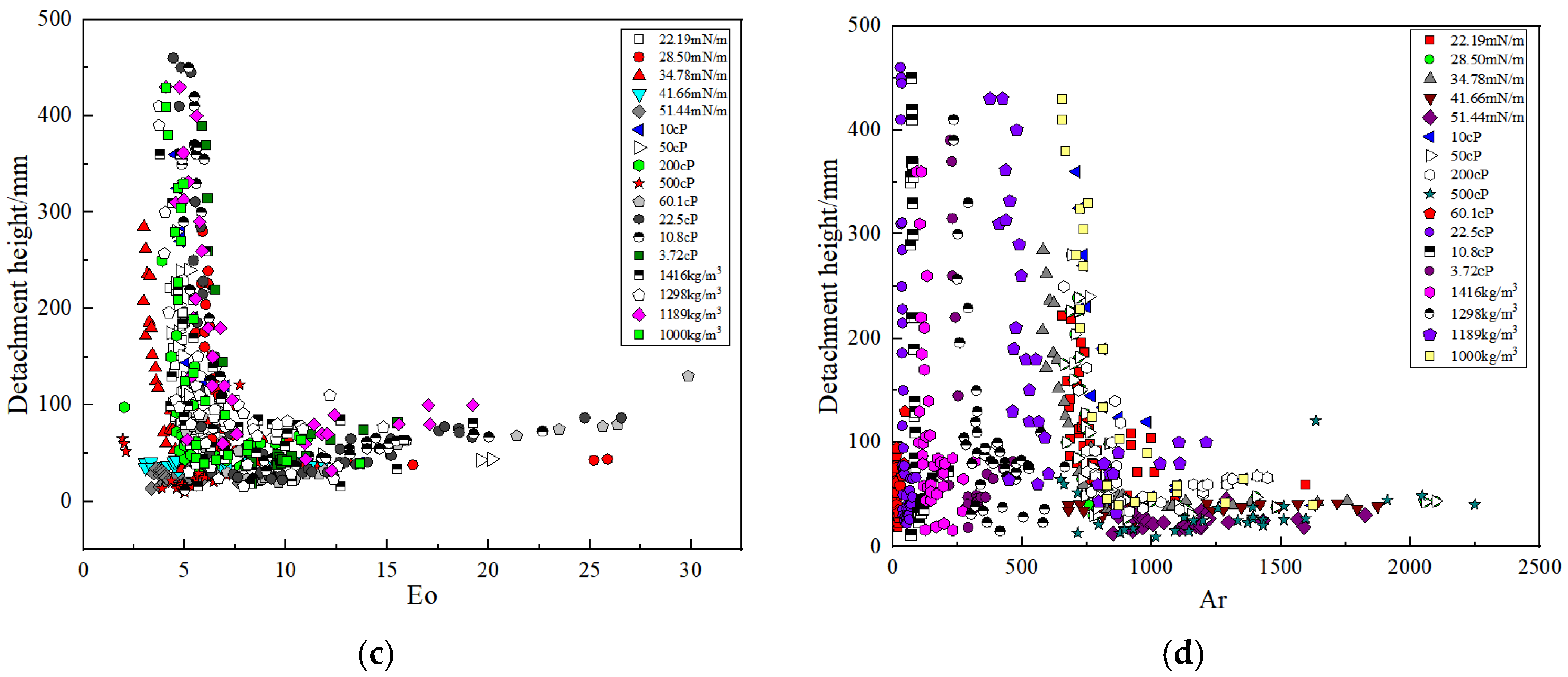
3.4. Correlation of the Detachment Height and Non-Dimensional Numbers
4. Industrial Applications
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rozario, A.; Viswanathan, N.; Basu, S. Rise of gas bubbles across the interface between two liquids. Metall. Mater. Trans. B 2019, 50, 10–15. [Google Scholar] [CrossRef]
- Xuan, C.; Persson, E.; Sevastopolev, R.; Nzotta, M. Motion and detachment behaviors of liquid inclusion at molten steel–slag interfaces. Metall. Mater. Trans. B 2019, 50, 1957–1973. [Google Scholar] [CrossRef]
- Silva, A.; Oliveira, M.; Peixoto, J.; Silva, C. Slag-steel emulsification on a modified RH degasser. Metall. Mater. Trans. B 2021, 52, 2111–2126. [Google Scholar] [CrossRef]
- Minto, R.; Davenport, W. Entrainment and flotation of matte in molten slags. Can. Min. Metall. Bull. 1972, 65, 36–42. [Google Scholar]
- Cheng, X.; Cui, Z.; Contreras, L.; Chen, M.; Nguyen, A.; Zhao, B. Matte entrainment by SO2 bubbles in copper smelting slag. JOM 2019, 71, 1897–1903. [Google Scholar] [CrossRef]
- Duangsuwan, W.; Tüzün, U.; Sermon, P. The dynamics of single air bubbles and alcohol drops in sunflower oil at various temperatures. AIChE J. 2011, 57, 897–910. [Google Scholar] [CrossRef]
- Greene, G.; Chen, J.; Conlin, M. Onset of entrainment between immiscible liquid layers due to rising gas bubbles. Int. J. Heat Mass Transf. 1988, 31, 1309–1317. [Google Scholar] [CrossRef]
- Greene, G.; Chen, J.; Conlin, M. Bubble induced entrainment between stratified liquid layers. Int. J. Heat Mass Transf. 1991, 34, 149–157. [Google Scholar] [CrossRef]
- Bonhomme, R.; Magnaudet, J.; Duval, F.; Piar, B. Inertial dynamics of air bubbles crossing a horizontal fluid–fluid interface. J. Fluid Mech. 2012, 707, 405–443. [Google Scholar] [CrossRef]
- Singh, K.; Bart, H. Passage of a Single Bubble through a Liquid–Liquid Interface. Ind. Eng. Chem. Res. 2015, 54, 9478–9493. [Google Scholar] [CrossRef]
- Hashimoto, H.; Kawano, S. A study on encapsulated liquid drop formation in liquid-liquid-gas systems. JSME Int. J. 1990, 33, 729–735. [Google Scholar]
- Zhang, L.; Taniguchi, S.; Cai, K. Fluid flow and inclusion removal in continuous casting tundish. Metall. Mater. Trans. B 2000, 31, 253–266. [Google Scholar] [CrossRef]
- Yang, H.; He, P.; Zhai, Y. Removal behavior of inclusions in molten steel by bubble wake flow based on water model experiment. ISIJ Int. 2014, 54, 578–581. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, T.; Zhang, N.; Wang, Z. Gas holdup, bubble behavior and mass transfer in a 5 m high internal-loop airlift reactor with non-Newtonian fluid. Chem. Eng. J. 2010, 160, 729–737. [Google Scholar] [CrossRef]
- Han, Z.; Holappa, L. Bubble bursting phenomenon in gas/metal/slag systems. ISIJ Int. 2003, 43, 292–297. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Z.; Sun, B.; Chen, L.; Zhang, J. Modeling of dynamic hydrate shell growth on bubble surface considering multiple factor interactions. Chem. Eng. J. 2018, 331, 221–233. [Google Scholar] [CrossRef]
- Tanno, M.; Liu, J.; Gao, X.; Kim, S.; Ueda, S.; Kitamura, S. Influence of the physical properties of liquids and diameter of bubble on the formation of liquid column at the interface of two liquid phases by the rising bubble. Metall. Mater. Trans. B 2017, 48, 2913–2921. [Google Scholar] [CrossRef]
- Reiter, G.; Schwerdtfeger, K. Characteristics rising bubbles of entrainment at liquid/liquid interfaces due to rising bubbles. ISIJ Int. 1992, 32, 57–65. [Google Scholar] [CrossRef]
- Rodrigues, R.; Ribeiro, C.; Lage, P. On the estimation of the gas-side mass-transfer coefficient during the formation and ascension of bubbles. Chem. Eng. J. 2008, 137, 282–293. [Google Scholar] [CrossRef]
- Uemura, T.; Ueda, Y.; Iguchi, M. Ripples on a rising bubble through an immiscible two-liquid interface generate numerous micro droplets. J. Vis. 2012, 15, 119–124. [Google Scholar] [CrossRef]
- Chevrier, V.; Cramb, A. In situ observations of inclusions at the (Mn, Si)-killed steel/CaO-Al2O3 interface. Metall. Mater. Trans. B 2000, 31, 37–40. [Google Scholar]
- Emery, T.; Raghupathi, P.; Kandlikar, S. Flow regimes and transition criteria during passage of bubbles through a liquid-liquid interface. Langmuir 2018, 34, 6766–6776. [Google Scholar] [CrossRef]
- Kupershtokh, A.; Ermanyuk, E.; Gavrilov, N. The rupture of thin liquid films placed on solid and liquid substrates in gravity body forces. Commun. Comput. Phys. 2015, 17, 1301–1319. [Google Scholar] [CrossRef]
- Ivanov, I.; Radoev, B.; Manev, E.; Scheludko, A. Theory of the critical thickness of rupture of thin liquid films. Trans. Faraday Soc. 1970, 66, 1262–1273. [Google Scholar] [CrossRef]
- Nguyen, A.; Schulze, H.; Stechemesser, H.; Zobel, G. Contact time during impact of a spherical particle against a plane gas-liquid interface: Theory. Int. J. Miner. Process. 1997, 50, 97–111. [Google Scholar] [CrossRef]
- Nguyen, A.; Schulze, H.; Stechemesser, H.; Zobel, G. Contact time during impact of a spherical particle against a plane gas-liquid interface: Experiment. Int. J. Miner. Process. 1997, 50, 113–125. [Google Scholar] [CrossRef]
- Mori, Y.; Komotori, K.; Higeta, K.; Inada, J. Rising behavior of air bubbles in superposed liquid layers. Can. J. Chem. Eng. 1977, 55, 9–12. [Google Scholar] [CrossRef]
- Mercier, J.; Cunha, F.; Teixeira, J.; Scofield, M. Influence of enveloping water layer on the rise of air bubbles in newtonian fluids. J. Appl. Mech. 1974, 41, 29–34. [Google Scholar] [CrossRef]
- Islam, M.; Nguyen, A. Bubble-assisted matte transportation into slag phase: A numerical investigation. Miner. Eng. 2023, 202, 108286. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, X.; Tian, Q.; Chen, M.; Zhao, B. Development and application of SKSSIM simulation software for the oxygen bottom blown copper smelting process. Metals 2017, 7, 431. [Google Scholar] [CrossRef]
- Natsui, S.; Suzuki, R. SPH simulations of the behavior of the interface between two immiscible liquid stirred by the movement of a gas bubble. Chem. Eng. Sci. 2016, 141, 342–355. [Google Scholar] [CrossRef]
- Kemiha, M.; Olmos, E.; Fei, W.; Poncin, S.; Li, H. Passage of a gas bubble through a liquid−liquid interface. Ind. Eng. Chem. Res. 2007, 46, 6099–6104. [Google Scholar] [CrossRef]
- Debrgeas, G.; De Gennes, P.G.; Brochard-Wyart, F. The life and death of “Bare” viscous bubbles. Science 1998, 279, 1704–1707. [Google Scholar] [CrossRef]
- Singh, K.; Bart, H. Passage of a bubble through the interface between a shear-thinning heavier liquid and a Newtonian lighter liquid. Chem. Eng. Commun. 2019, 207, 790–807. [Google Scholar] [CrossRef]
- Mao, N.; Kang, C.; Mulbah, C. Quantitative characterization of bubble evolution in mineral oil for different air-injection nozzles. Asia-Pac J Chem Eng. 2020, 15, e2446. [Google Scholar] [CrossRef]
- Mao, N.; Kang, C.; Teng, S.; Mulbah, C. Formation and detachment of the enclosing water film as a bubble passes through the water-oil interface. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124236. [Google Scholar] [CrossRef]
- Orvalho, S.; Hashida, M.; Zednikova, M.; Stanovsky, P.; Ruzicka, P.; Sasaki, S.; Tomiyama, A. Flow regimes in slurry bubble column: Effect of column height and particle concentration. Chem. Eng. 2020, 351, 799–815. [Google Scholar] [CrossRef]
- Cheng, X.; Zhao, B.; Zhang, F.; Qing, G.; Zhao, Z. Investigation of Bubble Penetration through Interface between Immiscible Liquids. In Proceedings of the 12th International Symposium on High-Temperature Metallurgical Processing, Anaheim, CA, USA, 4 February 2022; Springer: Cham, Switzerland, 2022; pp. 225–236. [Google Scholar]
- Amaya-Bower, L.; Lee, T. Single bubble rising dynamics for moderate Reynolds number using lattice boltzmann method. Comput. Fluids 2010, 39, 1191–1207. [Google Scholar] [CrossRef]



| Lower Phase | Upper Phase | |||||
|---|---|---|---|---|---|---|
| Material | Viscosity/cP | Density/ g·cm−3 | Surface Tension/ mN·m−1 | Material | Density/ g·cm−3 | Surface Tension/ mN·m−1 |
| Glycerol 80% | 60.1 | 1.21 | 48.06 | 10 cP silicone | 0.930 | 20.1 |
| Glycerol 70% | 22.5 | 1.19 | 48.26 | 50 cP silicone | 0.959 | 20.7 |
| Glycerol 60% | 10.8 | 1.16 | 48.11 | 200 cP silicone | 0.970 | 21.1 |
| Glycerol 40% | 3.72 | 1.10 | 48.66 | 500 cP silicone | 0.970 | 21.1 |
| CaCl2 40% | 8.48 | 1.42 | 58.03 | |||
| CaCl2 30% | 3.33 | 1.30 | 58.28 | |||
| CaCl2 20% | 1.81 | 1.19 | 57.00 | |||
| Water (SDS) | 1.00 | 1.00 | 42.89 | |||
| 1.00 | 1.00 | 49.20 | ||||
| 1.00 | 1.00 | 55.48 | ||||
| 1.00 | 1.00 | 62.36 | ||||
| 1.00 | 1.00 | 72.14 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, X.; Qing, G.; Zhao, Z.; Zhao, B. Fluid Dynamics Studies on Bottom Liquid Detachment from a Rising Bubble Crossing a Liquid–Liquid Interface. Metals 2024, 14, 1005. https://doi.org/10.3390/met14091005
Cheng X, Qing G, Zhao Z, Zhao B. Fluid Dynamics Studies on Bottom Liquid Detachment from a Rising Bubble Crossing a Liquid–Liquid Interface. Metals. 2024; 14(9):1005. https://doi.org/10.3390/met14091005
Chicago/Turabian StyleCheng, Xiangfeng, Gele Qing, Zhixing Zhao, and Baojun Zhao. 2024. "Fluid Dynamics Studies on Bottom Liquid Detachment from a Rising Bubble Crossing a Liquid–Liquid Interface" Metals 14, no. 9: 1005. https://doi.org/10.3390/met14091005





